Analysis of Clinical Phenotypes through Machine Learning of First-Line H. pylori Treatment in Europe during the Period 2013–2022: Data from the European Registry on H. pylori Management (Hp-EuReg)
Abstract
:1. Introduction
2. Results
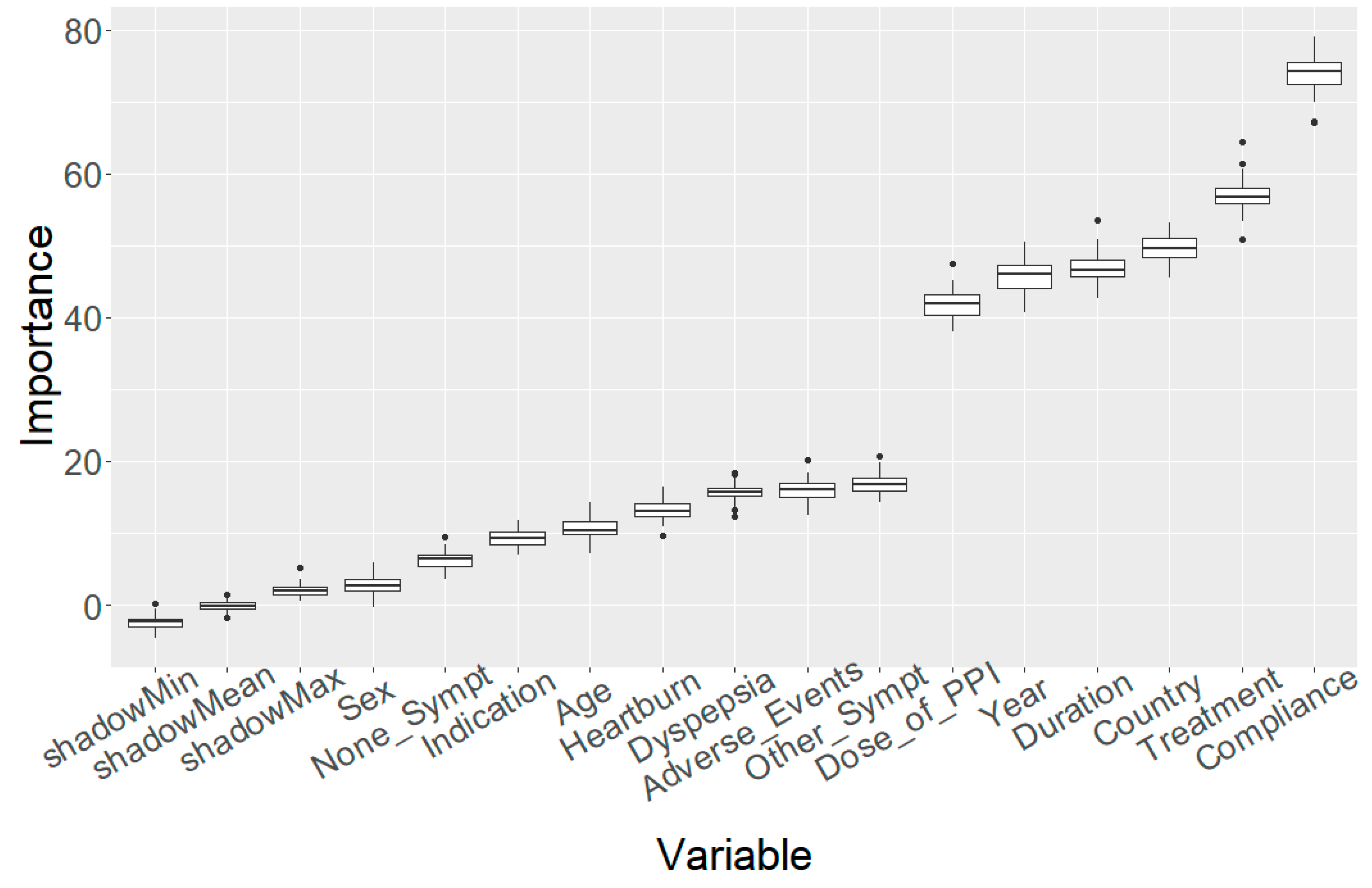
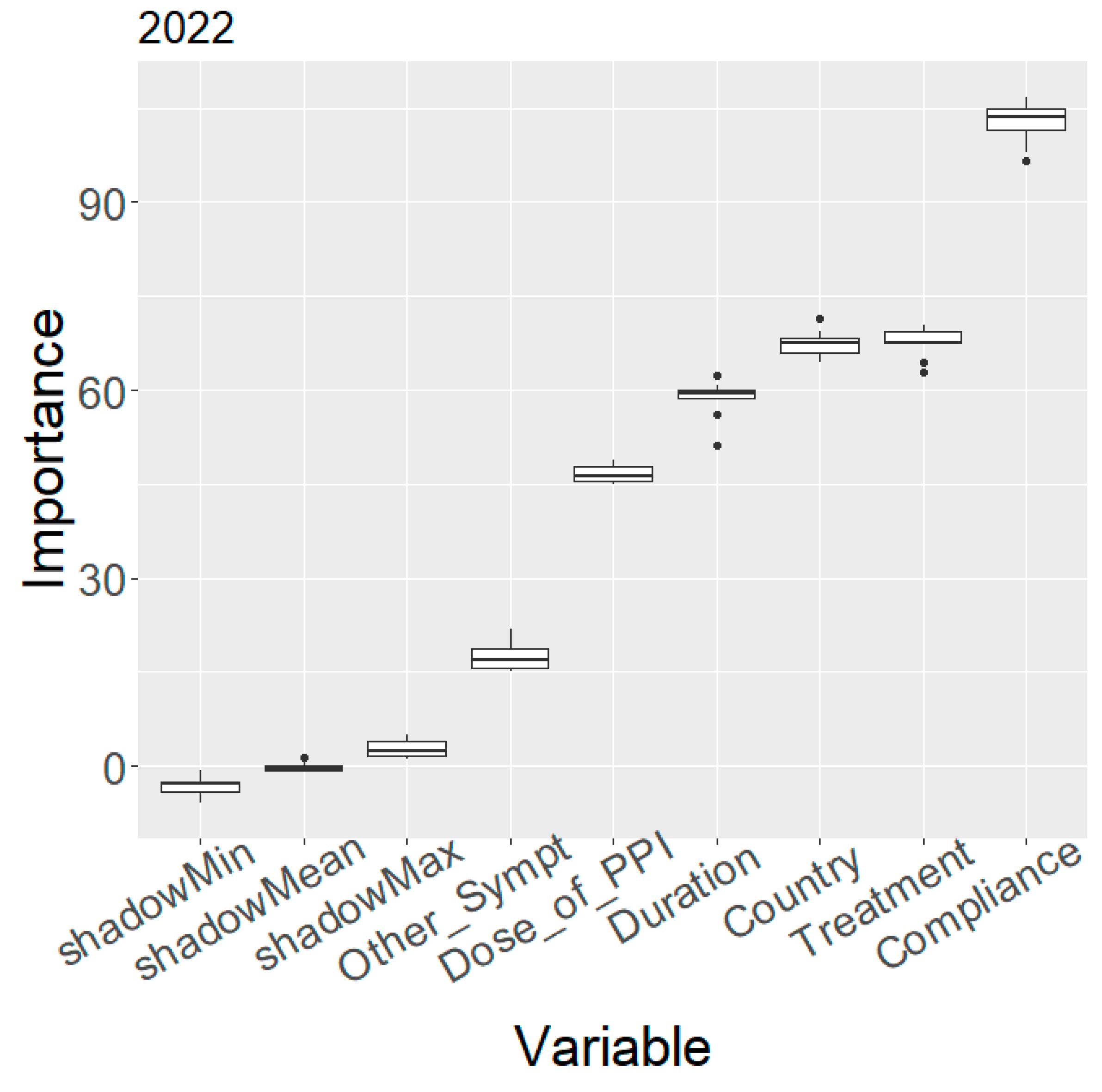
2.1. Variable Importance
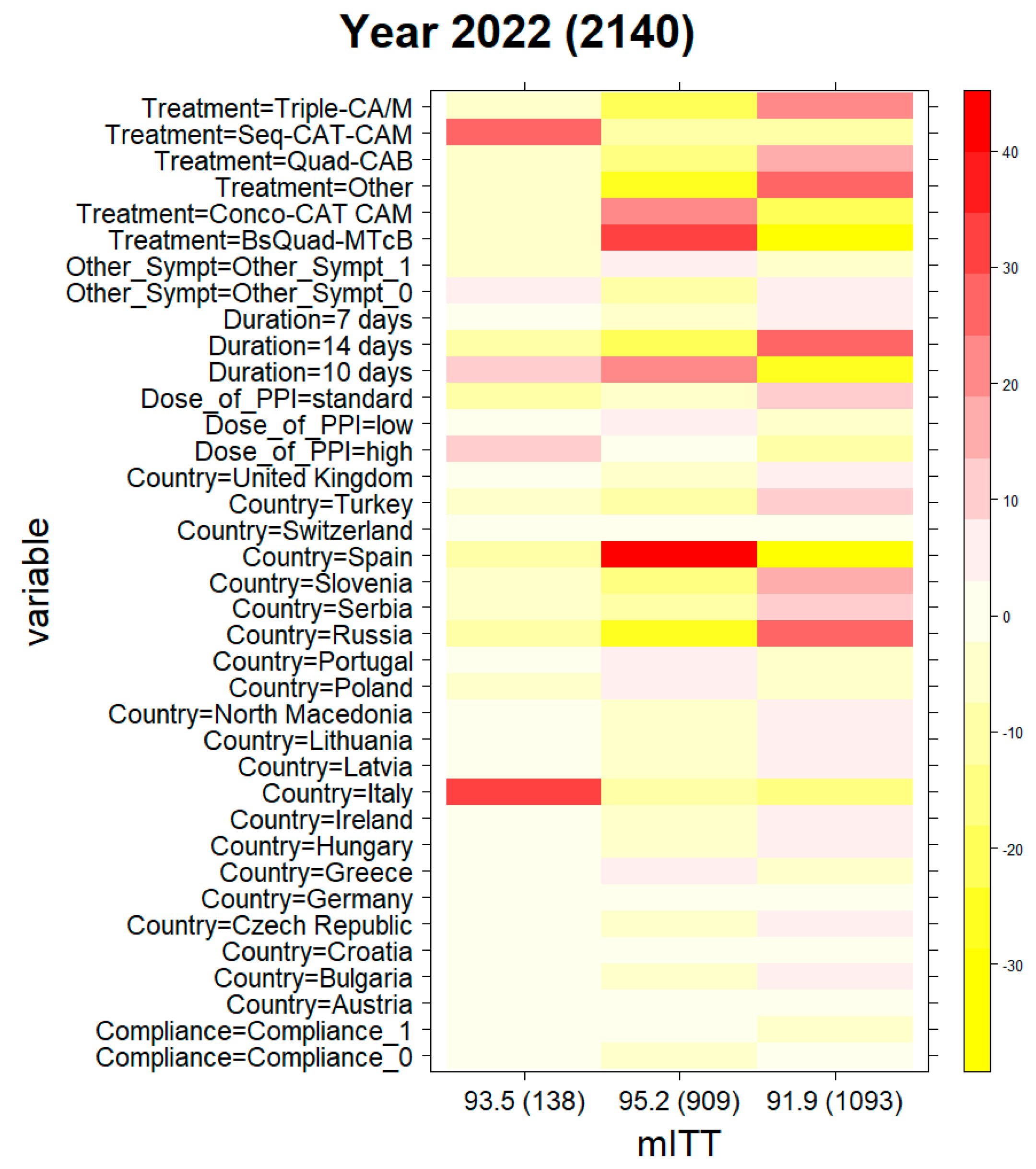
2.2. Clinical Phenotyping
2.3. Evolution of Treatment Effectiveness by Country
3. Discussion
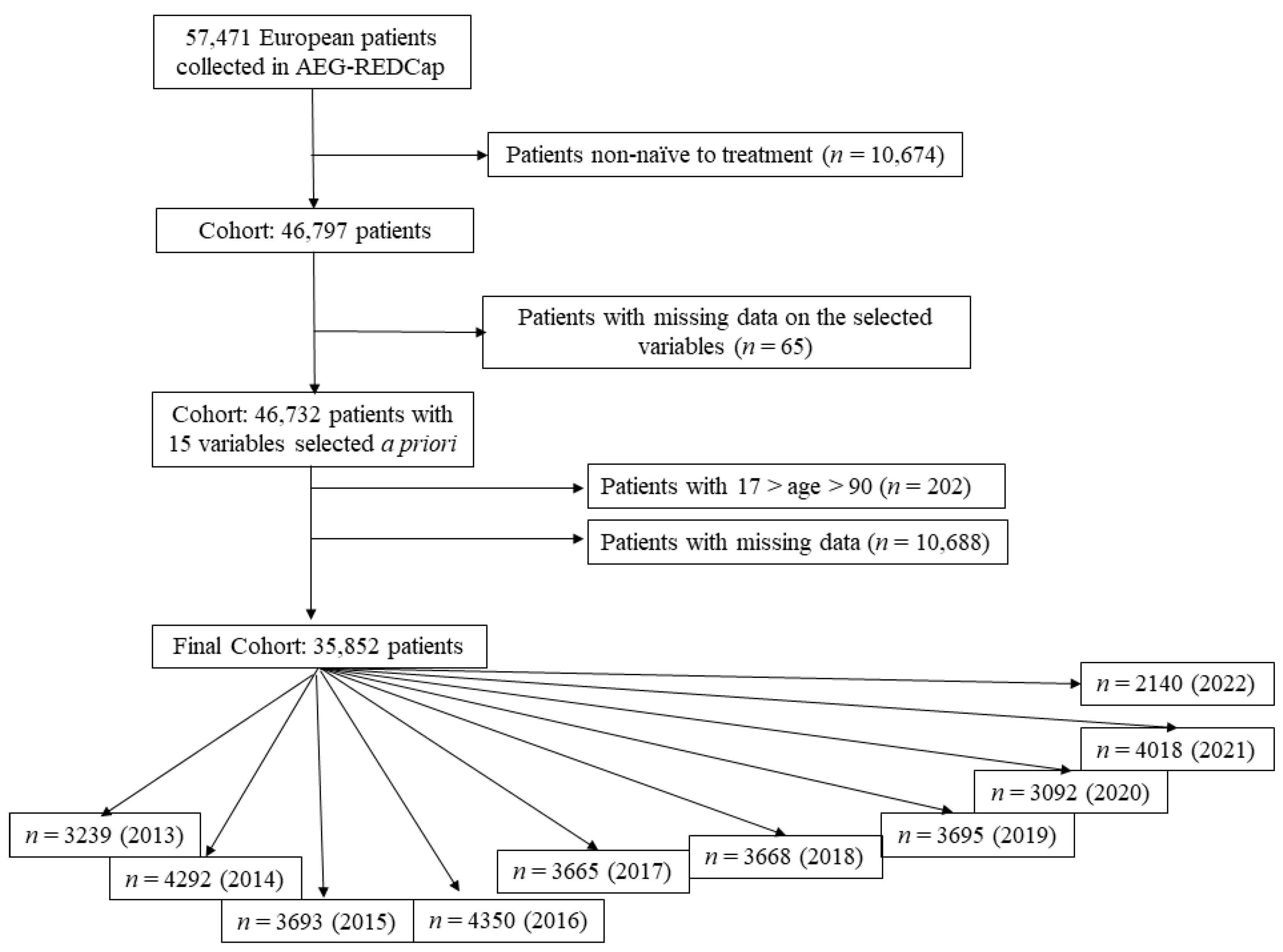
4. Materials and Methods
4.1. European Registry on H. pylori Management (Hp-EuReg)
4.2. Data Collection
4.3. Data Management
4.4. Statistical Analysis
4.4.1. Variable Categorisation and Definitions
4.4.2. Data Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Hooi, J.K.Y.; Lai, W.Y.; Ng, W.K.; Suen, M.M.Y.; Underwood, F.E.; Tanyingoh, D.; Malfertheiner, P.; Graham, D.Y.; Wong, V.W.S.; Wu, J.C.Y.; et al. Global Prevalence of Helicobacter pylori Infection: Systematic Review and Meta-Analysis. Gastroenterology 2017, 153, 420–429. [Google Scholar] [CrossRef]
- de Martel, C.; Georges, D.; Bray, F.; Ferlay, J.; Clifford, G.M. Global burden of cancer attributable to infections in 2018: A worldwide incidence analysis. Lancet Glob. Health 2020, 8, e180–e190. [Google Scholar] [CrossRef]
- McColl, K.E. Clinical practice. Helicobacter pylori infection. N. Engl. J. Med. 2010, 362, 1597–1604. [Google Scholar] [PubMed]
- Malfertheiner, P.; Megraud, F.; Rokkas, T.; Gisbert, J.P.; Liou, J.-M.; Schulz, C.; Gasbarrini, A.; Hunt, R.H.; Leja, M.; O’Morain, C.; et al. Management of Helicobacter pylori infection: The Maastricht VI/Florence consensus report. Gut 2022, 71, 1724–1762. [Google Scholar] [CrossRef] [PubMed]
