A Prospective, Randomized, Comparative Study of Topical Minocycline Gel 4% with Topical Clindamycin Phosphate Gel 1% in Indian Patients with Acne Vulgaris
Abstract
1. Introduction
2. Results
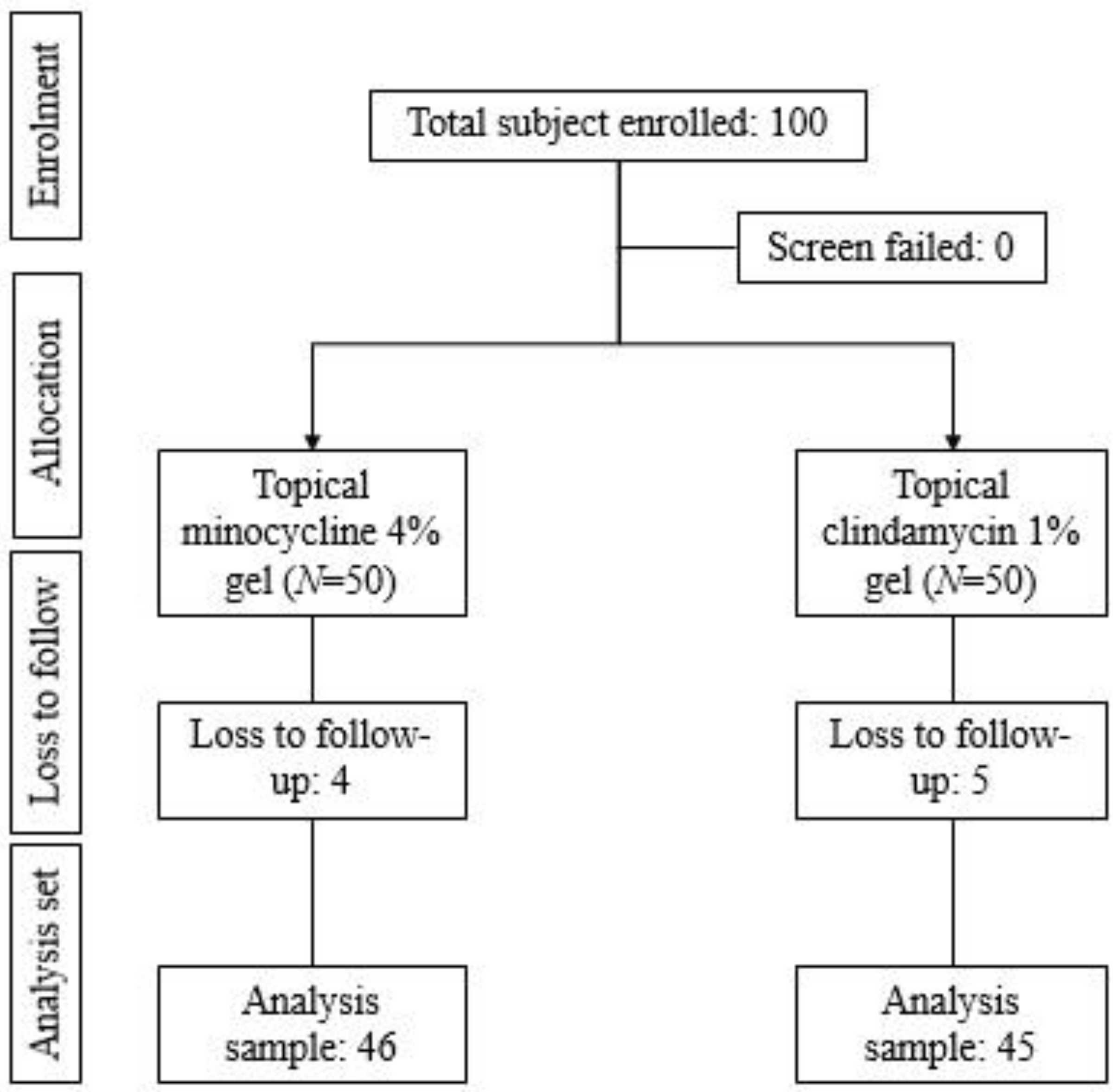
2.1. Patient Disposition
2.2. Demographic and Disease Characteristics at Baseline
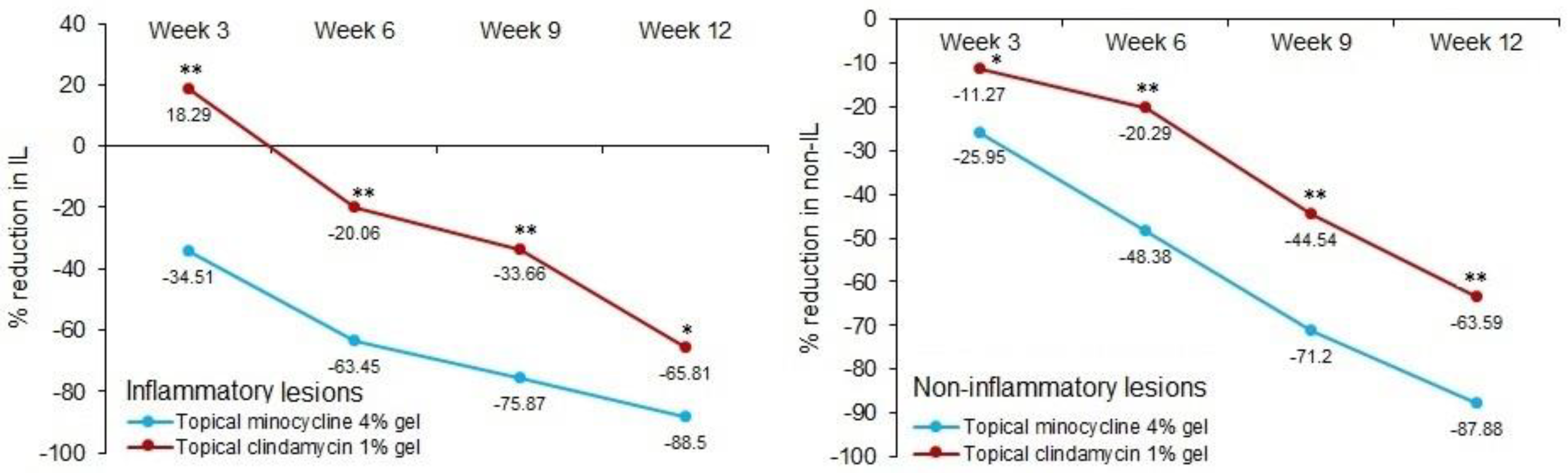
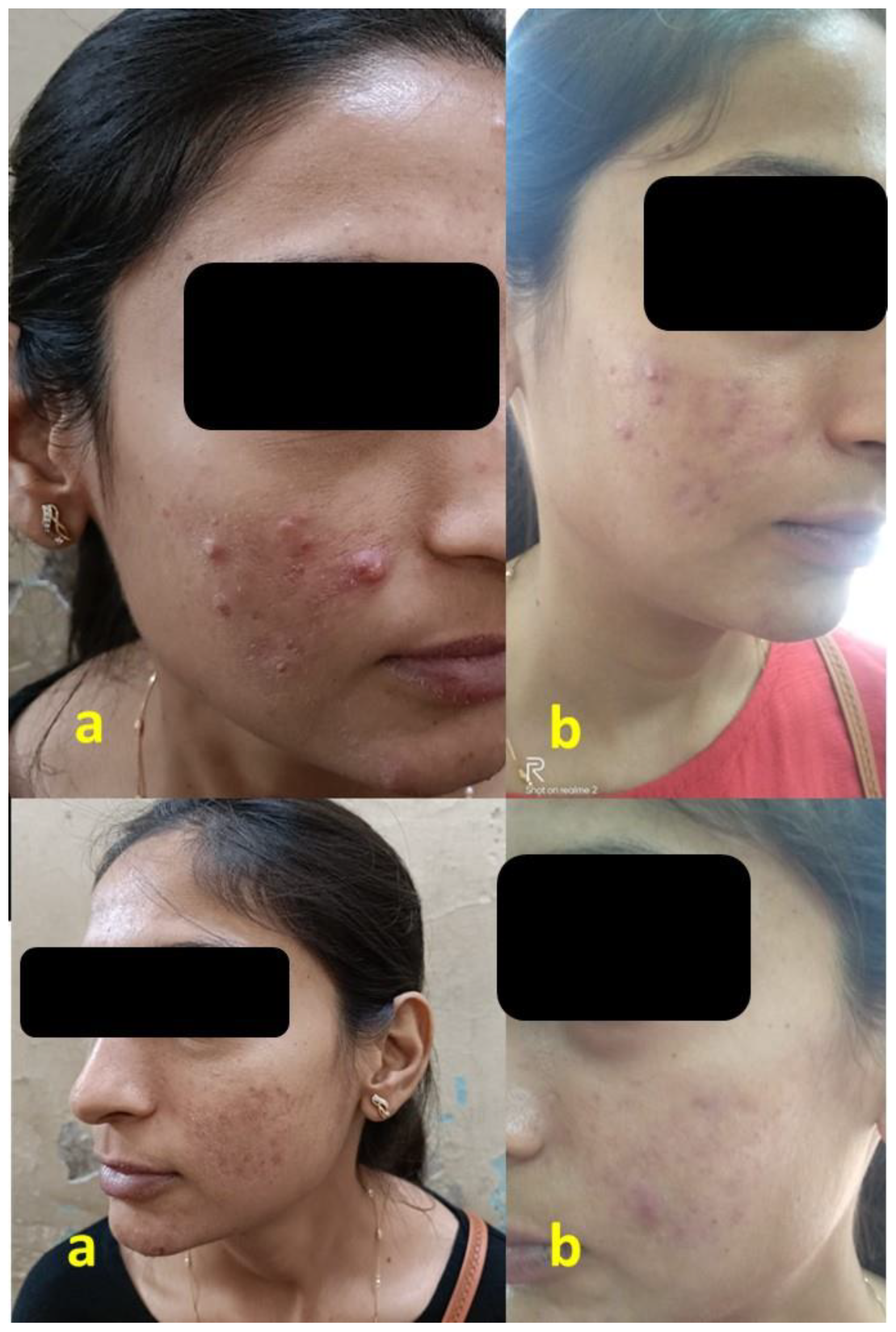
2.3. Efficacy Analysis
2.4. Efficacy among Young Adults and Adolescents
2.5. Safety and Tolerability Assessment
3. Discussion
4. Materials and Methods
4.1. Study Design
4.2. Participant’s Eligibility Criteria
4.3. Drug Administration
4.4. Efficacy and Safety Endpoints
4.5. Statistical Methods
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Heng, A.H.S.; Chew, F.T. Systematic review of the epidemiology of acne vulgaris. Sci. Rep. 2020, 10, 5754. [Google Scholar] [CrossRef]
- Chen, H.; Zhang, T.C.; Yin, X.L.; Man, J.Y.; Yang, X.R.; Lu, M. Magnitude and temporal trend of acne vulgaris burden in 204 countries and territories from 1990 to 2019: An analysis from the Global Burden of Disease Study 2019. Br. J. Dermatol. 2022, 186, 673–683. [Google Scholar] [CrossRef]
- Budamakuntla, L.; Parasramani, S.; Dhoot, D.; Deshmukh, G.; Barkate, H. Acne in Indian population: An epidemiological study evaluating multiple factors. IP Indian J. Clin. Exp. Dermatol. 2020, 6, 237–242. [Google Scholar]
- Makrantonaki, E.; Ganceviciene, R.; Zouboulis, C. An update on the role of the sebaceous gland in the pathogenesis of acne. Derm.-Endocrinol. 2011, 3, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Zouboulis, C.C.; Schagen, S.; Alestas, T. The sebocyte culture: A model to study the pathophysiology of the sebaceous gland in sebostasis, seborrhoea and acne. Arch Dermatol. Res. 2008, 300, 397–413. [Google Scholar] [CrossRef] [PubMed]
