Urinary Tract Infection and Antimicrobial Resistance Patterns: 5-Year Experience in a Tertiary Pediatric Nephrology Center in the Southwestern Region of Poland
Abstract
:1. Introduction
2. Results
2.1. Study Group Characteristic
2.2. Etiology of a UTI
2.3. Antibiotic Resistance Patterns
2.3.1. Antibiotic Resistance Patterns for E. coli
2.3.2. E. coli Antibiotic Resistance in Patients with No Abnormalities in the Urinary Tract, with CAKUT and a Neurogenic Bladder
2.3.3. Antibiotic Resistance Patterns for Klebsiella spp.
2.4. Antibiotic Resistance Risk Factors Analysis
2.5. Prescribed Antibiotic Therapy
3. Discussion
4. Materials and Methods
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- ‘t Hoen, L.A.; Bogaert, G.; Radmayr, C.; Dogan, H.S.; Nijman, R.J.M.; Quaedackers, J.; Rawashdeh, Y.F.; Silay, M.S.; Tekgul, S.; Bhatt, N.R.; et al. Update of the EAU/ESPU guidelines on urinary tract infections in children. J. Pediatr. Urol. 2021, 17, 200–207. [Google Scholar] [CrossRef] [PubMed]
- Leung, A.K.C.; Wong, A.H.C.; Leung, A.A.M.; Hon, K.L. Urinary Tract Infection in Children. Recent Pat. Inflamm. Allergy Drug Discov. 2019, 13, 2–18. [Google Scholar] [CrossRef] [PubMed]
- Simões E Silva, A.C.; Oliveira, E.A.; Mak, R.H. Urinary tract infection in pediatrics: An overview. J. Pediatr. Rio J. 2020, 96 (Suppl. S1), 65–79. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.; Jhaveri, R.; Seed, P.C.; Arshad, M. Update on Associated Risk Factors, Diagnosis, and Management of Recurrent Urinary Tract Infections in Children. J. Pediatr. Infect. Dis. Soc. 2019, 8, 152–159. [Google Scholar] [CrossRef]
- Boev, C.; Kiss, E. Hospital-Acquired Infections: Current Trends and Prevention. Crit. Care Nurs. Clin. N. Am. 2017, 29, 51–65. [Google Scholar] [CrossRef]
- Iacovelli, V.; Gaziev, G.; Topazio, L.; Bove, P.; Vespasiani, G.; Finazzi Agrò, E. Nosocomial urinary tract infections: A review. Urologia 2014, 81, 222–227. [Google Scholar] [CrossRef]
- Raoofi, S.; Pashazadeh Kan, F.; Rafiei, S.; Hosseinipalangi, Z.; Noorani Mejareh, Z.; Khani, S.; Abdollahi, B.; Seyghalani Talab, F.; Sanaei, M.; Zarabi, F.; et al. Global prevalence of nosocomial infection: A systematic review and meta-analysis. PLoS ONE 2023, 18, e0274248. [Google Scholar] [CrossRef]
