Saponin-Derived Silver Nanoparticles from Phoenix dactylifera (Ajwa Dates) Exhibit Broad-Spectrum Bioactivities Combating Bacterial Infections
Abstract
:1. Introduction
2. Materials and Methods
2.1. Collection of Ajwa Dates
2.2. Extraction of Crude Saponins
2.3. Foam Test for Saponins
2.4. Spectrophotometric Analysis of Saponins
2.5. High-Performance Thin-Layer Chromatography (HPTLC)
2.6. Derivatization with p-Anisaldehyde Sulphuric Acid after Chromatography
2.7. Biosynthesis of Silver Nanoparticles Using Extracted Saponins (AgNPs-S)
2.8. Characterization of AgNPs-S
2.9. Antibacterial Activity of AgNPs-S
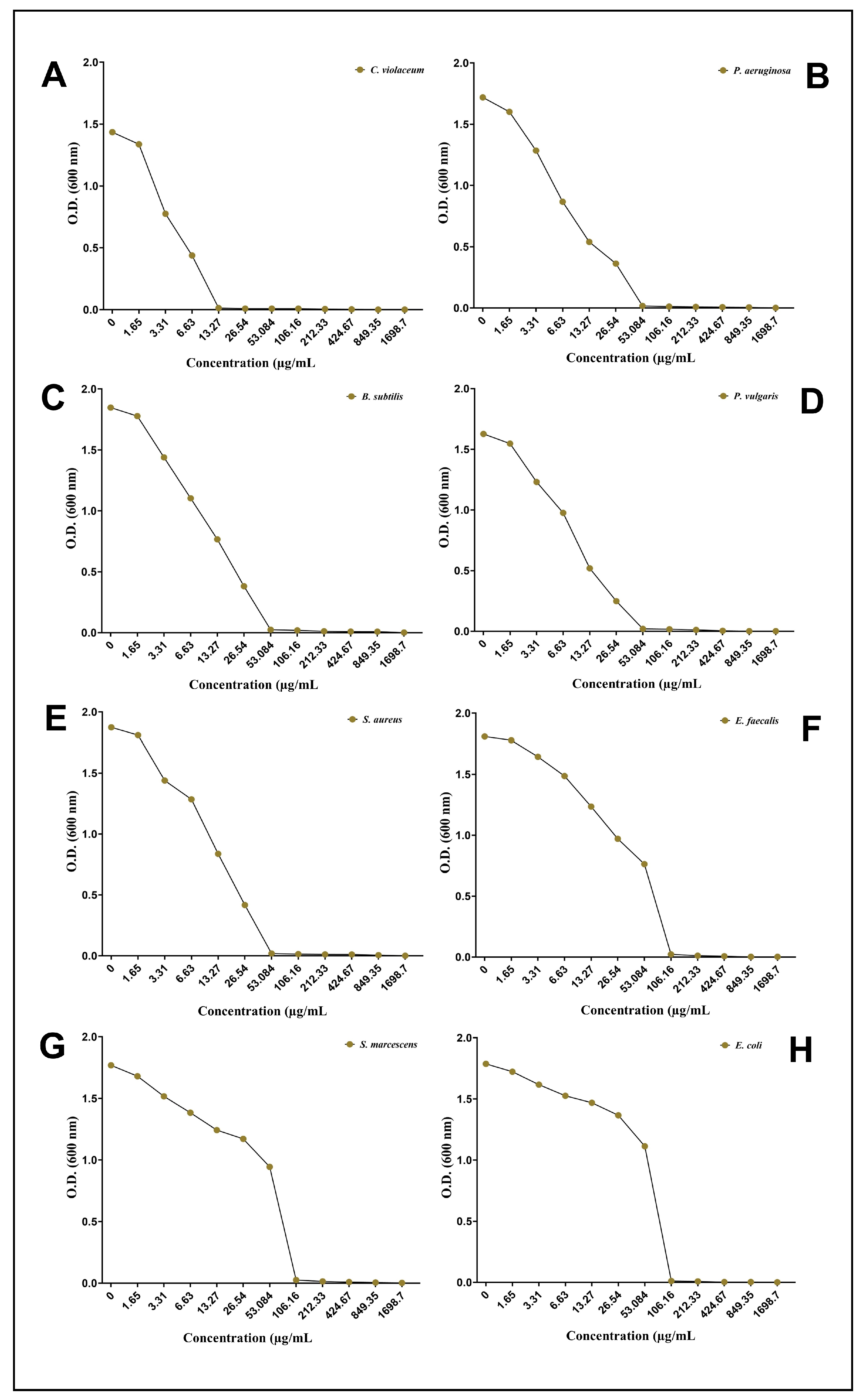
2.10. Determination of Minimum Inhibitory Concentration (MIC)
2.11. Determination of Nucleic Acid Leakage
2.12. Determination of Protein Leakage
2.13. Determination of Antibiofilm Activity
2.14. Determination of the Anti-QS Activity of AgNPs-S
2.15. Assessment of Violacein Pigment Production in C. violaceum
2.16. Assessment of Pyocyanin Pigment Production in P. aeruginosa
2.17. Assessment of Prodigiosin Pigment Production in S. marcescens
2.18. Determination of DPPH Free-Radical-Scavenging Activity
2.19. Determination of the Cytotoxic Potential of AgNPs-S
2.20. Statistical Analysis
3. Results
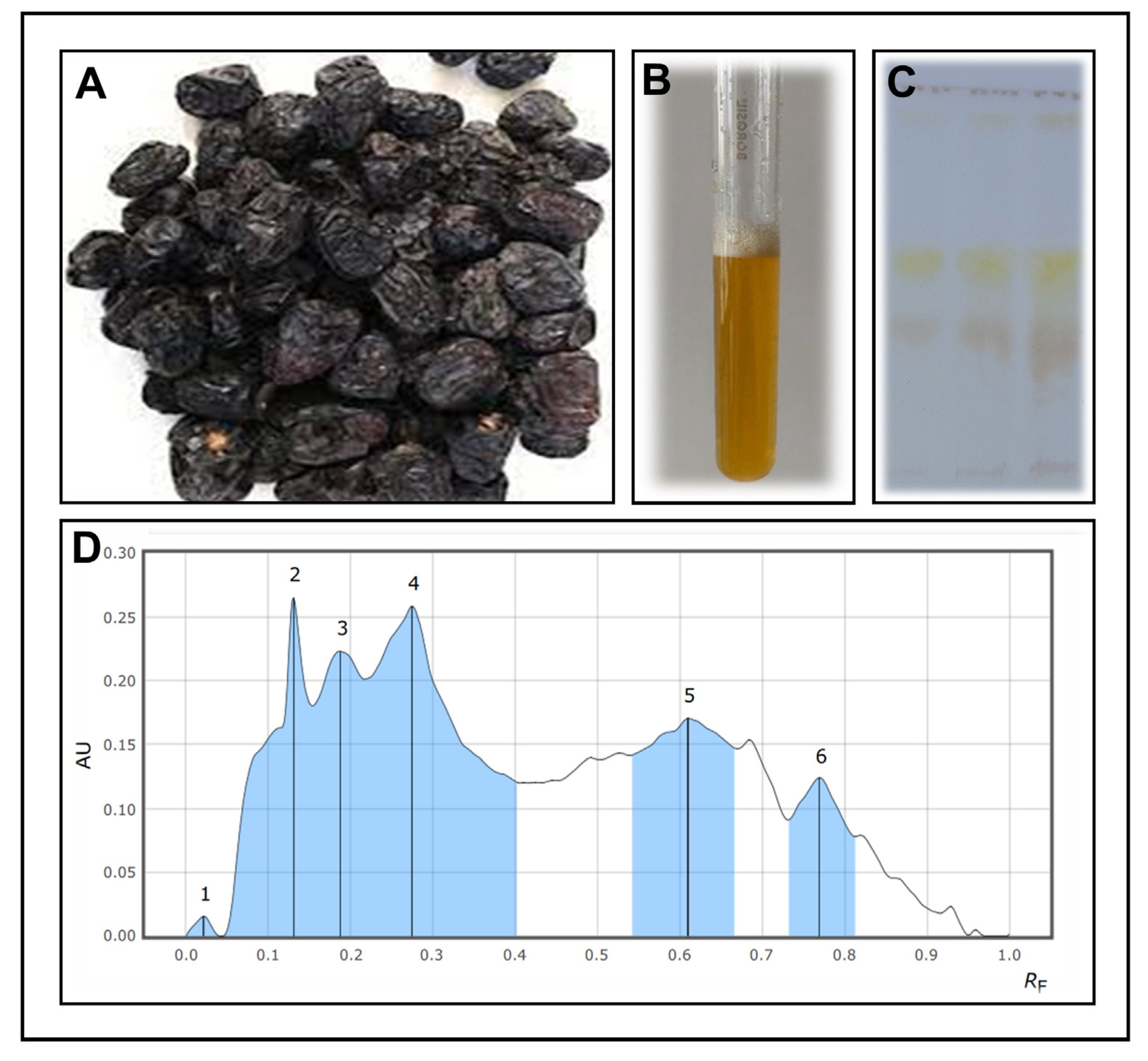
3.1. Extraction and Confirmation of Saponins
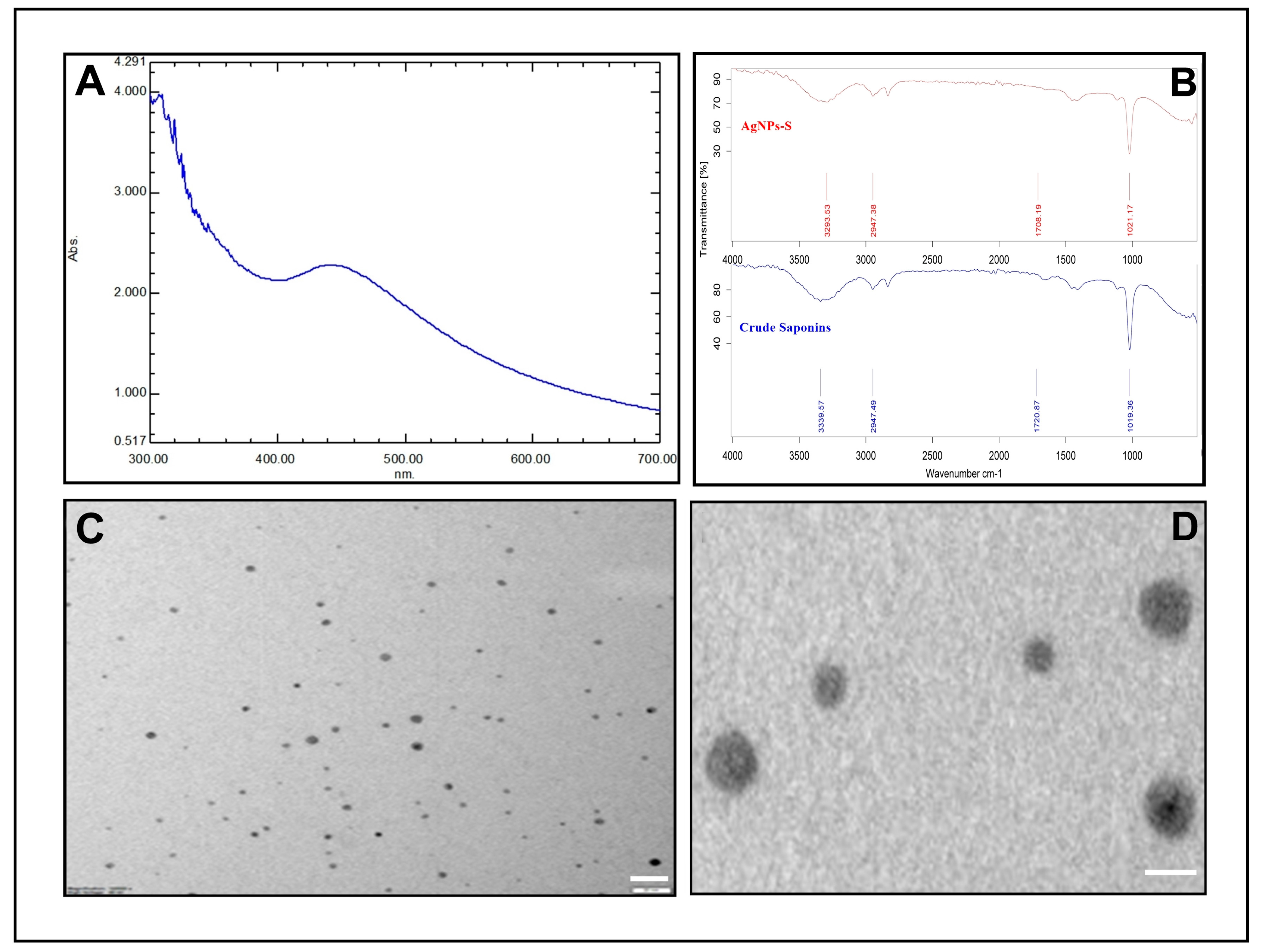
3.2. Synthesis and Characterization of AgNPs-S
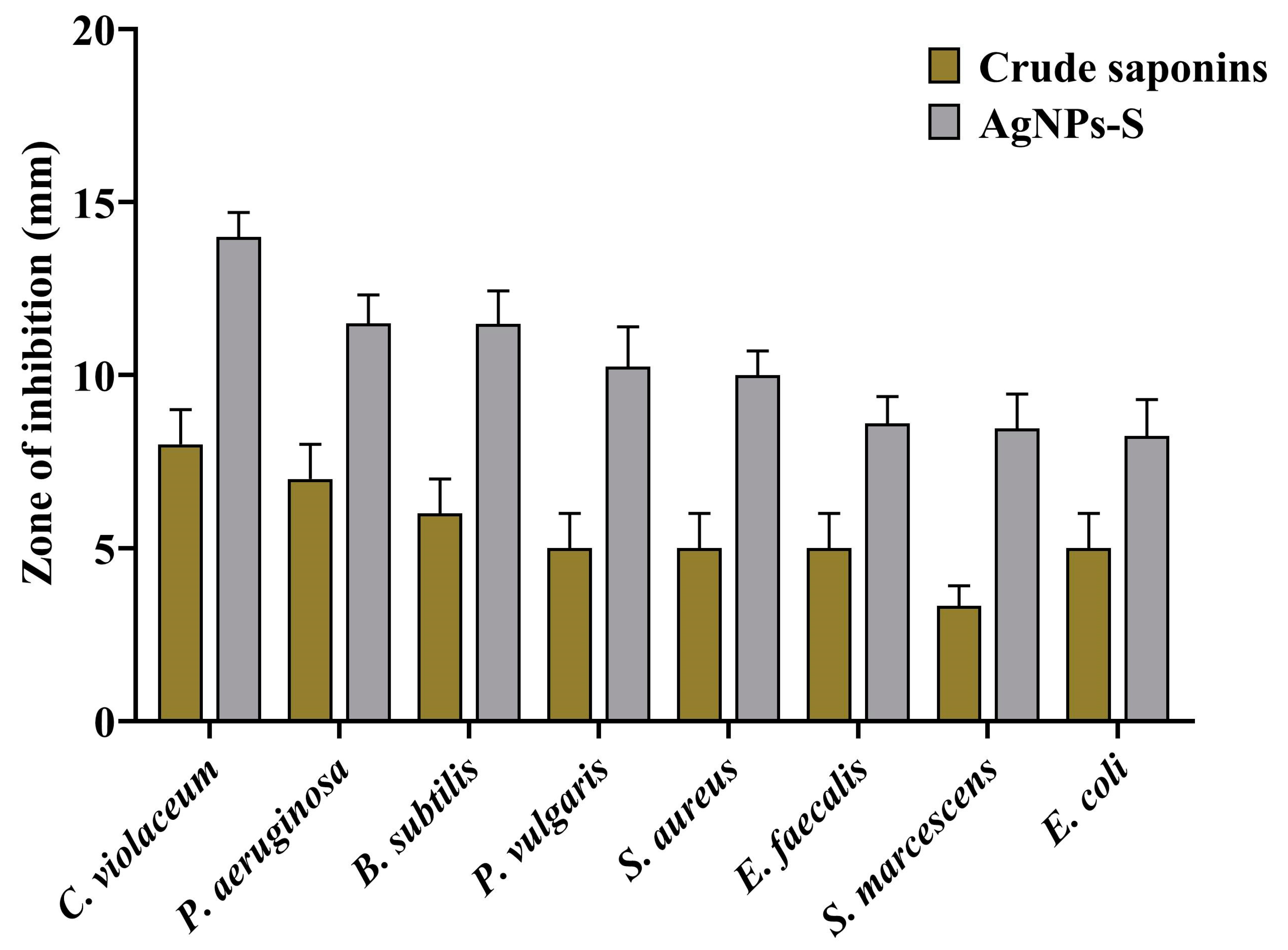
3.3. Antibacterial Potential of AgNPs-S
3.4. Determination of the Effect of AgNPs-S on Nucleic Acids and Protein Leakage
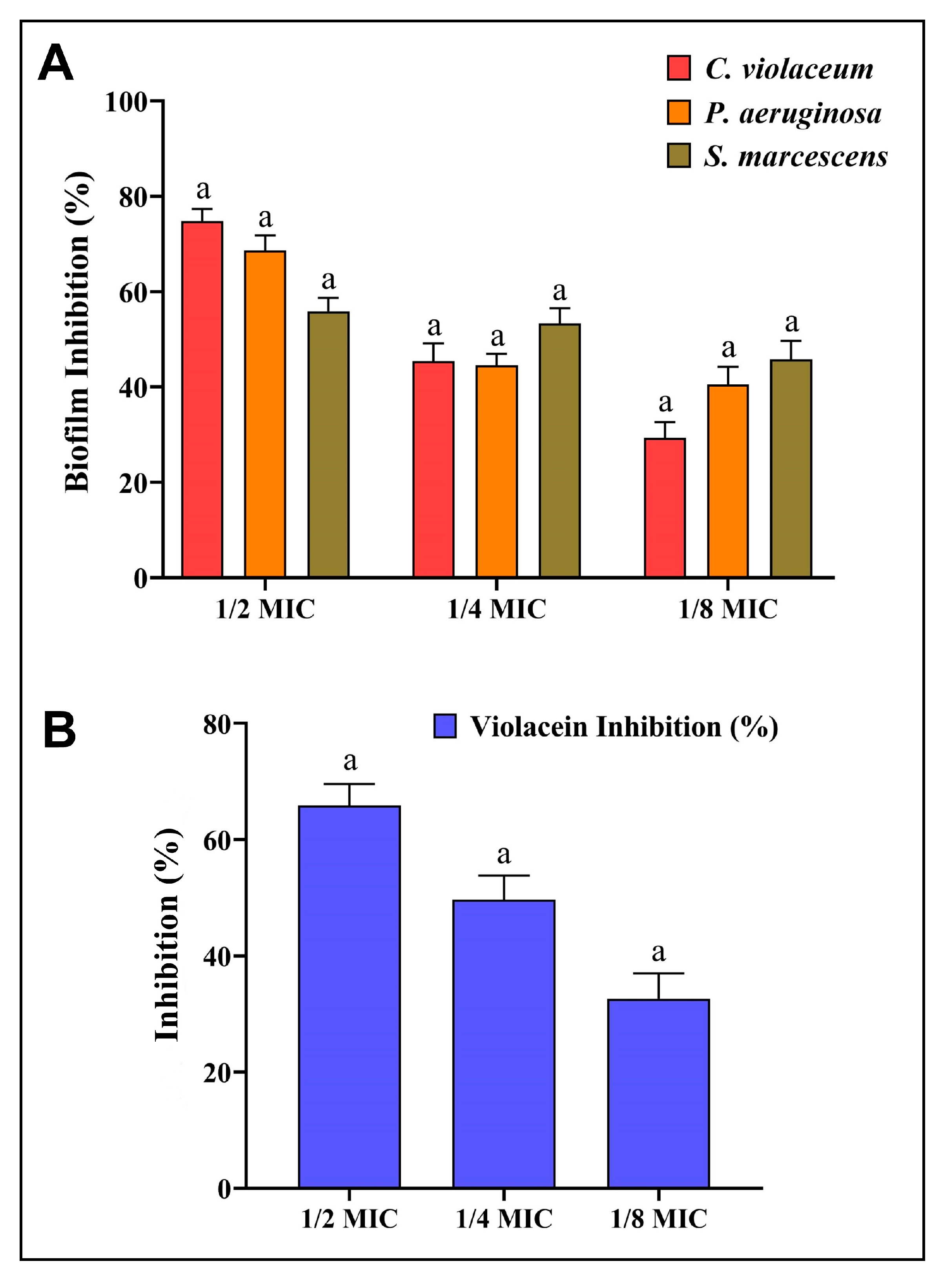
3.5. Antibiofilm Potential of AgNPs-S
3.6. Anti-QS Potential of AgNPs-S
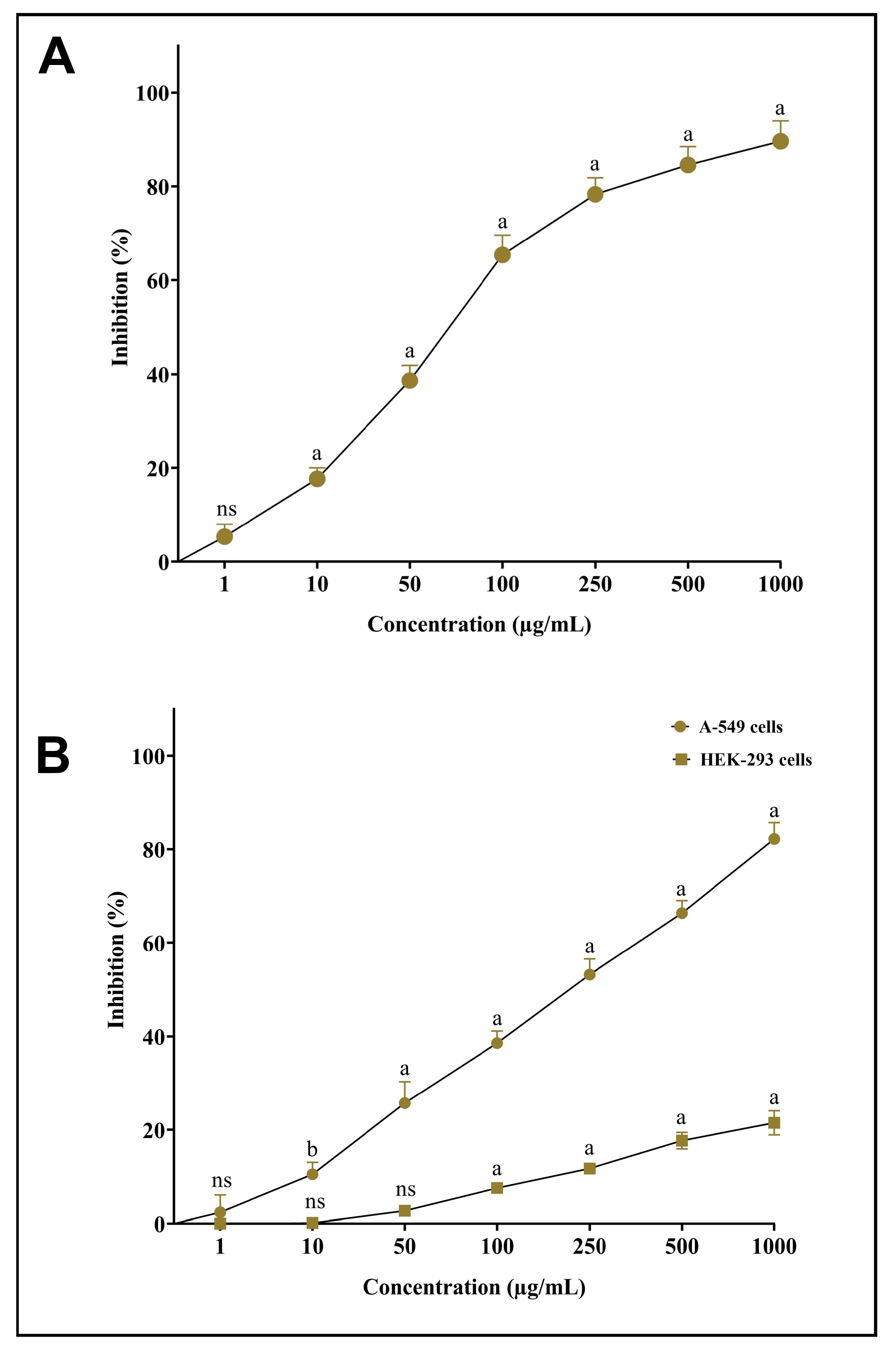
3.7. In Vitro Antioxidant and Cytotoxic Potential of AgNPs-S
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bayda, S.; Adeel, M.; Tuccinardi, T.; Cordani, M.; Rizzolio, F. The history of nanoscience and nanotechnology: From chemical–physical applications to nanomedicine. Molecules 2019, 25, 112. [Google Scholar] [CrossRef] [PubMed]
- Soliman, M.K.; Salem, S.S.; Abu-Elghait, M.; Azab, M.S. Biosynthesis of silver and gold nanoparticles and their efficacy towards antibacterial, antibiofilm, cytotoxicity, and antioxidant activities. Appl. Biochem. Biotechnol. 2023, 195, 1158–1183. [Google Scholar] [CrossRef] [PubMed]
- Alsaba, M.T.; Al Dushaishi, M.F.; Abbas, A.K. A comprehensive review of nanoparticles applications in the oil and gas industry. J. Pet. Explor. Prod. Technol. 2020, 10, 1389–1399. [Google Scholar] [CrossRef]
- Kumar, H.; Bhardwaj, K.; Kuča, K.; Kalia, A.; Nepovimova, E.; Verma, R.; Kumar, D. Flower-based green synthesis of metallic nanoparticles: Applications beyond fragrance. Nanomaterials 2020, 10, 766. [Google Scholar] [CrossRef] [PubMed]
- Martínez, G.; Merinero, M.; Pérez-Aranda, M.; Pérez-Soriano, E.M.; Ortiz, T.; Villamor, E.; Begines, B.; Alcudia, A. Environmental impact of nanoparticles’ application as an emerging technology: A review. Materials 2020, 14, 166. [Google Scholar] [CrossRef] [PubMed]
- Lines, M. Nanomaterials for practical functional uses. J. Alloys Compd. 2008, 449, 242–245. [Google Scholar] [CrossRef]
- Ozak, S.T.; Ozkan, P. Nanotechnology and dentistry. Eur. J. Dent. 2013, 7, 145–151. [Google Scholar]
