Bloodstream Infection Due to Coagulase-Negative Staphylococci: Impact of Species on Prevalence of Infective Endocarditis
Abstract
:1. Introduction
2. Results
3. Material and Methods
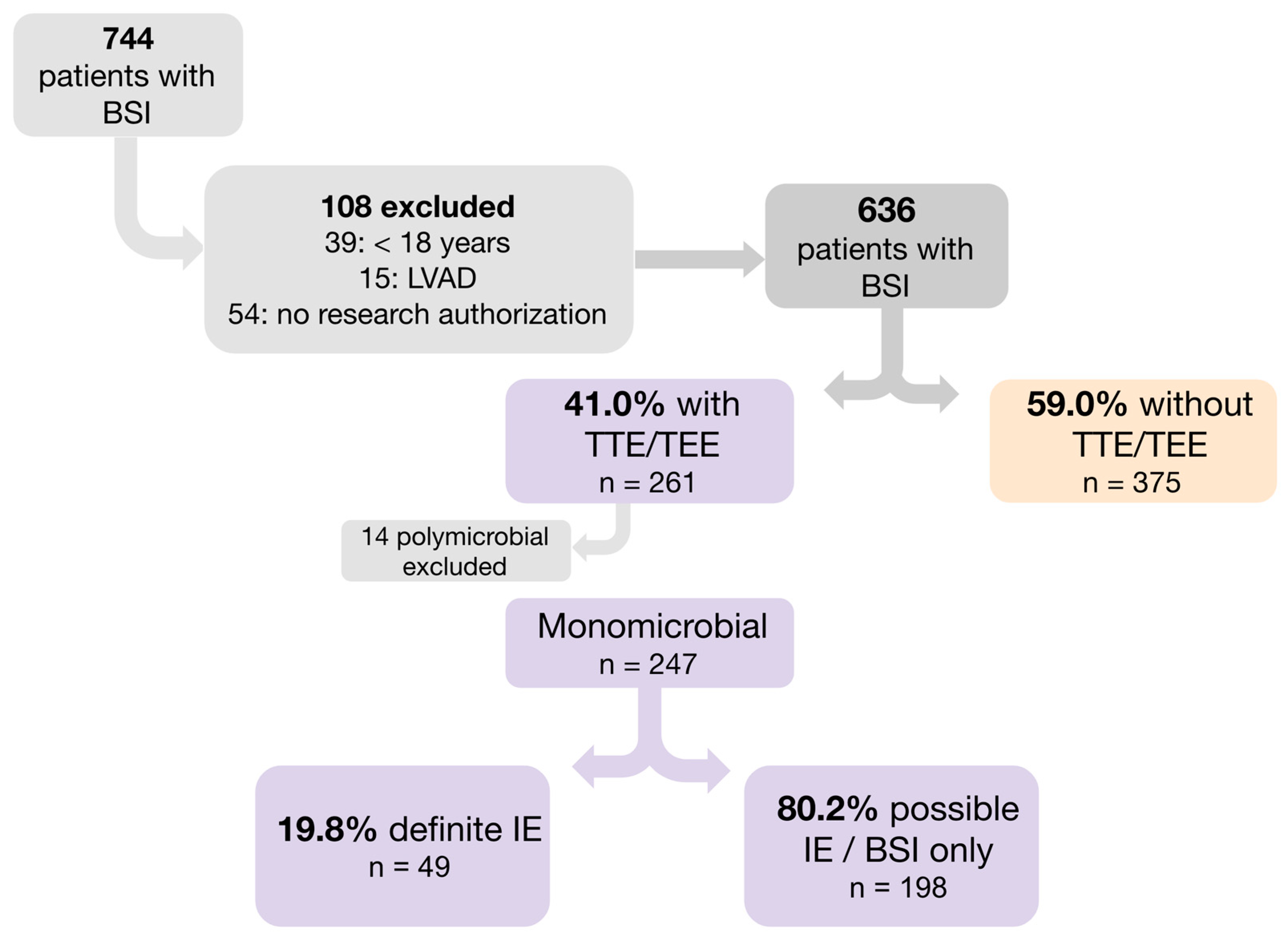
3.1. Study Design and Participant
3.2. Microbiologic Methods
3.3. Definitions
- CoNS BSI episode: Only a patient’s initial CoNS BSI episode during the study period was included if they had at least two or more positive blood cultures for the same CoNS species within 48 h.
- Community-onset BSI: positive blood culture collected from patients at the time of hospital admission or within 48 h of admission.
