New Fe3O4-Based Coatings with Enhanced Anti-Biofilm Activity for Medical Devices
Abstract
1. Introduction
2. Results
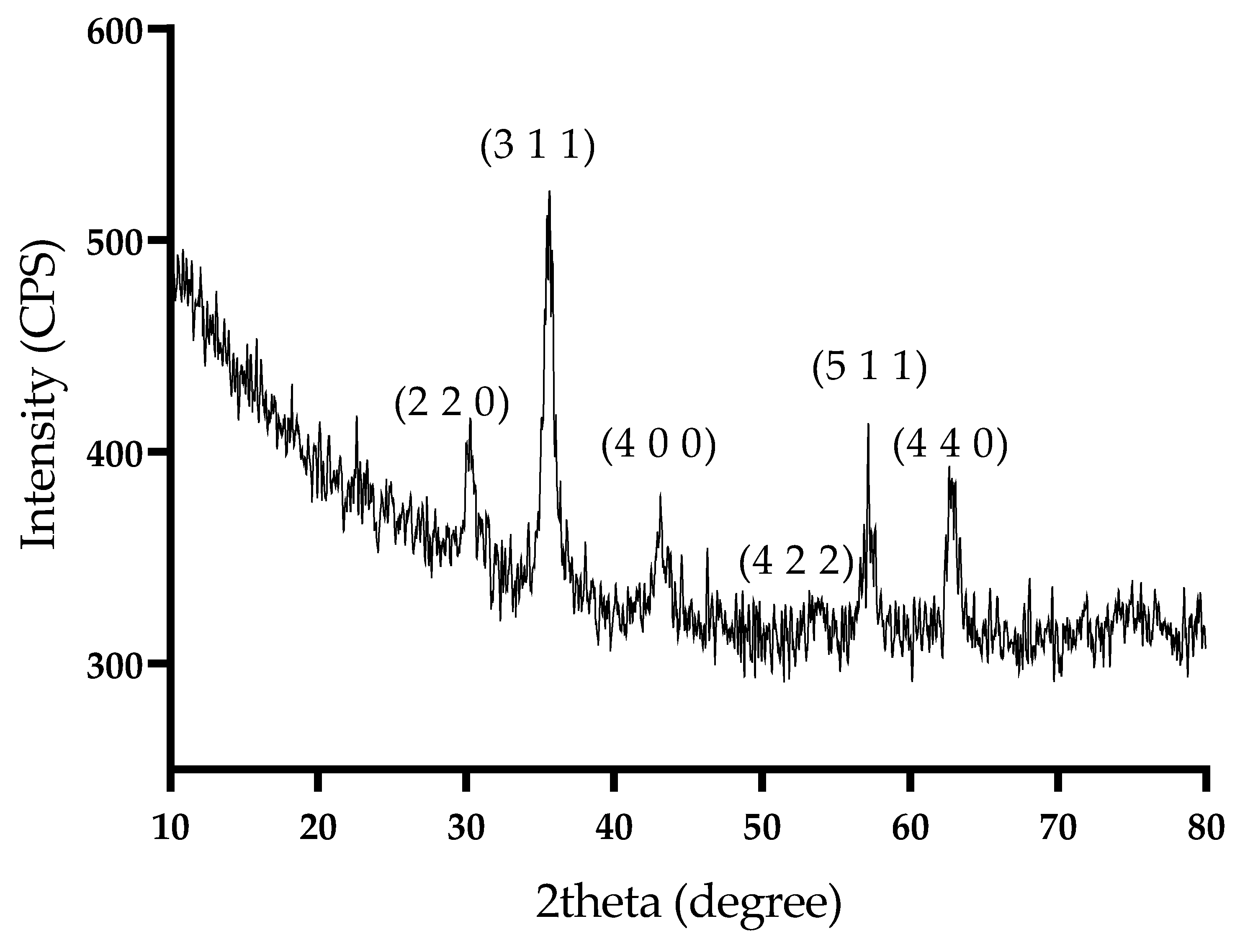
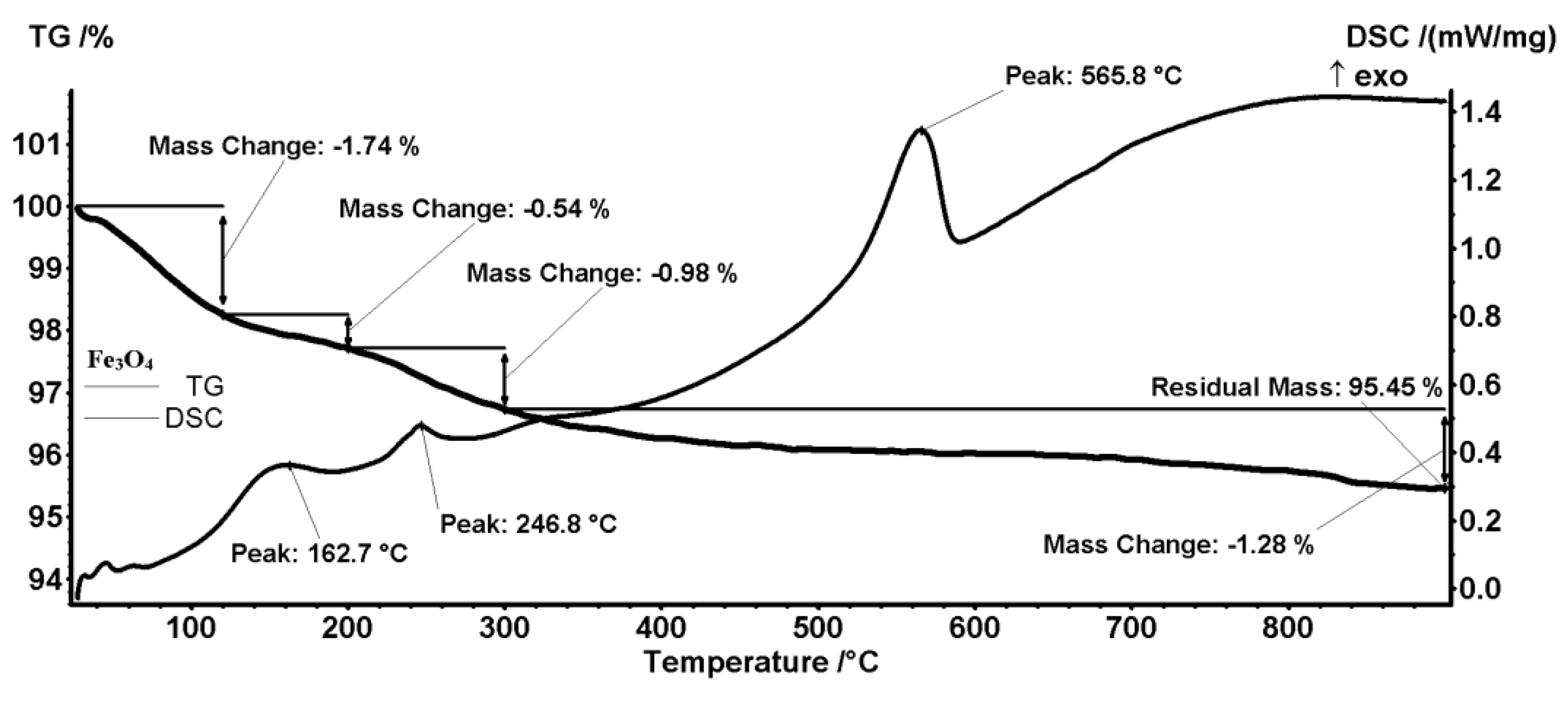
2.1. Physicochemical Characterization of Magnetite Nanoparticles
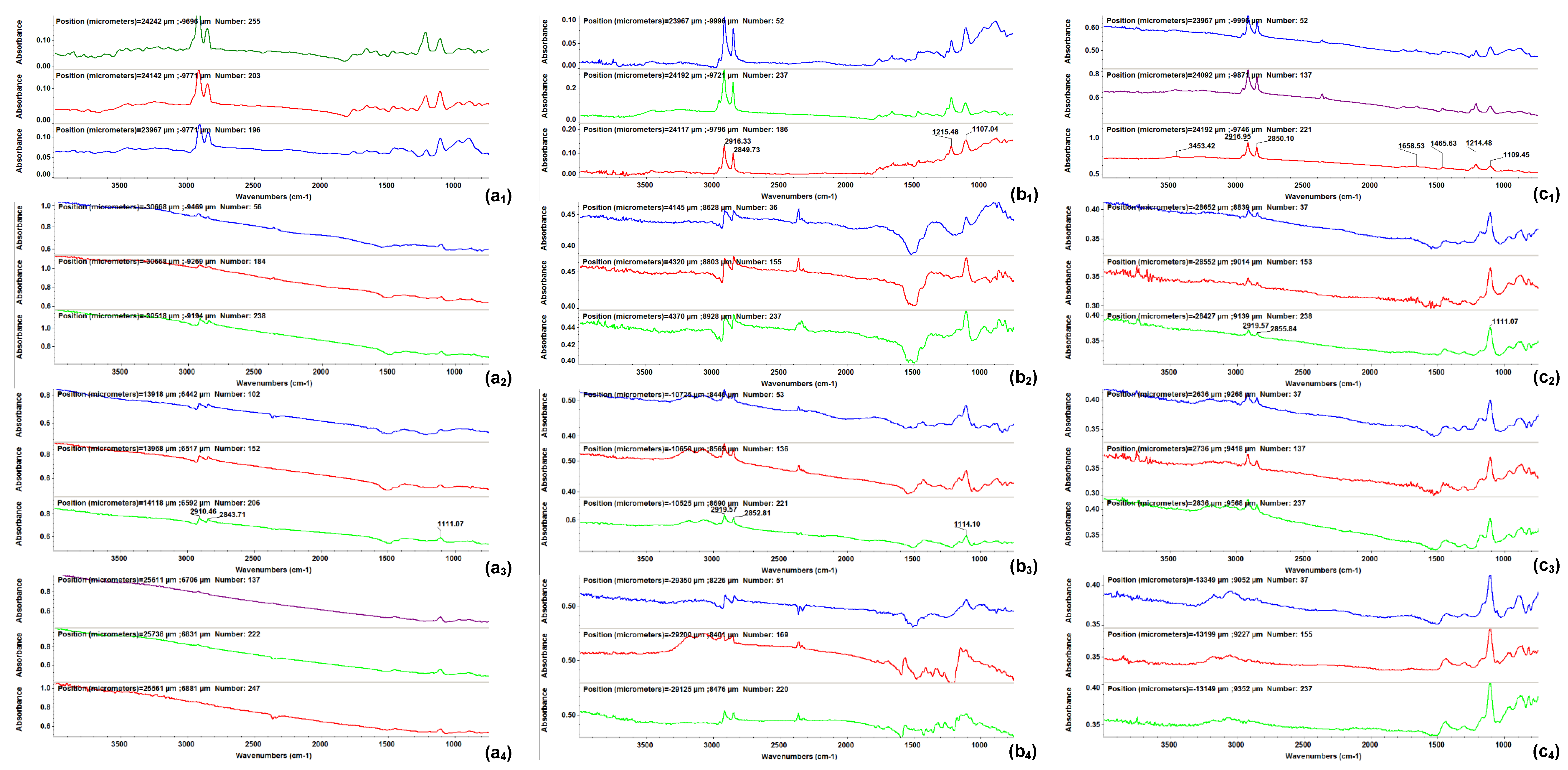
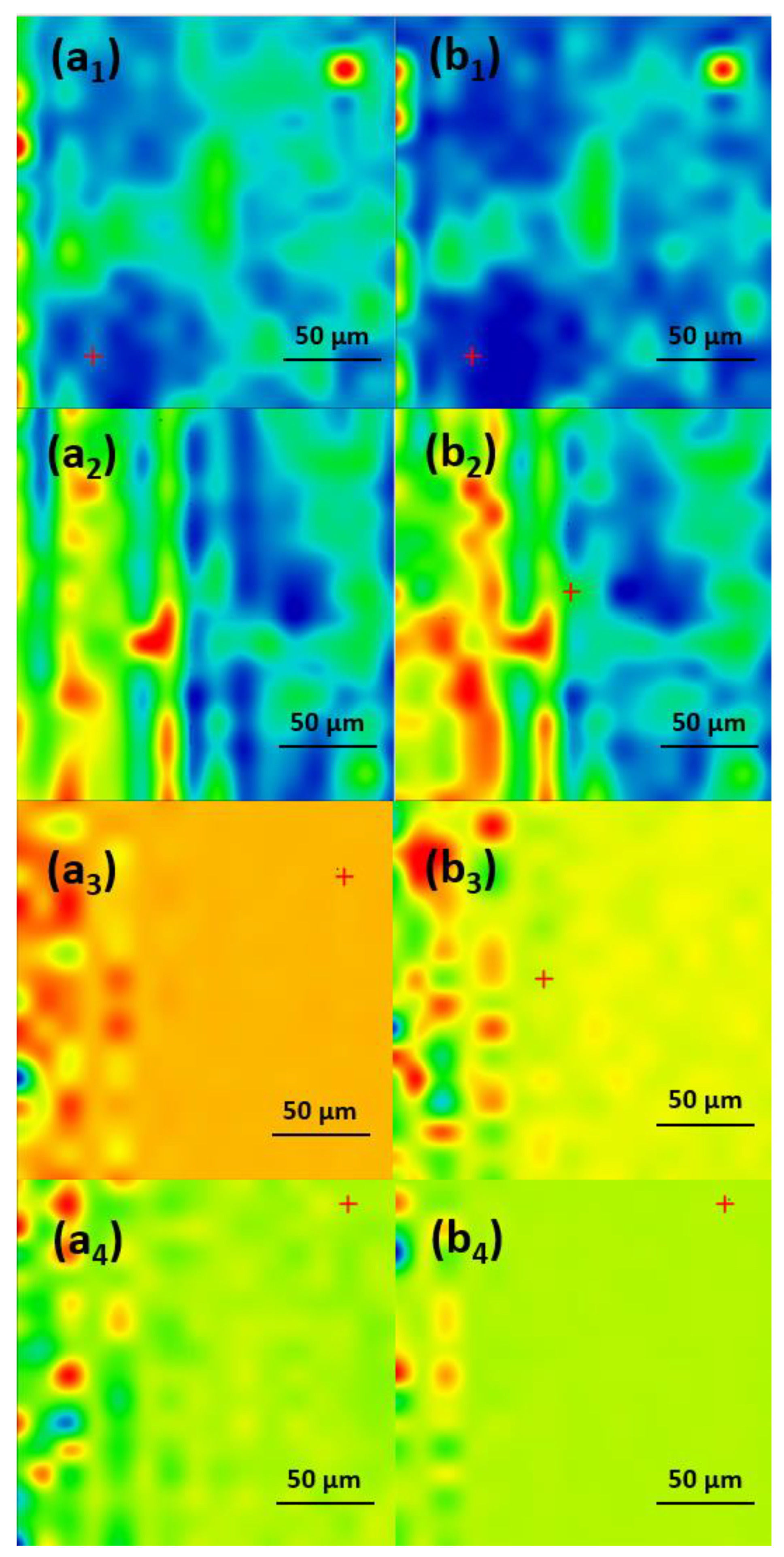
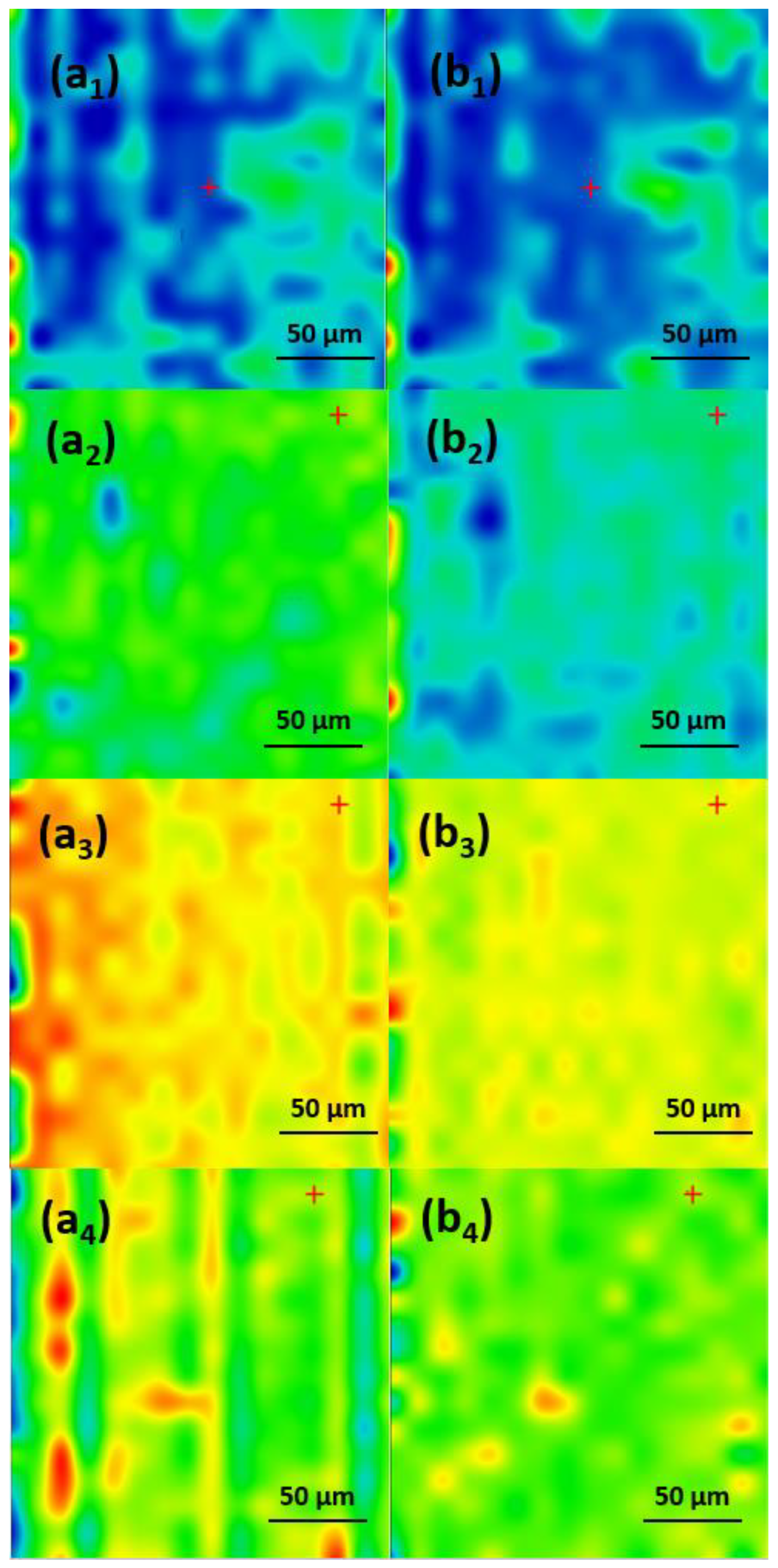
2.2. Physicochemical Characterization of the Coatings
2.3. Biological Evaluation
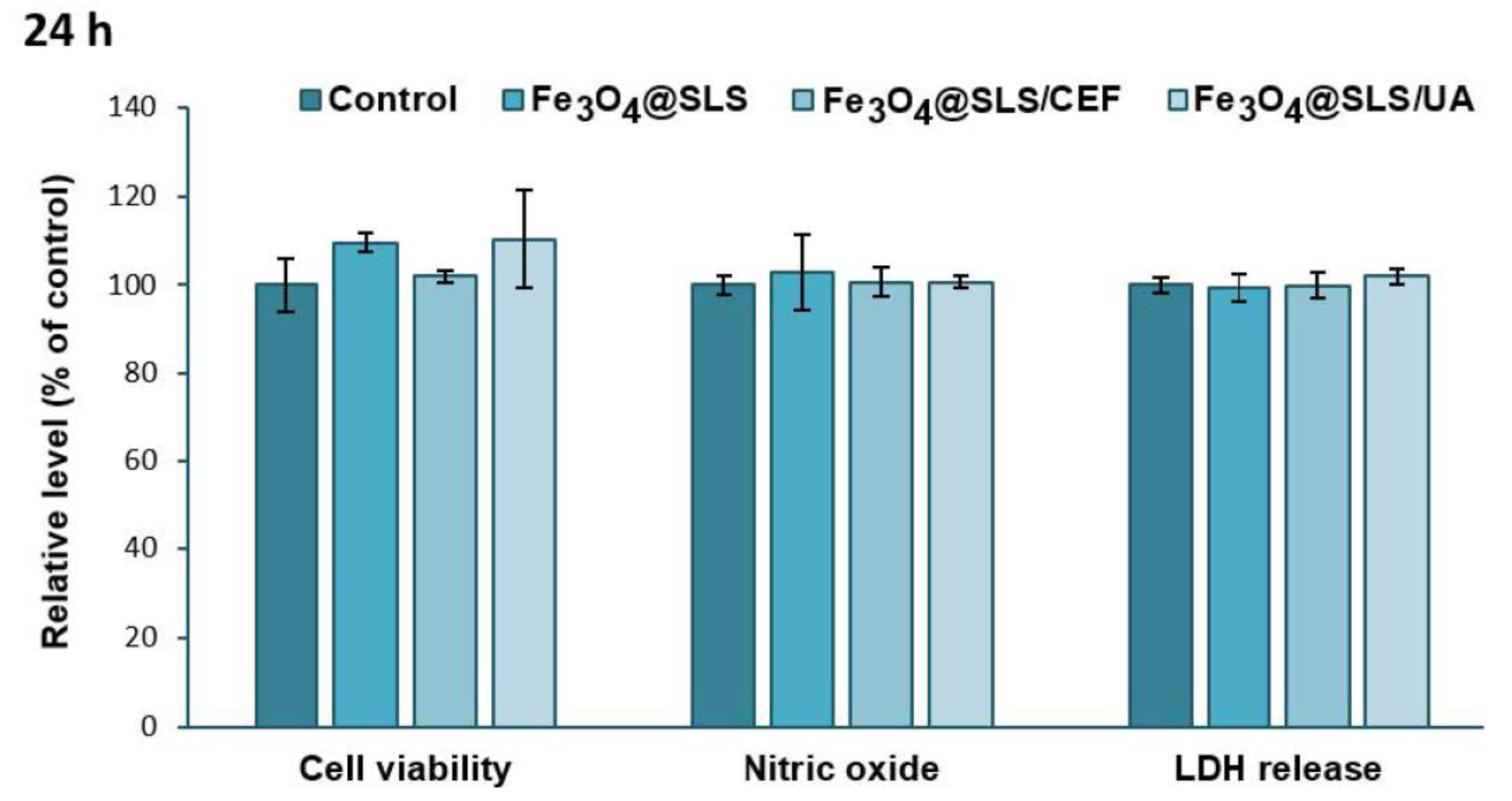
2.3.1. Biocompatibility on Pre-Osteoblasts
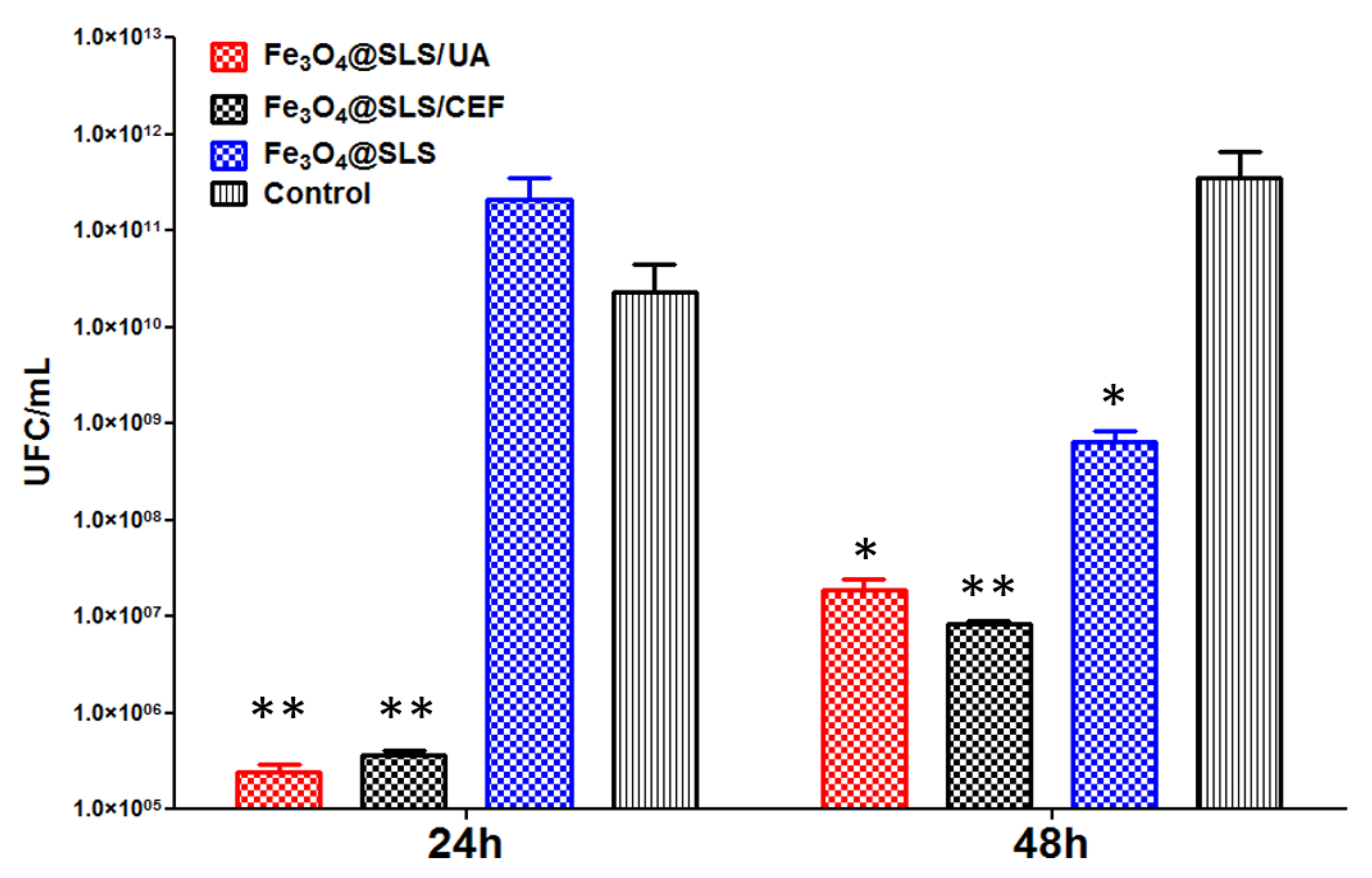
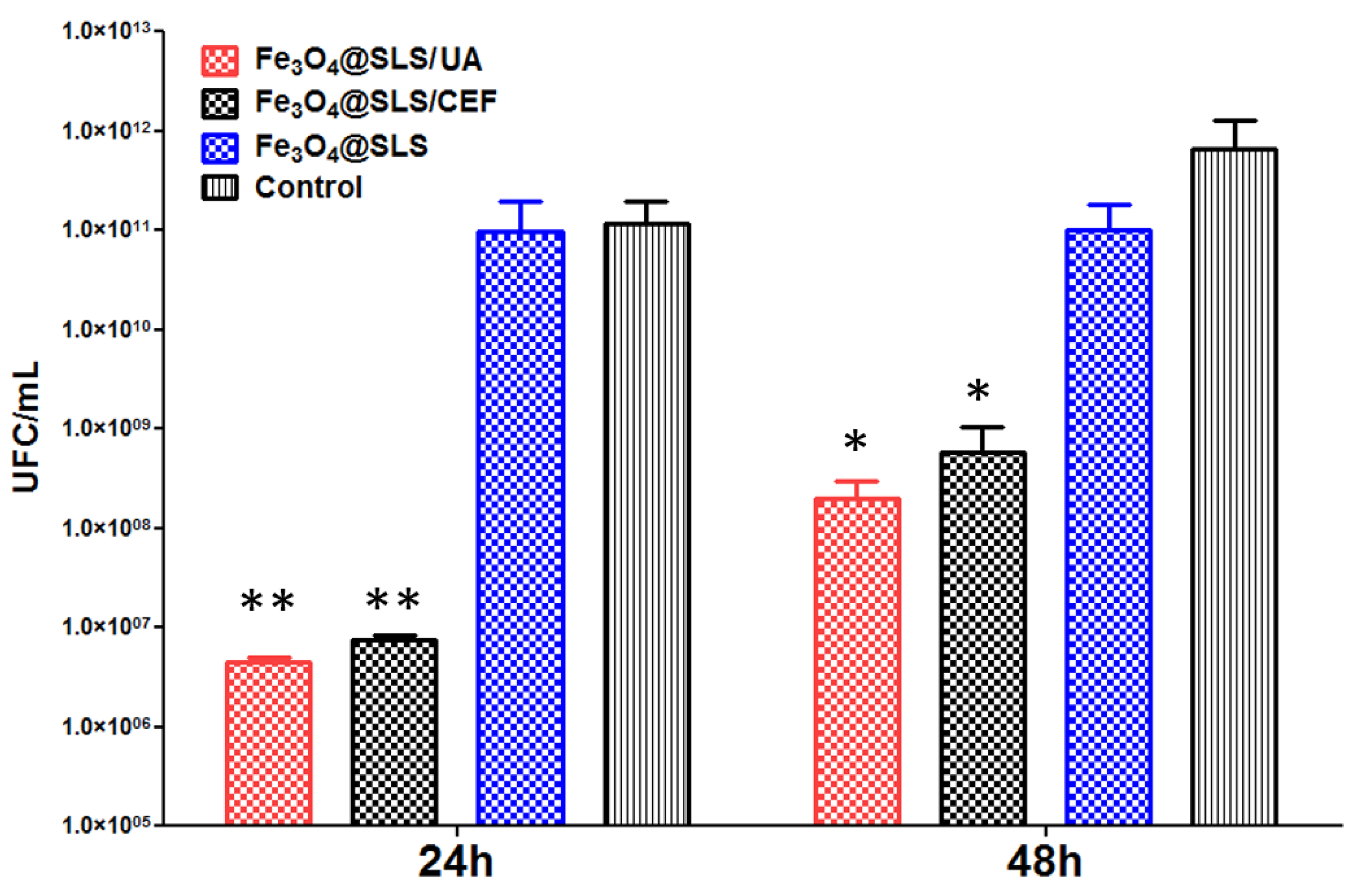
2.3.2. Effects on Pathogenic Biofilms Growth
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Chemical Synthesis of Sodium Lauryl Sulfate Covered Magnetite Fe3O4@SLS Nanoparticles
4.3. Chemical Syntheses of Magnetite NPs Functionalized with Antimicrobial Agents UA and CEF
4.4. MAPLE Experimental Conditions for Obtained Coatings
4.5. Physicochemical Characterization
4.6. Biological Tests
4.6.1. Assessment of Biocompatibility on Pre-Osteoblast Cells
4.6.2. Anti-Biofilm Activity
