A High-Performance Antibacterial Nanostructured ZnO Microfluidic Device for Controlled Bacterial Lysis and DNA Release
Abstract
1. Introduction
2. Results and Discussion
2.1. Surface Morphology
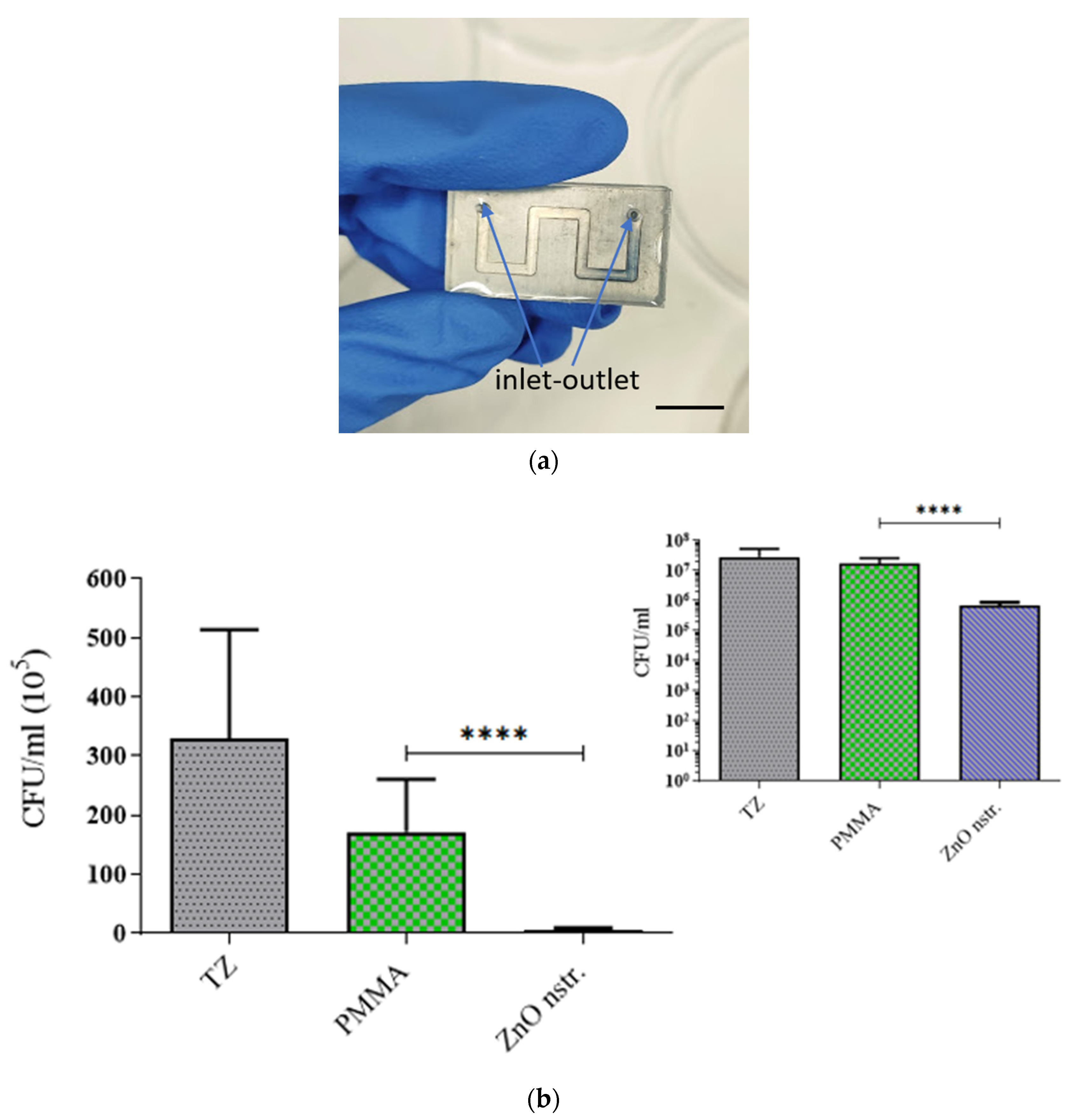
2.2. Antibacterial Activity of Nanotextured ZnO Substrates and Microchannel Walls
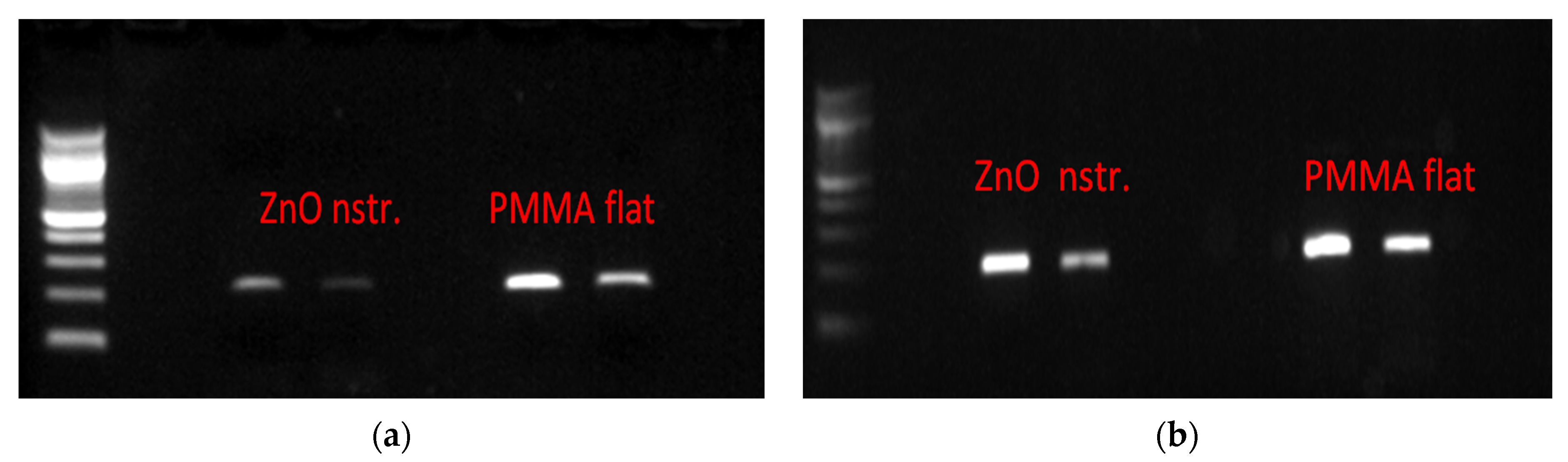
2.3. Bacterial DNA Release Following Bacterial Cells Exposure to ZnO Surfaces
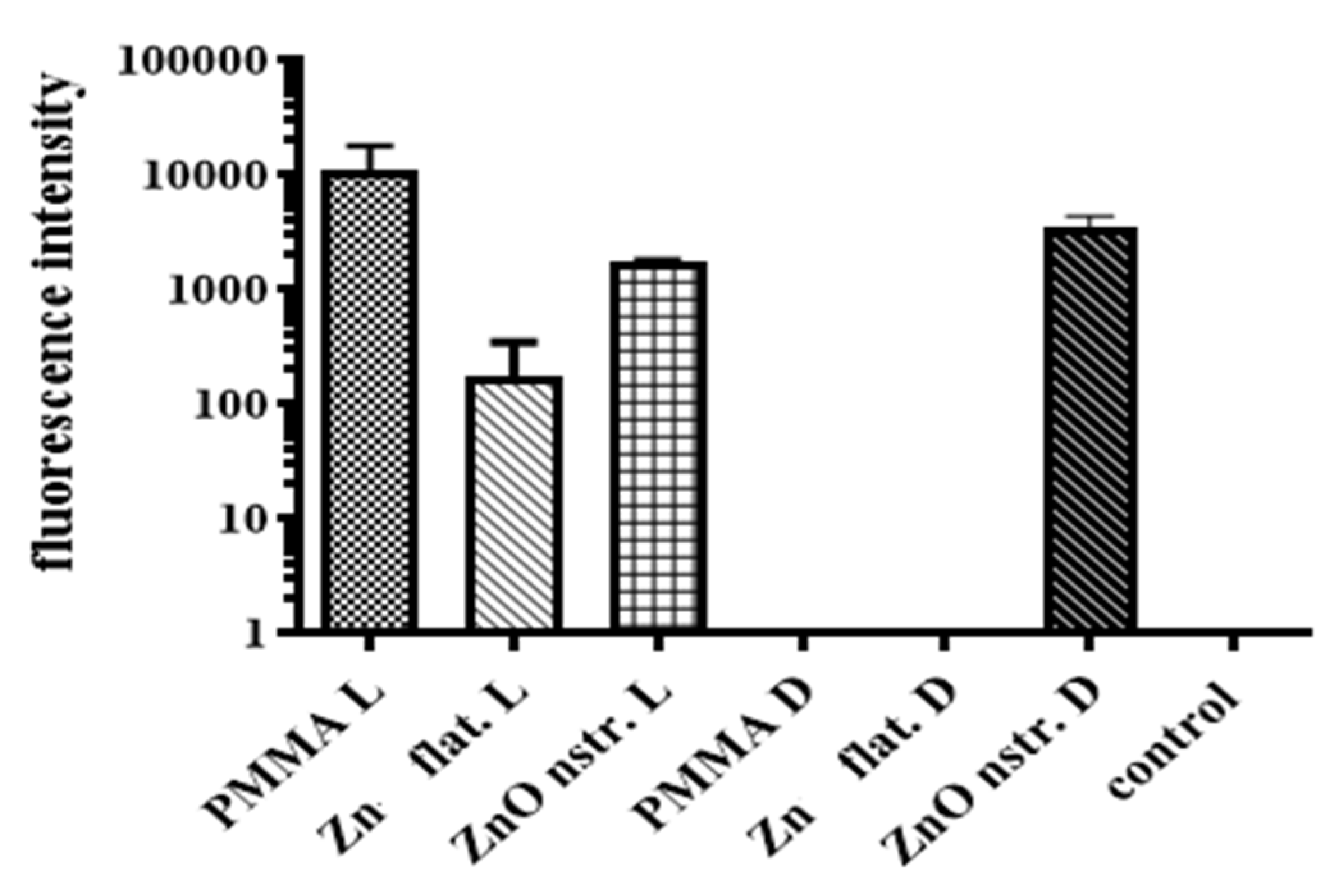
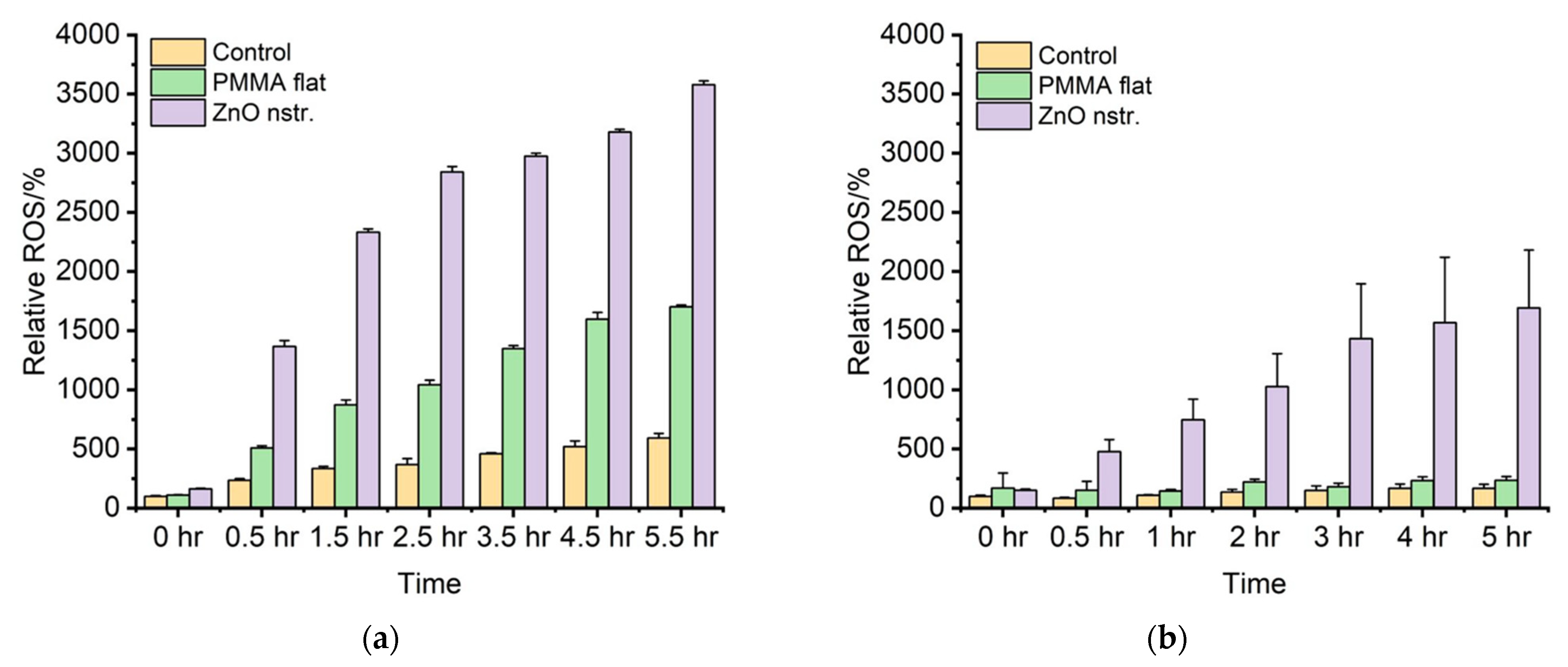
2.4. Reactive Oxygen Species (ROS)
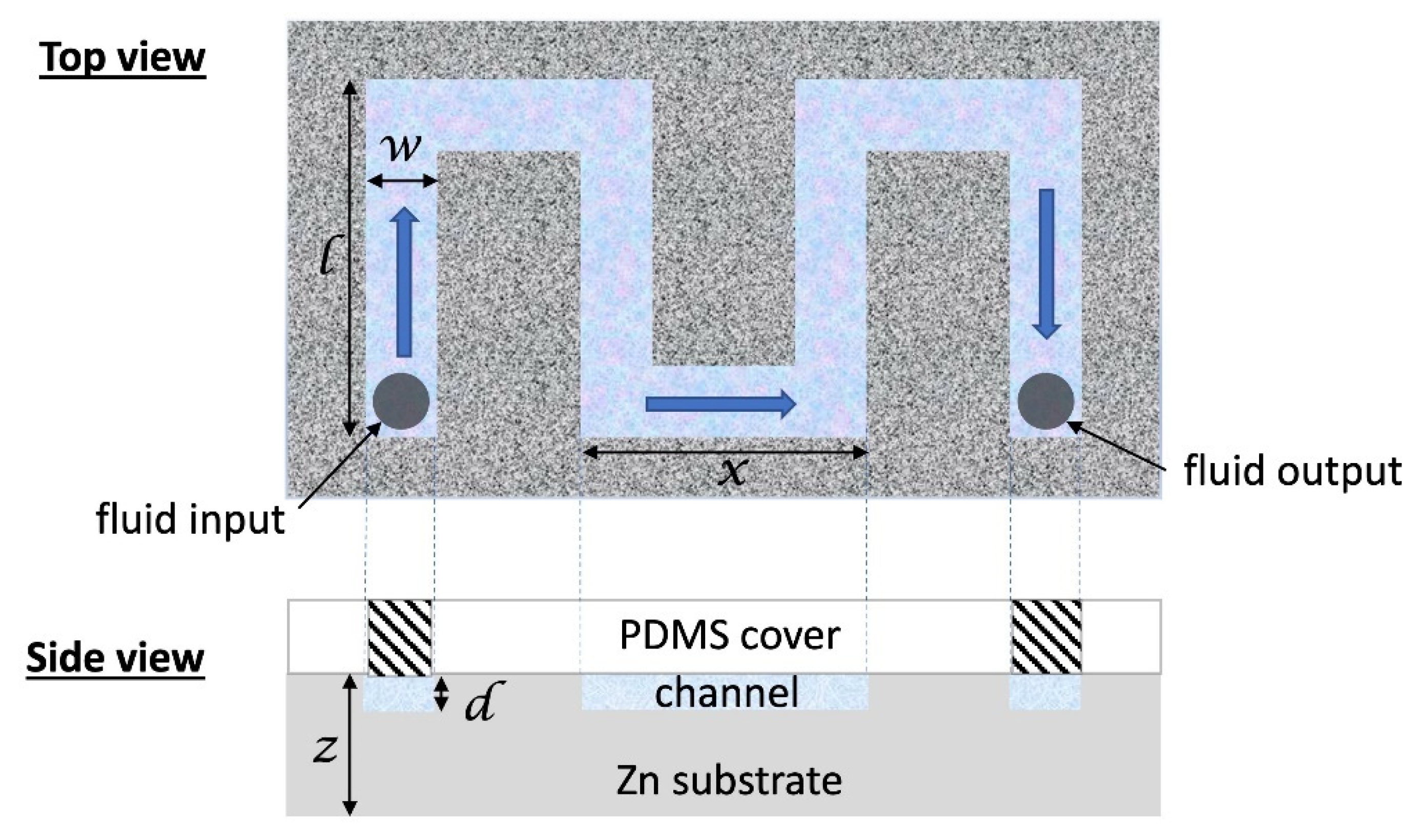
2.5. DNA Decontamination in Microchannels with Nanostructured ZnO Walls
3. Materials and Methods
3.1. Materials
3.2. Surface Modification and Characterization
3.3. Bacterial Viability on Various Substrates
3.4. Bacterial Viability in Microchannels
3.5. DNA Measurements
3.6. ROS Quantification
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liu, W.-M.; LI, L.; Ren, L.; Wang, J.-C.; Tu, Q.; Wang, X.-Q.; Wang, J.-Y. Diversification of Microfluidic Chip for Applications in Cell-Based Bioanalysis. Chin. J. Anal. Chem. 2012, 40, 24–31. [Google Scholar] [CrossRef]
- Sackmann, E.K.; Fulton, A.L.; Beebe, D.J. The Present and Future Role of Microfluidics in Biomedical Research. Nature 2014, 507, 181–189. [Google Scholar] [CrossRef] [PubMed]
- Ebadati, A. Microfluidics: Future perspectives. In Biomedical Applications of Microfluidic Devices; Academic Press: Cambridge, MA, USA, 2021; pp. 319–323. [Google Scholar] [CrossRef]
- Sohn, L.L.; Schwille, P.; Hierlemann, A.; Tay, S.; Samitier, J.; Fu, J.; Loskill, P. How Can Microfluidic and Microfabrication Approaches Make Experiments More Physiologically Relevant? Cell Syst. 2020, 11, 209–211. [Google Scholar] [CrossRef]
- Wang, J.; Yang, L.; Wang, H.; Wang, L. Application of microfluidic chips in the detection of airborne microorganisms. Micromachines 2022, 13, 1576. [Google Scholar] [CrossRef]
- Oblath, E.A.; Henley, W.H.; Alarie, J.P.; Ramsey, J.M. A Microfluidic Chip Integrating DNA Extraction and Real-Time PCR for the Detection of Bacteria in Saliva. Lab A Chip 2013, 13, 1325. [Google Scholar] [CrossRef]