- Health care-associated BSI: BSI that occurred in patients with prior healthcare exposure such as intravenous therapy or chemotherapy, wound care, hemodialysis, or a specialized nursing care in the 30 days before developing BSI, a hospitalization for two or more days in the 3 months preceding the BSI, or a residence in a nursing home or long-term care facility.
- Nosocomial BSI: A positive blood culture acquired after two days of hospitalization [3].
- Cardiovascular implantable electronic device (CIED): included pacemaker, implantable cardioverter defibrillator, cardiac resynchronization therapy device.
- Definite CIED-IE: clinical evidence of a pocket or generator infection, with two major criteria, or one major criterion plus three minor criteria [4].
- Immunosuppressive therapy: patients receiving immunosuppressive treatment ≤ 2 months after solid organ transplantation, patients receiving daily corticosteroid therapy with a dose ≥ 20 mg of prednisone or equivalent for ≥14 consecutive days, and patients receiving biologic immune modulators [5]. HIV and cancer (solid organ and hematological) were collected as separate variables.
- Native, congenital or acquired valvular disease: moderate to severe stenosis, regurgitation, or atresia of any valve.
- Charlson comorbidity index (CCI): comorbid conditions incorporated in CCI were not chart-abstracted like the other clinical data, rather the patients’ ICD9/10 codes were extracted from their electronic medical record and used to define presence/absence of each condition [6].
3.4. Objective
3.5. Statistical Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Michels, R.; Last, K.; Becker, S.L.; Papan, C. Update on Coagulase-Negative Staphylococci—What the Clinician Should Know. Microorganisms 2021, 9, 830. [Google Scholar] [CrossRef] [PubMed]
- Li, J.S.; Sexton, D.J.; Mick, N.; Nettles, R.; Fowler, V.G., Jr.; Ryan, T.; Bashore, T.; Corey, G.R. Proposed modifications to the Duke criteria for the diagnosis of infective endocarditis. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2000, 30, 633–638. [Google Scholar] [CrossRef]
- Friedman, N.D.; Kaye, K.S.; Stout, J.E.; McGarry, S.A.; Trivette, S.L.; Briggs, J.P.; Lamm, W.; Clark, C.; MacFarquhar, J.; Walton, A.L.; et al. Health care—Associated bloodstream infections in adults: A reason to change the accepted definition of community-acquired infections. Ann. Intern. Med. 2002, 137, 791–797. [Google Scholar] [CrossRef]
- Blomström-Lundqvist, C.; Traykov, V.; Erba, P.A.; Burri, H.; Nielsen, J.C.; Bongiorni, M.G.; Poole, J.; Boriani, G.; Costa, R.; Deharo, J.-C.; et al. European Heart Rhythm Association (EHRA) international consensus document on how to prevent, diagnose, and treat cardiac implantable electronic device infections-endorsed by the Heart Rhythm Society (HRS), the Asia Pacific Heart Rhythm Society (APHRS), the Latin American Heart Rhythm Society (LAHRS), International Society for Cardiovascular Infectious Diseases (ISCVID) and the European Society of Clinical Microbiology and Infectious Diseases (ESCMID) in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS). Europace 2020, 22, 515–549. [Google Scholar]
- Cimmino, G.; Bottino, R.; Formisano, T.; Orlandi, M.; Molinari, D.; Sperlongano, S.; Castaldo, P.; D’elia, S.; Carbone, A.; Palladino, A.; et al. Current Views on Infective Endocarditis: Changing Epidemiology, Improving Diagnostic Tools and Centering the Patient for Up-to-Date Management. Life Basel Switz. 2023, 13, 377. [Google Scholar] [CrossRef]
- Deyo, R.A.; Cherkin, D.C.; Ciol, M.A. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J. Clin. Epidemiol. 1992, 45, 613–619. [Google Scholar] [CrossRef] [PubMed]
- Ursi, M.P.; Durante Mangoni, E.; Rajani, R.; Hancock, J.; Chambers, J.B.; Prendergast, B. Infective Endocarditis in the Elderly: Diagnostic and Treatment Options. Drugs Aging. 2019, 36, 115–124. [Google Scholar] [CrossRef] [PubMed]
- Llopis, J.; Jiménez-Exposito, M.J.; Kourany, W.M.; Almirante, B.; Carosi, G.; Durante-Mangoni, E.; Fortes, C.Q.; Giannitsioti, E.; Lerakis, S.; Montagna-Mella, R.; et al. Infective Endocarditis in Patients on Chronic Hemodialysis. J. Am. Coll. Cardiol. 2021, 77, 1629–1640. [Google Scholar]
- Khan, Z.A.; Hollenberg, S.M. Valvular Heart Disease in Adults: Infective Endocarditis. FP Essent. 2017, 457, 30–38. [Google Scholar]
- An Arsenal of R Functions for Large-Scale Statistical Summaries [Internet]. Available online: https://mayoverse.github.io/arsenal/ (accessed on 8 August 2023).