- Sheehan-Dare, R.A.; Papworth-Smith, J.; Cunliffe, W.J. A double-blind comparison of topical clindamycin and oral minocycline in the treatment of acne vulgaris. Acta Derm. Venereol. 1990, 70, 534–537. [Google Scholar] [CrossRef]
- Biswal, I.; Gaind, R.; Kumar, N.; Mohanty, S.; Manchanda, V.; Khunger, N.; Ramesh, V.; Deb, M. In vitro antimicrobial susceptibility patterns of Propionibacterium acnes isolated from patients with acne vulgaris. J. Infect. Dev. Ctries 2016, 10, 1140–1145. [Google Scholar] [CrossRef][Green Version]
- Jones, T.M.; Ellman, H.; deVries, T. Pharmacokinetic comparison of once-daily topical minocycline foam 4% vs oral minocycline for moderate-to-severe acne. J. Drugs Dermatol. 2017, 16, 1022. [Google Scholar]
- Gold, L.S.; Dhawan, S.; Weiss, J.; Draelos, Z.D.; Ellman, H.; Stuart, I.A. A novel topical minocycline foam for the treatment of moderate-to-severe acne vulgaris: Results of 2 randomized, double-blind, phase 3 studies. J. Am. Acad. Dermatol. 2019, 80, 168–177. [Google Scholar] [CrossRef] [PubMed]
- Paik, J. Topical Minocycline Foam 4%: A Review in Acne Vulgaris. Am. J. Clin. Dermatol. 2020, 21, 449–456. [Google Scholar] [CrossRef]
- Gold, L.S.; Dhawan, S.; Weiss, J.; Draelos, Z.D.; Ellman, H.; Stuart, I. Open-label extension study evaluating long-term safety and efficacy of FMX101 4% minocycline foam for moderate-to-severe acne vulgaris. J. Clin. Aesthet. Dermatol. 2019, 12, 16–23. [Google Scholar]
- Available online: https://cdsco.gov.in/opencms/resources/UploadCDSCOWeb/2018/UploadCommitteeFiles/54th%20%20Dermatology%20Recommendation%2012.01.2021.pdf (accessed on 30 June 2023).
- Szymańska, A.; Budzisz, E.; Erkiert-Polguj, A. The Anti-Acne Effect of Near-Infrared Low-Level Laser Therapy. Clin. Cosmet. Investig. Dermatol. 2021, 14, 1045–1051. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.I.; Hino, P.D.; Parker, L.; Stephens, T.J.; Mccook, J.; Gotz, V. Efficacy and tolerability of an acne treatment regimen with antiaging benefits in adult women. J. Clin. Aesthet. Dermatol. 2018, 11, 46–51. [Google Scholar]