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Suetens, A.; Latour, K.; Kärki, T.; Ricchizzi, E.; Kinross, P.; Moro, M.L.; Jans, B.; Hopkins, S.; Hansen, S.; Lyytikäinen, O.; et al. Prevalence of healthcare-associated infections, estimated incidence and composite antimicrobial resistance index in acute care hospitals and long-term care facilities: Results from two European point prevalence surveys, 2016 to 2017. Eurosurveillance 2018, 23, 1800516. [Google Scholar] [CrossRef] [PubMed]
- Balaure, P.C.; Grumezescu, A.M. Recent Advances in Surface Nanoengineering for Biofilm Prevention and Control. Part I: Molecular Basis of Biofilm Recalcitrance. Passive Anti-Biofouling Nanocoatings. Nanomaterials 2020, 10, 1230. [Google Scholar] [CrossRef]
- Balaure, P.C.; Grumezescu, A.M. Recent Advances in Surface Nanoengineering for Biofilm Prevention and Control. Part II: Active, Combined Active and Passive, and Smart Bacteria-Responsive Antibiofilm Nanocoatings. Nanomaterials 2020, 10, 1527. [Google Scholar] [CrossRef]
- Cai, S.; Wu, C.; Yang, W.; Liang, W.; Yu, H.; Liu, L. Recent advance in surface modification for regulating cell adhesion and behaviors. Nanotechnol. Rev. 2020, 9, 971–989. [Google Scholar] [CrossRef]
- Wei, J.; Yoshinari, M.; Takemoto, S.; Hattori, M.; Kawada, E.; Liu, B.; Oda, Y. Adhesion of mouse fibroblasts on hexamethyldisiloxane surfaces with wide range of wettability. J. Biomed. Mater. Res. Part B. Appl. Biomater. 2007, 81, 66–75. [Google Scholar] [CrossRef] [PubMed]