- Escobar, A.; Chiu, P.; Qu, J.; Zhang, Y.; Xu, C. Integrated Microfluidic-Based Platforms for On-Site Detection and Quantification of Infectious Pathogens: Towards On-Site Medical Translation of SARS-CoV-2 Diagnostic Platforms. Micromachines 2021, 12, 1079. [Google Scholar] [CrossRef]
- Obino, D.; Vassalli, M.; Franceschi, A.; Alessandrini, A.; Facci, P.; Viti, F. An Overview on Microfluidic Systems for Nucleic Acids Extraction from Human Raw Samples. Sensors 2021, 21, 3058. [Google Scholar] [CrossRef] [PubMed]
- Alexandre, L.; Pereiro, I.; Bendali, A.; Tabnaoui, S.; Srbova, J.; Bilkova, Z.; Deegan, S.; Joshi, L.; Viovy, J.L.; Malaquin, L.; et al. A microfluidic fluidized bed to capture, amplify and detect bacteria from raw samples. In Methods in Cell Biology; Academic Press: Cambridge, MA, USA, 2018; Volume 147, pp. 59–75. [Google Scholar] [CrossRef]
- Kim, J.; Johnson, M.; Hill, P.; Gale, B.K. Microfluidic Sample Preparation: Cell Lysis and Nucleic Acid Purification. Integr. Biol. 2009, 1, 574. [Google Scholar] [CrossRef]
- Fradique, R.; Azevedo, A.M.; Chu, V.; Conde, J.P.; Aires-Barros, M.R. Microfluidic platform for rapid screening of bacterial cell lysis. J. Chromatogr. A 2020, 1610, 460539. [Google Scholar] [CrossRef]
- Nan, L.; Jiang, Z.; Wei, X. Emerging Microfluidic Devices for Cell Lysis: A Review. Lab A Chip 2014, 14, 1060. [Google Scholar] [CrossRef]
- Grigorov, E.; Kirov, B.; Marinov, M.B.; Galabov, V. Review of Microfluidic Methods for Cellular Lysis. Micromachines 2021, 12, 498. [Google Scholar] [CrossRef] [PubMed]
- Carlo, D.D.; Jeong, K.-H.; Lee, L.P. Reagentless Mechanical Cell Lysis by Nanoscale Barbs in Microchannels for Sample Preparation. Lab A Chip 2003, 3, 287. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Xing, X.; Ng, C.N.; Yobas, L. Single-Cell Point Constrictions for Reagent-Free High-Throughput Mechanical Lysis and Intact Nuclei Isolation. Micromachines 2019, 10, 488. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Wang, Y.; Wang, Z.; Huang, L.; Bi, M.; Xu, W.; Wang, W.; Ye, X. A Mechanical Cell Disruption Microfluidic Platform Based on an On-Chip Micropump. Biomicrofluidics 2017, 11, 024112. [Google Scholar] [CrossRef] [PubMed]