- HMISC: Harrell Miscellaneous [Internet]. 2023. Available online: https://cran.r-project.org/web/packages/Hmisc/index.html (accessed on 8 August 2023).
- RMS: Regression Modeling Strategies [Internet]. 2023. Available online: https://cran.r-project.org/web/packages/rms/index.html (accessed on 8 August 2023).
- R: The R Project for Statistical Computing [Internet]. Available online: https://www.r-project.org/ (accessed on 8 August 2023).
- Rasmussen, R.V.; Høst, U.; Arpi, M.; Hassager, C.; Johansen, H.K.; Korup, E.; Schønheyder, H.C.; Berning, J.; Gill, S.; Rosenvinge, F.S.; et al. Prevalence of infective endocarditis in patients with Staphylococcus aureus bacteraemia: The value of screening with echocardiography. Eur. J. Echocardiogr. 2011, 12, 414–420. [Google Scholar] [CrossRef]
- Fowler, V.G.; Durack, D.T.; Selton-Suty, C.; Athan, E.; Bayer, A.S.; Chamis, A.L.; Dahl, A.; DiBernardo, L.; Durante-Mangoni, E.; Duval, X.; et al. The 2023 Duke-ISCVID Criteria for Infective Endocarditis: Updating the Modified Duke Criteria. Clin. Infect. Dis. 2023, 77, 518–526. [Google Scholar] [CrossRef]
- Yukawa, S.; Noguchi, T.; Shinohara, K.; Tsuchido, Y.; Yamamoto, M.; Matsumura, Y.; Nagao, M. Characteristics and outcomes in adult patients with Staphylococcus lugdunensis bacteremia compared to patients with Staphylococcus epidermidis and Staphylococcus aureus bacteremia: A retrospective study in a 16-year period at the university hospital, Japan. BMC Infect. Dis. 2023, 23, 269. [Google Scholar] [CrossRef] [PubMed]
- Andersen, M.H.; Holle, S.L.K.; Klein, C.F.; Bruun, N.E.; Arpi, M.; Bundgaard, H.; Tønder, N.; Iversen, K.K. Risk for infective endocarditis in bacteremia with Gram positive cocci. Infection 2020, 48, 905–912. [Google Scholar] [CrossRef] [PubMed]
- Østergaard, L.; Bruun, N.E.; Voldstedlund, M.; Arpi, M.; Andersen, C.; Schønheyder, H.C.; Lemming, L.; Rosenvinge, F.; Valeur, N.; Søgaard, P.; et al. Prevalence of infective endocarditis in patients with positive blood cultures: A Danish nationwide study. Eur. Heart J. 2019, 40, 3237–3244. [Google Scholar] [CrossRef]
- Non, L.R.; Santos, C.A.Q. The occurrence of infective endocarditis with Staphylococcus lugdunensis bacteremia: A retrospective cohort study and systematic review. J. Infect. 2017, 74, 179–186. [Google Scholar] [CrossRef]
- Stortecky, S.; Heg, D.; Tueller, D.; Pilgrim, T.; Muller, O.; Noble, S.; Jeger, R.; Toggweiler, S.; Ferrari, E.; Taramasso, M.; et al. Infective Endocarditis After Transcatheter Aortic Valve Replacement. J. Am. Coll. Cardiol. 2020, 75, 3020–3030. [Google Scholar] [CrossRef] [PubMed]
- Regueiro, A.; Linke, A.; Latib, A.; Ihlemann, N.; Urena, M.; Walther, T.; Husser, O.; Herrmann, H.C.; Nombela-Franco, L.; Cheema, A.N.; et al. Association Between Transcatheter Aortic Valve Replacement and Subsequent Infective Endocarditis and In-Hospital Death. JAMA 2016, 316, 1083–1092. [Google Scholar] [CrossRef] [PubMed]
- Chu, V.H.; Cabell, C.H.; Abrutyn, E.; Corey, G.R.; Hoen, B.; Miro, J.M.; Olaison, L.; Stryjewski, M.E.; Pappas, P.; Anstrom, K.J.; et al. Native valve endocarditis due to coagulase-negative staphylococci: Report of 99 episodes from the International Collaboration on Endocarditis Merged Database. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2004, 39, 1527–1530. [Google Scholar] [CrossRef]