- Cheng, G.; Zhang, Z.; Chen, S.; Bryers, J.D.; Jiang, S. Inhibition of bacterial adhesion and biofilm formation on zwitterionic surfaces. Biomaterials 2007, 28, 4192–4199. [Google Scholar] [CrossRef] [PubMed]
- Sangaiya, P.; Jayaprakash, R. A review on iron oxide nanoparticles and their biomedical applications. J. Supercond. Nov. Magn. 2018, 31, 3397–3413. [Google Scholar] [CrossRef]
- Ryu, C.; Lee, H.; Kim, H.; Hwang, S.; Hadadian, Y.; Mohanty, A.; Park, I.-K.; Cho, B.; Yoon, J.; Lee, J.Y. Highly Optimized Iron Oxide Embedded Poly (Lactic Acid) Nanocomposites for Effective Magnetic Hyperthermia and Biosecurity. Int. J. Nanomed. 2022, 17, 31–44. [Google Scholar] [CrossRef]
- Karimi, M.; Ghasemi, A.; Zangabad, P.S.; Rahighi, R.; Moosavi Basri, S.M.; Mirshekari, H.; Amiri, M.; Shafaei Pishabad, Z.; Aslani, A.; Bozorgomid, M.; et al. Smart micro/nanoparticles in stimulus-responsive drug/gene delivery systems. Chem. Soc. Rev. 2016, 45, 1457–1501. [Google Scholar] [CrossRef]
- Hayashi, K.; Ono, K.; Suzuki, H.; Sawada, M.; Moriya, M.; Sakamoto, W.; Yogo, T. High-Frequency, Magnetic-Field-Responsive Drug Release from Magnetic Nanoparticle/Organic Hybrid Based on Hyperthermic Effect. ACS Appl. Mater. Interfaces 2010, 2, 1903–1911. [Google Scholar] [CrossRef]
