Abstract
Rapid and accurate detection of tuberculosis (TB) drug resistance is critical for the successful treatment and control of TB. Here, we investigated resistance to anti-TB drugs and genetic variations in 215 drug-resistant Mycobacterium tuberculosis isolates in Korea. Genetic variations were observed in rpoB Ser531Leu, katG Ser315Thr, and gyrA Asp94Gly; however, the minimum inhibitory concentrations varied, which can be attributed to other resistance mechanisms. Examination of genetic relatedness among drug-resistant isolates revealed that the cluster size of resistant bacteria was less than six strains, suggesting no evidence of a large-scale epidemic caused by a specific strain. However, rpoC mutants of the rifampicin-resistant isolates were composed of five types of clusters, suggesting that these compensatory mutations advance propagation. In the present study, more than 90% of the resistance mechanisms to major anti-TB drugs were identified, and the effect of each mutation on drug resistance was estimated. With the clinical application of recent next-generation sequencing-based susceptibility testing, the present study is expected to improve the clinical utilization of genotype-based drug susceptibility testing for the diagnosis and treatment of patients with drug-resistant TB.
1. Introduction
A major public health challenge associated with tuberculosis (TB) is the emergence of multidrug-resistant TB (MDR-TB) and extensive drug-resistant TB (XDR-TB). Treatment of MDR-TB is associated with a longer treatment period, higher cost, and more adverse drug events than drug-sensitive TB, while XDR-TB is more difficult to treat and has a relatively higher mortality rate than MDR-TB [1,2].
Patients with drug-susceptible TB undergo an initial six-month treatment utilizing first-line drugs, namely rifampicin (RIF), isoniazid (INH), ethambutol (EMB), and pyrazinamide (PZA). If they become resistant to RIF and/or INH, they are treated for an extended period with second-line drugs, such as fluoroquinolones (FQ) and aminoglycosides (AG). RIF interferes with the transcription process and kills Mycobacterium tuberculosis. rpoB, encoding the beta subunit of RNA polymerase, is involved in the resistance to RIF [3,4]. Moreover, the degree of resistance varies according to the position of the mutation. Globally, the highest rpoB mutation rate is observed in Ser531 (56.19 ± 9.14%), followed by His526 (17.81 ± 6.33%) and Asp516 (12.94 ± 6.95%) [5,6,7,8,9,10,11]. In particular, in the case of Ser531, more than 90% of single mutations are associated with high resistance to RIF [12], and more than 85% of Ser531 and His526 mutations are simultaneously involved in resistance to rifabutin. Mutations in rpoB may inhibit the growth of resistant bacteria; the phenomenon is referred to as the ‘fitness cost’ effect, which can be compensated for by mutations in rpoA or rpoC [13,14,15,16,17,18,19,20,21].
Although the mechanism of action of INH is not understood comprehensively, it is generally known to inhibit cell wall synthesis and various processes in cells. Genes related to resistance to INH, katG, inhA, ahpC, and others, have been reported [22,23,24,25]. Mutations related to INH resistance have been demonstrated to vary based on the country of identification, with a distribution of 47.7–91.7% for katG Ser315, followed by inhA C(-15)T mutations, at 7.0–35.5%. The katG Ser315 mutation is also considered to affect fitness cost [13,26]. In addition, the katG mutation is mainly related to high INH tolerance, and the inhA mutation is associated with low tolerance if it is a single mutation.
Another primary drug, EMB, interferes with cell wall composition and kills bacteria. The embCAB operon is associated with resistance to EMB, and mutations most related to resistance have occurred in embB [27,28,29].
Patients resistant to the primary drugs RIF and INH are subsequently administered secondary drugs, specifically AG and FQ, for their treatment. AG-based drugs used this way include streptomycin (STR), amikacin (AMK), kanamycin (KAN), and capreomycin (CAP), and related resistance genes include rrs, eis, and rpsL [30,31,32,33,34,35,36,37,38].
FQ targets enzymes essential for the survival and proliferation of bacteria, such as DNA gyrase and topoisomerase IV. Resistance to the drugs is most often due to mutations in gyrA, gyrB, parC, and parE that encode targets of the drug. However, resistance to FQ in M. tuberculosis primarily arises from mutations in gyrA and gyrB, as the bacterium lacks topoisomerase IV [39,40,41,42,43,44,45,46,47].
In Korea, the number of new patients with TB has been decreasing continuously since 2012, and the number of patients with MDR-TB had decreased to approximately one-third (399) by 2020 compared with that in 2012 (1212). The percentage of patients with MDR-TB in Korea is approximately 1.57%, which is lower than the global average (3.4%); however, caution should be exercised considering the increasing trend (1.3–7.7%) of MDR-TB among foreign patients in Korea [48].
Molecular DST determines drug resistance based on mutations in resistance-associated genes. Massive data are required to understand the impact of such resistance mutations on susceptibility to specific anti-TB drugs. Recently, the World Health Organization (WHO) announced recommendations on direct drug susceptibility testing (DST) using next-generation sequencing (NGS), indicating the increasing utilization of molecular DST [49,50,51]. To facilitate the exploration of the correlation between M. tuberculosis complex mutations and drug resistance, DST’s PPV (positive predictive value of mutation) was established based on the resistance gene mutations identified in several studies. Based on the catalog, the susceptibility and resistance of anti-tuberculosis drugs can be predicted according to the gene mutation location. In the present study, the distribution of genetic variations related to drug resistance was investigated, and the levels of resistance among TB strains isolated in Korea were compared based on the types of resistance-related mutations. To assess the possibility of an outbreak of a particular drug-resistant TB strain, the genetic relatedness of TB strains isolated in Korea was investigated.
2. Results
2.1. Anti-TB DST
Among the 215 tested isolates, 190 were resistant to at least one anti-TB drug, and 25 were susceptible to all tested anti-TB drugs. Sixty-four isolates corresponded to MDR-TB, and seventy were pre-extensively drug-resistant (pre-XDR)-TB, the phase prior to XDR. Isoniazid (INH)-resistant strains were the most common, with 155 isolates, followed by 152 RIF-resistant and 95 ofloxacin (OFX)-resistant strains.
2.2. RIF Resistance
Table 1 represents the minimum inhibitory concentrations (MICs) and DNA sequencing results of RIF and rpoB against 195 M. tuberculosis isolates. One hundred and fifty-one isolates had point mutations concentrated in ten different codons; among them, one isolate also had an insertion, whereas deletion was only identified in one isolate. Single mutations were found in 145 isolates, and 7 isolates showed double mutations.

Table 1.
Detection of point mutations in rpoB for rifampicin MIC in 152 M. tuberculosis isolates.