- Mégraud, F.; Graham, D.Y.; Howden, C.W.; Trevino, E.; Weissfeld, A.; Hunt, B.; Smith, N.; Leifke, E.; Chey, W.D. Rates of Antimicrobial Resistance in Helicobacter pylori Isolates from Clinical Trial Patients Across the US and Europe. Am. J. Gastroenterol. 2023, 118, 269–275. [Google Scholar] [CrossRef] [PubMed]
- Nyssen, O.P.; Moreira, L.; García-Morales, N.; Cano-Català, A.; Puig, I.; Mégraud, F.; O’Morain, C.; Gisbert, J.P. European Registry on Helicobacter pylori Management (Hp-EuReg): Most relevant results for clinical practice. Front. Gastroenterol. 2022, 1, 1–20. [Google Scholar] [CrossRef]
- Nyssen, O.P.; Bordin, D.; Tepes, B.; Pérez-Aisa, Á.; Vaira, D.; Caldas, M.; Bujanda, L.; Castro-Fernandez, M.; Lerang, F.; Leja, M.; et al. European Registry on Helicobacter pylori management (Hp-EuReg): Patterns and trends in first-line empirical eradication prescription and outcomes of 5 years and 21 533 patients. Gut 2021, 70, 40–54. [Google Scholar] [CrossRef]
- Fallone, C.A.; Moss, S.F.; Malfertheiner, P. Reconciliation of Recent Helicobacter pylori Treatment Guidelines in a Time of Increasing Resistance to Antibiotics. Gastroenterology 2019, 157, 44–53. [Google Scholar] [CrossRef]
- Megraud, F.; Bruyndonckx, R.; Coenen, S.; Wittkop, L.; Huang, T.-D.; Hoebeke, M.; Bénéjat, L.; Lehours, P.; Goossens, H.; Glupczynski, Y. Helicobacter pylori resistance to antibiotics in Europe in 2018 and its relationship to antibiotic consumption in the community. Gut 2021, 70, 1815–1822. [Google Scholar] [CrossRef]
