Therapeutic Potential of Chlorhexidine-Loaded Calcium Hydroxide-Based Intracanal Medications in Endo-Periodontal Lesions: An Ex Vivo and In Vitro Study
Abstract
1. Introduction
2. Results
2.1. Antimicrobial Properties of CH + CHX Intracanal Medications
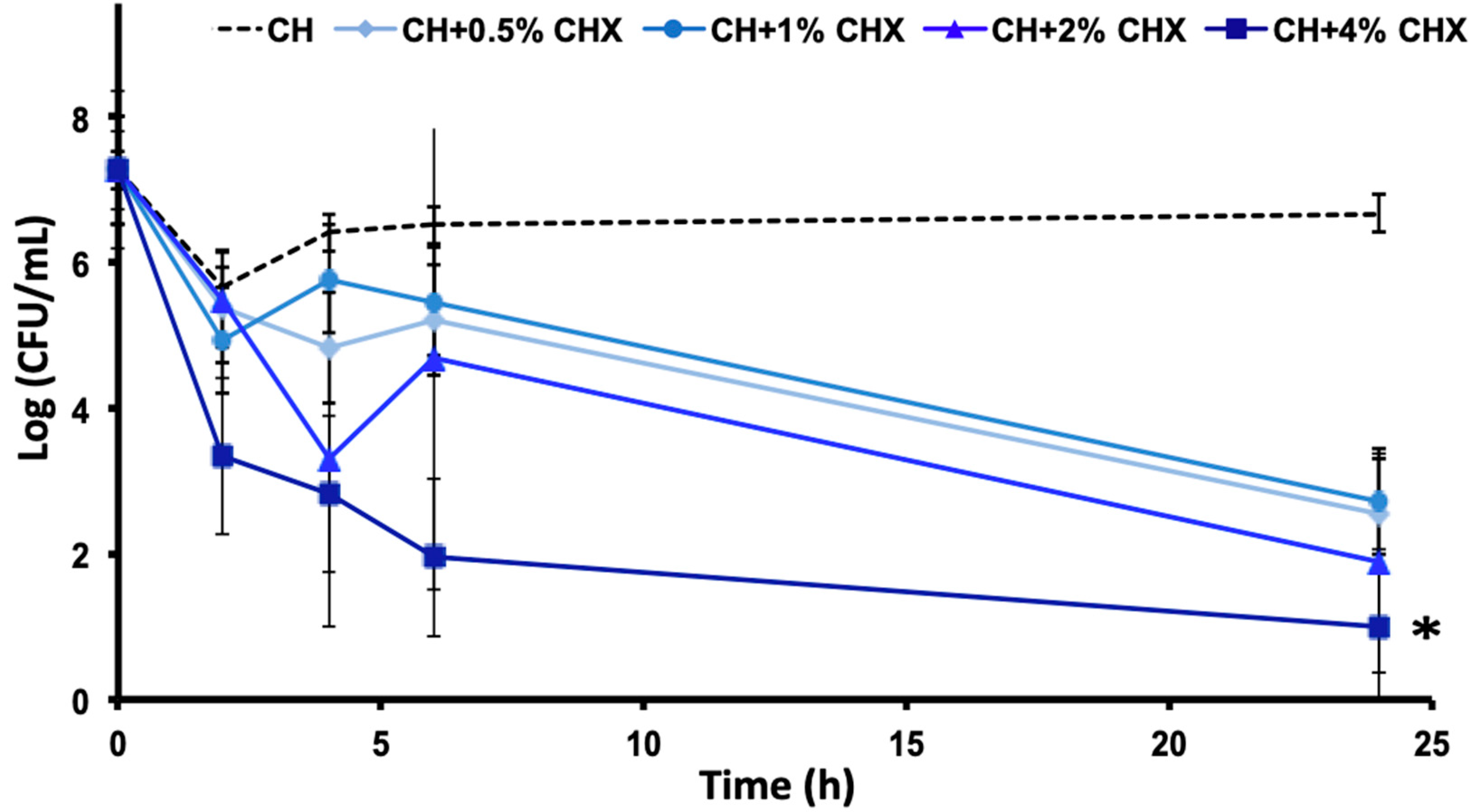
2.1.1. Bacterial Susceptibility to Chlorhexidine (Minimal Inhibitory Concentrations [MIC] and Minimal Bactericidal Concentrations [MBC]) and Time-Kill Assay
2.1.2. Antibiofilm Activity
2.2. Diffusion of Active Components across the Dental Root
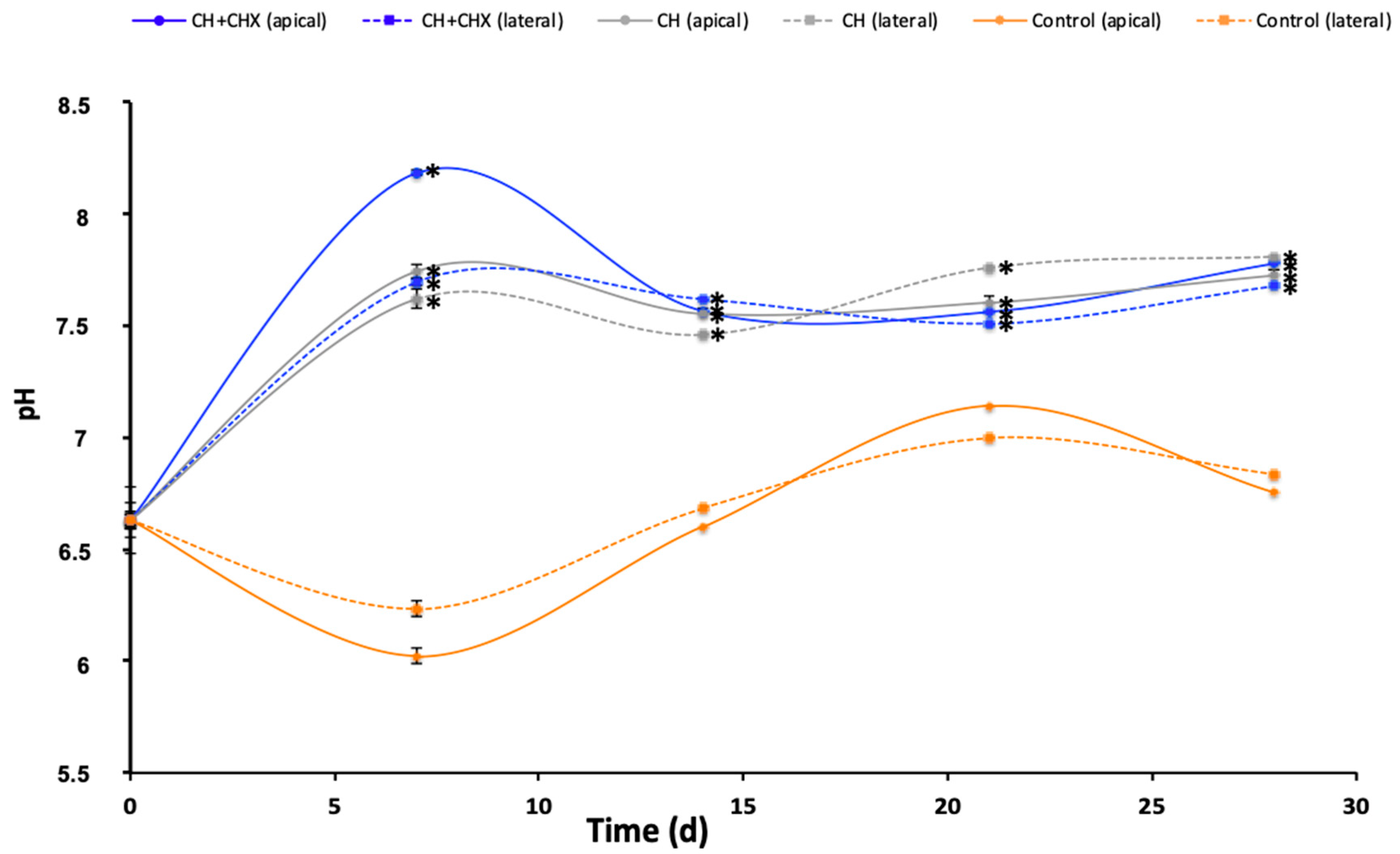
2.2.1. Diffusion of Hydroxyl Ions
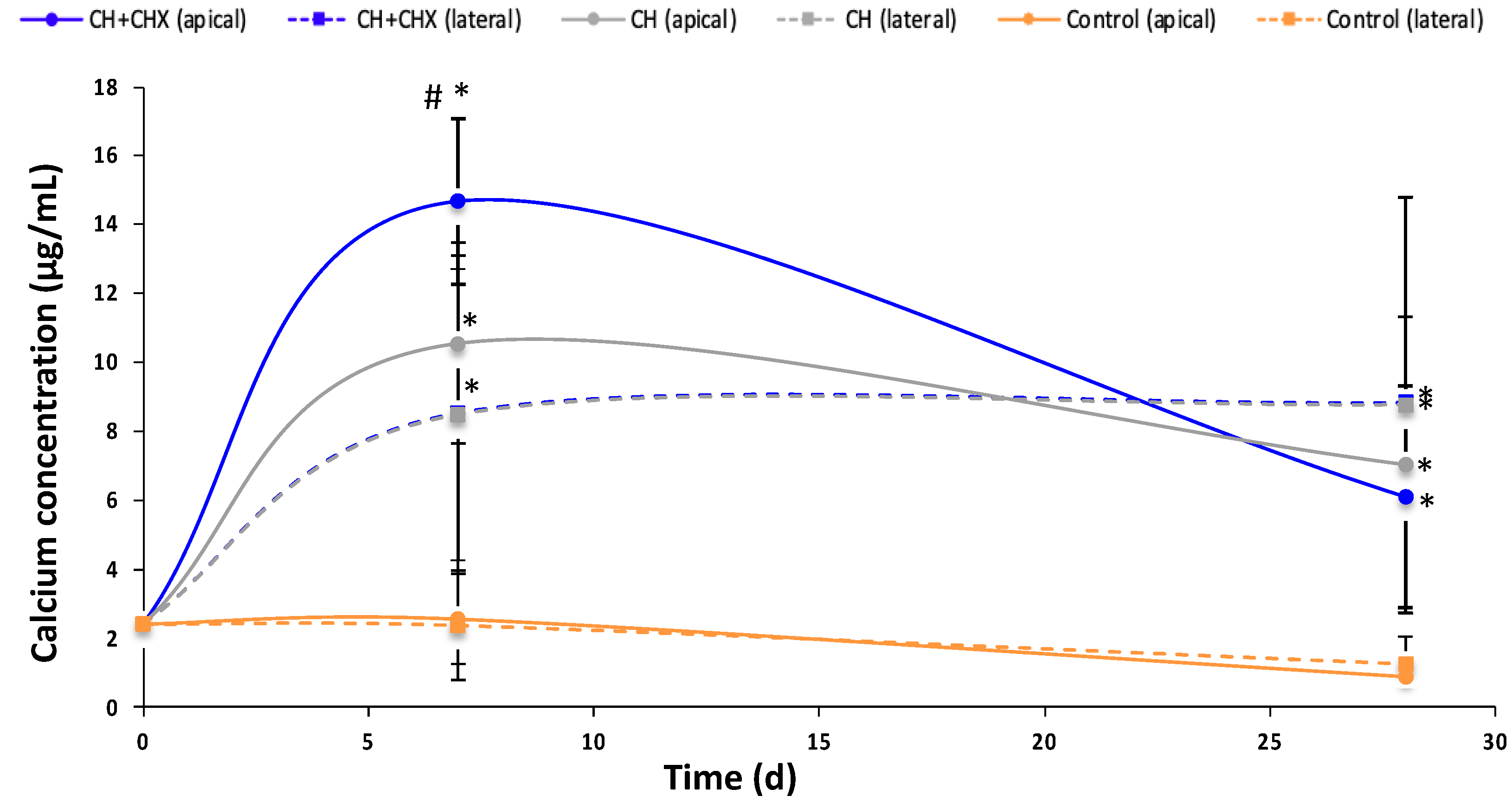
2.2.2. Diffusion of Calcium Ions
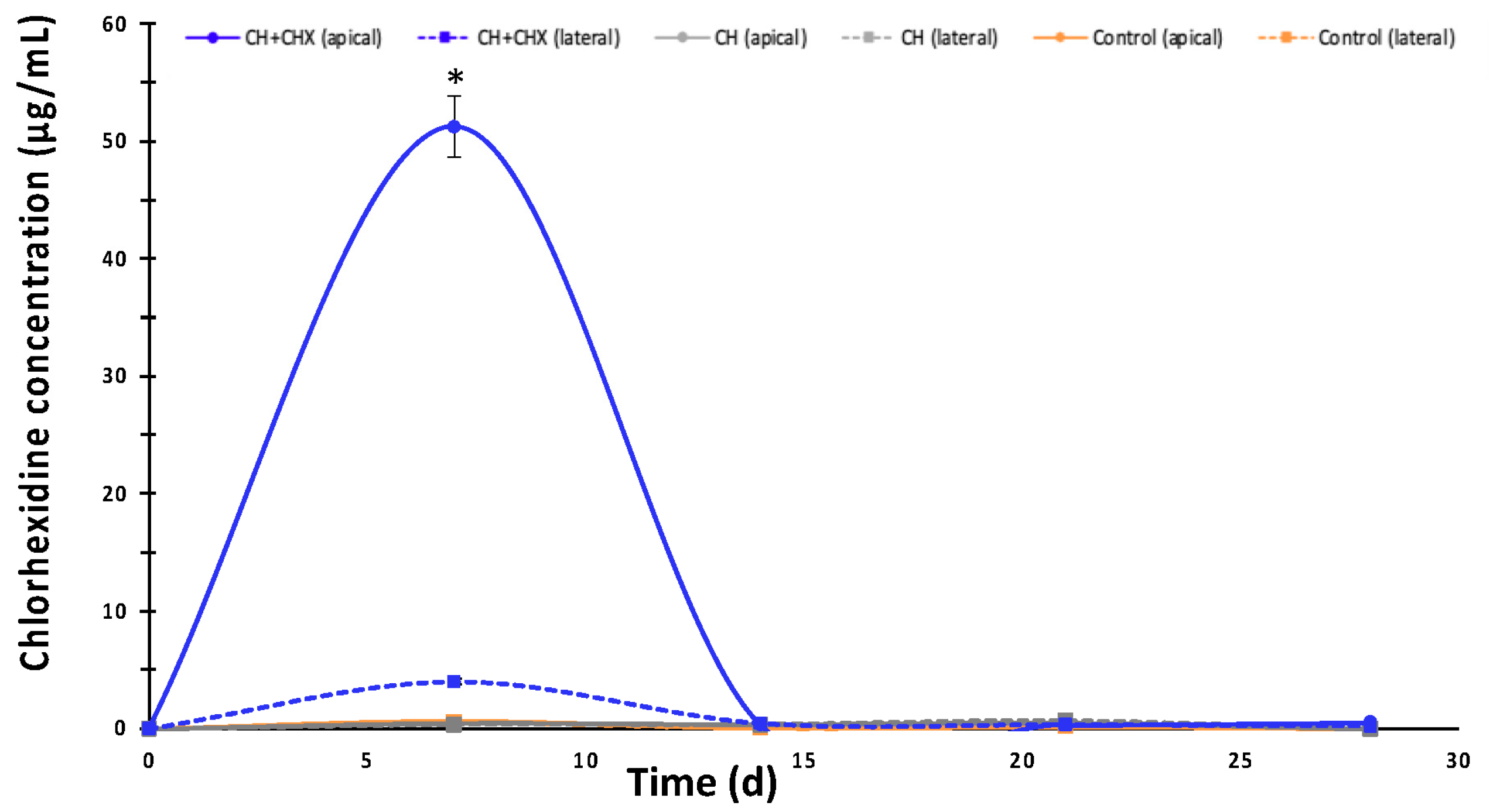
2.2.3. Diffusion of Chlorhexidine
2.3. Biological Effects
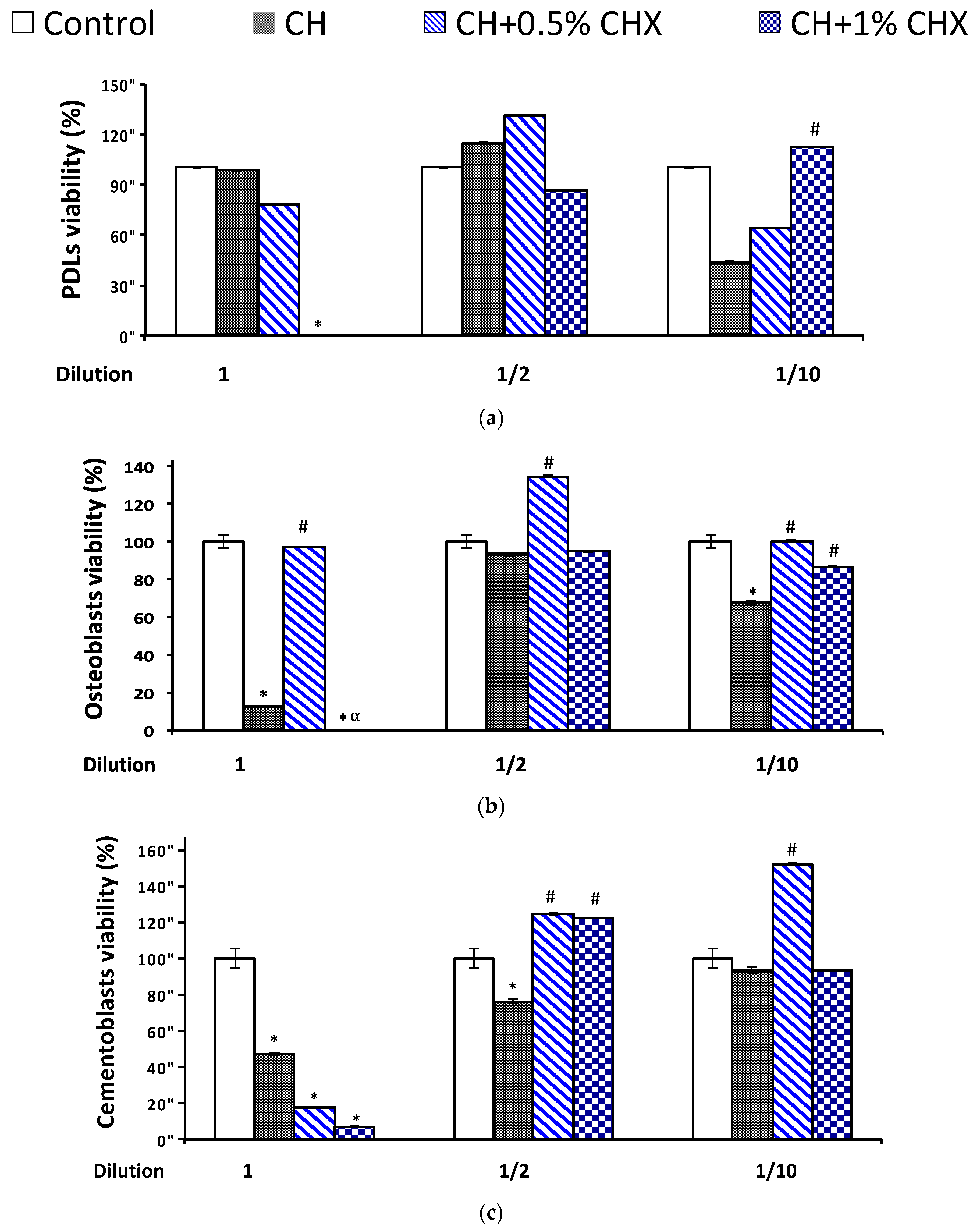
2.3.1. Cell Viability
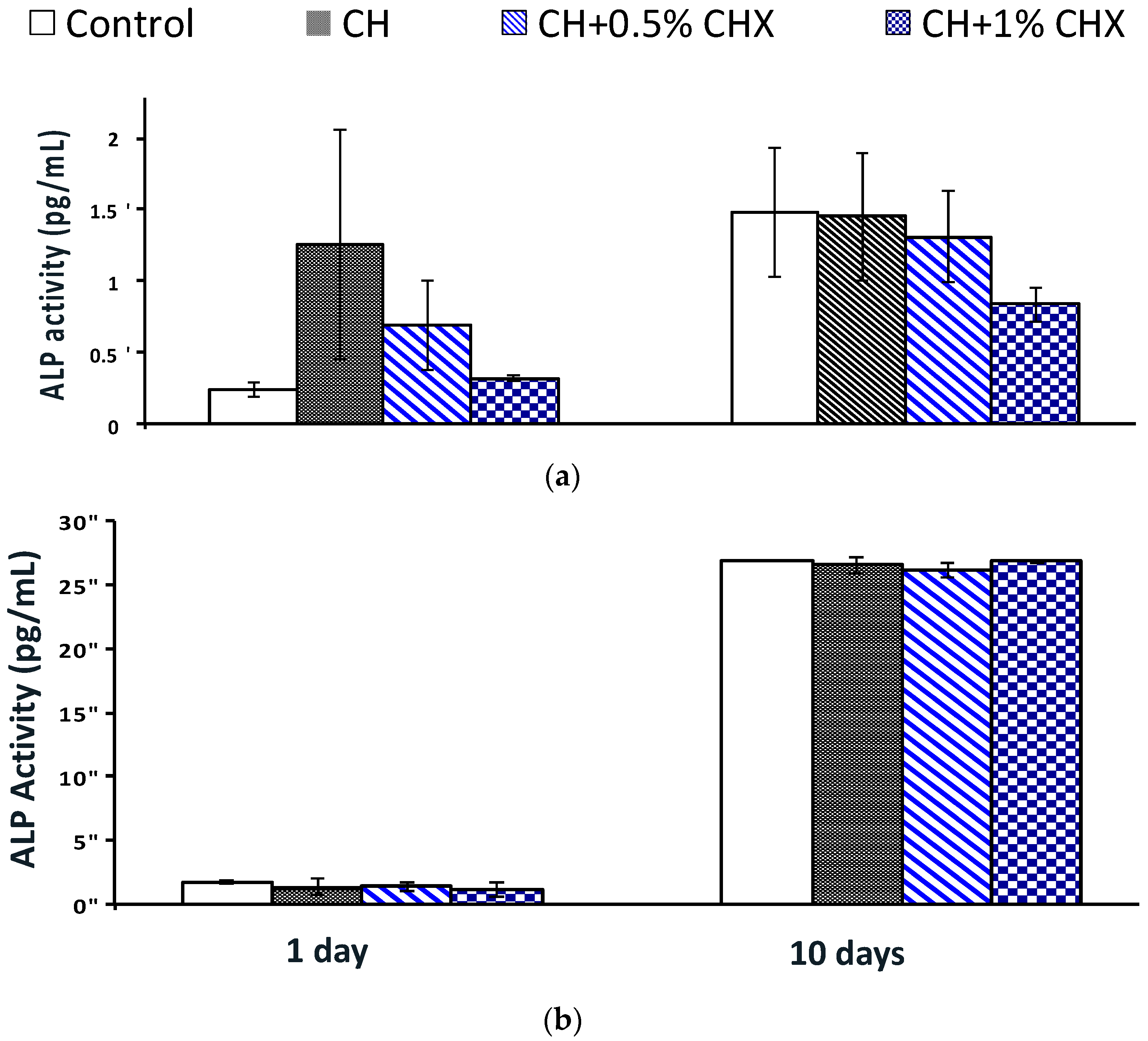
2.3.2. Alkaline Phosphatase (ALP) Activity
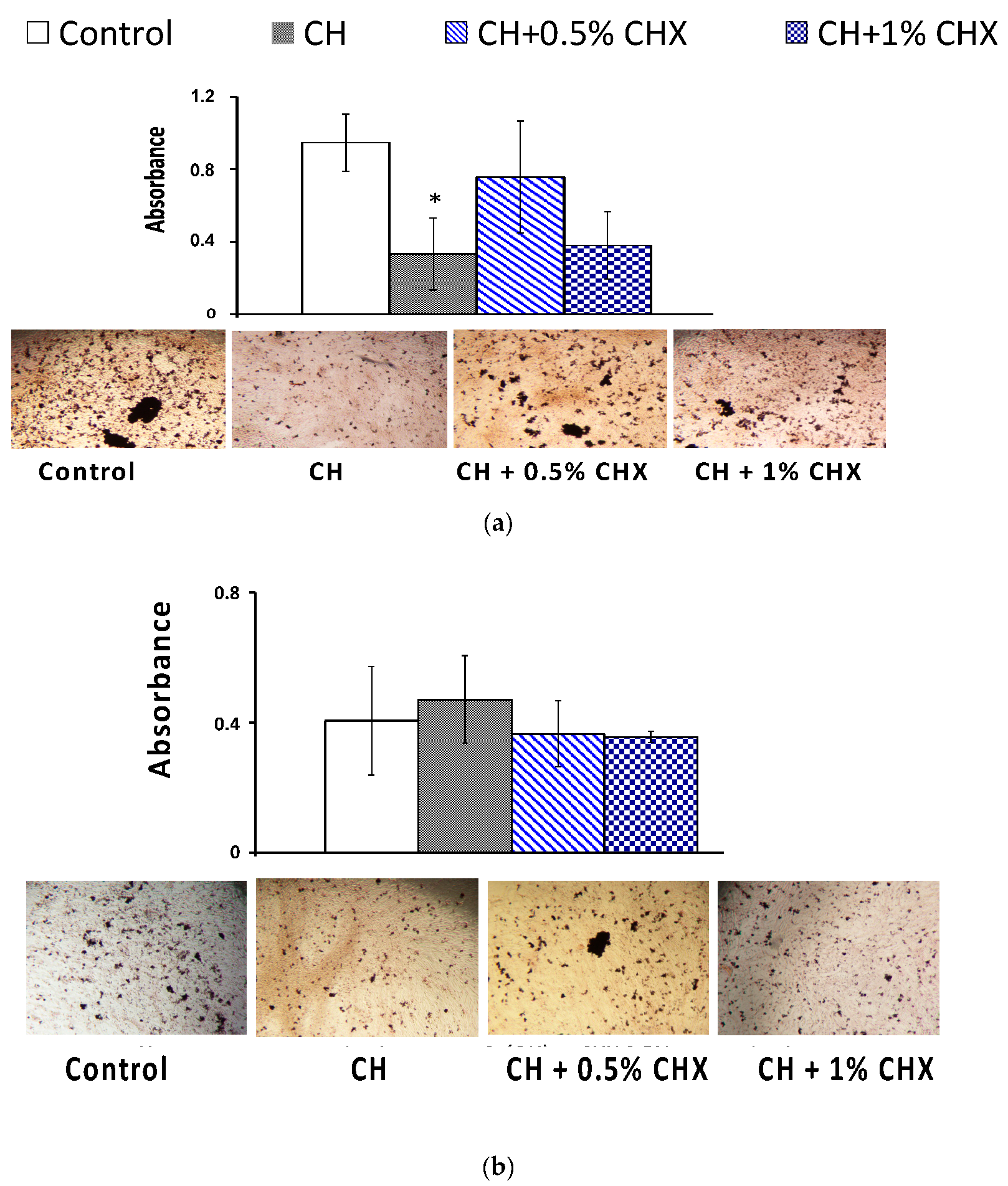
2.3.3. Mineralized-Bone-Like Nodule Formation
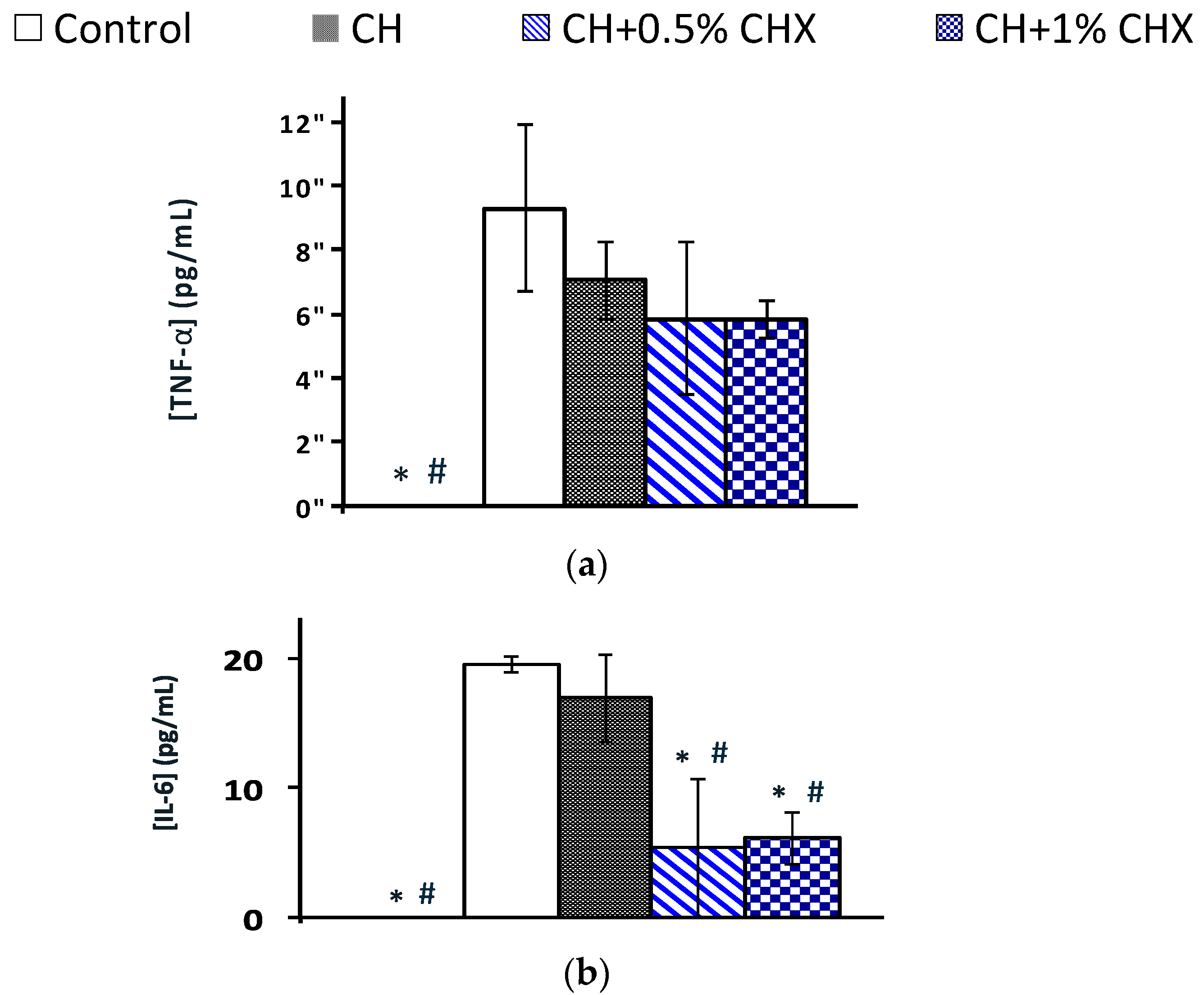
2.3.4. Anti-Inflammatory Activity: TNF-α and IL-6 Levels
3. Discussion
4. Materials and Methods
4.1. Intracanal Medications Formulations
4.1.1. Calcium Hydroxide Paste Formulation
4.1.2. Intracanal Medication Extract Preparation
4.2. Antimicrobial Properties of ICMs
4.2.1. Minimal Inhibitory Concentrations and Minimal Bactericidal Concentrations
4.2.2. Time–Kill Kinetics Assay
4.2.3. Biofilm Tests
Antimicrobial Effect on Biofilm Formation and on Mature Biofilms
Biofilm Quantification
4.3. Transradicular Release
4.3.1. Tooth Preparation
4.3.2. Hydroxide Ion Release Analysis
4.3.3. Calcium Ion Release Analysis
4.3.4. Chlorhexidine Release Analysis
4.4. Biological Assessment
4.4.1. Cell Isolation and Primary Cell Cultures
4.4.2. Periodontal Cell Stimulation with Calcium Hydroxide Paste Extracts Containing Chlorhexidine
4.4.3. Cell Viability
4.4.4. Alkaline Phosphatase Activity
4.4.5. Mineralized Bone-like Nodule Formation
4.4.6. Anti-Inflammatory Activity: Quantitative Cytokine (TNF-α and IL-6) Analysis
4.5. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Herrera, D.; Retamal-Valdes, B.; Alonso, B.; Feres, M. Acute periodontal lesions (periodontal abscesses and necrotizing periodontal diseases) and endo-periodontal lesions. J. Periodontol. 2018, 89, S85–S102. [Google Scholar] [CrossRef]
- Papapanou, P.N.; Sanz, M.; Buduneli, N.; Dietrich, T.; Feres, M.; Fine, D.H.; Flemmig, T.F.; Garcia, R.; Giannobile, W.V.; Graziani, F.; et al. Periodontitis: Consensus report of workgroup 2 of the 2017 World Workshop on the Classification of Periodontal and Peri-Implant Diseases and Conditions. J. Clin. Periodontol. 2018, 45, S162–S170. [Google Scholar] [CrossRef] [PubMed]
- Altaf, D.A.; Jeelani, D.M.; Basher, D.A. Assessment of prevalence of Endo-perio lesions among patients of known population: An observational study. Int. J. Appl. Dent. Sci. 2019, 5, 111–113. [Google Scholar]
- Prashaanthi, N.; Rajasekar, A.; Shantha Sundari, K.K. Prevalence of endo perio lesion-an institutional study. Int. J. Dent. Oral Sci. 2021, 8, 2858–2862. [Google Scholar]
- Ruetters, M.; Gehrig, H.; Kronsteiner, D.; Schuessler, D.L.; Kim, T.S. Prevalence of endo-perio lesions according to the 2017 World Workshop on the Classification of Periodontal and Peri-Implant Disease in a university hospital. Quintessence Int. 2022, 53, 134–142. [Google Scholar]