- Medina-Polo, J.; Naber, K.G.; Bjerklund Johansen, T.E. Healthcare-associated urinary tract infections in urology. GMS. Infect. Dis. 2021, 30, Doc05. [Google Scholar] [CrossRef]
- Devrim, F.; Serdaroğlu, E.; Çağlar, İ.; Oruç, Y.; Demiray, N.; Bayram, N.; Ağın, H.; Çalkavur, S.; Sorguç, Y.; Dinçel, N.; et al. The Emerging Resistance in Nosocomial Urinary Tract Infections: From the Pediatrics Perspective. Mediterr. J. Hematol. Infect. Dis. 2018, 10, e2018055. [Google Scholar] [CrossRef]
- Mongkonsritragoon, W.; Anugulruengkitt, S.; Chanakul, A. Incidence of healthcare-associated urinary tract infections in Thai children. Pediatr. Int. 2023, 65, e15467. [Google Scholar] [CrossRef]
- van Duin, D.; Paterson, D.L. Multidrug-Resistant Bacteria in the Community: An Update. Infect. Dis. Clin. N. Am. 2020, 34, 709–722. [Google Scholar] [CrossRef] [PubMed]
- Esposito, S.; Biasucci, G.; Pasini, A.; Predieri, B.; Vergine, G.; Crisafi, A.; Malaventura, C.; Casadio, L.; Sella, M.; Pierantoni, L.; et al. Antibiotic Resistance in Paediatric Febrile Urinary Tract Infections. J. Glob. Antimicrob. Resist. 2022, 29, 499–506. [Google Scholar] [CrossRef] [PubMed]
- Nabal Díaz, S.G.; Algara Robles, O.; García-Lechuz Moya, J.M. New definitions of susceptibility categories EUCAST 2019: Clinic application. Rev. Esp. Quimioter. 2022, 35 (Suppl. S3), 84–88. [Google Scholar] [CrossRef] [PubMed]
- Alsaywid, B.S.; Alyami, F.A.; Alqarni, N.; Neel, K.F.; Almaddah, T.O.; Abdulhaq, N.M.; Alajmani, L.B.; Hindi, M.O.; Alshayie, M.A.; Alsufyani, H.; et al. Urinary tract infection in children: A narrative review of clinical practice guidelines. Urol. Ann. 2023, 15, 113–132. [Google Scholar] [CrossRef]
- Parry, C.M.; Taylor, A.; Williams, R.; Lally, H.; Corbett, H.J. Antimicrobial resistance of breakthrough urinary tract infections in young children receiving continual antibiotic prophylaxis. Eur. J. Pediatr. 2023. [Google Scholar] [CrossRef]
- Zhang, K.; Zhang, Y.; Chao, M.; Hao, Z. Prevalence, Pathogenic Bacterial Profile and Antimicrobial Susceptibility Pattern of Urinary Tract Infection among Children with Congenital Anomalies of the Kidney and Urinary Tract. Infect. Drug Resist. 2023, 16, 4101–4112. [Google Scholar] [CrossRef]