- Bujanda, L.; Nyssen, O.P.; Vaira, D.; Saracino, I.M.; Fiorini, G.; Lerang, F.; Georgopoulos, S.; Tepes, B.; Heluwaert, F.; Gasbarrini, A.; et al. Antibiotic Resistance Prevalence and Trends in Patients Infected with Helicobacter pylori in the Period 2013–2020: Results of the European Registry on H. pylori Management (Hp-EuReg). Antibiotics 2021, 10, 1058. [Google Scholar] [CrossRef]
- Megraud, F. Antibiotic Resistance Is the Key Element in Treatment of Helicobacter pylori Infection. Gastroenterology 2018, 155, 1300–1302. [Google Scholar] [CrossRef] [PubMed]
- Mégraud, F.; Alix, C.; Charron, P.; Bénéjat, L.; Ducournau, A.; Bessède, E.; Lehours, P. Survey of the antimicrobial resistance of Helicobacter pylori in France in 2018 and evolution during the previous 5 years. Helicobacter 2021, 26, e12767. [Google Scholar] [CrossRef]
- Bordin, D.S.; Embutnieks, Y.V.; Vologzhanina, L.G.; Il’chishina, T.A.; Voinovan, I.N.; Sarsenbaeva, A.S.; Alekseenko, S.A.; Zaitsev, O.V.; Abdulkhakov, R.A.; Osipenko, M.F.; et al. European Registry on the management of Helicobacter pylori infection (Hp-EuReg): Analysis of 2360 patients receiving first-line therapy in Russia. Ter. Arkhiv 2018, 90, 35–42. [Google Scholar]
- Bordin, D.S.; Embutnieks, Y.V.; Vologzhanina, L.G.; Ilchishina, T.A.; Voynovan, I.N.; Sarsenbaeva, A.S.; Zaitsev, O.V.; Alekseenko, S.A.; Abdulkhakov, R.A.; Dehnich, N.N.; et al. European registry Helicobacter pylori (Hp-EuReg): How has clinical practice changed in Russia from 2013 to 2018 years. Ter. Arkhiv 2019, 91, 16–24. [Google Scholar] [CrossRef]