- Ahmed, H.M.A. Different perspectives in understanding the pulp and periodontal intercommunications with a new proposed classification for endo-perio lesions. ENDO—Endod. Pract. Today 2012, 6, 87–104. [Google Scholar]
- Rotstein, I. Interaction between endodontics and periodontics. Periodontol. 2000 2017, 74, 11–39. [Google Scholar] [CrossRef]
- Siew, K.L.; Goh, V.; Goo, C.L.; Corbet, E.; Leung, W.K. The Periodontal-Endodontic Relationship, What Do We Know? Periodontol. Dent. Implantol. 2019, 13, 231–252. [Google Scholar]
- Karteva, T.; Manchorova-Veleva, N. The Role of the Immune Response in Chronic Marginal and Apical Periodontitis. Folia Med. 2020, 62, 238–243. [Google Scholar] [CrossRef]
- Gomes, B.P.F.A.; Montagner, F.; Berber, V.B.; Zaia, A.A.; Ferraz, C.C.R.; de Almeida, J.F.A.; Souza-Filho, F.J. Antimicrobial action of intracanal medicaments on the external root surface. J. Dent. 2009, 37, 76–81. [Google Scholar] [CrossRef]
- Singh, P. Endo-perio dilemma: A brief review. Dent. Res. J. 2011, 8, 39–47. [Google Scholar]
- Solomon, C.; Chalfin, H.; Kellert, M.; Weseley, P. The endodontic-periodontal lesion: A rational approach to treatment. J. Am. Dent. Assoc. 1995, 126, 473–479. [Google Scholar] [CrossRef] [PubMed]
- Gambin, D.J.; Vitali, F.C.; De Carli, J.P.; Mazzon, R.R.; Gomes, B.P.F.A.; Duque, T.M.; Trentin, M.S. Prevalence of red and orange microbial complexes in endodontic-periodontal lesions: A systematic review and meta-analysis. Clin. Oral Investig. 2021, 25, 6533–6546. [Google Scholar] [CrossRef] [PubMed]
- Rovai, E.d.S.; Matos, F.d.S.; Kerbauy, W.D.; Cardoso, F.G.d.R.; Martinho, F.C.; Oliveira, L.D.; Valera, M.C.; Carvalho, C.A.T. Microbial Profile and Endotoxin Levels in Primary Periodontal Lesions with Secondary Endodontic Involvement. Braz. Dent. J. 2019, 30, 356–362. [Google Scholar] [CrossRef]
- Slots, J. Periodontitis: Facts; fallacies and the future. Periodontol. 2000 2017, 75, 7–23. [Google Scholar] [CrossRef]
- Xia, M.; Qi, Q. Bacterial analysis of combined periodontal-endodontic lesions by polymerase chain reaction-denaturing gradient gel electrophoresis. J. Oral Sci. 2013, 55, 287–291. [Google Scholar] [CrossRef]
- Cucolo, F.; Bonvalente, M.C.; Barroso, E.M.; de Toledo, B.E.C.; Souza, A.A.; Zuza, E.P. Endo-perio lesions prevalence in non-molar and molar teeth: A pilot study. Rev. Odontol. UNESP 2021, 50, e20210037. [Google Scholar] [CrossRef]
- Didilescu, A.C.; Rusu, D.; Anghel, A.; Nica, L.; Iliescu, A.; Greabu, M.; Bancescu, G.; Stratul, S.I. Investigation of six selected bacterial species in endo-periodontal lesions. Int. Endod. J. 2012, 45, 282–293. [Google Scholar] [CrossRef]
- Fan, X.; Xu, X.; Yu, S.; Liu, P.; Chen, C.; Pan, Y.; Lin, L.; Li, C. Prognostic Factors of Grade 2-3 Endo-Periodontal Lesions Treated Nonsurgically in Patients with Periodontitis: A Retrospective Case-Control Study. Biomed. Res. Int. 2020, 8, 1592910. [Google Scholar] [CrossRef]
- Tewari, S.; Sharma, G.; Tewari, S.; Mittal, S.; Bansal, S. Effect of immediate periodontal surgical treatment on periodontal healing in combined endodontic-periodontal lesions with communication-A randomized clinical trial. J. Oral Biol. Craniofacial Res. 2018, 8, 105–112. [Google Scholar] [CrossRef]
- Schmidt, J.C.; Walter, C.; Amato, M.; Weiger, R. Treatment of periodontal-endodontic lesions—A systematic review. J. Clin. Periodontol. 2014, 41, 779–790. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, Z.; Dummer, P.M.H. Properties and applications of calcium hydroxide in endodontics and dental traumatology. Int. Endod. J. 2011, 44, 697–730. [Google Scholar] [CrossRef]