- Isac, R.; Basaca, D.G.; Olariu, I.C.; Stroescu, R.F.; Ardelean, A.M.; Steflea, R.M.; Gafencu, M.; Chirita-Emandi, A.; Bagiu, I.C.; Horhat, F.G.; et al. Antibiotic Resistance Patterns of Uropathogens Causing Urinary Tract Infections in Children with Congenital Anomalies of Kidney and Urinary Tract. Children 2021, 8, 585. [Google Scholar] [CrossRef]
- Daniel, M.; Szymanik-Grzelak, H.; Sierdziński, J.; Podsiadły, E.; Kowalewska-Młot, M.; Pańczyk-Tomaszewska, M. Epidemiology and Risk Factors of UTIs in Children-A Single-Center Observation. J. Pers. Med. 2023, 13, 138. [Google Scholar] [CrossRef]
- Wasilewska, A. (Ed.) Recommendations of Polish Society for Pediatric Nephrology in the Management of Children with the Urinary Tract Infection. Poland 2021. Available online: https://ptnfd.org/site/resource/1323,zalecenia-zum_2021.pdf (accessed on 10 July 2023).
- Vazouras, K.; Velali, K.; Tassiou, I.; Anastasiou-Katsiardani, A.; Athanasopoulou, K.; Barbouni, A.; Jackson, C.; Folgori, L.; Zaoutis, T.; Basmaci, R.; et al. Antibiotic treatment and antimicrobial resistance in children with urinary tract infections. J. Glob. Antimicrob. Resist. 2020, 20, 4–10. [Google Scholar] [CrossRef]
- Montagnani, C.; Tersigni, C.; D’Arienzo, S.; Miftode, A.; Venturini, E.; Bortone, B.; Bianchi, L.; Chiappini, E.; Forni, S.; Gemmi, F.; et al. Resistance Patterns from Urine Cultures in Children Aged 0 to 6 Years: Implications for Empirical Antibiotic Choice. Infect. Drug Resist. 2021, 14, 2341–2348. [Google Scholar] [CrossRef]
- Sorlózano-Puerto, A.; Gómez-Luque, J.M.; Luna-Del-Castillo, J.D.; Navarro-Marí, J.M.; Gutiérrez-Fernández, J. Etiological and Resistance Profile of Bacteria Involved in Urinary Tract Infections in Young Children. BioMed Res. Int. 2017, 2017, 4909452. [Google Scholar] [CrossRef] [PubMed]
- Hrbacek, J.; Cermak, P.; Zachoval, R. Current Antibiotic Resistance Trends of Uropathogens in Central Europe: Survey from a Tertiary Hospital Urology Department 2011–2019. Antibiotics 2020, 9, 630. [Google Scholar] [CrossRef] [PubMed]
- Dejonckheere, Y.; Desmet, S.; Knops, N. A study of the 20-year evolution of antimicrobial resistance patterns of pediatric urinary tract infections in a single center. Eur. J. Pediatr. 2022, 181, 3271–3281. [Google Scholar] [CrossRef] [PubMed]
- Werbel, K.; Jankowska, D.; Wasilewska, A.; Taranta-Janusz, K. Clinical and Epidemiological Analysis of Children’s Urinary Tract Infections in Accordance with Antibiotic Resistance Patterns of Pathogens. J. Clin. Med. 2021, 10, 5260. [Google Scholar] [CrossRef] [PubMed]