The most common mutation, Ser531Leu, was identified in 85 isolates. Irrespective of the presence of other mutations, the Ser531Leu mutation showed an MIC > 16 µg/mL for RIF.
The second most common mutation occurred in codons 516 (27 isolates) and 526 (24 isolates). Codons 516 and 526 showed four and eight different amino acid changes, respectively. Among these, 13 isolates harbored the Asp516Val mutation, which is commonly observed after the Ser531Leu mutation.
Seven isolates had Leu533Pro, a representative disputed mutation, and exhibited an MIC of 1–2 µg/mL for RIF. Nine isolates had another disputed mutation, Asp516Tyr, five of which showed a low MIC (≤2 µg/mL), and four of which showed a high MIC (≥8 µg/mL) for RIF in the absence of accompanying mutations. Another disputed mutation, His526Leu, found in three isolates, showed an MIC of ≥8 µg/mL for RIF.
2.3. INH Resistance
The nucleotide sequences of katG, inhA, and ahpC were analyzed, and the MIC for INH was compared in 199 isolates in association with the mutations (Table 2).

Table 2.
Detection of point mutations in katG, inhA, and ahpC for isoniazid MIC in 177 M. tuberculosis isolates.
In 15 INH-resistant isolates, among a total of 153, no mutations were detected in katG, inhA, and ahpC. However, 19 susceptible isolates harbored mutations in katG and inhA.
Mutations in katG were identified in 111 isolates, including two susceptible strains. Among them, 16 katG mutants harbored mutations in inhA.
Variations in katG showed 14 types of mutations in 13 codons, and 97 isolates of katG mutants had the Ser315Thr mutation. Most Ser315Thr-associated MICs for INH were higher than 2 µg/mL, but five isolates showed an MIC ranging from 0.5 to 1 µg/mL.
Mutations in the inhA promoter were identified in five types at four positions. Of these, the C-to-T change at position -15 of the inhA promoter was the most frequent, with 54 isolates harboring this mutation. Among the C(-15)T mutants, eight occurred simultaneously with the Ser315Thr mutation of katG in the isolates.
In addition, the ahpC mutation was confirmed in nine isolates, and seven mutation types were found at six positions. One isolate harbored additional mutations in katG.
2.4. Ethambutol (EMB) Resistance
A total of 116 strains were examined for mutations in embB, and the MIC for EMB was calculated (Table 3). An embB mutation was identified in 91 isolates, 24 of which were susceptible. Among the resistant isolates, seven had no mutations.

Table 3.
Detection of point mutations in embB for ethambutol MIC in 91 M. tuberculosis isolates.
Nineteen different types of mutations were found in 11 codons. In addition, four isolates had double mutations, and eighty-seven had a single mutation.
The most frequent mutations were substitutions in the codon of Met306 of embB. We identified 57 isolates with a mutation in the codon; 30 isolates had a Met306Val mutation, 26 isolates had a Met306Ile mutation, and 1 isolate had a Met306Leu mutation. The MIC50 of the isolate differed depending on the type of mutation present. The MIC50 values of isolates with Met-to-Val mutation and Met-to-Ile mutation were 8 µg/mL and 4 µg/mL, respectively. Twelve isolates with a Met306Ile mutation were in the susceptible range for EMB (4 µg/mL).
2.5. Fluoroquinolone (FQ) Resistance
Based on the MIC of OFX, mutations in gyrA and gyrB were investigated in 170 isolates (75 OFX-susceptible and 95 OFX-resistant strains), and the MIC values of all strains were compared (Table 4). Mutations in codons 21 and/or 95 of gyrA were excluded from the analysis, as they have been reported to be natural polymorphisms that are not related to FQ resistance.

Table 4.
Detection of point mutations in gyrA and gyrB for ofloxacin MIC in 79 and 25 M. tuberculosis isolates.
Mutations in gyrA were concentrated in four codons in 81 isolates. Mutations in gyrB occurred in seven codons of 26 isolates.
Sixty-three isolates had only a single mutation, and three had double mutations within gyrA. Eleven isolates had a single mutation in gyrB, fourteen isolates had single mutations in both gyrA and gyrB, and one isolate had a three-point mutation: one in gyrA and two in gyrB (Table 5).

Table 5.
Number of point mutations in gyrA and gyrB.
The most common mutation occurred in gyrA at codon 94 Asp in 54 isolates, and all strains were transformed into five different codons; among these, codon 94 Asp-to-Gly mutants were the most prevalent, with 31 isolates. The Ala90Val mutation, observed in 21 isolates of gyrA mutants, was the second most frequent variation. The most common mutation in gyrB was Asp500Asn, which was detected in five isolates.
Even with a single mutation in gyrA, the MIC for OFX was 8 µg/mL or higher. When a single mutation was present in gyrB, the MIC for OFX was lower (4 µg/mL or below). When simultaneous mutations were present in gyrA and gyrB, the MIC was significantly higher (16 µg/mL or higher).
2.6. Aminoglycoside (AG) Resistance
The sequence of rrs was analyzed in 125 isolates, and among them, the eis gene sequence was examined in 89 isolates. The sequencing results and MIC for amikacin (AMK) and kanamycin (KAN) were compared. Of the 125 isolates analyzed, 87 were susceptible, and 38 were resistant to KAN (Table 6).

Table 6.
Detection of point mutations in rrs and eis for amikacin MIC in 35 and 10 M. tuberculosis isolates, respectively.
Sequencing of rrs revealed mutations in 35 out of the 125 isolates. Mutations were identified in seven codons with single mutations, except for one isolate with a double mutation. The most prevalent mutation was the A1401G substitution in rrs, and the 24 isolates with the mutation induced a strong resistance of at least 40 µg/mL to KAN and 16 µg/mL to AMK. However, mutations in other positions, such as positions 514 and 517, either did not affect drug susceptibility or resulted in a slight decrease in sensitivity.
Mutations in the eis promoter were identified at three positions in 10 isolates. The mutations result in decreased sensitivity or low levels of resistance to KAN at its MIC. However, for AMK, most mutations showed an MIC of ≤1 µg/mL, which did not affect the sensitivity.
The rpsL sequence was analyzed in 118 isolates containing 59 streptomycin (STR)-resistant strains and compared with the sequencing results of the previously analyzed rrs (Table 7). Mutations were found in 38 isolates, all of which were single mutations identified in five types at three codon positions. Among them, 38 isolates were within the resistance range, and 1 isolate showed an MIC, indicating susceptibility for STR but near the critical concentration. The most common mutation of rpsL, Lys43Arg, occurred in 28 isolates. Regardless of the presence of another mutation, all Lys43Arg mutations induced an MIC of >32 µg/mL for STR. Two isolates, mutated to methionine at the same position, were not accompanied by mutations of other genes and had an MIC of >32 µg/mL for STR. Lys88Arg, the second most common mutation, was not accompanied by other site mutations and induced an MIC range of 8 to 32 µg/mL.