- Siqueira, J.F.; Lopes, H.P. Mechanisms of antimicrobial activity of calcium hydroxide: A critical review. Int. Endod. J. 1999, 32, 361–369. [Google Scholar] [CrossRef] [PubMed]
- Leonardo, M.R.; Hernandez, M.E.F.T.; Silva, L.A.B.; Tanomaru-Filho, M. Effect of a calcium hydroxide-based root canal dressing on periapical repair in dogs: A histological study. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2006, 102, 680–685. [Google Scholar] [CrossRef] [PubMed]
- De Rossi, A.; Silva, L.A.B.; Leonardo, M.R.; Rocha, L.B.; Rossi, M.A. Effect of rotary or manual instrumentation; with or without a calcium hydroxide/1% chlorhexidine intracanal dressing; on the healing of experimentally induced chronic periapical lesions. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2005, 99, 628–636. [Google Scholar] [CrossRef] [PubMed]
- Gomes, B.P.F.A.; Vianna, M.E.; Zaia, A.A.; Almeida, J.F.A.; Souza-Filho, F.J.; Ferraz, C.C.R. Chlorhexidine in endodontics. Braz. Dent. J. 2013, 24, 89–102. [Google Scholar] [CrossRef]
- Mohammadi, Z.; Jafarzadeh, H.; Shalavi, S.; Sahebalam, R.; Kinoshita, J.I. Additive and reducing Effects between Calcium Hydroxide and Current Irrigation Solutions. J. Contemp. Dent. Pract. 2017, 18, 246–249. [Google Scholar]
- Sy, K.; Agossa, K.; Maton, M.; Chijcheapaza-Flores, H.; Martel, B.; Siepmann, F.; Deveaux, E.; Blanchemain, N.; Neut, C. How Adding Chlorhexidine or Metallic Nanoparticles Affects the Antimicrobial Performance of Calcium Hydroxide Paste as an Intracanal Medication: An In Vitro Study. Antibiotics 2021, 10, 1352. [Google Scholar] [CrossRef]
- Raheja, J.; Tewari, S.; Tewari, S.; Duhan, J. Evaluation of efficacy of chlorhexidine intracanal medicament on the periodontal healing of concomitant endodontic-periodontal lesions without communication: An interventional study. J. Periodontol. 2014, 85, 1019–1026. [Google Scholar] [CrossRef]
- Shao, W.; Xiao, F.; Xu, Z.X.; Ren, R.H.; Wang, Y.; Wu, Y.Q. Treatment of severe periodontic-endodontic combined lesions with minocycline hydrochloride ointment combined with mineral trioxide aggregate. Exp. Ther. Med. 2018, 16, 1389–1396. [Google Scholar] [CrossRef]
- Duque, T.M.; Prado, M.; Herrera, D.R.; Gomes, B.P.F.A. Periodontal and endodontic infectious/inflammatory profile in primary periodontal lesions with secondary endodontic involvement after a calcium hydroxide-based intracanal medication. Clin. Oral Investig. 2019, 23, 53–63. [Google Scholar] [CrossRef]
- Carvalho, C.N.; Freire, L.G.; Carvalho, A.P.; Duarte, M.A.H.; Bauer, J.; Gavini, G. Ions Release and pH of Calcium Hydroxide-; Chlorhexidine- and Bioactive Glass-Based Endodontic Medicaments. Braz. Dent. J. 2016, 27, 325–331. [Google Scholar] [CrossRef] [PubMed]
- Chamberlain, T.M.; Kirkpatrick, T.C.; Rutledge, R.E. pH changes in external root surface cavities after calcium hydroxide is placed at 1; 3 and 5 mm short of the radiographic apex. Dent. Traumatol. 2009, 25, 470–474. [Google Scholar] [CrossRef]
- Esberard, R.M.; Carnes, D.L.; del Rio, C.E. Changes in pH at the dentin surface in roots obturated with calcium hydroxide pastes. J. Endod. 1996, 22, 402–405. [Google Scholar] [CrossRef] [PubMed]
- Miñana, M.; Carnes, D.L.; Walker, W.A. pH Changes at the Surface of Root Dentin after Intracanal Dressing with Calcium Oxide and Calcium Hydroxide. J. Endod. 2001, 27, 43–45. [Google Scholar] [CrossRef]