- World Health Organization. Adolescent Health. 2023. Available online: https://www.who.int/health-topics/adolescent-health#tab=tab_1 (accessed on 30 June 2023).
- Mendoza, N.; Hernandez, P.O.; Tyring, S.K.; Haitz, K.A.; Motta, A. Antimicrobial susceptibility of Propionibacterium acnes isolates from acne patients in Colombia. Int. J. Dermatol. 2013, 52, 688–692. [Google Scholar] [CrossRef]
- Garner, S.E.; Eady, A.; Bennett, C.; Newton, J.N.; Thomas, K.; Popescu, C.M. Minocycline for acne vulgaris: Efficacy and safety. Cochrane Database Syst. Rev. 2012, 2012, CD002086. [Google Scholar] [CrossRef]
- Raoof, T.J.; Hooper, D.; Moore, A.; Zaiac, M.; Sullivan, T.; Kircik, L.; Lain, E.; Jankicevic, J.; Stuart, I. Efficacy and safety of a novel topical minocycline foam for the treatment of moderate to severe acne vulgaris: A phase 3 study. J. Am. Acad. Dermatol. 2020, 82, 832–837. [Google Scholar] [CrossRef]
- Kircik, L.; Del Rosso, J.Q.; Weiss, J.S.; Stakias, V.; London, A.; Keynan, R.; Hazot, Y.; Elliott, R.; Stuart, I. Formulation and Profile of FMX101 4% Minocycline Topical Foam for the Treatment of Acne Vulgaris. J. Clin. Aesthet. Dermatol. 2020, 13, 14–21. [Google Scholar]
- Onge, E.S.; Mobley, W.C. Minocycline Topical Foam: A New Drug for the Treatment of Acne. Ann. Pharmacother. 2021, 55, 105–110. [Google Scholar] [CrossRef]
- Del Rosso, J.Q.; Schmidt, N.F. A review of the anti-inflammatory properties of clindamycin in the treatment of acne vulgaris. Cutis 2010, 85, 15–24. [Google Scholar]
- Bhavsar, B.; Choksi, B.; Sanmukhani, J.; Dogra, A.; Haq, R.; Mehta, S.; Mukherjee, S.; Subramanian, V.; Sheikh, S.; Mittal, R. Clindamycin 1% Nano-emulsion Gel Formulation for the Treatment of Acne Vulgaris: Results of a Randomized, Active Controlled, Multicentre, Phase IV Clinical Trial. J. Clin. Diagn. Res. 2014, 8, YC05–YC09. [Google Scholar]