- Caldas, M.; Pérez-Aisa, A.; Castro-Fernández, M.; Bujanda, L.; Lucendo, A.J.; Rodrigo, L.; Huguet, J.M.; Pérez-Lasala, J.; Molina-Infante, J.; Barrio, J.; et al. European Registry on Helicobacter pylori Management: Effectiveness of First and Second-Line Treatment in Spain. Antibiotics 2020, 10, 13. [Google Scholar] [CrossRef]
- Tepes, B.; Jurecic, N.B.; Tepes, K.; Sanchez, M.E.; Nyssen, O.P.; O’Morain, C.; Mégraud, F.; Gisbert, J. Helicobacter pylori Eradication Rates in Slovenia in the Period from 2017 to 2019: Data from the European Registry on H. pylori Management. Dig. Dis. 2021, 39, 318–324. [Google Scholar] [CrossRef]
- Gatta, L.; Nyssen, O.P.; Fiorini, G.; Saracino, I.M.; Pavoni, M.; Romano, M.; Gravina, A.G.; Granata, L.; Pellicano, R.; Gasbarrini, A.; et al. Effectiveness of first and second-line empirical treatment in Italy: Results of the European registry on Helicobacter pylori management. United Eur. Gastroenterol. J. 2023, 11, 103–113. [Google Scholar] [CrossRef] [PubMed]
- Bordin, D.S.; Voynovan, I.N.; Embutnieks, Y.V.; Nyssen, O.P.; Megraud, F.; Francis, M.; Colm, O.; Colm, O.; Perez-Gisbert, J.; Javier, P.G. European registry on Helicobacter pylori management (Hp-EuReg) as a tool to evaluate and improve clinical practice in Moscow. Ter. Arkhiv 2020, 92, 12–18. [Google Scholar] [CrossRef] [PubMed]
- Tepes, B.; Kastelic, M.; Vujasinovic, M.; Lampic, P.; Seruga, M.; Jurecic, N.B.; Nyssen, O.P.; Donday, M.G.; O’Morain, C.; Megraud, F.; et al. Helicobacter pylori Treatment Results in Slovenia in the Period 2013-2015 as a Part of European Registry on Helicobacter pylori Management. Radiol. Oncol. 2018, 52, 1–6. [Google Scholar] [CrossRef]