- Morfis, A.; Sylaras, S.N.; Georgopoulou, M.; Kernani, M.; Prountzos, F. Study of the apices of human permanent teeth with the use of a scanning electron microscope. Oral Surg. Oral Med. Oral Pathol. 1994, 77, 172–176. [Google Scholar] [CrossRef] [PubMed]
- Moreinos, D.; Front, E.; Lin, S. Perio-endo interaction: A review. Oral Health Care 2021, 6, 1–8. [Google Scholar]
- Barbin, L.E.; Estrela, C.; Guedes, D.F.C.; Spanó, J.C.E.; Sousa-Neto, M.D.; Pécora, J.D. Detection of para-chloroaniline; reactive oxygen species; and 1-chloro-4-nitrobenzene in high concentrations of chlorhexidine and in a mixture of chlorhexidine and calcium hydroxide. J. Endod. 2013, 39, 664–668. [Google Scholar] [CrossRef] [PubMed]
- Below, H.; Assadian, O.; Baguhl, R.; Hildebrandt, U.; Jäger, B.; Meissner, K.; Leaper, D.J.; Kramer, A. Measurements of chlorhexidine; p-chloroaniline; and p-chloronitrobenzene in saliva after mouth wash before and after operation with 0.2% chlorhexidine digluconate in maxillofacial surgery: A randomised controlled trial. Br. J. Oral Maxillofac. Surg. 2017, 55, 150–155. [Google Scholar] [CrossRef]
- Câmara De Bem, S.H.; Estrela, C.; Guedes, D.F.C.; Sousa-Neto, M.D.; Pécora, J.D. Determination of chemical components derived from 2% chlorhexidine gel degradation using gas chromatography-mass spectrometry. Acta Odontol. Scand. 2014, 72, 630–638. [Google Scholar] [CrossRef]
- Khatib, M.S.; Ameer, B.; Ajit Mannur, N.; Ramalingaiahsetty, A.M.; Peerzade, S.M.; Bambawale, A. Decoding the Perplexing Mystery of Para-Chloroaniline Formation: A Systematic Review. J. Int. Soc. Prev. Community Dent. 2020, 10, 142–147. [Google Scholar] [CrossRef] [PubMed]
- Selvamani, M.; Madhushankari, G.S.; Basandi, S.P.; Donoghue, M.; Nayak, V.; Diwakar, G. Effect of Vitality on Translucent Dentine—A Study. J. Int. Oral Health 2013, 5, 1–7. [Google Scholar]
- Weber, D.F. Human dentine sclerosis: A microradiographic survey. Arch. Oral Biol. 1974, 19, 163–169. [Google Scholar] [CrossRef]
- Jacinto, R.C.; Linhares-Farina, G.; Sposito, O.d.S.; Zanchi, C.H.; Cenci, M.S. Influence of 2% chlorhexidine on pH; calcium release and setting time of a resinous MTA-based root-end filling material. Braz. Oral Res. 2015, 29, S1806-83242015000100240. [Google Scholar] [CrossRef] [PubMed]
- Krüger, H.C.; Francio, J.; Silva, A.S.; da Oliveira, G.S.N.; de Brancher, J.A.; Dantas, L.R.; Oliveira, R.C.; Tuon, F.F.; Carneiro, E. Antimicrobial action; cytotoxicity; calcium ion release; and pH variation of a calcium hydroxide-based paste associated with Myracrodruon urundeuva Allemão extract. Aust. Endod. J. J. Aust. Soc. Endodontology Inc. 2021, 48, 170–178. [Google Scholar] [CrossRef] [PubMed]
- Misra, P.; Bains, R.; Loomba, K.; Singh, A.; Sharma, V.P.; Murthy, R.C.; Kumar, R. Measurement of pH and calcium ions release from different calcium hydroxide pastes at different intervals of time: Atomic spectrophotometric analysis. J. Oral Biol. Craniofac. Res. 2017, 7, 36–41. [Google Scholar] [CrossRef] [PubMed]
- Ximenes, M.; Cardoso, M. Assessment of diffusion of hydroxyl and calcium ions of root canal filling materials in primary teeth. Pediatr. Dent. 2012, 34, 122–126. [Google Scholar] [PubMed]
- Guerreiro-Tanomaru, J.M.; Chula, D.G.; de Pontes Lima, R.K.; Berbert, F.L.V.C.; Tanomaru-Filho, M. Release and diffusion of hydroxyl ion from calcium hydroxide-based medicaments. Dent. Traumatol. 2012, 28, 320–323. [Google Scholar] [CrossRef] [PubMed]