- Ellinas, K.; Kefallinou, D.; Stamatakis, K.; Gogolides, E.; Tserepi, A. Is There a Threshold in the Antibacterial Action of Superhydrophobic Surfaces? ACS Appl. Mater. Interfaces 2017, 9, 39781–39789. [Google Scholar] [CrossRef]
- Vadillo-Rodríguez, V.; Guerra-García-Mora, A.I.; Perera-Costa, D.; Gónzalez-Martín, M.L.; Fernández-Calderón, M.C. Bacterial response to spatially organized microtopographic surface patterns with nanometer scale roughness. Colloids Surf. B Biointerfaces 2018, 169, 340–347. [Google Scholar] [CrossRef]
- Shimada, T.; Yasui, T.; Akihiro, Y.; Yanagida, T.; Kaji, N.; Kanai, M.; Nagashima, K.; Kawai, T.; Baba, Y. Mechanical Rupture-Based Antibacterial and Cell-Compatible ZnO/SiO2 Nanowire Structures Formed by Bottom-up Approaches. Micromachines 2020, 11, 610. [Google Scholar] [CrossRef]
- Diu, T.; Faruqui, N.; Sjöström, T.; Lamarre, B.; Jenkinson, H.F.; Su, B.; Ryadnov, M.G. Cicada-Inspired Cell-Instructive Nanopatterned Arrays. Sci. Rep. 2014, 4, 7122. [Google Scholar] [CrossRef]
- Bourkoula, A.; Constantoudis, V.; Kontziampasis, D.; Petrou, P.S.; Kakabakos, S.E.; Tserepi, A.; Gogolides, E. Roughness Threshold for Cell Attachment and Proliferation on Plasma Micro-Nanotextured Polymeric Surfaces: The Case of Primary Human Skin Fibroblasts and Mouse Immortalized 3T3 Fibroblasts. J. Phys. D Appl. Phys. 2016, 49, 304002. [Google Scholar] [CrossRef]
- Shehadul Islam, M.; Aryasomayajula, A.; Selvaganapathy, P. A Review on Macroscale and Microscale Cell Lysis Methods. Micromachines 2017, 8, 83. [Google Scholar] [CrossRef]
- Yasui, T.; Yanagida, T.; Shimada, T.; Otsuka, K.; Takeuchi, M.; Nagashima, K.; Rahong, S.; Naito, T.; Takeshita, D.; Yonese, A.; et al. Engineering Nanowire-Mediated Cell Lysis for Microbial Cell Identification. ACS Nano 2019, 13, 2262–2273. [Google Scholar] [CrossRef] [PubMed]