- Yaqoob, A.A.; Umar, K.; Ibrahim, M.N.M. Silver nanoparticles: Various methods of synthesis, size affecting factors and their potential applications–a review. Appl. Nanosci. 2020, 10, 1369–1378. [Google Scholar] [CrossRef]
- Shanmuganathan, R.; Karuppusamy, I.; Saravanan, M.; Muthukumar, H.; Ponnuchamy, K.; Ramkumar, V.S.; Pugazhendhi, A. Synthesis of silver nanoparticles and their biomedical applications—A comprehensive review. Curr. Pharm. Des. 2019, 25, 2650–2660. [Google Scholar] [CrossRef]
- Some, S.; Sen, I.K.; Mandal, A.; Aslan, T.; Ustun, Y.; Yilmaz, E.Ş.; Katı, A.; Demirbas, A.; Mandal, A.K.; Ocsoy, I. Biosynthesis of silver nanoparticles and their versatile antimicrobial properties. Mater. Res. Express 2018, 6, 012001. [Google Scholar] [CrossRef]
- Keat, C.L.; Aziz, A.; Eid, A.M.; Elmarzugi, N.A. Biosynthesis of nanoparticles and silver nanoparticles. Bioresour. Bioprocess. 2015, 2, 47. [Google Scholar] [CrossRef]
- Syafiuddin, A.; Salmiati; Salim, M.R.; Beng Hong Kueh, A.; Hadibarata, T.; Nur, H. A review of silver nanoparticles: Research trends, global consumption, synthesis, properties, and future challenges. J. Chin. Chem. Soc. 2017, 64, 732–756. [Google Scholar] [CrossRef]
- Yusuf, M. Silver nanoparticles: Synthesis and applications. Handb. Ecomater. 2019, 2343. [Google Scholar] [CrossRef]
- Mohammadlou, M.; Maghsoudi, H.; Jafarizadeh-Malmiri, H. A review on green silver nanoparticles based on plants: Synthesis, potential applications and eco-friendly approach. Int. Food Res. J. 2016, 23, 446. [Google Scholar]
- Walusansa, A.; Asiimwe, S.; Nakavuma, J.; Ssenku, J.; Katuura, E.; Kafeero, H.; Aruhomukama, D.; Nabatanzi, A.; Anywar, G.; Tugume, A.K.; et al. Antibiotic-resistance in medically important bacteria isolated from commercial herbal medicines in Africa from 2000 to 2021: A systematic review and meta-analysis. Antimicrob. Resist. Infect. Control 2022, 11, 11. [Google Scholar] [CrossRef]
- Ayukekbong, J.A.; Ntemgwa, M.; Atabe, A.N. The threat of antimicrobial resistance in developing countries: Causes and control strategies. Antimicrob. Resist. Infect. Control 2017, 6, 47. [Google Scholar] [CrossRef]
- Alfred Ngenge, T.; Kucukaydin, S.; Ceylan, O.; Duru, M.E. Evaluation of enzyme inhibition and anti-quorum sensing potentials of Melaleuca alternifolia and Citrus sinensis essential oils. Nat. Prod. Commun. 2021, 16, 1934578X211044565. [Google Scholar] [CrossRef]
- Yum, S.-j.; Kwon, J.H.; Lee, K.-T.; Park, J.-T.; Jeong, H.-G. Efficacy of pristimerin against Staphylococcus aureus planktonic cultures and biofilms. LWT 2022, 164, 113627. [Google Scholar] [CrossRef]
- Abebe, G.M. The role of bacterial biofilm in antibiotic resistance and food contamination. Int. J. Microbiol. 2020, 2020, 1705814. [Google Scholar] [CrossRef]
- Skandamis, P.N.; Nychas, G.-J.E. Quorum sensing in the context of food microbiology. Appl. Environ. Microbiol. 2012, 78, 5473–5482. [Google Scholar] [CrossRef]
- Tamfu, A.N.; Ceylan, O.; Cârâc, G.; Talla, E.; Dinica, R.M. Antibiofilm and anti-quorum sensing potential of cycloartane-type triterpene acids from cameroonian grassland propolis: Phenolic profile and antioxidant activity of crude extract. Molecules 2022, 27, 4872. [Google Scholar] [CrossRef] [PubMed]
- Mittal, A.K.; Chisti, Y.; Banerjee, U.C. Synthesis of metallic nanoparticles using plant extracts. Biotechnol. Adv. 2013, 31, 346–356. [Google Scholar] [CrossRef] [PubMed]