- Whitener, C.; Caputo, G.M.; Weitekamp, M.R.; Karchmer, A.W. Endocarditis due to coagulase-negative staphylococci. Microbiologic, epidemiologic, and clinical considerations. Infect. Dis. Clin. North Am. 1993, 7, 81–96. [Google Scholar] [CrossRef]
- Etienne, J.; Eykyn, S.J. Increase in native valve endocarditis caused by coagulase negative staphylococci: An Anglo-French clinical and microbiological study. Br. Heart J. 1990, 64, 381–384. [Google Scholar] [CrossRef]
- Miele, P.S.; Kogulan, P.K.; Levy, C.S.; Goldstein, S.; Marcus, K.A.; Smith, M.A.; Rosenthal, J.; Croxton, M.; Gill, V.J.; Lucey, D.R. Seven cases of surgical native valve endocarditis caused by coagulase-negative staphylococci: An underappreciated disease. Am. Heart J. 2001, 142, 571–576. [Google Scholar] [CrossRef]
- Al-Tamtami, N.; Al-Lawati, J.; Al-Abri, S. Native Valve Endocarditis Caused by Coagulase Negative Staphylococci; an Appeal to Start Outpatient Antimicrobial Therapy: An Unusual Case Report. Oman Med. J. 2011, 26, 269–270. [Google Scholar] [CrossRef]
- Popa-Fotea, N.-M.; Scafa-Udriste, A.; Iulia, G.; Scarlatescu, A.I.; Oprescu, N.; Mihai, C.; Micheu, M.M. Increasing clinical impact and microbiological difficulties in diagnosing coagulase-negative staphylococci in infective endocarditis - a review starting from a series of cases. Curr. Med. Res. Opin. 2022, 38, 2077–2083. [Google Scholar] [CrossRef] [PubMed]

| Characteristic | Overall (n = 247) | Definite IE (n = 49) | Possible IE/BSI Only (n = 198) | p Value |
|---|---|---|---|---|
| Male | 165 (66.8%) | 35 (71.4%) | 130 (65.7%) | 0.442 1 |
| Age, years | 64.6 (53.8–72.6) | 69.5 (59.7–75.7) | 62.8 (53.0–70.8) | 0.017 2 |
| Race | 0.918 1 | |||
| 224 (90.7%) | 45 (91.8%) | 179 (90.4%) | |
| 10 (4.0%) | 2 (4.1%) | 8 (4.0%) | |
| 13 (5.3%) | 2 (4.1%) | 11 (5.6%) | |
| Category of CoNS BSI | <0.001 1 | |||
| 32 (13.0%) | 12 (24.5%) | 20 (10.1%) | |
| 123 (49.8%) | 30 (61.2%) | 93 (47.0%) | |
| 92 (37.2%) | 7 (14.3%) | 85 (42.9%) | |
| Charlson Comorbidity Index | ||||
| 2 (0–7) | 3 (1–8) | 2 (0–7) | 0.196 2 |
| 5 (2–9) | 5 (3–10) | 4 (2–9) | 0.085 2 |
| Diabetes mellitus | 63 (25.5%) | 17 (34.7%) | 46 (23.2%) | 0.099 1 |
| Moderate/severe renal disease | 65 (26.3%) | 17 (34.7%) | 48 (24.2%) | 0.137 1 |
| Chronic heart failure | 74 (30.0%) | 26 (53.1%) | 48 (24.2%) | <0.001 1 |
| Chronic pulmonary disease | 70 (28.3%) | 13 (26.5%) | 57 (28.8%) | 0.754 1 |
| Other type of cancer | 71 (28.7%) | 9 (18.4%) | 62 (31.3%) | 0.073 1 |
| Metastatic solid tumor | 13 (5.3%) | 2 (4.1%) | 11 (5.6%) | 0.679 1 |
| Injection drug use | 2 (0.8%) | 1 (2.0%) | 1 (0.5%) | 0.283 1 |
| Hemodialysis | 63 (25.5%) | 11 (22.4%) | 52 (26.3%) | 0.583 1 |
| 56 (88.9%) | 9 (81.8%) | 47 (90.4%) | |
| 1 (1.6%) | 1 (9.1%) | 0 (0.0%) | |
| 6 (9.5%) | 1 (9.1%) | 5 (9.6%) | |
| Immunosuppressive therapy | 73 (29.6%) | 11 (22.4%) | 62 (31.3%) | 0.223 1 |
| Valve disease | 83 (33.6%) | 32 (65.3%) | 51 (25.8%) | <0.001 1 |
| Prosthetic valve | 41 (16.6%) | 20 (40.8%) | 21 (10.6%) | <0.001 1 |