- Michno, M.; Sydor, A.; Wałaszek, M.; Sułowicz, W. Microbiology and Drug Resistance of Pathogens in Patients Hospitalized at the Nephrology Department in the South of Poland. Pol. J. Microbiol. 2018, 67, 517–524. [Google Scholar] [CrossRef]
- Raupach, T.; Held, J.; Prokosch, H.U.; Rascher, W.; Zierk, J. Resistance to antibacterial therapy in pediatric febrile urinary tract infections-a single-center analysis. J. Pediatr. Urol. 2020, 16, 71–79. [Google Scholar] [CrossRef]
- Budnik, T.V.; Bevzenko, T.B. A ten-year analysis of changes in the sensitivity of the leading uropathogen to antibacterial agents in children with urinary tract infection in the nephrology department. Wiad. Lek. 2020, 73, 1360–1364. [Google Scholar] [CrossRef]
- Wanke-Rytt, M.; Sobierajski, T.; Lachowicz, D.; Seliga-Gąsior, D.; Podsiadły, E. Analysis of Etiology of Community-Acquired and Nosocomial Urinary Tract Infections and Antibiotic Resistance of Isolated Strains: Results of a 3-Year Surveillance (2020–2022) at the Pediatric Teaching Hospital in Warsaw. Microorganisms 2023, 11, 1438. [Google Scholar] [CrossRef]
- Esposito, S.; Maglietta, G.; Di Costanzo, M.; Ceccoli, M.; Vergine, G.; La Scola, C.; Malaventura, C.; Falcioni, A.; Iacono, A.; Crisafi, A.; et al. Retrospective 8-Year Study on the Antibiotic Resistance of Uropathogens in Children Hospitalised for Urinary Tract Infection in the Emilia-Romagna Region, Italy. Antibiotics 2021, 10, 1207. [Google Scholar] [CrossRef]
- Choi, U.; Kim, E.; Lyu, D.H.; Kim, K.S.; Park, B.H.; Chung, H.; Han, C.H.; Bae, S. The change of antibiotic susceptibility in febrile urinary tract infection in childhood and adolescence during the last decade. Investig. Clin. Urol. 2022, 63, 99–106. [Google Scholar] [CrossRef]
- Erol, B.; Culpan, M.; Caskurlu, H.; Sari, U.; Cag, Y.; Vahaboglu, H.; Özumut, S.H.; Karaman, M.I.; Caskurlu, T. Changes in antimicrobial resistance and demographics of UTIs in pediatric patients in a single institution over a 6-year period. J. Pediatr. Urol. 2018, 14, e1–e176. [Google Scholar] [CrossRef] [PubMed]