- Pacios, M.G.; de la Casa, M.L.; de Bulacio, M.l.; López, M.E. Influence of different vehicles on the pH of calcium hydroxide pastes. J. Oral Sci. 2004, 46, 107–111. [Google Scholar] [CrossRef]
- Tanomaru, J.M.G.; Leonardo, M.R.; Tanomaru Filho, M.; Bonetti Filho, I.; Silva, L.a.B. Effect of different irrigation solutions and calcium hydroxide on bacterial LPS. Int. Endod. J. 2003, 36, 733–739. [Google Scholar] [CrossRef]
- Duarte, M.A.H.; Midena, R.Z.; Zeferino, M.A.; Vivan, R.R.; Weckwerth, P.H.; Dos Santos, F.; Guerreiro-Tanomaru, J.M.; Tanomaru-Filho, M. Evaluation of pH and calcium ion release of calcium hydroxide pastes containing different substances. J. Endod. 2009, 35, 1274–1277. [Google Scholar] [CrossRef] [PubMed]
- Signoretti, F.G.C.; Gomes, B.P.F.; Montagner, F.; Barrichello Tosello, F.; Jacinto, R.C. Influence of 2% chlorhexidine gel on calcium hydroxide ionic dissociation and its ability of reducing endotoxin. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2011, 111, 653–658. [Google Scholar] [CrossRef] [PubMed]
- Lima, T.F.R.; Ascendino, J.F.; Cavalcante, I.d.O.D.; Assunção, F.L.C.; Salazar-Silva, J.R.; da Silva, E.J.N.L.; Lemos, S.G.; Soares, A.J. Influence of chlorhexidine and zinc oxide in calcium hydroxide pastes on pH changes in external root surface. Braz. Oral Res. 2019, 33, e005. [Google Scholar] [CrossRef] [PubMed]
- Delgado, R.J.R.; Gasparoto, T.H.; Sipert, C.R.; Pinheiro, C.R.; Moraes, I.G.; Garcia, R.B.; Bramante, C.M.; Campanelli, A.P.; Bernardineli, N. Antimicrobial effects of calcium hydroxide and chlorhexidine on Enterococcus faecalis. J. Endod. 2010, 36, 1389–1393. [Google Scholar] [CrossRef]
- Balto, H.; Bukhary, S.; Al-Omran, O.; BaHammam, A.; Al-Mutairi, B. Combined Effect of a Mixture of Silver Nanoparticles and Calcium Hydroxide against Enterococcus faecalis Biofilm. J. Endod. 2020, 46, 1689–1694. [Google Scholar] [CrossRef] [PubMed]
- Blanscet, M.L.; Tordik, P.A.; Goodell, G.G. An agar diffusion comparison of the antimicrobial effect of calcium hydroxide at five different concentrations with three different vehicles. J. Endod. 2008, 34, 1246–1248. [Google Scholar] [CrossRef]
- da Silva, R.A.B.; Leonardo, M.R.; da Silva, L.A.B.; de Castro, L.M.S.; Rosa, A.L.; de Oliveira, P.T. Effects of the association between a calcium hydroxide paste and 0.4% chlorhexidine on the development of the osteogenic phenotype in vitro. J. Endod. 2008, 34, 1485–1489. [Google Scholar] [CrossRef]
- Voel, D.; Voel, J.G.; Pratt, C.W. Fundamentals of Biochemistry, 1st ed.; John Wiley and Sons: Hobokken, NJ, USA, 2016; pp. 93–123. [Google Scholar]
- De-Deus, G.; Canabarro, A.; Alves, G.; Linhares, A.; Senne, M.I.; Granjeiro, J.M. Optimal cytocompatibility of a bioceramic nanoparticulate cement in primary human mesenchymal cells. J. Endod. 2009, 35, 1387–1390. [Google Scholar] [CrossRef]
- Garg, A.; Mala, K.; Kamath, P.M. Biofilm models in endodontics—A narrative review. J. Conserv. Dent. 2021, 24, 2–9. [Google Scholar]
- Tülü, G.; Kaya, B.Ü.; Çetin, E.S.; Köle, M. Antibacterial effect of silver nanoparticles mixed with calcium hydroxide or chlorhexidine on multispecies biofilms. Odontology 2021, 109, 802–811. [Google Scholar] [CrossRef]
- Kim, D.; Kim, E. Antimicrobial effect of calcium hydroxide as an intracanal medicament in root canal treatment: A literature review—Part I. In vitro studies. Restor. Dent. Endod. 2014, 39, 241–252. [Google Scholar] [CrossRef] [PubMed]
- Swimberghe, R.C.D.; Coenye, T.; De Moor, R.J.G.; Meire, M.A. Biofilm model systems for root canal disinfection: A literature review. Int. Endod. J. 2019, 52, 604–628. [Google Scholar] [CrossRef] [PubMed]