- Zaenglein, A.L.; Pathy, A.L.; Schlosser, B.J.; Alikhan, A.; Baldwin, H.E.; Berson, D.S.; Bowe, W.P.; Graber, E.M.; Harper, J.C.; Kang, S.; et al. Guidelines of care for the management of acne vulgaris. J. Am. Acad. Dermatol. 2016, 74, 945–973.e33. [Google Scholar] [CrossRef] [PubMed]
- Ross, J.I.; Snelling, A.M.; Carnegie, E.; Coates, P.; Cunliffe, W.J.; Bettoli, V.; Tosti, G.; Katsambas, A.; Galvan Peréz Del Pulgar, J.I.; Rollman, O.; et al. Antibiotic resistant acne: Lessons from Europe. Br. J. Dermatol. 2003, 148, 467–478. [Google Scholar] [CrossRef] [PubMed]
- Shemer, A.; Shiri, J.; Mashiah, J.; Farhi, R.; Gupta, A.K. Topical minocycline foam for moderate to severe acne vulgaris: Phase 2 randomized double-blind, vehicle-controlled study results. J. Am. Acad. Dermatol. 2016, 74, 1251–1252. [Google Scholar] [CrossRef] [PubMed]
- Austin, B.A.; Fleischer, A.B., Jr. The extinction of topical erythromycin therapy for acne vulgaris and concern for the future of topical clindamycin. J. Dermatol. Treat. 2017, 28, 145–148. [Google Scholar] [CrossRef]
| Characteristics | Treatment | p-Value | ||
|---|---|---|---|---|
| Topical Minocycline | Topical Clindamycin | |||
| 4% Gel (N = 50) | 1% Gel (N = 50) | |||
| Age (years) 1 | 19.76 ± 4.38; 18.0; (14, 31) | 20.58 ± 4.59; 20.0; {14, 32) | 0.363 † | |
| Gender 2 | Male | 18 (36.0) | 16 (32) | 0.673 * |
| Female | 32 (64.0) | 34 (68) | ||
| Grade of acne 2 | 1 | 10 (20.0) | 18 (36.0) | 0.069 * |
| 2 | 31 (62.0) | 29 (58.0) | ||
| 3 | 9 (18.0) | 3 (6.0) | ||
| Duration of acne (months) 1 | 12.24 ± 13.63; 7.00; (1, 60) | 8.08 ± 9.91; 4.00; (1, 48) | 0.084 † | |
| Number of inflammatory lesions 1 | 6.22 ± 5.03; 5.00; (0, 20) | 4.32 ± 3.89; 3.00; (0, 15) | 0.066 ‡ | |
| Number of non-inflammatory lesions 1 | 14.06 ± 8.22; 12.00; (2, 42) | 12.74 ± 6.21; 12.00; (0, 35) | 0.618 ‡ | |