Table 7.
Detection of point mutations in rpsL and rrs for streptomycin MIC in 38 and 35 M. tuberculosis isolates, respectively.
The correlation between AG resistance and mutations of the common rrs was that the rrs A1401G mutation induced resistance to KM and AK but not to STR. Conversely, mutations in bases 514, 517, and 908 induced the resistance to STR but did not affect resistance to KM and AK.
2.7. Compensatory Mutation
Mutations in rpoC were identified in 35 out of the 181 isolates. Mutations were identified as 20 types in the 17 codons. Of the 35 isolates, 1 isolate had a double mutation, and the remaining isolates had a single mutation. In addition, all rpoC mutants harbored mutations in rpoB. The mutation types in the rpoB were Ser531Leu, with 33 isolates, and His526Asp and His526Arg in the remaining isolates.
The most prevalent mutation was Phe452Leu, with six isolates harboring this mutation. The second was Pro434Val, which was detected in five isolates. Codon 491 isoleucine was mutated into two types: three isolates had a substitution for threonine, and the other three were substituted with valine. The Gly388Ala and Lys445Arg mutants contained His526Asp and His526Arg mutations in rpoB, respectively.
An examination of the genetic association of rpoC mutants using 24 locus mycobacterial interspersed repetitive unit–variable number of tandem repeats (MIRU-VNTR) of the 35 strains in which rpoC was mutated revealed that 14 isolates formed five clusters with a size of 2–5 strains. Four isolates of rpoC Phe452Leu mutants composed a cluster, and all five Phe452Leu-mutant isolates formed their own clusters. All three isolates of Ile491Thr mutants also composed clusters (Figure 1).
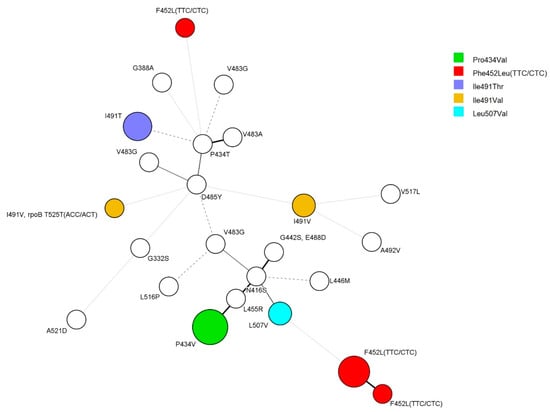
Figure 1.
Minimum spanning tree (MST) based on 24-locus MIRU-VNTR. A total of 34 strains were analyzed for rpoC mutations. Except for the two strains with Ile491Val and Phe452Leu, each color represents the same mutation. The size of the circles indicates the number of isolates: five strains, Pro434Val; six strains, Phe452Leu; two strains, Leu507Val; two strains, Ile491Val; three strains, Ile491Thr.
2.8. Genetic Relevance of Resistant Strains
Genotypic lineages were identified by spoligotyping 190 isolates that were resistant to at least one of the anti-TB drugs investigated in the present study (Figure S1). A total of 162 isolates (85.3%) were classified as the East Asian lineage (lineage 2), including the Beijing family, and 21 isolates belonged to the Euro-American lineage (lineage 4).
Genetic association analysis of the resistant strains using 24- locus MIRU-VNTR identified 163 types, of which 16 formed cluster sizes consisting of two to six isolates, while the remaining 143 isolates had unique types.
It was difficult to observe a correlation between each MIRU-VNTR type with anti-tuberculosis drug susceptibility pattern or region and time of isolation. However, there was a correlation with the mutation type of the rpoC gene (Figure 1).
3. Discussion
In the present study, we investigated variations in genes related to anti-TB drug resistance in isolates from Korea. The results of this study provide evidence that could improve the accuracy of anti-TB resistance determination using molecular DST. It is vital to know the extent to which a mutation affects anti-TB resistance, in addition to determining whether the mutation is in a related gene. Therefore, the anti-TB susceptibility levels of the isolates used in the present study were determined using the MIC test, and the gene sequence related to the resistance to each anti-TB agent was analyzed to confirm its association with the MIC.
The WHO recently revised the definition of XDR-TB, according to which pre-XDR-TB refers to TB that meets the criteria for XDR-TB and shows RIF resistance, as well as resistance to FQs. However, because the study did not provide drug susceptibility test results for bedaquiline and linezolid, accurately classifying the bacteria as XDR was challenging.
Genetic mutations associated with drug-resistant TB showed patterns similar to those reported in previous studies [50]. However, some mutations exhibit slightly different effects on drug resistance.
Several disputed mutations that are susceptible to DST but are known to affect treatment outcomes were detected in the present study. Excluding Leu533Pro and Asp516Tyr, the disputed mutations showed high MIC for RIF in the absence of accompanying mutations, and Asp516Tyr-mutant strains were divided into two groups based on the MIC for RIF: five isolates of 2 µg/mL or lower and four isolates of 8 µg/mL or higher. According to the WHO mutation catalogue, the positive predictive value of mutation (PPV) for antibiotic resistance was 69.5% for Leu533Pro and 78.6% for Asp516Tyr. In the case of Ser531Leu, the PPV was 98.9%, and the results of the present study also confirmed resistance, with an MIC of 8 µg/mL or higher. Thus, the contributions of other factors, such as efflux pumps, are expected. As data accumulate in the future, the predictive chart will be able to produce more accurate results.
The mutations were concentrated in His codon 526 and Asp codon 516. In contrast to previous studies, codon 516 exhibited more mutations than codon 526. The detection rate for codon 516 (19.1%) in the present study was more than twice that reported in China and Taiwan (less than 10%) [52,53].
Mutations in ahpC are associated with mutations in the katG gene. However, in the present study, among the eight isolates of ahpC mutants, only one exhibited co-occurrence of a mutation in katG. Although C(-52)A, C(-52)T, and ins(-88)GT of ahpC have not been reported elsewhere and can be considered natural polymorphisms, G(-48)A, G(-51)A, and C(-81)T are widely recognized mutations that occur in conjunction with katG mutations. However, it is challenging to determine whether mutations in ahpC indeed affect INH resistance, and it is not yet widely accepted that the ahpC mutation alone is involved in INH resistance in M. tuberculosis [54].