- Zhao, S.; Yu, X.; Qian, Y.; Chen, W.; Shen, J. Multifunctional magnetic iron oxide nanoparticles: An advanced platform for cancer theranostics. Theranostics 2020, 10, 6278–6309. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhang, H. Fe3O4-based nanotheranostics for magnetic resonance imaging-synergized multifunctional cancer management. Nanomedicine 2019, 14, 1493–1512. [Google Scholar] [CrossRef]
- Chen, S.; Haung, B.; Pei, W.; Xu, Y.; Jiang, Z.; Li, J.; Wang, L.; Niu, C. Magnetically targeted nanoparticles for imaging guided photothermal therapy of cancer. RSC Adv. 2019, 9, 38154–38163. [Google Scholar] [CrossRef] [PubMed]
- Arias, L.S.; Pessan, J.P.; Vieira, A.P.M.; Toito de Lima, T.M.; Botazzo Delbem, A.C.; Monteiro, D.R. Iron Oxide Nanoparticles for Biomedical Applications: A Perspective on Synthesis, Drugs, Antimicrobial Activity, and Toxicity. Antibiotics 2018, 7, 46. [Google Scholar] [CrossRef]
- Gao, L.; Fan, K.; Yan, X. Iron Oxide Nanozyme: A Multifunctional Enzyme Mimetic for Biomedical Applications. Theranostics 2017, 7, 3207–3227. [Google Scholar] [CrossRef] [PubMed]