| Investigator’s global score 1 | 2.64 ± 0.9; 3.00; (1, 5) | 2.44 ± 1.15; 2.00; (1, 9) | 0.059 ‡ | |
| Parameter | Visit | Treatment | p-Value 2 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Topical Minocycline | Topical Clindamycin | |||||||||
| 4% Gel (N = 50) | 1% Gel (N = 50) | |||||||||
| n | Mean | SD | Median | n | Mean | SD | Median | |||
| Number of inflammatory lesions | Baseline | 50 | 6.22 | 5.03 | 5 | 50 | 4.32 | 3.89 | 3 | 0.066 |
| Week 3 | 50 | 4.54 | 4.36 | 4 | 50 | 4.48 | 3.59 | 4 | 0.7 | |
| Week 6 | 50 | 2.8 | 3.02 | 2 | 50 | 3.36 | 2.96 | 3 | 0.221 | |
| Week 9 | 49 | 1.8 | 2.26 | 1 | 49 | 2.71 | 2.61 | 2 | 0.047 | |
| Week 12 | 46 | 0.98 | 1.51 | 0 | 45 | 1.69 | 1.84 | 1 | 0.038 | |
| p-value 1 | <0.0001 | <0.0001 | ||||||||
| Number of non-inflammatory lesions | Baseline | 50 | 14.06 | 8.22 | 12 | 50 | 12.74 | 6.21 | 12 | 0.618 |
| Week 3 | 50 | 10.42 | 6.27 | 10 | 50 | 10.8 | 4.9 | 10 | 0.274 | |
| Week 6 | 50 | 7.52 | 5.1 | 7 | 50 | 9.26 | 4.21 | 8 | 0.01 | |
| Week 9 | 49 | 4.39 | 3.7 | 4 | 49 | 6.69 | 3.7 | 6 | 0.001 | |
| Week 12 | 46 | 2.07 | 2.45 | 1.5 | 45 | 4.49 | 2.84 | 4 | <0.0001 | |
| p-value 1 | <0.0001 | <0.0001 | ||||||||
| Parameter | Visit | Treatment | p-Value 2 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Topical Minocycline | Topical Clindamycin | |||||||||
| 4% Gel (N = 50) | 1% Gel (N = 50) | |||||||||
| n | Mean | SD | Median | n | Mean | SD | Median | |||
| Investigator’s global assessment score | Baseline | 50 | 2.64 | 0.9 | 3 | 50 | 2.44 | 1.15 | 2 | 0.059 |
| Week 3 | 50 | 2.20 | 0.86 | 2 | 50 | 2.26 | 0.56 | 2 | 0.808 | |
| Week 6 | 50 | 1.76 | 0.85 | 2 | 50 | 2.02 | 0.59 | 2 | 0.080 | |
| Week 9 | 49 | 1.22 | 0.65 | 1 | 49 | 1.71 | 0.65 | 2 | 0.001 | |
| Week 12 | 46 | 0.74 | 0.71 | 1 | 45 | 1.02 | 0.87 | 1 | 0.113 | |
| p-value 1 | <0.0001 | <0.0001 | ||||||||
| Investigator’s Global Assessment—Treatment success at (YES) | n (%) | n (%) | p-value 3 | |||||||