- Gisbert, J.P.; Alcedo, J.; Amador, J.; Bujanda, L.; Calvet, X.; Castro-Fernández, M.; Fernández-Salazar, L.; Gené, E.; Lanas, A.; Lucendo, A.; et al. V Spanish Consensus Conference on Helicobacter pylori infection treatment. Gastroenterol. Hepatol. 2022, 45, 392–417. [Google Scholar] [CrossRef]
- McNicholl, A.G.; O’Morain, C.A.; Megraud, F.; Gisbert, J.P.; As Scientific Committee of the Hp-Eureg on Behalf of the National C. Protocol of the European Registry on the management of Helicobacter pylori infection (Hp-EuReg). Helicobacter 2019, 24, e12630. [Google Scholar] [CrossRef]
- Graham, D.Y.; Lu, H.; Dore, M.P. Relative potency of proton-pump inhibitors, Helicobacter pylori therapy cure rates, and meaning of double-dose PPI. Helicobacter 2019, 24, e12554. [Google Scholar] [CrossRef] [PubMed]
- Kirchheiner, J.; Glatt, S.; Fuhr, U.; Klotz, U.; Meineke, I.; Seufferlein, T.; Brockmöller, J. Relative potency of proton-pump inhibitors-comparison of effects on intragastric pH. Eur. J. Clin. Pharmacol. 2009, 65, 19–31. [Google Scholar] [CrossRef] [PubMed]
- Kursa, M.B.; Rudnicki, W.R. Feature Selection with the Boruta Package. J. Stat. Softw. 2010, 36, 1–13. [Google Scholar] [CrossRef]
- Breiman, L. Random Forests. Mach. Lang. 2001, 45, 5–32. [Google Scholar]
- R-Core-Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021. [Google Scholar]

| Year | Cluster #1, % mITT (Number of Patients) | Cluster #2, % mITT (Number of Patients) | Cluster #3, % mITT (Number of Patients) |
|---|---|---|---|
| 2013 | 86.4 (2011) | 88.4 (224) | 84.5 (1004) |