Met306Ile mutations in embB have also been identified in susceptible strains [55,56]. In the present study, 12 isolates of the 26 Met306Ile mutants were in the susceptible range for EMB, but all had an MIC of 4 µg/mL, which was near the critical concentration; therefore, this mutation did not affect resistance but was inferred to decrease susceptibility.
The findings of the present study on resistance to FQ and some gyrB mutations differed from the results of other studies. The MIC according to the Arg485Cys mutation of gyrB was 4 µg/mL in the present study, albeit with little difference from the results of studies by Farhat et al. (2 µg/mL) and Malik et al. (1 μg/mL) [43,46]. In addition, the Glu540Asp mutation induced slightly higher resistance at levels of 1–2 µg/mL in the present study than at 0.5 µg/mL in the two other abovementioned studies. The Glu540Asp mutants had an MIC for MFX that was 2–4 µg/mL higher than that for OFX. Additionally, in the two studies above, the MIC for MFX was confirmed to be 2 µg/mL, and the mutation had a greater effect on MFX than OFX, unlike other variations. The Ser486Phe mutation was the first clinical isolate identified in the present study, whereas the Asn538Asp and Thr539Asn mutations induced the same level of resistance reported in other studies.
In the present study, the percentages of isolates that did not have mutations related to anti-TB drug resistance were 3.3%, 9.8%, 7.4%, 9.4%, and 7.9% for RIF, INH, FQ, EMB, and AG, respectively. This is because of the contribution of other factors, such as heteroresistance and efflux pumps. However, more than 90% of the resistance mechanisms against major anti-TB drugs could be identified using only the genes identified in the present study.
A secondary aim of this study was to investigate whether there has been an MDR-TB epidemic caused by a specific strain in South Korea. Examination of the genetic relationships of the resistant isolates revealed that all cluster sizes were less than six isolates, and even in the same cluster; except for the rpoC mutants, the drug susceptibility patterns were different. Hence, there is no evidence of large-scale epidemics caused by specifically resistant strains. However, the five types of rpoC mutations formed clusters consisting of two to five isolates, showing a higher frequency of genetic cluster formation than other resistant strains. This trend has also been reported in other studies [57,58]. Therefore, it is presumed that rpoC mutants caused by the rpoB mutations are more advantageous in propagation than RIF-resistant strains without the mutation, owing to the compensatory effect of rpoC mutations on the fitness cost of rpoB mutations.
The recent application of NGS-based DST for TB in clinical settings provides evidence supporting the utility of genotype-based DST. The results obtained using this method will serve as a basis for understanding the effects of specific mutations on drug resistance. Analyzing the effects of each mutation on resistance is expected to further enhance the effectiveness of genetics-based DST.
4. Materials and Methods
4.1. Mycobacterium tuberculosis Culture and DNA Extraction
A total of 215 M. tuberculosis strains were selected from the strains isolated from patients with TB in the Masan National Hospital and the public health network in Korea. Clinical isolates were collected between October 2009 and December 2014. No duplicate isolates of the same origin were detected. Strains were incubated in 10 mL 7H9 broth containing 0.05% (v/v) Tween 80 and 0.2% (v/v) glycerol at 37 °C with shaking at 150 rpm. Harvested bacterial cells were allowed to stand for 15 min. The precipitated bacterial cells were transferred to a 1.5 mL microcentrifuge tube in 1 mL volume. The bacterial cells were centrifuged for 2 min at 13,000 rpm, and the supernatant was discarded. DNA was extracted using the InstaGene™ Matrix (Bio-Rad, Hercules, CA, USA) according to the manufacturer’s instructions.
4.2. Resistance Gene Sequencing
PCR amplification was performed using the AmpliTaq Gold 360 Master Mix (Applied Biosystems, Foster City, CA, USA). The thermal cycling protocol for rpoB, inhA, ahpC, gyrA, gyrB, embB, rpsL, and rrs was as follows: predenaturation at 94 °C for 5 min; 25 cycles at 94 °C for 30 s, 58 °C for 30 s, 72 °C for 1 min; and a final extension step at 72 °C for 10 min. The thermal cycling protocol for katG was as follows: predenaturation at 94 °C for 5 min; 25 cycles at 94 °C for 30 s, 62 °C for 30 s, 72 °C for 1 min; and a final extension step at 72 °C for 10 min. The thermal cycling protocol for eis was as follows: predenaturation at 94 °C for 5 min; 25 cycles at 94 °C for 30 s, 50 °C for 30 s, 72 °C for 1 min; and a final extension step at 72 °C for 10 min. Genomic DNA from the M. tuberculosis isolates and reference strains was subjected to PCR. The primer sets used for the amplification and sequencing of resistance-related genes are shown in Table S1. The PCR amplification products of each gene were sequenced using the same primers as those used by Biofact Co. (Biofact Co. Deajeon, Republic of Korea).
The sequences were analyzed using CLC Main Workbench 6 (CLC bio, Prismet, Aarhus, Denmark). Gene polymorphisms were identified by alignment with the reference strain M. tuberculosis H37Rv (GenBank accession no. NC_000962).
4.3. Spoligotyping
Spoligotyping is a PCR-based method performed using a spoligotyping kit (Mapmygenome, Hyderabad, India), which was performed according to the manufacturer’s instructions. Genomic DNA was used at a concentration of 100 ng/µL. The spoligotypes were assigned a Spoligo International Type (SIT) and analyzed using SITVITWEB (http://www.pasteur-guadeloupe.fr.8081/SITVIT2_ONLINE/, accessed on 20 June 2020). The SITs were analyzed to similar values using BioNumerics 7.1 (Applied Maths, Sint-Martens-Latem, Belgium).
4.4. 24 Locus MIRU-VNTR
The MIRU-VNTR assay was used to describe the triplex PCR-based 24-locus MIRU-VNTR according to Supply et al. [59]. In the present study, we used six sets of quadruplex PCR methods established by Jeon et al. [60]. The amplicons were analyzed using a Genetic Analyzer 3500xL (Applied Biosystems, Waltham, MA, USA). The MIRU-VNTR types are referred to as MV types in this paper. MIRU-VNTR results were analyzed using the BioNumerics 7.1 software (Applied Maths, Sint-Martens-Latem, Belgium).
4.5. Antibiotic Susceptibility Testing
M. tuberculosis MIC was determined on a Sensititre™ MYCOTBI MIC plate (Trek Diagnostic Systems, Inc., Cleveland, OH, USA) because it is reasonable, rapid, and simple to perform [61,62].