| Week 3 | 0 (0) | 0 (0) | - | |||||||
| Week 6 | 4 (8.0) | 0 (0) | 0.126 | |||||||
| Week 9 | 15 (30.6) | 2 (4.1) | 0.001 | |||||||
| Week 12 | 34 (73.9) | 21 (46.7) | 0.015 | |||||||
| Change from Baseline to 12 Months | Treatment | p-Value *,1 (Young Adult) | p-Value *,2 (Adolescent) | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Topical Minocycline 4% Gel | Topical Clindamycin 1% Gel | |||||||||||||||
| Age > 19 (Young Adult) | Age ≤ 19 (Adolescent) | p-Value * | Age > 19 (Young Adult) | Age ≤ 19 (Adolescent) | p-Value * | |||||||||||
| n | Mean | SD | n | Mean | SD | n | Mean | SD | n | Mean | SD | |||||
| % reduction in inflammatory lesions | 20 | 88.24 | 13.89 | 26 | 88.71 | 14.47 | 0.988 | 24 | 66.90 | 36.41 | 21 | 64.60 | 30.54 | 0.645 | 0.086 | 0.004 |
| % reduction in non-inflammatory lesions | 20 | 87.30 | 14.59 | 26 | 88.32 | 10.35 | 0.899 | 24 | 65.00 | 21.16 | 21 | 61.91 | 21.10 | 0.697 | 0.001 | <0.0001 |
| IGA score | 20 | 1.90 | 0.64 | 26 | 1.92 | 0.84 | 0.797 | 24 | 1.17 | 0.92 | 21 | 1.71 | 1.59 | 0.383 | 0.001 | 0.037 |
| Tolerability Assessment Parameter | Visit | Topical Minocycline 4% Gel (N = 50) | Topical Clindamycin 1% Gel (N = 50) | p-Value | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| None | Mild | Moderate | Intense | None | Mild | Moderate | Intense | |||
| Erythema | Week 3 | 40 | 9 | 1 | 0 | 32 | 17 | 1 | 0 | 0.187 |
| Week 6 | 47 | 3 | 0 | 0 | 43 | 7 | 0 | 0 | 0.317 | |
| Week 9 | 47 | 2 | 0 | 0 | 40 | 9 | 0 | 0 | 0.055 | |
| Week 12 | 46 | 0 | 0 | 0 | 40 | 5 | 0 | 0 | 0.062 | |
| Dryness | Week 3 | 44 | 6 | 0 | 0 | 30 | 19 | 1 | 0 | 0.005 |
| Week 6 | 47 | 3 | 0 | 0 | 31 | 19 | 0 | 0 | <0.0001 | |
| Week 9 | 45 | 4 | 0 | 0 | 35 | 14 | 0 | 0 | 0.019 | |
| Week 12 | 40 | 6 | 0 | 0 | 22 | 23 | 0 | 0 | <0.0001 | |
| Hyperpigmentation | Week 3 | 50 | 0 | 0 | 0 | 50 | 0 | 0 | 0 | - |
| Week 6 | 49 | 1 | 0 | 0 | 50 | 0 | 0 | 0 | 0.999 | |