- Kartsev, V.; Lichitsky, B.; Geronikaki, A.; Petrou, A.; Smiljkovic, M.; Kostic, M.; Radanovic, O.; Soković, M. Design, synthesis and antimicrobial activity of usnic acid derivatives. Med. Chem. Commun. 2018, 9, 870–882. [Google Scholar] [CrossRef]
- Andrade de Araújo, H.D.; Fagundes Silva, H.A.; da Silva Júnior, J.G.; Pessoa de Azevedo Albuquerque, M.C.; Breitenbach Barroso Coelho, L.C.; de Lima Aires, A. The Natural Compound Hydrophobic Usnic Acid and Hydrophilic Potassium Usnate Derivative: Applications and Comparisons. Molecules 2021, 26, 5995. [Google Scholar] [CrossRef] [PubMed]
- Taresco, V.; Francolini, I.; Padella, F.; Bellusci, M.; Boni, A.; Innocenti, C.; Martinelli, A.; D’Ilario, L.; Piozzi, A. Design and characterization of antimicrobial usnic acid loaded-core/shell magnetic nanoparticles. Mat. Sci. Eng. C 2015, 52, 72–81. [Google Scholar] [CrossRef] [PubMed]
- Nithyanand, P.; Shafreen, R.M.B.; Muthamil, S.; Pandian, S.K. Usnic acid inhibits biofilm formation and virulent morphological traits of Candida albicans. Microbiol. Res. 2015, 179, 20–28. [Google Scholar] [CrossRef]
- Pompilio, A.; Pomponio, S.; Di Vincenzo, V.; Crocetta, V.; Nicoletti, M.; Piovano, M.; Garbarino, J.A.; Di Bonaventura, G. Antimicrobial and antibiofilm activity of secondary metabolites of lichens against methicillin-resistant Staphylococcus aureus strains from cystic fibrosis patients. Future Microbiol. 2013, 8, 281–292. [Google Scholar] [CrossRef] [PubMed]