- Dauthal, P.; Mukhopadhyay, M. Noble metal nanoparticles: Plant-mediated synthesis, mechanistic aspects of synthesis, and applications. Ind. Eng. Chem. Res. 2016, 55, 9557–9577. [Google Scholar] [CrossRef]
- Singh, P.; Kim, Y.-J.; Zhang, D.; Yang, D.-C. Biological synthesis of nanoparticles from plants and microorganisms. Trends Biotechnol. 2016, 34, 588–599. [Google Scholar] [CrossRef]
- Ahmed, S.; Ahmad, M.; Swami, B.L.; Ikram, S. A review on plants extract mediated synthesis of silver nanoparticles for antimicrobial applications: A green expertise. J. Adv. Res. 2016, 7, 17–28. [Google Scholar] [CrossRef]
- Zhang, C.-R.; Aldosari, S.A.; Vidyasagar, P.S.; Nair, K.M.; Nair, M.G. Antioxidant and anti-inflammatory assays confirm bioactive compounds in Ajwa date fruit. J. Agric. Food Chem. 2013, 61, 5834–5840. [Google Scholar] [CrossRef]
- Al-Shahib, W.; Marshall, R.J. The fruit of the date palm: Its possible use as the best food for the future? Int. J. Food Sci. Nutr. 2003, 54, 247–259. [Google Scholar] [CrossRef]
- Al-Farsi, M.A.; Lee, C.Y. Nutritional and functional properties of dates: A review. Crit. Rev. Food Sci. Nutr. 2008, 48, 877–887. [Google Scholar] [CrossRef]
- Hasan, N.S.; Amom, Z.H.; Nor, A.; Norhafizah, M.; Norhaizan, M.E.; Azrina, A. Nutritional composition and in vitro evaluation of the antioxidant properties of various dates extracts (Phoenix dactylifera L.) from Libya. Asian J. Clin. Nutr. 2010, 2, 208–214. [Google Scholar] [CrossRef]
- Khalid, S.; Khalid, N.; Khan, R.S.; Ahmed, H.; Ahmad, A. A review on chemistry and pharmacology of Ajwa date fruit and pit. Trends Food Sci. Technol. 2017, 63, 60–69. [Google Scholar] [CrossRef]
- Edeoga, H.O.; Okwu, D.; Mbaebie, B. Phytochemical constituents of some Nigerian medicinal plants. Afr. J. Biotechnol. 2005, 4, 685–688. [Google Scholar] [CrossRef]
- Uematsu, Y.; Hirata, K.; Saito, K.; Kudo, I. Spectrophotometric determination of saponin in Yucca extract used as food additive. J. AOAC Int. 2000, 83, 1451–1454. [Google Scholar] [CrossRef] [PubMed]
- Bobby, M.N.; Wesely, E.; Johnson, M. High performance thin layer chromatography profile studies on the alkaloids of Albizia lebbeck. Asian Pac. J. Trop. Biomed. 2012, 2, S1–S6. [Google Scholar] [CrossRef]
- Venkatesh, P.; Mukherjee, P.K.; Kumar, N.S.; Bandyopadhyay, A.; Fukui, H.; Mizuguchi, H.; Islam, N. Anti-allergic activity of standardized extract of Albizia lebbeck with reference to catechin as a phytomarker. Immunopharmacol. Immunotoxicol. 2010, 32, 272–276. [Google Scholar] [CrossRef]
- Atarod, M.; Nasrollahzadeh, M.; Sajadi, S.M. Green synthesis of Pd/RGO/Fe3O4 nanocomposite using Withania coagulans leaf extract and its application as magnetically separable and reusable catalyst for the reduction of 4-nitrophenol. J. Colloid Interface Sci. 2016, 465, 249–258. [Google Scholar] [CrossRef]
- Liu, C.; Xu, B.; McClements, D.J.; Xu, X.; Cui, S.; Gao, L.; Zhou, L.; Xiong, L.; Sun, Q.; Dai, L. Properties of curcumin-loaded zein-tea saponin nanoparticles prepared by antisolvent co-precipitation and precipitation. Food Chem. 2022, 391, 133224. [Google Scholar] [CrossRef]
- Geethalakshmi, R.; Sarada, D. Characterization and antimicrobial activity of gold and silver nanoparticles synthesized using saponin isolated from Trianthema decandra L. Ind. Crops Prod. 2013, 51, 107–115. [Google Scholar]