- Afkhami, F.; Ahmadi, P.; Chiniforush, N.; Sooratgar, A. Effect of different activations of silver nanoparticle irrigants on the elimination of Enterococcus faecalis. Clin. Oral Investig. 2021, 25, 6893–6899. [Google Scholar] [CrossRef] [PubMed]
- Choi, M.J.; Kim, M.A.; Choi, Y.; Neelakantan, P.; Yu, M.K.; Min, K.S. A novel three-dimensionally printed model to assess biofilm removal by ultrasonically activated irrigation. Int. Endod. J. 2021, 54, 1871–1877. [Google Scholar] [CrossRef]
- Hoedke, D.; Kaulika, N.; Dommisch, H.; Schlafer, S.; Shemesh, H.; Bitter, K. Reduction of dual-species biofilm after sonic- or ultrasonic-activated irrigation protocols: A laboratory study. Int. Endod. J. 2021, 54, 2219–2228. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Tong, Z.; Ling, J.; Liu, H.; Wei, X. The effects of sodium hypochlorite and chlorhexidine irrigants on the antibacterial activities of alkaline media against Enterococcus faecalis. Arch. Oral Biol. 2015, 60, 1075–1081. [Google Scholar] [CrossRef]
- Li, Y.; Wang, Y.; Chen, X.; Jiang, W.; Jiang, X.; Zeng, Y.; Li, X.; Feng, Z.; Luo, J.; Zhang, L. Antimicrobial peptide GH12 as root canal irrigant inhibits biofilm and virulence of Enterococcus faecalis. Int. Endod. J. 2020, 53, 948–961. [Google Scholar] [CrossRef] [PubMed]
- Qaeed, M.A.; Hendi, A.; Obaid, A.S.; Thahe, A.A.; Osman, A.M.; Ismail, A.; Mindil, A.; Eid, A.A.; Aqlan, F.; Osman, N.M.A.; et al. The effect of different aqueous solutions ratios of Ocimum basilicum utilized in AgNPs synthesis on the inhibition of bacterial growth. Sci. Rep. 2023, 13, 5866. [Google Scholar] [CrossRef]
- Bekhouche, M.; Bolon, M.; Charriaud, F.; Lamrayah, M.; Da Costa, D.; Primard, C.; Costantini, A.; Pasdeloup, M.; Gobert, S.; Mallein-Gerin, F.; et al. Development of an antibacterial nanocomposite hydrogel for human dental pulp engineering. J. Mater. Chem. B 2020, 8, 8422–8432. [Google Scholar] [CrossRef]
- Pintor, A.V.B.; Queiroz, L.D.; Sancas, M.C.; Brochado, A.C.B.; Spoladore, J.; Fonseca-Gonçalves, A.; Fidalgo, T.K.S.; Freitas-Fernandes, L.B.; Valente, A.P.; de Souza, I.P.R.; et al. Cytocompatibility of filling pastes by primary teeth root simulating model. Odontology 2021, 109, 174–183. [Google Scholar] [CrossRef]
- Kranz, S.; Guellmar, A.; Braeutigam, F.; Tonndorf-Martini, S.; Heyder, M.; Reise, M.; Sigusch, B. Antibacterial Effect of Endodontic Disinfections on Enterococcus faecalis in Dental Root Canals-An In-Vitro Model Study. Materials 2021, 14, 2427. [Google Scholar] [CrossRef] [PubMed]
- Mounir, M.M.F.; Rashed, F.M.; Bukhary, S.M. Amelogenin as a regenerative endodontic molecule for immature teeth with apical periodontitis. An experimental study. J. Oral Biol. Craniofac. Res. 2022, 12, 721–726. [Google Scholar] [CrossRef]
- Li, M.; Yang, Y.; Lin, C.; Zhang, Q.; Gong, L.; Wang, Y.; Zhang, X. Antibacterial Properties of Small-Size Peptide Derived from Penetratin against Oral Streptococci. Materials 2021, 14, 2730. [Google Scholar] [CrossRef] [PubMed]
- Abedini, A.; Roumy, V.; Mahieux, S.; Gohari, A.; Farimani, M.M.; Rivière, C.; Samaillie, J.; Sahpaz, S.; Bailleul, F.; Neut, C.; et al. Antimicrobial activity of selected Iranian medicinal plants against a broad spectrum of pathogenic and drug multiresistant micro-organisms. Lett. Appl. Microbiol. 2014, 59, 412–421. [Google Scholar] [CrossRef]