- Autore, G.; Neglia, C.; Di Costanzo, M.; Ceccoli, M.; Vergine, G.; La Scola, C.; Malaventura, C.; Falcioni, A.; Iacono, A.; Crisafi, A.; et al. Clinical Outcome of Discordant Empirical Therapy and Risk Factors Associated to Treatment Failure in Children Hospitalized for Urinary Tract Infections. Children 2022, 9, 128. [Google Scholar] [CrossRef] [PubMed]
- Jerardi, K.E.; Auger, K.A.; Shah, S.S.; Hall, M.; Hain, P.D.; Myers, A.L.; Williams, D.J.; Tieder, J.S. Discordant antibiotic therapy and length of stay in children hospitalized for urinary tract infection. J. Hosp. Med. 2012, 7, 622–627. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.E.; Lee, V.; Greenhow, T.L.; Beck, J.; Bendel-Stenzel, M.; Hames, N.; McDaniel, C.E.; King, E.E.; Sherry, W.; Parmar, D.; et al. Clinical Response to Discordant Therapy in Third-Generation Cephalosporin-Resistant UTIs. Pediatrics 2020, 145, e20191608. [Google Scholar] [CrossRef] [PubMed]
- Sihra, N.; Goodman, A.; Zakri, R.; Sahai, A.; Malde, S. Nonantibiotic prevention and management of recurrent urinary tract infection. Nat. Rev. Urol. 2018, 15, 750–776. [Google Scholar] [CrossRef] [PubMed]
- Meena, J.; Thomas, C.C.; Kumar, J.; Raut, S.; Hari, P. Non-antibiotic interventions for prevention of urinary tract infections in children: A systematic review and meta-analysis of randomized controlled trials. Eur. J. Pediatr. 2021, 180, 3535–3545. [Google Scholar] [CrossRef] [PubMed]
- Sadeghi-Bojd, S.; Naghshizadian, R.; Mazaheri, M.; Ghane Sharbaf, F.; Assadi, F. Efficacy of Probiotic Prophylaxis after the First Febrile Urinary Tract Infection in Children with Normal Urinary Tracts. J. Pediatr. Infect. Dis. Soc. 2020, 9, 305–310. [Google Scholar] [CrossRef]
- Hosseini, M.; Yousefifard, M.; Ataei, N.; Oraii, A.; Mirzay Razaz, J.; Izadi, A. The efficacy of probiotics in prevention of urinary tract infection in children: A systematic review and meta-analysis. J. Pediatr. Urol. 2017, 13, 581–591. [Google Scholar] [CrossRef]
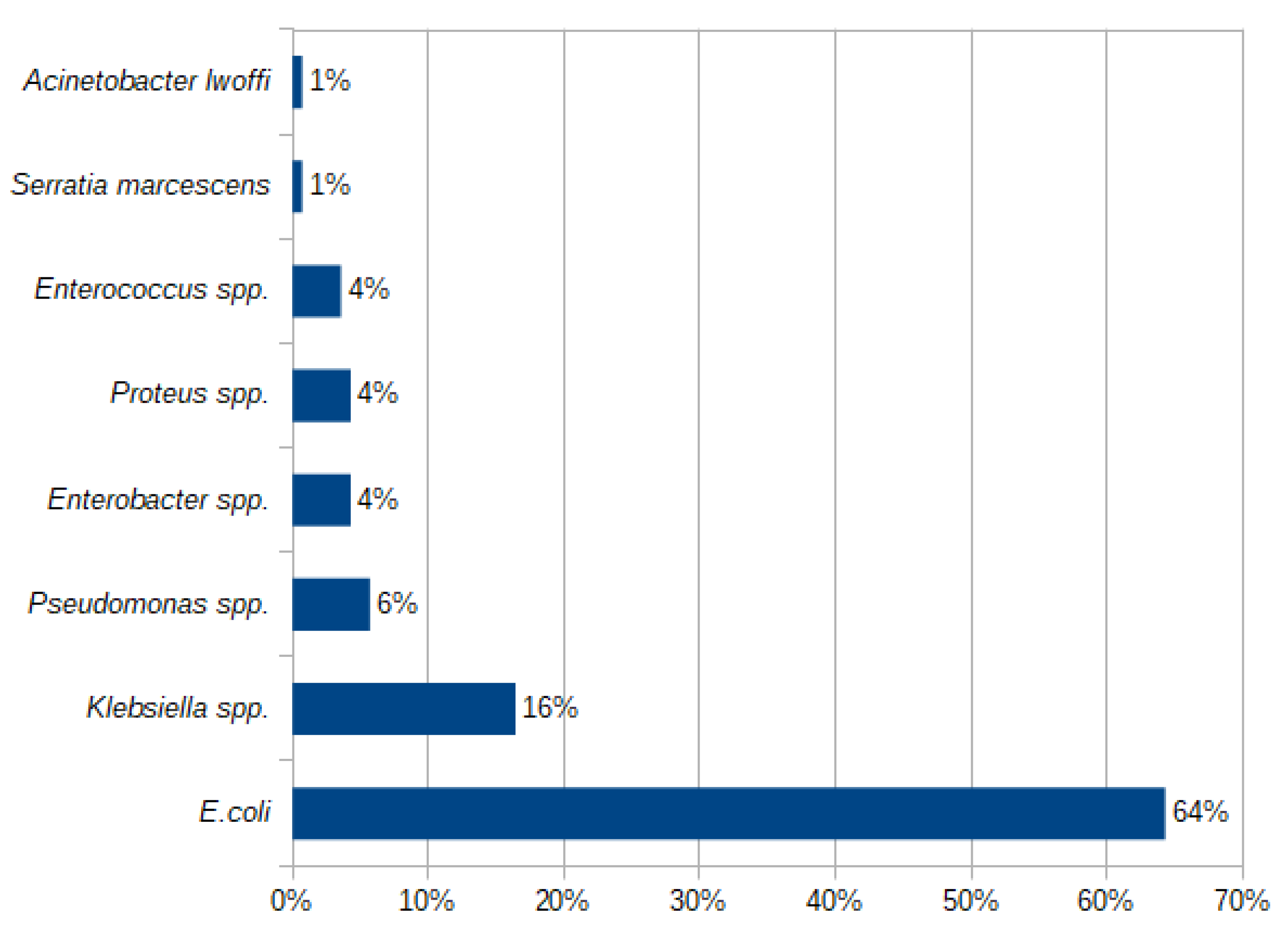




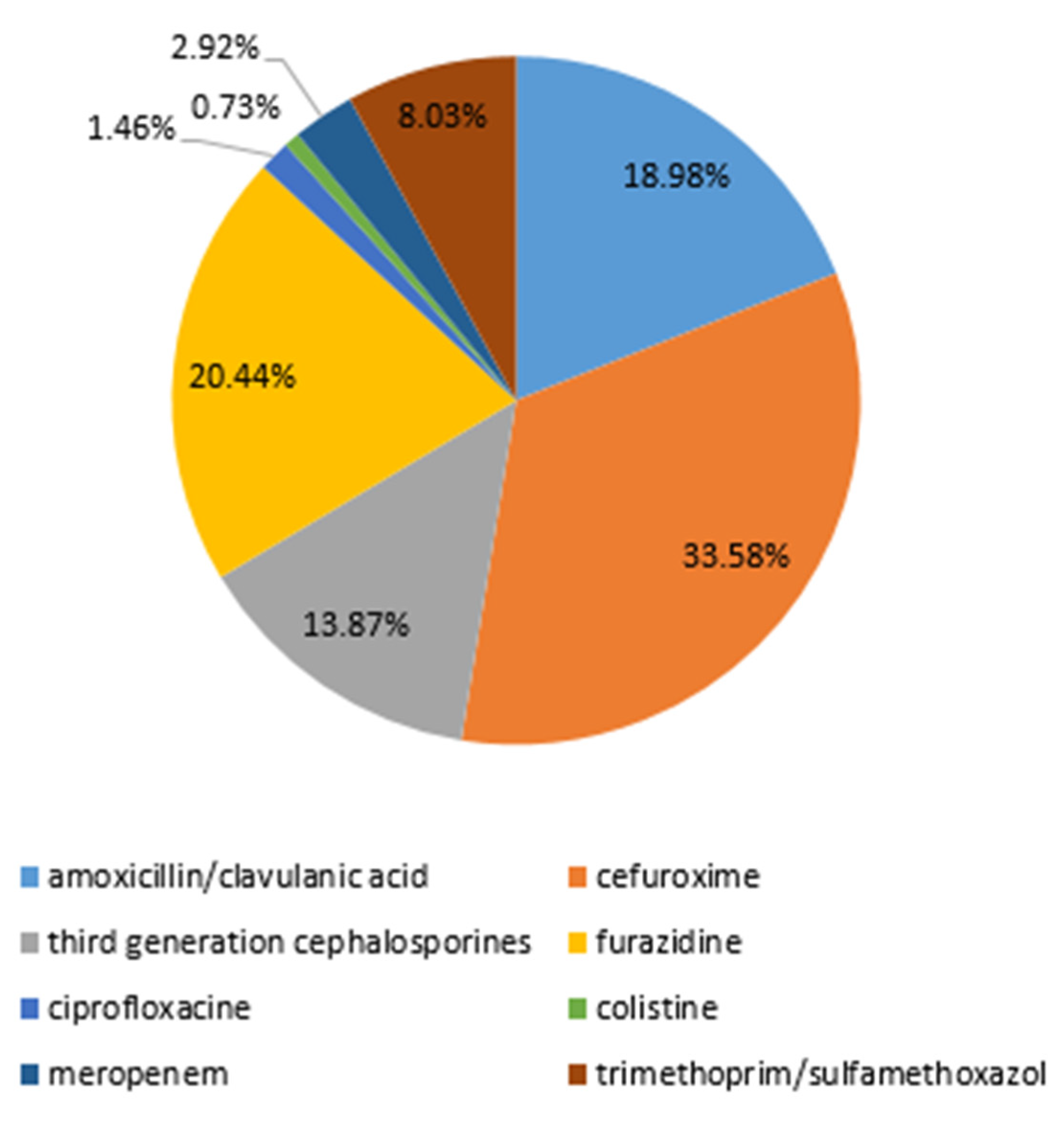
| Patients’ Subgroup | Number of Patients in the Subgroup | E. coli | non-E. coli | p Value |
|---|---|---|---|---|
| First episode of UTI | 49 | 36 (73.5%) | 13 (26.5%) | 0.096 |
| >1 UTI in the past | 91 | 54 (59.3%) | 37 (40.7%) | |
| CAKUT | 62 | 28 (45.2%) | 34 (54.8%) | <0.001 |
| No abnormalities in urinary tract | 61 | 54 (88.5%) | 7 (11.5%) | |
| Neurogenic bladder | 17 | 8 (47.1%) | 9 (52.9%) | <0.001 |