- Muniyan, A.; Ravi, K.; Mohan, U.; Panchamoorthy, R. Characterization and in vitro antibacterial activity of saponin-conjugated silver nanoparticles against bacteria that cause burn wound infection. World J. Microbiol. Biotechnol. 2017, 33, 147. [Google Scholar] [CrossRef]
- Shameli, K.; Ahmad, M.B.; Yunus, W.M.Z.W.; Ibrahim, N.A.; Rahman, R.A.; Jokar, M.; Darroudi, M. Silver/poly (lactic acid) nanocomposites: Preparation, characterization, and antibacterial activity. Int. J. Nanomed. 2010, 5, 573–579. [Google Scholar] [CrossRef]
- Biedenbach, D.; Lob, S.; Badal, R.; Sahm, D. Variability of Susceptibility and Multidrug Resistance among K. pneumoniae from IAI in Asia/Pacific Countries–SMART 2012–2013. Int. J. Antimicrob. Agents PO BOX 2015, 211, 1000. [Google Scholar]
- Chen, C.Z.; Cooper, S.L. Interactions between dendrimer biocides and bacterial membranes. Biomaterials 2002, 23, 3359–3368. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Ghaima, K.K.; Rasheed, S.F.; Ahmed, E.F. Antibiofilm, antibacterial and antioxidant activities of water extract of Calendula officinalis flowers. Int. J. Biol. Pharm. Res. 2013, 4, 465–470. [Google Scholar]
- Zahin, M.; Hasan, S.; Aqil, F.; Khan, M.; Ahmad, S.; Husain, F.M.; Ahmad, I. Screening of certain medicinal plants from India for their anti-quorum sensing activity. Indian J. Exp. Boil. 2010, 48, 1219–1224. [Google Scholar]
- Matz, C.; Deines, P.; Boenigk, J.; Arndt, H.; Eberl, L.; Kjelleberg, S.; Jürgens, K. Impact of violacein-producing bacteria on survival and feeding of bacterivorous nanoflagellates. Appl. Environ. Microbiol. 2004, 70, 1593–1599. [Google Scholar] [CrossRef]
- Essar, D.W.; Eberly, L.; Hadero, A.; Crawford, I. Identification and characterization of genes for a second anthranilate synthase in Pseudomonas aeruginosa: Interchangeability of the two anthranilate synthases and evolutionary implications. J. Bacteriol. 1990, 172, 884–900. [Google Scholar] [CrossRef]
- Slater, H.; Crow, M.; Everson, L.; Salmond, G.P. Phosphate availability regulates biosynthesis of two antibiotics, prodigiosin and carbapenem, in Serratia via both quorum-sensing-dependent and-independent pathways. Mol. Microbiol. 2003, 47, 303–320. [Google Scholar] [CrossRef]
- Bhakya, S.; Muthukrishnan, S.; Sukumaran, M.; Muthukumar, M. Biogenic synthesis of silver nanoparticles and their antioxidant and antibacterial activity. Appl. Nanosci. 2016, 6, 755–766. [Google Scholar] [CrossRef]
- Kareem, O.; Ali, T.; Dar, L.A.; Mir, S.A.; Rashid, R.; Nazli, N.; Gulzar, T.; Bader, G. Positive Health Benefits of Saponins from Edible Legumes: Phytochemistry and Pharmacology. In Edible Plants in Health and Diseases: Volume II: Phytochemical and Pharmacological Properties; Springer: Singapore, 2022; pp. 279–298. [Google Scholar]
- Oleszek, M.; Oleszek, W. Saponins in Food. In Handbook of Dietary Phytochemicals; Springer: Singapore, 2020. [Google Scholar]
- Kora, A.J. Plant saponin biosurfactants used as soap, hair cleanser, and detergent in India. Appl. Next Gener. Biosurfactants Food Sect. 2023, 459–477. [Google Scholar] [CrossRef]
- Desai, S.D.; Desai, D.G.; Kaur, H. Saponins and their biological activities. Pharma Times 2009, 41, 13–16. [Google Scholar]