| CIED | 56 (22.7%) | 23 (46.9%) | 33 (16.7%) | <0.001 1 |
| PICC/central line | 122 (49.4%) | 16 (32.7%) | 106 (53.5%) | 0.009 1 |
| Vascular graft/stent | 27 (10.9%) | 6 (12.2%) | 21 (10.6%) | 0.742 1 |
| Prosthetic joint | 33 (13.4%) | 13 (26.5%) | 20 (10.1%) | 0.002 1 |
| Neurologic device | 1 (0.4%) | 0 (0.0%) | 1 (0.5%) | 0.618 1 |
| Characteristic | Overall (n = 247) | Definite IE (n = 49) | Possible IE/BSI Only (n = 198) | p Value |
|---|---|---|---|---|
| CoNS species | <0.001 1 | |||
| S. epidermidis | 196 (79.4%) | 33 (67.3%) | 163 (82.3%) | |
| S. lugdunensis | 22 (8.9%) | 13 (26.5%) | 9 (4.5%) | |
| S. devriesei/haemolyticus | 11 (4.5%) | 1 (2.0%) | 10 (5.1%) | |
| S. capitis | 7 (2.8%) | 1 (2.0%) | 6 (3.0%) | |
| S. hominis | 6 (2.4%) | 0 (0.0%) | 6 (3.0%) | |
| Other CoNS * | 5 (2.0%) | 1 (2.0%) | 4 (2.0%) | |
| Methicillin resistance | 163 (66.5%) | 25 (52.1%) | 138 (70.1%) | 0.018 1 |
| Time to positivity, hours | 19.0 (15.0–22.5) | 21.0 (16.0–24.0) | 19.0 (15.0–22.0) | 0.035 2 |
| Odds Ratio (95% Confidence Interval) | p Value | ||
|---|---|---|---|
| Model 1 | |||
| Age | (per 10 years) | 1.18 (0.92–1.52) | 0.196 |
| Charlson Comorbidity Index | (per 1 point) | 0.98 (0.89–1.08) | 0.681 |
| Hemodialysis | 0.99 (0.41–2.40) | 0.980 | |
| Valve disease | 3.24 (1.54–6.80) | 0.002 | |
| Foreign device | 6.28 (2.98–13.26) | <0.001 | |
| Model 2 | |||
| Charlson Comorbidity Index * | (per 1 point) | 0.98 (0.90–1.06) | 0.585 |
| Hemodialysis | 1.27 (0.52–3.12) | 0.595 | |
| Valve disease | 3.27 (1.51–7.07) | 0.003 | |
| Foreign device | 6.51 (2.98–14.21) | <0.001 | |
| CoNS Species | 0.002 | ||
| S. lugdunensis | vs. S. epidermidis | 8.02 (2.40–26.90) | |
| S. lugdunensis | vs. other CoNS species | 12.89 (2.34–70.90) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Haddad, S.F.; Lahr, B.D.; Patarroyo, S.S.; Chesdachai, S.; Kies, K.D.; O’Horo, J.C.; DeSimone, D.C.; Sendi, P.; Baddour, L.M. Bloodstream Infection Due to Coagulase-Negative Staphylococci: Impact of Species on Prevalence of Infective Endocarditis. Antibiotics 2023, 12, 1453. https://doi.org/10.3390/antibiotics12091453
Haddad SF, Lahr BD, Patarroyo SS, Chesdachai S, Kies KD, O’Horo JC, DeSimone DC, Sendi P, Baddour LM. Bloodstream Infection Due to Coagulase-Negative Staphylococci: Impact of Species on Prevalence of Infective Endocarditis. Antibiotics. 2023; 12(9):1453. https://doi.org/10.3390/antibiotics12091453
Chicago/Turabian StyleHaddad, Sara F., Brian D. Lahr, Sebastian Santos Patarroyo, Supavit Chesdachai, Kami D. Kies, John C. O’Horo, Daniel C. DeSimone, Parham Sendi, and Larry M. Baddour. 2023. "Bloodstream Infection Due to Coagulase-Negative Staphylococci: Impact of Species on Prevalence of Infective Endocarditis" Antibiotics 12, no. 9: 1453. https://doi.org/10.3390/antibiotics12091453
APA StyleHaddad, S. F., Lahr, B. D., Patarroyo, S. S., Chesdachai, S., Kies, K. D., O’Horo, J. C., DeSimone, D. C., Sendi, P., & Baddour, L. M. (2023). Bloodstream Infection Due to Coagulase-Negative Staphylococci: Impact of Species on Prevalence of Infective Endocarditis. Antibiotics, 12(9), 1453. https://doi.org/10.3390/antibiotics12091453