- Gupta, V.K.; Verma, S.; Gupta, S.; Singh, A.; Pal, A.; Srivastava, S.K.; Srivastava, P.K.; Singh, S.C.; Darokar, M.P. Membrane-damaging potential of natural L-(−)-usnic acid in Staphylococcus aureus. Eur. J. Clin. Microbiol. Infect. Dis. 2012, 31, 3375–3383. [Google Scholar] [CrossRef] [PubMed]
- Grumezescu, V.; Holban, A.M.; Grumezescu, A.M.; Socol, G.; Ficai, A.; Vasile, B.S.; Truscă, R.; Bleotu, C.; Lazar, V.; Chifiriuc, C.M. Usnic acid-loaded biocompatible magnetic PLGA-PVA microsphere coatings fabricated by MAPLE with increased resistance to staphylococcal colonization. Biofabrication 2014, 6, 035002. [Google Scholar] [CrossRef] [PubMed]
- Grumezescu, A.M.; Saviuc, C.; Chifiriuc, M.C.; Hristu, R.; Mihaiescu, D.E.; Balaure, P.; Stanciu, G.A.; Lazar, V. Inhibitory Activity of Fe3O4/Oleic Acid/Usnic Acid-Core/Shell/Extra-Shell Nanofluid on S. aureus Biofilm Development. IEEE Trans. Nanobiosci. 2011, 10I, 269–274. [Google Scholar] [CrossRef]
- Yue, Q.; Shen, T.; Wang, C.; Gao, C.; Liu, J. Study on the Interaction of Bovine Serum Albumin with Ceftriaxone and the Inhibition Effect of Zinc (II). Int. J. Spectrosc. 2012, 2012, 284173. [Google Scholar] [CrossRef]