| 2014 | 88.5 (435) | 86.5 (2644) | 84.3 (1213) |
| 2015 | 89.0 (346) | 87.1 (2656) | 83.5 (691) |
| 2016 | 91.6 (2523) | 85.5 (1152) | 80.3 (675) * |
| 2017 | 85.4 (323) | 91.6 (1969) | 82.4 (1373) |
| 2018 | 90.1 (1974) | 90.9 (1122) | 90.6 (352) |
| 2019 | 90.1 (1225) | 86.7 (721) | 89.6 (1749) |
| 2020 | 86.1 (1783) | 87.4 (585) | 92.3 (724) |
| 2021 | 87.4 (310) | 91.1 (1882) | 90.4 (1826) |
| 2022 | 93.5 (138) | 95.2 (909) | 91.9 (1093) |
| 1 (n = 138) | 2 (n = 909) | 3 (n = 1093) | Overall p-Value | N | |
|---|---|---|---|---|---|
| Gastrointestinal symptoms | <0.001 | 2140 | |||
| Absence (none) | 137 (99.3%) | 756 (83.2%) | 1019(93.2%) | ||
| Other symptoms1 | 1 (0.72%) | 153 (16.8%) | 74 (6.77%) | ||
| Compliance | 0.022 | 2140 | |||
| No (<90% drug intake) | 3 (2.17%) | 9 (0.99%) | 28 (2.56%) | ||
| Yes (>90% drug intake) | 135 (97.8%) | 900 (99.0%) | 1065 (97.4%) | ||
| Duration (days) | 2140 | ||||
| 7 | 1 (0.72%) | 1 (0.11%) | 55 (5.03%) | ||
| 10 | 126 (91.3%) | 633 (69.6%) | 168 (15.4%) | ||
| 14 | 11 (7.97%) | 275 (30.3%) | 870 (79.6%) | ||
| Dose of PPI (mg OE)2 | <0.001 | 2140 | |||
| Low | 30 (21.7%) | 308 (33.9%) | 265 (24.2%) | ||
| Standard | 1 (0.72%) | 253 (27.8%) | 534 (48.9%) | ||
| High | 107 (77.5%) | 348 (38.3%) | 294 (26.9%) | ||
| Country | 2140 | ||||
| Austria | 0 (0.00%) | 5 (0.55%) | 0 (0.00%) | ||
| Bulgaria | 0 (0.00%) | 0 (0.00%) | 15 (1.37%) | ||
| Croatia | 0 (0.00%) | 19 (2.09%) | 26 (2.38%) | ||
| Czech Republic | 0 (0.00%) | 0 (0.00%) | 16 (1.46%) | ||
| Germany | 0 (0.00%) | 25 (2.75%) | 22 (2.01%) | ||