- Shetty, S.; Sekar, P.; Shetty, R.M.; Abou Neel, E.A. Antibacterial and Antibiofilm Efficacy of Copper-Doped Phosphate Glass on Pathogenic Bacteria. Molecules 2023, 28, 3179. [Google Scholar] [CrossRef] [PubMed]
- Bago Jurič, I.; Plečko, V.; Anić, I.; Pleško, S.; Jakovljević, S.; Rocca, J.P.; Medioni, E. Antimicrobial efficacy of photodynamic therapy; Nd:YAG laser and QMiX solution against Enterococcus faecalis biofilm. Photodiagnosis Photodyn. Ther. 2016, 13, 238–243. [Google Scholar] [CrossRef]
- Raoof, M.; Khaleghi, M.; Siasar, N.; Mohannadalizadeh, S.; Haghani, J.; Amanpour, S. Antimicrobial Activity of Methanolic Extracts of Myrtus communis L. and Eucalyptus Galbie and their Combination with Calcium Hydroxide Powder against Enterococcus faecalis. J. Dent. Shiraz Iran. 2019, 20, 195–202. [Google Scholar]
- Song, Z.M.; Zhang, J.L.; Zhou, K.; Yue, L.M.; Zhang, Y.; Wang, C.Y.; Wang, K.L.; Xu, Y. Anthraquinones as Potential Antibiofilm Agents Against Methicillin-Resistant Staphylococcus aureus. Front. Microbiol. 2021, 12, 709826. [Google Scholar] [CrossRef]
- Eftekhar, B.; Moghimipour, E.; Eini, E.; Jafarzadeh, M.; Behrooz, N. Evaluation of hydroxyl ion diffusion in dentin and injectable forms and a simple powder-water calcium hydroxide paste: An in vitro study. Jundishapur J. Nat. Pharm. Prod. 2014, 9, e14029. [Google Scholar] [CrossRef] [PubMed]
- Forghani, M.; Mashhoor, H.; Rouhani, A.; Jafarzadeh, H. Comparison of pH changes induced by calcium enriched mixture and those of calcium hydroxide in simulated root resorption defects. J. Endod. 2014, 40, 2070–2073. [Google Scholar] [CrossRef] [PubMed]
- Shetty, S.; Manjunath, M.K.; Tejaswi, S. An In-vitro Evaluation of the pH Change Through Root Dentin Using Different Calcium Hydroxide Preparations as an Intracanal Medicament. J. Clin. Diagn. Res. JCDR 2014, 8, ZC13–ZC16. [Google Scholar] [CrossRef]
- Yazdanpanahi, N.; Behzadi, A.; Zare Jahromi, M. Long-term pH Alterations in the Periradicular Area Following the Application of Calcium Hydroxide and MTA. J. Dent. Shiraz Iran. 2021, 22, 90–95. [Google Scholar]
- Lizzi, F.; Goutaudier, C.; Attik, N.; Jackson, P.; Campbell, I.; Mokbel, I.; Grosgogeat, B.; Villat, C. Ion release characterization in phase separated borosilicate glass powders. J. Non-Cryst. Solids 2020, 534, 119934. [Google Scholar] [CrossRef]
- Kudo, K.; Ikeda, N.; Kiyoshima, A.; Hino, Y.; Nishida, N.; Inoue, N. Toxicological analysis of chlorhexidine in human serum using HPLC on a polymer-coated ODS column. J. Anal. Toxicol. 2002, 26, 119–122. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Xue, Y.; Tang, M.; Hieda, Y.; Fujihara, J.; Takayama, K.; Takatsuka, H.; Takeshita, H. High-performance liquid chromatographic determination of chlorhexidine in whole blood by solid-phase extraction and kinetics following an intravenous infusion in rats. J. Anal. Toxicol. 2009, 33, 85–91. [Google Scholar] [CrossRef]
- Cardoso, M.A.; Fávero, M.L.D.; Gasparetto, J.C.; Hess, B.S.; Stremel, D.P.; Pontarolo, R. Development and Validation of an Rp-Hplc Method for the Determination of Chlorhexidine and P-Chloroaniline in Various Pharmaceutical Formulations. J. Liq. Chromatogr. Relat. Technol. 2011, 34, 1556–1567. [Google Scholar] [CrossRef]
- Peng, W.; Huan, Z.; Pei, G.; Li, J.; Cao, Y.; Jiang, L.; Zhu, Y. Silicate bioceramics elicit proliferation and odonto-genic differentiation of human dental pulp cells. Dent. Mater. J. 2022, 41, 27–36. [Google Scholar] [CrossRef]