- Fleck, J.D.; Betti, A.H.; Da Silva, F.P.; Troian, E.A.; Olivaro, C.; Ferreira, F.; Verza, S.G. Saponins from Quillaja saponaria and Quillaja brasiliensis: Particular chemical characteristics and biological activities. Molecules 2019, 24, 171. [Google Scholar] [CrossRef] [PubMed]
- Rai, S.; Acharya-Siwakoti, E.; Kafle, A.; Devkota, H.P.; Bhattarai, A. Plant-derived saponins: A review of their surfactant properties and applications. Sci 2021, 3, 44. [Google Scholar] [CrossRef]
- Sparg, S.; Light, M.; Van Staden, J. Biological activities and distribution of plant saponins. J. Ethnopharmacol. 2004, 94, 219–243. [Google Scholar] [CrossRef] [PubMed]
- Sani, I. Proximate Analysis, Phytochemical Screening And Antioxidant Potential Of Ajwa Date From Medina, Saudi Arabia. Int. Res. J. Pharm. Biosci. 2015, 2, 12–17. [Google Scholar]
- Debnath, B.; Das, R. Controlled synthesis of saponin-capped silver nanotriangles and their optical properties. Plasmonics 2019, 14, 1365–1375. [Google Scholar] [CrossRef]
- Srivastava, N.; Choudhary, M.; Singhal, G.; Bhagyawant, S.S. SEM studies of saponin silver nanoparticles isolated from leaves of Chenopodium album L. for in vitro anti-acne activity. Proc. Natl. Acad. Sci. India Sect. B Biol. Sci. 2020, 90, 333–341. [Google Scholar] [CrossRef]
- Singh, J.; Bajaj, R.; Harpreet, K.; Harjot, K.; Navneet, K.; Sukhmeen, K.; Muhit, R. Chemo-bio synthesis of silver nanoparticles. J. Nanomed. Res. 2016, 4, 00092. [Google Scholar]
- Nguyen, D.H.; Vo, T.N.N.; Le, N.T.T.; Thi, D.P.N.; Thi, T.T.H. Evaluation of saponin-rich/poor leaf extract-mediated silver nanoparticles and their antifungal capacity. Green Process. Synth. 2020, 9, 429–439. [Google Scholar] [CrossRef]
- Paramesh, C.C.; Halligudra, G.; Gangaraju, V.; Sriramoju, J.B.; Shastri, M.; Rangappa, D.; Subbegowda, R.K.; Shivaramu, P.D. Silver nanoparticles synthesized using saponin extract of Simarouba glauca oil seed meal as effective, recoverable and reusable catalyst for reduction of organic dyes. Results Surf. Interfaces 2021, 3, 100005. [Google Scholar] [CrossRef]
- Uddin, T.M.; Chakraborty, A.J.; Khusro, A.; Zidan, B.R.M.; Mitra, S.; Emran, T.B.; Dhama, K.; Ripon, M.K.H.; Gajdács, M.; Sahibzada, M.U.K.; et al. Antibiotic resistance in microbes: History, mechanisms, therapeutic strategies and future prospects. J. Infect. Public Health 2021, 14, 1750–1766. [Google Scholar] [CrossRef]
- Adnan, M.; Patel, M.; Deshpande, S.; Alreshidi, M.; Siddiqui, A.J.; Reddy, M.N.; Emira, N.; De Feo, V. Effect of Adiantum philippense extract on biofilm formation, adhesion with its antibacterial activities against foodborne pathogens, and characterization of bioactive metabolites: An in vitro-in silico approach. Front. Microbiol. 2020, 11, 823. [Google Scholar] [CrossRef] [PubMed]
- Awadelkareem, A.M.; Siddiqui, A.J.; Noumi, E.; Ashraf, S.A.; Hadi, S.; Snoussi, M.; Badraoui, R.; Bardakci, F.; Ashraf, M.S.; Danciu, C. Biosynthesized Silver Nanoparticles Derived from Probiotic Lactobacillus rhamnosus (AgNPs-LR) Targeting Biofilm Formation and Quorum Sensing-Mediated Virulence Factors. Antibiotics 2023, 12, 986. [Google Scholar] [CrossRef] [PubMed]
- Patel, M.; Ashraf, M.S.; Siddiqui, A.J.; Ashraf, S.A.; Sachidanandan, M.; Snoussi, M.; Adnan, M.; Hadi, S. Profiling and role of bioactive molecules from puntius sophore (Freshwater/brackish fish) skin mucus with its potent antibacterial, antiadhesion, and antibiofilm activities. Biomolecules 2020, 10, 920. [Google Scholar] [CrossRef]