- Grumezescu, V.; Socol, G.; Grumezescu, A.M.; Holban, A.M.; Ficai, A.; Trusca, R.; Bleotu, C.; Balaure, P.C.; Cristescu, R.; Chifiriuc, M.C. Functionalized antibiofilm thin coatings based on PLA-PVA microspheres loaded with usnic acid natural compounds fabricated by MAPLE. Appl. Surf. Sci. 2014, 302, 262–267. [Google Scholar] [CrossRef]
- Matei, E.; Predescu, C.; Berbecaru, A.; Predescu, A.; Truşcă, R. Leaching tests for synthesized magnetite nanoparticles used as Adsorbent for metal ions from liquid solutions. Dig. J. Nanomater. Biostruct. 2011, 6, 1701–1708. [Google Scholar]
- Puiu, R.A.; Balaure, P.C.; Constantinescu, E.; Grumezescu, A.M.; Andronescu, E.; Oprea, O.-C.; Vasile, B.S.; Grumezescu, V.; Negut, I.; Nica, I.C.; et al. Anti-Cancer Nanopowders and MAPLE-Fabricated Thin Coatings Based on SPIONs Surface Modified with Paclitaxel Loaded β-Cyclodextrin. Pharmaceutics 2021, 13, 1356. [Google Scholar] [CrossRef] [PubMed]
- Quaroni, L.; Pogoda, K.; Wiltowska-Zubera, J.; Kwiatek, W.M. Mid-infrared spectroscopy and microscopy of subcellular structures in eukaryotic cells withatomic force microscopy—Infrared spectroscopy. RSC Adv. 2018, 8, 2786–2794. [Google Scholar] [CrossRef]