- Mukhtar-Fayyad, D. Cytocompatibility of new bioceramic-based materials on human fibroblast cells (MRC-5). Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2011, 112, e137–e142. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.H.; Xie, Q. Total glycosides from Eucommia ulmoides seed promoted osteogenic differentiation of adipose-derived mesenchymal stem cells and bone formation in ovariectomized rats through regulating Notch signaling pathway. J. Orthop. Surg. 2021, 16, 660. [Google Scholar] [CrossRef]
- Guerreiro, J.C.M.; Ochoa-Rodrígez, V.M.; Rodrigues, E.M.; Chavez-Andrade, G.M.; Tanomaru-Filho, M.; Guerreiro-Tanomaru, J.M.; Faria, G. Antibacterial activity; cytocompatibility and effect of Bio-C Temp bioceramic intracanal medicament on osteoblast biology. Int. Endod. J. 2021, 54, 1155–1165. [Google Scholar] [CrossRef] [PubMed]
- Narita, H.; Itoh, S.; Imazato, S.; Yoshitake, F.; Ebisu, S. An explanation of the mineralization mechanism in osteoblasts induced by calcium hydroxide. Acta Biomater. 2010, 6, 586–590. [Google Scholar] [CrossRef] [PubMed]
- Morand, D.N.; Huck, O.; Keller, L.; Jessel, N.; Tenenbaum, H.; Davideau, J.L. Active Nanofibrous Membrane Effects on Gingival Cell Inflammatory Response. Materials 2015, 8, 7217–7229. [Google Scholar] [CrossRef] [PubMed]
- Jeanneau, C.; Giraud, T.; Laurent, P.; About, I. BioRoot RCS Extracts Modulate the Early Mechanisms of Periodontal Inflammation and Regeneration. J. Endod. 2019, 45, 1016–1023. [Google Scholar] [CrossRef] [PubMed]

| A | Negative Control Samples | Positive Control Samples | Test Samples | |||
|---|---|---|---|---|---|---|
| CH | CH + CHX | CH | CH + CHX | CH | CH + CHX | |
| Week 1 | – | – | + | + | – | – |
| Week 2 | – | – | + | + | – | – |
| Week 3 | – | – | + | + | – | – |
| Week 4 | – | – | + | + | – | – |
| B | Negative Control Samples | Positive Control Samples | Test Samples | |||
| CH | CH + CHX | CH | CH + CHX | CH | CH + CHX | |
| Week 1 | – | – | + | + | – | – |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sy, K.; Chevalier, C.; Maton, M.; Mokbel, I.; Mahieux, S.; Houcke, I.; Neut, C.; Grosgogeat, B.; Deveaux, E.; Gritsch, K.; et al. Therapeutic Potential of Chlorhexidine-Loaded Calcium Hydroxide-Based Intracanal Medications in Endo-Periodontal Lesions: An Ex Vivo and In Vitro Study. Antibiotics 2023, 12, 1416. https://doi.org/10.3390/antibiotics12091416
Sy K, Chevalier C, Maton M, Mokbel I, Mahieux S, Houcke I, Neut C, Grosgogeat B, Deveaux E, Gritsch K, et al. Therapeutic Potential of Chlorhexidine-Loaded Calcium Hydroxide-Based Intracanal Medications in Endo-Periodontal Lesions: An Ex Vivo and In Vitro Study. Antibiotics. 2023; 12(9):1416. https://doi.org/10.3390/antibiotics12091416
Chicago/Turabian StyleSy, Kadiatou, Charlène Chevalier, Mickaël Maton, Ilham Mokbel, Séverine Mahieux, Isabelle Houcke, Christel Neut, Brigitte Grosgogeat, Etienne Deveaux, Kerstin Gritsch, and et al. 2023. "Therapeutic Potential of Chlorhexidine-Loaded Calcium Hydroxide-Based Intracanal Medications in Endo-Periodontal Lesions: An Ex Vivo and In Vitro Study" Antibiotics 12, no. 9: 1416. https://doi.org/10.3390/antibiotics12091416
APA StyleSy, K., Chevalier, C., Maton, M., Mokbel, I., Mahieux, S., Houcke, I., Neut, C., Grosgogeat, B., Deveaux, E., Gritsch, K., & Agossa, K. (2023). Therapeutic Potential of Chlorhexidine-Loaded Calcium Hydroxide-Based Intracanal Medications in Endo-Periodontal Lesions: An Ex Vivo and In Vitro Study. Antibiotics, 12(9), 1416. https://doi.org/10.3390/antibiotics12091416