- Aly, M.A.S.; Gauthier, M.; Yeow, J. Lysis of Gram-Positive and Gram-Negative Bacteria by Antibacterial Porous Polymeric Monolith Formed in Microfluidic Biochips for Sample Preparation. Anal. Bioanal. Chem. 2014, 406, 5977–5987. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Bhadra, C.M.; Yen Dang, T.H.; Buividas, R.; Wang, J.; Crawford, R.J.; Ivanova, E.P.; Juodkazis, S. A Bactericidal Microfluidic Device Constructed Using Nano-Textured Black Silicon. RSC Adv. 2016, 6, 26300–26306. [Google Scholar] [CrossRef]
- Li, L.; Tian, F.; Chang, H.; Zhang, J.; Wang, X.; Rao, W.; Hu, H. Interactions of Bacteria with Monolithic Lateral Silicon Nanospikes inside a Microfluidic Channel. Front. Chem. 2019, 7, 483. [Google Scholar] [CrossRef] [PubMed]
- Ge, X.; Ren, C.; Ding, Y.; Chen, G.; Tang, Y.; Wang, K.; Ren, F.; Yang, M.; Wang, Z.; Li, J.; et al. Micro/Nano-Structured TiO2 Surface with Dual-Functional Antibacterial Effects for Biomedical Applications. Bioact. Mater. 2019, 4, 346–357. [Google Scholar] [CrossRef] [PubMed]
- Linklater, D.P.; Juodkazis, S.; Crawford, R.J.; Ivanova, E.P. Mechanical Inactivation of Staphylococcus Aureus and Pseudomonas Aeruginosa by Titanium Substrata with Hierarchical Surface Structures. Materialia 2019, 5, 100197. [Google Scholar] [CrossRef]
- Kim, J.; Hong, J.W.; Kim, D.P.; Shin, J.H.; Park, I. Nanowire-Integrated Microfluidic Devices for Facile and Reagent-Free Mechanical Cell Lysis. Lab A Chip 2012, 12, 2914. [Google Scholar] [CrossRef]
- Wang, L.; Hu, C.; Shao, L. The Antimicrobial Activity of Nanoparticles: Present Situation and Prospects for the Future. Int. J. Nanomed. 2017, 12, 1227–1249. [Google Scholar] [CrossRef]
- Imlay, J.A. The Molecular Mechanisms and Physiological Consequences of Oxidative Stress: Lessons from a Model Bacterium. Nat. Rev. Microbiol. 2013, 11, 443–454. [Google Scholar] [CrossRef]
- Cooke, M.S.; Evans, M.D.; Dizdaroglu, M.; Lunec, J. Oxidative DNA Damage: Mechanisms, Mutation, and Disease. FASEB J. 2003, 17, 1195–1214. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, W.; Niu, J.; Chen, Y. Mechanism of Photogenerated Reactive Oxygen Species and Correlation with the Antibacterial Properties of Engineered Metal-Oxide Nanoparticles. ACS Nano 2012, 6, 5164–5173. [Google Scholar] [CrossRef]