- Dufour, D.; Leung, V.; Lévesque, C.M. Bacterial biofilm: Structure, function, and antimicrobial resistance. Endod. Top. 2010, 22, 2–16. [Google Scholar] [CrossRef]
- Alam, A.; Kumar, A.; Tripathi, P.; Ehtesham, N.Z.; Hasnain, S.E. Biofilms: A phenotypic mechanism of bacteria conferring tolerance against stress and antibiotics. In Mycobacterium Tuberculosis: Molecular Infection Biology, Pathogenesis, Diagnostics and New Interventions; Springer: Singapore, 2019; pp. 315–333. [Google Scholar]
- Nirwati, H.; Sinanjung, K.; Fahrunissa, F.; Wijaya, F.; Napitupulu, S.; Hati, V.P.; Hakim, M.S.; Meliala, A.; Aman, A.T.; Nuryastuti, T. Biofilm formation and antibiotic resistance of Klebsiella pneumoniae isolated from clinical samples in a tertiary care hospital, Klaten, Indonesia. BMC Proc. 2019, 13, 20. [Google Scholar] [CrossRef]
- Vestby, L.K.; Grønseth, T.; Simm, R.; Nesse, L.L. Bacterial biofilm and its role in the pathogenesis of disease. Antibiotics 2020, 9, 59. [Google Scholar] [CrossRef]
- Bhattacharya, M.; Wozniak, D.J.; Stoodley, P.; Hall-Stoodley, L. Prevention and treatment of Staphylococcus aureus biofilms. Expert Rev. Anti-Infect. Ther. 2015, 13, 1499–1516. [Google Scholar] [CrossRef]
- Khatoon, Z.; McTiernan, C.D.; Suuronen, E.J.; Mah, T.-F.; Alarcon, E.I. Bacterial biofilm formation on implantable devices and approaches to its treatment and prevention. Heliyon 2018, 4, e01067. [Google Scholar] [CrossRef]
- Koo, H.; Allan, R.N.; Howlin, R.P.; Stoodley, P.; Hall-Stoodley, L. Targeting microbial biofilms: Current and prospective therapeutic strategies. Nat. Rev. Microbiol. 2017, 15, 740–755. [Google Scholar] [CrossRef]
- Markowska, K.; Grudniak, A.; Wolska, K. Silver nanoparticles as an alternative strategy against bacterial biofilms. Acta Biochim. Pol. 2013, 60, 523–530. [Google Scholar] [CrossRef]
- Römling, U.; Balsalobre, C. Biofilm infections, their resilience to therapy and innovative treatment strategies. J. Intern. Med. 2012, 272, 541–561. [Google Scholar] [CrossRef] [PubMed]
- Paluch, E.; Rewak-Soroczyńska, J.; Jędrusik, I.; Mazurkiewicz, E.; Jermakow, K. Prevention of biofilm formation by quorum quenching. Appl. Microbiol. Biotechnol. 2020, 104, 1871–1881. [Google Scholar] [CrossRef] [PubMed]
- Preda, V.G.; Săndulescu, O. Communication is the key: Biofilms, quorum sensing, formation and prevention. Discoveries 2019, 7, e10. [Google Scholar] [CrossRef] [PubMed]
- Tagousop, C.N.; Tamokou, J.-d.-D.; Kengne, I.C.; Ngnokam, D.; Voutquenne-Nazabadioko, L. Antimicrobial activities of saponins from Melanthera elliptica and their synergistic effects with antibiotics against pathogenic phenotypes. Chem. Cent. J. 2018, 12, 97. [Google Scholar] [CrossRef] [PubMed]
- Zhong, J.; Tan, L.; Chen, M.; He, C. Pharmacological activities and molecular mechanisms of Pulsatilla saponins. Chin. Med. 2022, 17, 59. [Google Scholar] [CrossRef]
- Parvekar, P.; Palaskar, J.; Metgud, S.; Maria, R.; Dutta, S. The minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC) of silver nanoparticles against Staphylococcus aureus. Biomater. Investig. Dent. 2020, 7, 105–109. [Google Scholar] [CrossRef]
- Van de Vel, E.; Sampers, I.; Raes, K. A review on influencing factors on the minimum inhibitory concentration of essential oils. Crit. Rev. Food Sci. Nutr. 2019, 59, 357–378. [Google Scholar] [CrossRef]