| Greece | 0 (0.00%) | 39 (4.29%) | 0 (0.00%) | ||
| Hungary | 0 (0.00%) | 0 (0.00%) | 21 (1.92%) | ||
| Ireland | 0 (0.00%) | 0 (0.00%) | 21 (1.92%) | ||
| Israel | 0 (0.00%) | 2 (0.22%) | 0 (0.00%) | ||
| Italy | 138 (100%) | 1 (0.11%) | 3 (0.27%) | ||
| Latvia | 0 (0.00%) | 0 (0.00%) | 13 (1.19%) | ||
| Lithuania | 0 (0.00%) | 0 (0.00%) | 26 (2.38%) | ||
| North Macedonia | 0 (0.00%) | 0 (0.00%) | 31 (2.84%) | ||
| Poland | 0 (0.00%) | 55 (6.05%) | 11 (1.01%) | ||
| Portugal | 0 (0.00%) | 20 (2.20%) | 0 (0.00%) | ||
| Russia | 0 (0.00%) | 0 (0.00%) | 437 (40.0%) | ||
| Serbia | 0 (0.00%) | 1 (0.11%) | 133 (12.2%) | ||
| Slovenia | 0 (0.00%) | 0 (0.00%) | 163 (14.9%) | ||
| Spain | 0 (0.00%) | 739 (81.3%) | 46 (4.21%) | ||
| Switzerland | 0 (0.00%) | 3 (0.33%) | 11 (1.01%) | ||
| Turkey | 0 (0.00%) | 0 (0.00%) | 78 (7.14%) | ||
| United Kingdom | 0 (0.00%) | 0 (0.00%) | 20 (1.83%) | ||
| Most frequent 1st line treatments | 2140 | ||||
| Triple-CA/M | 3 (2.17%) | 9 (0.99%) | 365 (33.4%) | ||
| Seq-CAT-CAM | 94 (68.1%) | 0 (0.00%) | 0 (0.00%) | ||
| Conco-CAT CAM | 9 (6.52%) | 305 (33.6%) | 2 (0.18%) | ||
| BsQuad-MTcB | 20 (14.5%) | 579 (63.7%) | 9 (0.82%) | ||
| Quadruple-CAB | 0 (0.00%) | 2 (0.22%) | 169 (15.5%) | ||
| Other3 | 12 (8.70%) | 14 (1.54%) | 548 (50.1%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nyssen, O.P.; Pratesi, P.; Spínola, M.A.; Jonaitis, L.; Pérez-Aísa, Á.; Vaira, D.; Saracino, I.M.; Pavoni, M.; Fiorini, G.; Tepes, B.; et al. Analysis of Clinical Phenotypes through Machine Learning of First-Line H. pylori Treatment in Europe during the Period 2013–2022: Data from the European Registry on H. pylori Management (Hp-EuReg). Antibiotics 2023, 12, 1427. https://doi.org/10.3390/antibiotics12091427