| Week 9 | 45 | 4 | 0 | 0 | 49 | 0 | 0 | 0 | 0.126 | |
| Week 12 | 44 | 2 | 0 | 0 | 45 | 0 | 0 | 0 | 0.484 | |
| Skin peeling | Week 3 | 44 | 6 | 0 | 0 | 38 | 12 | 0 | 0 | 0.193 |
| Week 6 | 45 | 5 | 0 | 0 | 34 | 16 | 0 | 0 | 0.014 | |
| Week 9 | 43 | 5 | 1 | 0 | 38 | 11 | 0 | 0 | 0.169 | |
| Week 12 | 45 | 1 | 0 | 0 | 38 | 7 | 0 | 0 | 0.060 | |
| Itching | Week 3 | 36 | 13 | 1 | 0 | 33 | 16 | 1 | 0 | 0.802 |
| Week 6 | 42 | 8 | 0 | 0 | 41 | 9 | 0 | 0 | 0.999 | |
| Week 9 | 49 | 0 | 0 | 0 | 36 | 13 | 0 | 0 | <0.0001 | |
| Week 12 | 44 | 2 | 0 | 0 | 30 | 15 | 0 | 0 | 0.001 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shah, B.; Mistry, D.; Gonsalves, N.; Vasani, P.; Dhoot, D.; Barkate, H. A Prospective, Randomized, Comparative Study of Topical Minocycline Gel 4% with Topical Clindamycin Phosphate Gel 1% in Indian Patients with Acne Vulgaris. Antibiotics 2023, 12, 1455. https://doi.org/10.3390/antibiotics12091455
Shah B, Mistry D, Gonsalves N, Vasani P, Dhoot D, Barkate H. A Prospective, Randomized, Comparative Study of Topical Minocycline Gel 4% with Topical Clindamycin Phosphate Gel 1% in Indian Patients with Acne Vulgaris. Antibiotics. 2023; 12(9):1455. https://doi.org/10.3390/antibiotics12091455
Chicago/Turabian StyleShah, Bela, Deval Mistry, Nelry Gonsalves, Presha Vasani, Dhiraj Dhoot, and Hanmant Barkate. 2023. "A Prospective, Randomized, Comparative Study of Topical Minocycline Gel 4% with Topical Clindamycin Phosphate Gel 1% in Indian Patients with Acne Vulgaris" Antibiotics 12, no. 9: 1455. https://doi.org/10.3390/antibiotics12091455
APA StyleShah, B., Mistry, D., Gonsalves, N., Vasani, P., Dhoot, D., & Barkate, H. (2023). A Prospective, Randomized, Comparative Study of Topical Minocycline Gel 4% with Topical Clindamycin Phosphate Gel 1% in Indian Patients with Acne Vulgaris. Antibiotics, 12(9), 1455. https://doi.org/10.3390/antibiotics12091455