Strains were incubated in 10 mL 7H9 broth containing 0.05% Tween 80 and 0.2% glycerol at 37 °C with shaking at 150 rpm. Harvested bacterial cells were allowed to stand for 15 min. MICs were calculated according to the manufacturer’s protocol. The antibiotics used were OFX, moxifloxacin (MXF), RIF, AMK, STR, rifabutin (RFB), para-aminosalicylic acid (PAS), ethionamide (ETH), cycloserine (CYC), INH, KAN, and EMB. The MIC results were read according to the guidelines of the Clinical Laboratory Standards Institute (CLSI).
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/antibiotics12081324/s1, Table S1: Primers used for amplification and sequencing [63,64,65]. Figure S1: Dendrogram of molecular typing with 24 loci MIRU-VNTR and Spoligotyping used UPGMA method.
Author Contributions
Conceptualization, S.K. and N.S.; formal analysis, N.-R.L. and S.-M.J.; resources, N.L. and J.J.; data curation, S.-M.J.; writing—original draft preparation, S.-M.J.; writing—review and editing, S.K. and S.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Korea National Institute of Health, grant number 2014-NG46001-00.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data available on request.
Acknowledgments
Thanks to the late Kilsoo Lee.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Orenstein, E.W.; Basu, S.; Shah, N.S.; Andrews, J.R.; Friedland, G.H.; Moll, A.P.; Gandhi, N.R.; Galvani, A.P. Treatment outcomes among patients with multidrug-resistant tuberculosis: Systematic review and meta-analysis. Lancet Infect. Dis. 2009, 9, 153–161. [Google Scholar] [CrossRef] [PubMed]
- Jacobson, K.R.; Tierney, D.B.; Jeon, C.Y.; Mitnick, C.D.; Murray, M.B. Treatment Outcomes among Patients with Extensively Drug-Resistant Tuberculosis: Systematic Review and Meta-Analysis. Clin. Infect. Dis. 2010, 51, 6–14. [Google Scholar] [CrossRef] [PubMed]
- Swain, S.S.; Sharma, D.; Hussain, T.; Pati, S. Molecular mechanisms of underlying genetic factors and associated mutations for drug resistance in Mycobacterium tuberculosis. Emerg. Microbes Infect. 2020, 9, 1651–1663. [Google Scholar] [CrossRef]
- Li, M.-C.; Lu, J.; Lu, Y.; Xiao, T.-Y.; Liu, H.-C.; Lin, S.-Q.; Xu, D.; Li, G.-L.; Zhao, X.-Q.; Liu, Z.-G.; et al. rpoB Mutations and Effects on Rifampin Resistance in Mycobacterium tuberculosis. Infect. Drug Resist. 2021, 14, 4119–4128. [Google Scholar] [CrossRef] [PubMed]
- Isakova, J.; Sovkhozova, N.; Vinnikov, D.; Goncharova, Z.; Talaibekova, E.; Aldasheva, N.; Aldashev, A. Mutations of rpoB, katG, inhA and ahp genes in rifampicin and isoniazid-resistant Mycobacterium tuberculosis in Kyrgyz Republic. BMC Microbiol. 2018, 18, 22. [Google Scholar] [CrossRef]
- Kim, B.J.; Oh, S.H.; Cho, E.J.; Park, S.K. Cross-resistance Between Rifampicin and Rifabutin and Its Relationship with rpoB Gene Mutations in Clinically Isolated MDR-TB Strains. Tuberc. Respir. Dis. 2006, 60, 171–179. [Google Scholar] [CrossRef][Green Version]
- Lew, W.J.; Kil Park, Y.; Kim, H.J.; Chang, C.; Bai, G.H.; Kim, S.K. The Proportion of Rifabutin-susceptible Strains among Rifampicin-resistant Isolates and Its Specific rpoB Mutations. Tuberc. Respir. Dis. 2005, 59, 257–265. [Google Scholar] [CrossRef]
- Prammananan, T.; Cheunoy, W.; Taechamahapun, D.; Yorsangsukkamol, J.; Phunpruch, S.; Phdarat, P.; Leechawengwong, M.; Chaiprasert, A. Distribution of rpoB mutations among multidrug-resistant Mycobacterium tuberculosis (MDRTB) strains from Thailand and development of a rapid method for mutation detection. Clin. Microbiol. Infect. 2008, 14, 446–453. [Google Scholar] [CrossRef][Green Version]
- Sharma, S. Detection of Mutations in rpob Gene of Clinically Isolated, M. Tuberculosis by DNA Sequencing. Mycobact. Dis. 2014, 4, 156. [Google Scholar] [CrossRef]
- Uddin, M.K.M.; Rahman, A.; Ather, F.; Ahmed, T.; Rahman, S.M.M.; Ahmed, S.; Banu, S. Distribution and Frequency of rpoB Mutations Detected by Xpert MTB/RIF Assay Among Beijing and Non-Beijing Rifampicin Resistant Mycobacterium tuberculosis Isolates in Bangladesh. Infect. Drug Resist. 2020, 13, 789–797. [Google Scholar] [CrossRef]
- Yoon, J.-H.; Nam, J.-S.; Kim, K.-J.; Choi, Y.; Lee, H.; Cho, S.-N.; Ro, Y.-T. Molecular characterization of drug-resistant and -susceptible Mycobacterium tuberculosis isolated from patients with tuberculosis in Korea. Diagn. Microbiol. Infect. Dis. 2012, 72, 52–61. [Google Scholar] [CrossRef] [PubMed]
- Hwang, H.-Y.; Chang, C.-Y.; Chang, L.-L.; Chang, S.-F.; Chang, Y.-H.; Chen, Y.-J. Characterization of rifampicin-resistant Mycobacterium tuberculosis in Taiwan. J. Med. Microbiol. 2003, 52, 239–245. [Google Scholar] [CrossRef] [PubMed]
- Brandis, G.; Pietsch, F.; Alemayehu, R.; Hughes, D. Comprehensive phenotypic characterization of rifampicin resistance mutations in Salmonella provides insight into the evolution of resistance in Mycobacterium tuberculosis. J. Antimicrob. Chemother. 2014, 70, 680–685. [Google Scholar] [CrossRef]
- De Vos, M.; Müller, B.; Borrell, S.; Black, P.A.; van Helden, P.D.; Warren, R.M.; Gagneux, S.; Victor, T.C. Putative Compensatory Mutations in the rpoC Gene of Rifampin-Resistant Mycobacterium tuberculosis Are Associated with Ongoing Transmission. Antimicrob. Agents Chemother. 2013, 57, 827–832. [Google Scholar] [CrossRef] [PubMed]