| No abnormalities in urinary tract | 61 | 54 (88.5%) | 7 (11.5%) | |
| Antibiotic prophylaxis | 22 | 6 (27.3%) | 16 (72.7%) | <0.001 |
| No antibiotic prophylaxis | 118 | 84 (71.2%) | 34 (28.8%) |
| Variable | B | Std. Err. of B | β | p Value |
|---|---|---|---|---|
| Gender | 0.103 | 0.089 | 0.103 | 0.253 |
| Age | 0.077 | 0.092 | 0.007 | 0.403 |
| First episode of UTI | 0.020 | 0.136 | 0.019 | 0.890 |
| CAKUT | 0.052 | 0.098 | 0.051 | 0.592 |
| Neurogenic bladder | 0.249 | 0.100 | 0.370 | 0.014 |
| Previous antibiotic therapy for a UTI | 0.031 | 0.132 | 0.031 | 0.811 |
| Previous antibiotic therapy for other infection | 0.041 | 0.084 | 0.091 | 0.629 |
| Antibiotic prophylaxis for a UTI | −0.193 | 0.091 | −0.258 | 0.036 |
| Urological surgery in the past | 0.217 | 0.092 | 0.256 | 0.021 |
| Year of Hospitalization | Number of Records Identified During Initial Data Search | Number of Records Included in the Final Analysis |
|---|---|---|
| 2018 | 65 | 40 |
| 2019 | 39 | 21 |
| 2020 | 45 | 26 |
| 2021 | 45 | 25 |
| 2022 | 48 | 28 |
| Total | 242 | 140 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kawalec, A.; Józefiak, J.; Kiliś-Pstrusińska, K. Urinary Tract Infection and Antimicrobial Resistance Patterns: 5-Year Experience in a Tertiary Pediatric Nephrology Center in the Southwestern Region of Poland. Antibiotics 2023, 12, 1454. https://doi.org/10.3390/antibiotics12091454
Kawalec A, Józefiak J, Kiliś-Pstrusińska K. Urinary Tract Infection and Antimicrobial Resistance Patterns: 5-Year Experience in a Tertiary Pediatric Nephrology Center in the Southwestern Region of Poland. Antibiotics. 2023; 12(9):1454. https://doi.org/10.3390/antibiotics12091454
Chicago/Turabian StyleKawalec, Anna, Justyna Józefiak, and Katarzyna Kiliś-Pstrusińska. 2023. "Urinary Tract Infection and Antimicrobial Resistance Patterns: 5-Year Experience in a Tertiary Pediatric Nephrology Center in the Southwestern Region of Poland" Antibiotics 12, no. 9: 1454. https://doi.org/10.3390/antibiotics12091454
APA StyleKawalec, A., Józefiak, J., & Kiliś-Pstrusińska, K. (2023). Urinary Tract Infection and Antimicrobial Resistance Patterns: 5-Year Experience in a Tertiary Pediatric Nephrology Center in the Southwestern Region of Poland. Antibiotics, 12(9), 1454. https://doi.org/10.3390/antibiotics12091454