- Kowalska-Krochmal, B.; Dudek-Wicher, R. The minimum inhibitory concentration of antibiotics: Methods, interpretation, clinical relevance. Pathogens 2021, 10, 165. [Google Scholar] [CrossRef]
- Walsh, C. Molecular mechanisms that confer antibacterial drug resistance. Nature 2000, 406, 775–781. [Google Scholar] [CrossRef]
- Mansoor, S.; Zahoor, I.; Baba, T.; Padder, S.; Bhat, Z.; Koul, A.; Jiang, L. Fabrication of silver nanoparticles against fungal pathogens. Front. Nanotechnol. 2021, 3, 679358. [Google Scholar] [CrossRef]
- Alavi, M.; Hamblin, M.R. Antibacterial silver nanoparticles: Effects on bacterial nucleic acids. Cell. Mol. Biomed. Rep. 2023, 3, 35–40. [Google Scholar] [CrossRef]
- Gurunathan, S.; Han, J.W.; Kwon, D.-N.; Kim, J.-H. Enhanced antibacterial and anti-biofilm activities of silver nanoparticles against Gram-negative and Gram-positive bacteria. Nanoscale Res. Lett. 2014, 9, 373. [Google Scholar] [CrossRef] [PubMed]
- Mohanta, Y.K.; Biswas, K.; Jena, S.K.; Hashem, A.; Abd_Allah, E.F.; Mohanta, T.K. Anti-biofilm and antibacterial activities of silver nanoparticles synthesized by the reducing activity of phytoconstituents present in the Indian medicinal plants. Front. Microbiol. 2020, 11, 1143. [Google Scholar] [CrossRef] [PubMed]
- Siddique, M.H.; Aslam, B.; Imran, M.; Ashraf, A.; Nadeem, H.; Hayat, S.; Khurshid, M.; Afzal, M.; Malik, I.R.; Shahzad, M. Effect of silver nanoparticles on biofilm formation and EPS production of multidrug-resistant Klebsiella pneumoniae. Biomed Res. Int. 2020, 2020, 6398165. [Google Scholar] [CrossRef]
- Ansari, M.A.; Khan, H.M.; Khan, A.A.; Cameotra, S.S.; Pal, R. Antibiofilm efficacy of silver nanoparticles against biofilm of extended spectrum β-lactamase isolates of Escherichia coli and Klebsiella pneumoniae. Appl. Nanosci. 2014, 4, 859–868. [Google Scholar] [CrossRef]
- Dos Santos, E.M.P.; Martins, C.C.B.; de Oliveira Santos, J.V.; da Silva, W.R.C.; Silva, S.B.C.; Pelagio-Flores, M.A.; Galembeck, A.; Cavalcanti, I.M.F. Silver nanoparticles–chitosan composites activity against resistant bacteria: Tolerance and biofilm inhibition. J. Nanoparticle Res. 2021, 23, 196. [Google Scholar] [CrossRef] [PubMed]
- Adwas, A.A.; Elsayed, A.; Azab, A.; Quwaydir, F. Oxidative stress and antioxidant mechanisms in human body. J. Appl. Biotechnol. Bioeng. 2019, 6, 43–47. [Google Scholar]
- Sharma, N. Free radicals, antioxidants and disease. Biol. Med. 2014, 6, 1. [Google Scholar] [CrossRef]
- López-Alarcón, C.; Denicola, A. Evaluating the antioxidant capacity of natural products: A review on chemical and cellular-based assays. Anal. Chim. Acta 2013, 763, 1–10. [Google Scholar] [CrossRef]
- Bedlovičová, Z.; Strapáč, I.; Baláž, M.; Salayová, A. A brief overview on antioxidant activity determination of silver nanoparticles. Molecules 2020, 25, 3191. [Google Scholar] [CrossRef]
- Keshari, A.K.; Srivastava, R.; Singh, P.; Yadav, V.B.; Nath, G. Antioxidant and antibacterial activity of silver nanoparticles synthesized by Cestrum nocturnum. J. Ayurveda Integr. Med. 2020, 11, 37–44. [Google Scholar] [CrossRef]
- White, P.A.; Oliveira, R.C.; Oliveira, A.P.; Serafini, M.R.; Araújo, A.A.; Gelain, D.P.; Moreira, J.C.; Almeida, J.R.; Quintans, J.S.; Quintans-Junior, L.J.; et al. Antioxidant activity and mechanisms of action of natural compounds isolated from lichens: A systematic review. Molecules 2014, 19, 14496–14527. [Google Scholar] [CrossRef]