- Horng, Y.-T.; Jeng, W.-Y.; Chen, Y.-Y.; Liu, C.-H.; Dou, H.-Y.; Lee, J.-J.; Chang, K.-C.; Chien, C.-C.; Soo, P.-C. Molecular Analysis of Codon 548 in the rpoB Gene Involved in Mycobacterium tuberculosis Resistance to Rifampin. Antimicrob. Agents Chemother. 2015, 59, 1542–1548. [Google Scholar] [CrossRef]
- McAlister, A.J.; Driscoll, J.; Metchock, B. DNA Sequencing for Confirmation of Rifampin Resistance Detected by Cepheid Xpert MTB/RIF Assay. J. Clin. Microbiol. 2015, 53, 1752–1753. [Google Scholar] [CrossRef]
- Meftahi, N.; Namouchi, A.; Mhenni, B.; Brandis, G.; Hughes, D.; Mardassi, H. Evidence for the critical role of a secondary site rpoB mutation in the compensatory evolution and successful transmission of an MDR tuberculosis outbreak strain. J. Antimicrob. Chemother. 2015, 71, 324–332. [Google Scholar] [CrossRef]
- Ocheretina, O.; Escuyer, V.E.; Mabou, M.-M.; Royal-Mardi, G.; Collins, S.; Vilbrun, S.C.; Pape, J.W.; Fitzgerald, D.W. Correlation between Genotypic and Phenotypic Testing for Resistance to Rifampin in Mycobacterium tuberculosis Clinical Isolates in Haiti: Investigation of Cases with Discrepant Susceptibility Results. PLoS ONE 2014, 9, e90569. [Google Scholar] [CrossRef]
- Rigouts, L.; Gumusboga, M.; de Rijk, W.B.; Nduwamahoro, E.; Uwizeye, C.; de Jong, B.; Van Deun, A. Rifampin Resistance Missed in Automated Liquid Culture System for Mycobacterium tuberculosis Isolates with Specific rpoB Mutations. J. Clin. Microbiol. 2013, 51, 2641–2645. [Google Scholar] [CrossRef]
- Schon, T.; Jureen, P.; Chryssanthou, E.; Giske, C.G.; Kahlmeter, G.; Hoffner, S.; Angeby, K. Rifampicin-resistant and rifabutin-susceptible Mycobacterium tuberculosis strains: A breakpoint artefact? J. Antimicrob. Chemother. 2013, 68, 2074–2077. [Google Scholar] [CrossRef]
- Strauss, O.J.; Warren, R.M.; Jordaan, A.; Streicher, E.M.; Hanekom, M.; Falmer, A.A.; Albert, H.; Trollip, A.; Hoosain, E.; van Helden, P.D.; et al. Spread of a Low-Fitness Drug-Resistant Mycobacterium tuberculosis Strain in a Setting of High Human Immunodeficiency Virus Prevalence. J. Clin. Microbiol. 2008, 46, 1514–1516. [Google Scholar] [CrossRef]
- Fenner, L.; Egger, M.; Bodmer, T.; Altpeter, E.; Zwahlen, M.; Jaton, K.; Pfyffer, G.E.; Borrell, S.; Dubuis, O.; Bruderer, T.; et al. Effect of Mutation and Genetic Background on Drug Resistance in Mycobacterium tuberculosis. Antimicrob. Agents Chemother. 2012, 56, 3047–3053. [Google Scholar] [CrossRef] [PubMed]
- Gagneux, S.; Burgos, M.V.; DeRiemer, K.; Enciso, A.; Muñoz, S.; Hopewell, P.C.; Small, P.M.; Pym, A.S. Impact of Bacterial Genetics on the Transmission of Isoniazid-Resistant Mycobacterium tuberculosis. PLoS Pathog. 2006, 2, e61. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-Y.; Park, Y.-J.; Kim, W.-I.; Lee, S.-H.; Chang, C.L.; Kang, S.-J.; Kang, C.-S. Molecular analysis of isoniazid resistance in Mycobacterium tuberculosis isolates recovered from South Korea. Diagn. Microbiol. Infect. Dis. 2003, 47, 497–502. [Google Scholar] [CrossRef]
- Unissa, A.N.; Selvakumar, N.; Narayanan, S.; Narayanan, P. Molecular analysis of isoniazid-resistant clinical isolates of Mycobacterium tuberculosis from India. Int. J. Antimicrob. Agents 2008, 31, 71–75. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Salvatore, P.P.; Becerra, M.C.; Wiesch, P.A.Z.; Hinkley, T.; Kaur, D.; Sloutsky, A.; Cohen, T. Fitness Costs of Drug Resistance Mutations in Multidrug-Resistant Mycobacterium tuberculosis: A Household-Based Case-Control Study. J. Infect. Dis. 2015, 213, 149–155. [Google Scholar] [CrossRef]
- Brossier, F.; Sougakoff, W.; Bernard, C.; Petrou, M.; Adeyema, K.; Pham, A.; de la Breteque, D.A.; Vallet, M.; Jarlier, V.; Sola, C.; et al. Molecular Analysis of the embCAB Locus and embR Gene Involved in Ethambutol Resistance in Clinical Isolates of Mycobacterium tuberculosis in France. Antimicrob. Agents Chemother. 2015, 59, 4800–4808. [Google Scholar] [CrossRef]
- Cuevas-Córdoba, B.; Juárez-Eusebio, D.M.; Almaraz-Velasco, R.; Muñiz-Salazar, R.; Laniado-Laborin, R.; Zenteno-Cuevas, R. Mutation at embB Codon 306, a Potential Marker for the Identification of Multidrug Resistance Associated with Ethambutol in Mycobacterium tuberculosis. Antimicrob. Agents Chemother. 2015, 59, 5455–5462. [Google Scholar] [CrossRef]
- Zhao, L.-L.; Liu, H.-C.; Sun, Q.; Xiao, T.-Y.; Zhao, X.-Q.; Li, G.-L.; Zeng, C.-Y.; Wan, K.-L. Identification of mutations conferring streptomycin resistance in multidrug-resistant tuberculosis of China. Diagn. Microbiol. Infect. Dis. 2015, 83, 150–153. [Google Scholar] [CrossRef]
- Alangaden, G.J.; Kreiswirth, B.N.; Aouad, A.; Khetarpal, M.; Igno, F.R.; Moghazeh, S.L.; Manavathu, E.K.; Lerner, S.A. Mechanism of Resistance to Amikacin and Kanamycin in Mycobacterium tuberculosis. Antimicrob. Agents Chemother. 1998, 42, 1295–1297. [Google Scholar] [CrossRef]
- Maus, C.E.; Plikaytis, B.B.; Shinnick, T.M. Mutation of tlyA Confers Capreomycin Resistance in Mycobacterium tuberculosis. Antimicrob. Agents Chemother. 2005, 49, 571–577. [Google Scholar] [CrossRef]