- Kessler, A.; Hedberg, J.; Blomberg, E.; Odnevall, I. Reactive Oxygen Species Formed by Metal and Metal Oxide Nanoparticles in Physiological Media—A Review of Reactions of Importance to Nanotoxicity and Proposal for Categorization. Nanomaterials 2022, 12, 1922. [Google Scholar] [CrossRef] [PubMed]
- Xin, Z.; He, Q.; Wang, S.; Han, X.; Fu, Z.; Xu, X.; Zhao, X. Recent Progress in ZnO-Based Nanostructures for Photocatalytic Antimicrobial in Water Treatment: A Review. Appl. Sci. 2022, 12, 7910. [Google Scholar] [CrossRef]
- Raha, S.; Ahmaruzzaman, M. ZnO Nanostructured Materials and Their Potential Applications: Progress, Challenges and Perspectives. Nanoscale Adv. 2022, 4, 1868–1925. [Google Scholar] [CrossRef] [PubMed]
- Sirelkhatim, A.; Mahmud, S.; Seeni, A.; Kaus, N.H.M.; Ann, L.C.; Bakhori, S.K.M.; Hasan, H.; Mohamad, D. Review on Zinc Oxide Nanoparticles: Antibacterial Activity and Toxicity Mechanism. Nano-Micro Lett. 2015, 7, 219–242. [Google Scholar] [CrossRef]
- Hao, N.; Zhang, M.Q.; Zhang, J.H. Microfluidics for ZnO Micro-/Nanomaterials Development: Rational Design, Controllable Synthesis, and On-Chip Bioapplications. Biomater. Sci. 2020, 8, 1783–1801. [Google Scholar] [CrossRef] [PubMed]
- Babayevska, N.; Przysiecka, Ł.; Iatsunskyi, I.; Nowaczyk, G.; Jarek, M.; Janiszewska, E.; Jurga, S. ZnO size and shape effect on antibacterial activity and cytotoxicity profile. Sci. Rep. 2022, 12, 8148. [Google Scholar] [CrossRef]
- Jimenez-Cadena, G.; Comini, E.; Ferroni, M.; Vomiero, A.; Sberveglieri, G. Synthesis of Different ZnO Nanostructures by Modified PVD Process and Potential Use for Dye-Sensitized Solar Cells. Mater. Chem. Phys. 2010, 124, 694–698. [Google Scholar] [CrossRef]
- Taunk, P.B.; Das, R.; Bisen, D.P.; Tamrakar, R.K.; Rathor, N. Synthesis and Optical Properties of Chemical Bath Deposited ZnO Thin Film. Karbala Int. J. Mod. Sci. 2015, 1, 159–165. [Google Scholar] [CrossRef]