- Bade, B.C.; Cruz, C.S.D. Lung cancer 2020: Epidemiology, etiology, and prevention. Clin. Chest Med. 2020, 41, 1–24. [Google Scholar] [CrossRef]
- Huang, C.-Y.; Ju, D.-T.; Chang, C.-F.; Reddy, P.M.; Velmurugan, B.K. A review on the effects of current chemotherapy drugs and natural agents in treating non–small cell lung cancer. Biomedicine 2017, 7, 23. [Google Scholar] [CrossRef] [PubMed]
- Huy, T.Q.; Huyen, P.; Le, A.-T.; Tonezzer, M. Recent advances of silver nanoparticles in cancer diagnosis and treatment. Anticancer Agents Med. Chem. 2020, 20, 1276–1287. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Ning, C.; Zhou, Z.; Yu, P.; Zhu, Y.; Tan, G.; Mao, C. Nanomaterials as photothermal therapeutic agents. Prog. Mater. Sci. 2019, 99, 1–26. [Google Scholar] [CrossRef] [PubMed]
- Gopinath, P.; Gogoi, S.K.; Sanpui, P.; Paul, A.; Chattopadhyay, A.; Ghosh, S.S. Signaling gene cascade in silver nanoparticle induced apoptosis. Colloids Surf. B Biointerfaces 2010, 77, 240–245. [Google Scholar] [CrossRef]
- Mao, B.-H.; Tsai, J.-C.; Chen, C.-W.; Yan, S.-J.; Wang, Y.-J. Mechanisms of silver nanoparticle-induced toxicity and important role of autophagy. Nanotoxicology 2016, 10, 1021–1040. [Google Scholar] [CrossRef]
- Gurunathan, S.; Lee, K.-J.; Kalishwaralal, K.; Sheikpranbabu, S.; Vaidyanathan, R.; Eom, S.H. Antiangiogenic properties of silver nanoparticles. Biomaterials 2009, 30, 6341–6350. [Google Scholar] [CrossRef]
- Ivanova, N.; Gugleva, V.; Dobreva, M.; Pehlivanov, I.; Stefanov, S.; Andonova, V. Silver Nanoparticles as Multifunctional Drug Delivery Systems; IntechOpen: London, UK, 2018. [Google Scholar]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Adnan, M.; Siddiqui, A.J.; Ashraf, S.A.; Ashraf, M.S.; Alomrani, S.O.; Alreshidi, M.; Tepe, B.; Sachidanandan, M.; Danciu, C.; Patel, M. Saponin-Derived Silver Nanoparticles from Phoenix dactylifera (Ajwa Dates) Exhibit Broad-Spectrum Bioactivities Combating Bacterial Infections. Antibiotics 2023, 12, 1415. https://doi.org/10.3390/antibiotics12091415
Adnan M, Siddiqui AJ, Ashraf SA, Ashraf MS, Alomrani SO, Alreshidi M, Tepe B, Sachidanandan M, Danciu C, Patel M. Saponin-Derived Silver Nanoparticles from Phoenix dactylifera (Ajwa Dates) Exhibit Broad-Spectrum Bioactivities Combating Bacterial Infections. Antibiotics. 2023; 12(9):1415. https://doi.org/10.3390/antibiotics12091415
Chicago/Turabian StyleAdnan, Mohd, Arif Jamal Siddiqui, Syed Amir Ashraf, Mohammad Saquib Ashraf, Sarah Owdah Alomrani, Mousa Alreshidi, Bektas Tepe, Manojkumar Sachidanandan, Corina Danciu, and Mitesh Patel. 2023. "Saponin-Derived Silver Nanoparticles from Phoenix dactylifera (Ajwa Dates) Exhibit Broad-Spectrum Bioactivities Combating Bacterial Infections" Antibiotics 12, no. 9: 1415. https://doi.org/10.3390/antibiotics12091415
APA StyleAdnan, M., Siddiqui, A. J., Ashraf, S. A., Ashraf, M. S., Alomrani, S. O., Alreshidi, M., Tepe, B., Sachidanandan, M., Danciu, C., & Patel, M. (2023). Saponin-Derived Silver Nanoparticles from Phoenix dactylifera (Ajwa Dates) Exhibit Broad-Spectrum Bioactivities Combating Bacterial Infections. Antibiotics, 12(9), 1415. https://doi.org/10.3390/antibiotics12091415