- Okamoto, S.; Tamaru, A.; Nakajima, C.; Nishimura, K.; Tanaka, Y.; Tokuyama, S.; Suzuki, Y.; Ochi, K. Loss of a conserved 7-methylguanosine modification in 16S rRNA confers low-level streptomycin resistance in bacteria. Mol. Microbiol. 2007, 63, 1096–1106. [Google Scholar] [CrossRef] [PubMed]
- Smittipat, N.; Juthayothin, T.; Billamas, P.; Jaitrong, S.; Rukseree, K.; Dokladda, K.; Chaiyasirinroje, B.; Disratthakit, A.; Chaiprasert, A.; Mahasirimongkol, S.; et al. Mutations in rrs, rpsL and gidB in streptomycin-resistant Mycobacterium tuberculosis isolates from Thailand. J. Glob. Antimicrob. Resist. 2015, 4, 5–10. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Zhang, C.; Xiang, L.; Pi, R.; Guo, Z.; Zheng, C.; Li, S.; Zhao, Y.; Tang, K.; Luo, M.; et al. Characterization of mutations in streptomycin-resistant Mycobacterium tuberculosis isolates in Sichuan, China and the association between Beijing-lineage and dual-mutation in gidB. Tuberculosis 2016, 96, 102–106. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, Y.; Katsukawa, C.; Tamaru, A.; Abe, C.; Makino, M.; Mizuguchi, Y.; Taniguchi, H. Detection of Kanamycin-Resistant Mycobacterium tuberculosis by Identifying Mutations in the 16S rRNA Gene. J. Clin. Microbiol. 1998, 36, 1220–1225. [Google Scholar] [CrossRef] [PubMed]
- Verma, J.S.; Gupta, Y.; Nair, D.; Manzoor, N.; Rautela, R.S.; Rai, A.; Katoch, V.M. Evaluation of gidB alterations responsible for streptomycin resistance in Mycobacterium tuberculosis. J. Antimicrob. Chemother. 2014, 69, 2935–2941. [Google Scholar] [CrossRef]
- Zaunbrecher, M.A.; Sikes, R.D.; Metchock, B.; Shinnick, T.M.; Posey, J.E. Overexpression of the chromosomally encoded aminoglycoside acetyltransferase eis confers kanamycin resistance in Mycobacterium tuberculosis. Proc. Natl. Acad. Sci. USA 2009, 106, 20004–20009. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.-L.; Sun, Q.; Liu, H.-C.; Wu, X.-C.; Xiao, T.-Y.; Zhao, X.-Q.; Li, G.-L.; Jiang, Y.; Zeng, C.-Y.; Wan, K.-L. Analysis of embCAB Mutations Associated with Ethambutol Resistance in Multidrug-Resistant Mycobacterium tuberculosis Isolates from China. Antimicrob. Agents Chemother. 2015, 59, 2045–2050. [Google Scholar] [CrossRef]
- Aubry, A.; Veziris, N.; Cambau, E.; Truffot-Pernot, C.; Jarlier, V.; Fisher, L.M. Novel Gyrase Mutations in Quinolone-Resistant and -Hypersusceptible Clinical Isolates of Mycobacterium tuberculosis: Functional Analysis of Mutant Enzymes. Antimicrob. Agents Chemother. 2006, 50, 104–112. [Google Scholar] [CrossRef]
- Bernard, C.; Veziris, N.; Brossier, F.; Sougakoff, W.; Jarlier, V.; Robert, J.; Aubry, A. Molecular Diagnosis of Fluoroquinolone Resistance in Mycobacterium tuberculosis. Antimicrob. Agents Chemother. 2015, 59, 1519–1524. [Google Scholar] [CrossRef]
- Chien, J.-Y.; Chiu, W.-Y.; Chien, S.-T.; Chiang, C.-J.; Yu, C.-J.; Hsueh, P.-R. Mutations in gyrA and gyrB among Fluoroquinolone- and Multidrug-Resistant Mycobacterium tuberculosis Isolates. Antimicrob. Agents Chemother. 2016, 60, 2090–2096. [Google Scholar] [CrossRef]
- Coeck, N.; de Jong, B.C.; Diels, M.; de Rijk, P.; Ardizzoni, E.; Van Deun, A.; Rigouts, L. Correlation of different phenotypic drug susceptibility testing methods for four fluoroquinolones in Mycobacterium tuberculosis. J. Antimicrob. Chemother. 2016, 71, 1233–1240. [Google Scholar] [CrossRef] [PubMed]
- Farhat, M.R.; Jacobson, K.R.; Franke, M.F.; Kaur, D.; Sloutsky, A.; Mitnick, C.D.; Murray, M. Gyrase Mutations Are Associated with Variable Levels of Fluoroquinolone Resistance in Mycobacterium tuberculosis. J. Clin. Microbiol. 2016, 54, 727–733. [Google Scholar] [CrossRef] [PubMed]
- Lau, R.W.T.; Ho, P.-L.; Kao, R.Y.T.; Yew, W.-W.; Lau, T.C.K.; Cheng, V.C.C.; Yuen, K.-Y.; Tsui, S.K.W.; Chen, X.; Yam, W.-C. Molecular Characterization of Fluoroquinolone Resistance in Mycobacterium tuberculosis: Functional Analysis of gyrA Mutation at Position 74. Antimicrob. Agents Chemother. 2011, 55, 608–614. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Liu, M.; Wang, Y.; Pang, Y.; Zhao, Z. Mechanisms of fluoroquinolone monoresistance in Mycobacterium tuberculosis. FEMS Microbiol. Lett. 2014, 353, 40–48. [Google Scholar] [CrossRef]
- Malik, S.; Willby, M.; Sikes, D.; Tsodikov, O.V.; Posey, J.E. New Insights into Fluoroquinolone Resistance in Mycobacterium tuberculosis: Functional Genetic Analysis of gyrA and gyrB Mutations. PLoS ONE 2012, 7, e39754. [Google Scholar] [CrossRef]
- Willby, M.; Sikes, R.D.; Malik, S.; Metchock, B.; Posey, J.E. Correlation between GyrA Substitutions and Ofloxacin, Levofloxacin, and Moxifloxacin Cross-Resistance in Mycobacterium tuberculosis. Antimicrob. Agents Chemother. 2015, 59, 5427–5434. [Google Scholar] [CrossRef]
- Lee, M.; Han, J.; Kim, Y.R.; Kwak, N.; Kim, J.H.; Park, O.; Shin, S.; Moon, H.S.; Kim, H.J.; Jang, M.-J.; et al. Multidrug-resistant tuberculosis in South Korea: A retrospective analysis of national registry data in 2011–2015. Int. J. Tuberc. Lung Dis. 2019, 23, 850–857. [Google Scholar] [CrossRef]
- World Health Organization. The Use of Next-Generation Sequencing Technologies for the Detection of Mutations Associated with Drug Resistance in Mycobacterium Tuberculosis Complex: Technical Guide; World Health Organization: Geneva, Switzerland, 2018. Available online: https://apps.who.int/iris/handle/10665/274443 (accessed on 24 July 2023).