Nyssen OP, Pratesi P, Spínola MA, Jonaitis L, Pérez-Aísa Á, Vaira D, Saracino IM, Pavoni M, Fiorini G, Tepes B, et al. Analysis of Clinical Phenotypes through Machine Learning of First-Line H. pylori Treatment in Europe during the Period 2013–2022: Data from the European Registry on H. pylori Management (Hp-EuReg). Antibiotics. 2023; 12(9):1427. https://doi.org/10.3390/antibiotics12091427
Chicago/Turabian StyleNyssen, Olga P., Pietro Pratesi, Miguel A. Spínola, Laimas Jonaitis, Ángeles Pérez-Aísa, Dino Vaira, Ilaria Maria Saracino, Matteo Pavoni, Giulia Fiorini, Bojan Tepes, and et al. 2023. "Analysis of Clinical Phenotypes through Machine Learning of First-Line H. pylori Treatment in Europe during the Period 2013–2022: Data from the European Registry on H. pylori Management (Hp-EuReg)" Antibiotics 12, no. 9: 1427. https://doi.org/10.3390/antibiotics12091427
APA StyleNyssen, O. P., Pratesi, P., Spínola, M. A., Jonaitis, L., Pérez-Aísa, Á., Vaira, D., Saracino, I. M., Pavoni, M., Fiorini, G., Tepes, B., Bordin, D. S., Voynovan, I., Lanas, Á., Martínez-Domínguez, S. J., Alfaro, E., Bujanda, L., Pabón-Carrasco, M., Hernández, L., Gasbarrini, A., ... on behalf of the Hp-EuReg Investigators. (2023). Analysis of Clinical Phenotypes through Machine Learning of First-Line H. pylori Treatment in Europe during the Period 2013–2022: Data from the European Registry on H. pylori Management (Hp-EuReg). Antibiotics, 12(9), 1427. https://doi.org/10.3390/antibiotics12091427