- Sportelli, M.C.; Picca, R.A.; Izzi, M.; Palazzo, G.; Gristina, R.; Innocenti, M.; Torsi, L.; Cioffi, N. ZnO Nanostructures with Antibacterial Properties Prepared by a Green Electrochemical-Thermal Approach. Nanomaterials 2020, 10, 473. [Google Scholar] [CrossRef]
- Znaidi, L. Sol–Gel-Deposited ZnO Thin Films: A Review. Mater. Sci. Eng. B 2010, 174, 18–30. [Google Scholar] [CrossRef]
- Franklin, J.B.; Zou, B.; Petrov, P.; McComb, D.W.; Ryan, M.P.; McLachlan, M.A. Optimised Pulsed Laser Deposition of ZnO Thin Films on Transparent Conducting Substrates. J. Mater. Chem. 2011, 21, 8178. [Google Scholar] [CrossRef]
- Saadi, N.S.; Hassan, L.B.; Karabacak, T. Metal Oxide Nanostructures by a Simple Hot Water Treatment. Sci. Rep. 2017, 7, 7158. [Google Scholar] [CrossRef]
- Tan, W.K.; Razak, K.A.; Lockman, Z.; Kawamura, G.; Muto, H.; Matsuda, A. Formation of Highly Crystallized ZnO Nanostructures by Hot-Water Treatment of Etched Zn Foils. Mater. Lett. 2013, 91, 111–114. [Google Scholar] [CrossRef]
- Chen, R.; Zou, C.; Yan, X.; Alyamani, A.Y.; Gao, W. Growth Mechanism of ZnO Nanostructures in Wet-Oxidation Process. Thin Solid Film. 2011, 519, 1837–1844. [Google Scholar] [CrossRef]
- Milionis, A.; Tripathy, A.; Donati, M.; Sharma, C.S.; Pan, F.; Maniura-Weber, K.; Ren, Q.; Poulikakos, D. Water-Based Scalable Methods for Self-Cleaning Antibacterial ZnO-Nanostructured Surfaces. Ind. Eng. Chem. Res. 2020, 59, 14323–14333. [Google Scholar] [CrossRef]
- Brugger, S.D.; Baumberger, C.; Jost, M.; Jenni, W.; Brugger, U.; Mühlemann, K. Automated Counting of Bacterial Colony Forming Units on Agar Plates. PLoS ONE 2012, 7, e33695. [Google Scholar] [CrossRef]
- Svensson, R.J.; Sabiiti, W.; Kibiki, G.S.; Ntinginya, N.E.; Bhatt, N.; Davies, G.; Gillespie, S.H.; Simonsson, U.S.H. Model-Based Relationship between the Molecular Bacterial Load Assay and Time to Positivity in Liquid Culture. Antimicrob. Agents Chemother. 2019, 63, e00652-e19. [Google Scholar] [CrossRef]