- World Health Organization. Catalogue of Mutations in Mycobacterium tuberculosis Complex and Their Association with Drug Resistance; World Health Organization: Geneva, Switzerland, 2021. Available online: https://www.who.int/publications/i/item/9789240028173 (accessed on 24 July 2023).
- World Health Organization. Global Tuberculosis Report 2022; World Health Organization: Geneva, Switzerland, 2022. Available online: https://www.who.int/publications/i/item/9789240061729 (accessed on 24 July 2023).
- Lin, Y.-H.; Tai, C.-H.; Li, C.-R.; Lin, C.-F.; Shi, Z.-Y. Resistance profiles and rpoB gene mutations of Mycobacterium tuberculosis isolates in Taiwan. J. Microbiol. Immunol. Infect. 2013, 46, 266–270. [Google Scholar] [CrossRef][Green Version]
- Wang, S.; Zhao, B.; Song, Y.; Zhou, Y.; Pang, Y.; Ou, X.; Li, Q.; Xia, H.; Zhao, Y. Molecular characterization of the rpoB gene mutations of Mycobacterium tuberculosis isolated from China. J. Tuberc. Res. 2013, 1, 1–8. [Google Scholar] [CrossRef][Green Version]
- Unissa, A.N.; Subbian, S.; Hanna, L.E.; Selvakumar, N. Overview on mechanisms of isoniazid action and resistance in Mycobacterium tuberculosis. Infect. Genet. Evol. 2016, 45, 474–492. [Google Scholar] [CrossRef] [PubMed]
- Kil Park, Y.; Ryoo, S.W.; Lee, S.H.; Jnawali, H.N.; Kim, C.-K.; Kim, H.J.; Kim, S.J. Correlation of the phenotypic ethambutol susceptibility of Mycobacterium tuberculosis with embB gene mutations in Korea. J. Med. Microbiol. 2012, 61, 529–534. [Google Scholar] [CrossRef] [PubMed]
- Yakrus, M.A.; Driscoll, J.; McAlister, A.; Sikes, D.; Hartline, D.; Metchock, B.; Starks, A.M. Molecular and Growth-Based Drug Susceptibility Testing of Mycobacterium tuberculosis Complex for Ethambutol Resistance in the United States. Tuberc. Res. Treat. 2016, 2016, 3404860. [Google Scholar] [CrossRef]
- Song, T.; Park, Y.; Shamputa, I.C.; Seo, S.; Lee, S.Y.; Jeon, H.-S.; Choi, H.; Lee, M.; Glynne, R.J.; Barnes, S.W.; et al. Fitness costs of rifampicin resistance in Mycobacterium tuberculosis are amplified under conditions of nutrient starvation and compensated by mutation in the β′ subunit of RNA polymerase. Mol. Microbiol. 2014, 91, 1106–1119. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Zhou, Y.; Zhao, B.; Ou, X.; Xia, H.; Zheng, Y.; Song, Y.; Cheng, Q.; Wang, X.; Zhao, Y. Characteristics of compensatory mutations in the rpoC gene and their association with compensated transmission of Mycobacterium tuberculosis. Front. Med. 2020, 14, 51–59. [Google Scholar] [CrossRef]
- Supply, P.; Allix, C.; Lesjean, S.; Cardoso-Oelemann, M.; Rüsch-Gerdes, S.; Willery, E.; Savine, E.; de Haas, P.; van Deutekom, H.; Roring, S.; et al. Proposal for Standardization of Optimized Mycobacterial Interspersed Repetitive Unit-Variable-Number Tandem Repeat Typing of Mycobacterium tuberculosis. J. Clin. Microbiol. 2006, 44, 4498–4510. [Google Scholar] [CrossRef] [PubMed]
- Jeon, S.; Lim, N.; Park, S.; Park, M.; Kim, S. Comparison of PFGE, IS6110-RFLP, and 24-Locus MIRU-VNTR for Molecular Epidemiologic Typing of Mycobacterium tuberculosis Isolates with Known Epidemic Connections. J. Microbiol. Biotechnol. 2018, 28, 338–346. [Google Scholar] [CrossRef]
- Lee, J.; Armstrong, D.T.; Ssengooba, W.; Park, J.-A.; Yu, Y.; Mumbowa, F.; Namaganda, C.; Mboowa, G.; Nakayita, G.; Armakovitch, S.; et al. Sensititre MYCOTB MIC Plate for Testing Mycobacterium tuberculosis Susceptibility to First- and Second-Line Drugs. Antimicrob. Agents Chemother. 2014, 58, 11–18. [Google Scholar] [CrossRef]
- Heysell, S.K.; Pholwat, S.; Mpagama, S.G.; Pazia, S.J.; Kumburu, H.; Ndusilo, N.; Gratz, J.; Houpt, E.R.; Kibiki, G.S. Sensititre MycoTB Plate Compared to Bactec MGIT 960 for First- and Second-Line Antituberculosis Drug Susceptibility Testing in Tanzania: A Call To Operationalize MICs. Antimicrob. Agents Chemother. 2015, 59, 7104–7108. [Google Scholar] [CrossRef]
- Campbell, P.J.; Morlock, G.P.; Sikes, R.D.; Dalton, T.L.; Metchock, B.; Starks, A.M.; Hooks, D.P.; Cowan, L.S.; Plikaytis, B.B.; Posey, J.E. Molecular detection of mutations associated with first- and second-line drug resistance compared with conventional drug susceptibility testing of Mycobacterium tuberculosis. Antimicrob. Agents Chemother. 2011, 55, 2032–2041. [Google Scholar] [CrossRef]
- Jnawali, H.N.; Hwang, S.C.; Park, Y.K.; Kim, H.; Lee, Y.S.; Chung, G.T.; Choe, K.H.; Ryoo, S. Characterization of mutations in multi- and extensive drug resistance among strains of Mycobacterium tuberculosis clinical isolates in Republic of Korea. Diagn. Microbiol. Infect. Dis. 2013, 76, 187–196. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, Y.; Nakajima, C.; Tamaru, A.; Kim, H.; Matsuba, T.; Saito, H. Sensitivities of ciprofloxacin-resistant Mycobacterium tuberculosis clinical isolates to fluoroquinolones: Role of mutant DNA gyrase subunits in drug resistance. Int. J. Antimicrob. Agents 2012, 39, 435–439. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).