- Georgoutsou-Spyridonos, M.; Filippidou, M.; Kaprou, G.D.; Mastellos, D.C.; Chatzandroulis, S.; Tserepi, A. Isothermal Recombinase Polymerase Amplification (RPA) of E. coli GDNA in Commercially Fabricated PCB-Based Microfluidic Platforms. Micromachines 2021, 12, 1387. [Google Scholar] [CrossRef]
- Azam, A.; Ahmed, A.S.; Oves, M.; Khan, M.S.; Habib, S.S.; Memic, A. Antimicrobial Activity of Metal Oxide Nanoparticles against Gram-Positive and Gram-Negative Bacteria: A Comparative Study. Int. J. Nanomed. 2012, 2012, 6003–6009. [Google Scholar] [CrossRef] [PubMed]
- Stanić, V.; Tanasković., S.B. Antibacterial activity of metal oxide nanoparticles. In Nanotoxicity; Elsevier: Amsterdam, The Netherlands, 2020; pp. 241–274. [Google Scholar] [CrossRef]
- Karsten, U.; Wollenberger, A. Improvements in the Ethidium Bromide Method for Direct Fluorometric Estimation of DNA and RNA in Cell and Tissue Homogenates. Anal. Biochem. 1977, 77, 464–470. [Google Scholar] [CrossRef] [PubMed]
- Vardevanyan, P.O.; Antonyan, A.P.; Parsadanyan, M.A.; Davtyan, H.G.; Karapetyan, A.T. The Binding of Ethidium Bromide with DNA: Interaction with Single- and Double-Stranded Structures. Exp. Mol. Med. 2003, 35, 527–533. [Google Scholar] [CrossRef] [PubMed]
- Guo, M.Y.; Ng, A.M.C.; Liu, F.; Djurišić, A.B.; Chan, W.K. Photocatalytic Activity of Metal Oxides—The Role of Holes and OH Radicals. Appl. Catal. B Environ. 2011, 107, 150–157. [Google Scholar] [CrossRef]
- Lipovsky, A.; Tzitrinovich, Z.; Friedmann, H.; Applerot, G.; Gedanken, A.; Lubart, R. EPR Study of Visible Light-Induced ROS Generation by Nanoparticles of ZnO. J. Phys. Chem. C 2009, 113, 15997–16001. [Google Scholar] [CrossRef]
- Fasnacht, M.; Polacek, N. Oxidative Stress in Bacteria and the Central Dogma of Molecular Biology. Front. Mol. Biosci. 2021, 8, 671037. [Google Scholar] [CrossRef]
- McQuillan, J.S.; Wilson, M.W. Recombinase Polymerase Amplification for Fast, Selective, DNA-Based Detection of Faecal Indicator E. coli. Lett. Appl. Microbiol. 2021, 72, 382–389. [Google Scholar] [CrossRef]

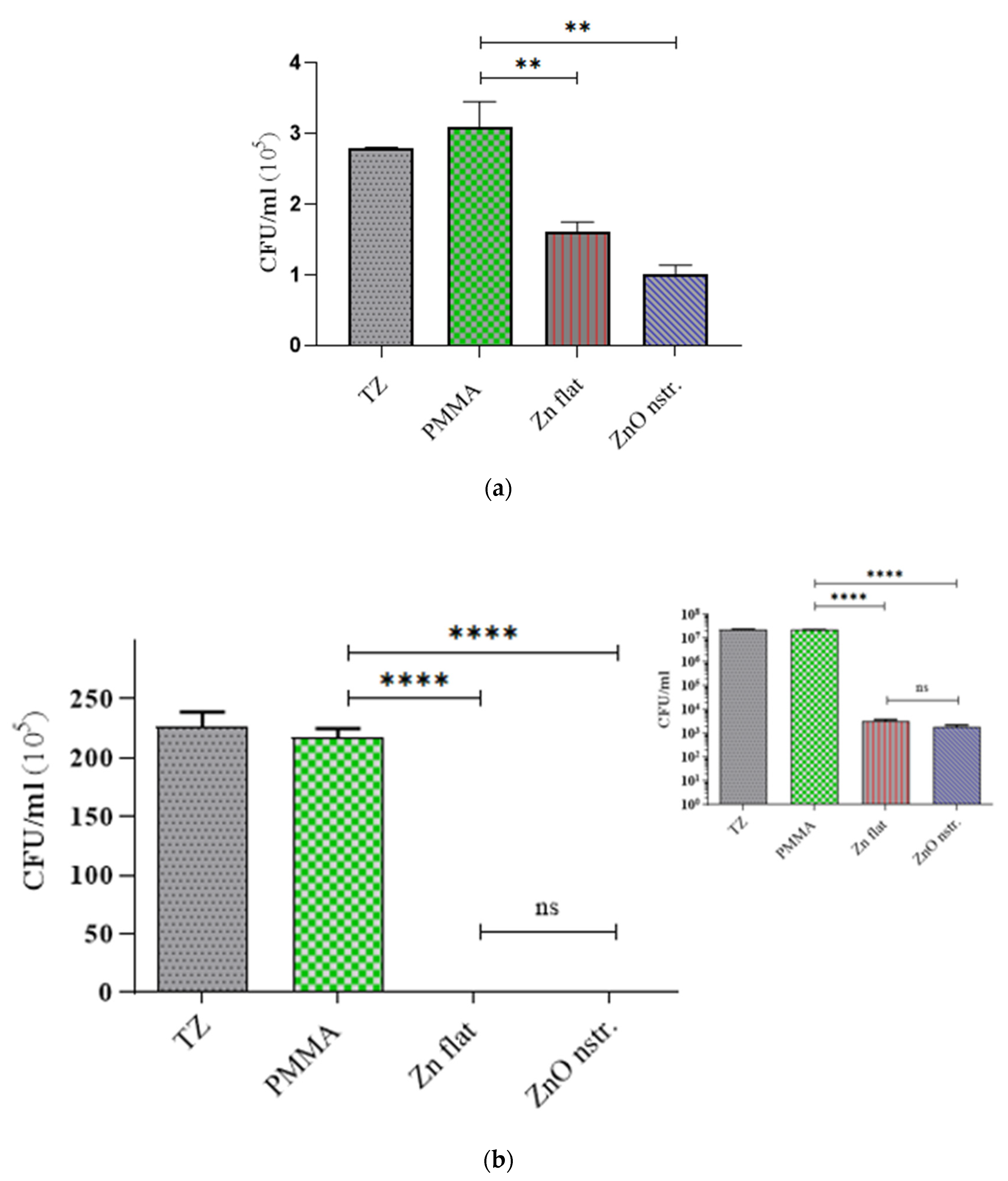

| Surface Area (cm2) | Initial Concentration (CFU/mL) | Incubation Time (h) | Killing Rate (CFU/cm2 h) | |
|---|---|---|---|---|
| Zn flat substrates | 1 × 1 | ~106 | 1 | (3.0 ± 0.5) ×10 5 |
| 1.5 × 1.5 (2.25) | ~108 | 0.25 | (7.8 ± 1.0) × 107 | |
| ZnO nanostructured substrates | 1 × 1 | ~106 | 1 | (4.1 ± 0.7) × 105 |
| 1.5 × 1.5 (2.25) | ~108 | 0.25 | (7.8 ± 1.0) × 107 | |
| ZnO nanostructured microchannel | 1.54 | ~108 | 0.25 | (1.9 ± 1.0) × 106 |
| DNA from Lysed Bacteria after 15 min Residence (ng/μL) | Purified DNA before Introduction in Microchannels (ng/μL) | Purified DNA after 15 min Residence (ng/μL) | |
|---|---|---|---|
| Flat PMMA microchannel (control) | 21 | 52 | 55 |
| Nanostructured ZnO microchannel | 6 | 52 | 25 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xesfyngi, Y.; Georgoutsou-Spyridonos, M.; Tripathy, A.; Milionis, A.; Poulikakos, D.; Mastellos, D.C.; Tserepi, A. A High-Performance Antibacterial Nanostructured ZnO Microfluidic Device for Controlled Bacterial Lysis and DNA Release. Antibiotics 2023, 12, 1276. https://doi.org/10.3390/antibiotics12081276
Xesfyngi Y, Georgoutsou-Spyridonos M, Tripathy A, Milionis A, Poulikakos D, Mastellos DC, Tserepi A. A High-Performance Antibacterial Nanostructured ZnO Microfluidic Device for Controlled Bacterial Lysis and DNA Release. Antibiotics. 2023; 12(8):1276. https://doi.org/10.3390/antibiotics12081276
Chicago/Turabian StyleXesfyngi, Yvonni, Maria Georgoutsou-Spyridonos, Abinash Tripathy, Athanasios Milionis, Dimos Poulikakos, Dimitrios C. Mastellos, and Angeliki Tserepi. 2023. "A High-Performance Antibacterial Nanostructured ZnO Microfluidic Device for Controlled Bacterial Lysis and DNA Release" Antibiotics 12, no. 8: 1276. https://doi.org/10.3390/antibiotics12081276
APA StyleXesfyngi, Y., Georgoutsou-Spyridonos, M., Tripathy, A., Milionis, A., Poulikakos, D., Mastellos, D. C., & Tserepi, A. (2023). A High-Performance Antibacterial Nanostructured ZnO Microfluidic Device for Controlled Bacterial Lysis and DNA Release. Antibiotics, 12(8), 1276. https://doi.org/10.3390/antibiotics12081276