- Sciutto, G.; Oliveri, P.; Prati, S.; Catelli, E.; Bonacini, I.; Mazzeo, R. A Multivariate Methodological Workflow forthe Analysis of FTIR Chemical Mapping Applied on Historic Paint Stratigraphies. Int. J. Anal. Chem. 2017, 2017, 4938145. [Google Scholar] [CrossRef]
- Gieroba, B.; Krysa, M.; Wojtowicz, K.; Wiater, A.; Pleszczyńska, M.; Tomczyk, M.; Sroka-Bart, A. The FT-IR and Raman Spectroscopies as Tools for Biofilm Characterization Created by Cariogenic Streptococci. Int. J. Mol. Sci. 2020, 21, 3811. [Google Scholar] [CrossRef]
- Gao, X.; Chorover, J. Adsorption of sodium dodecyl sulfate (SDS) at ZnSe and a-Fe2O3 surfaces: Combining infrared spectroscopy and batch uptake studies. J. Colloid Interface Sci. 2010, 348, 167–176. [Google Scholar] [CrossRef] [PubMed]
- Kartha, V.B.; . Leitch, L.C.; Mantsch, H.H. Infrared and Raman spectra of alkali palmityl sulfates. Can. J. Chem. 1984, 62, 128–132. [Google Scholar] [CrossRef]
- Araújo, H.D.A.; Silva Júnior, J.G.; Saturnino Oliveira, J.R.; Ribeiro, M.H.M.L.; Barroso Martins, M.C.; Cavalcanti Bezerra, M.A.; Aires, A.L.; Azevedo Albuquerque, M.C.P.; Melo-Júnior, M.R.; Pontes Filho, N.T.; et al. Usnic Acid Potassium Salt: Evaluation of the Acute Toxicity and Antinociceptive Effect in Murine Model. Molecules 2019, 24, 2042. [Google Scholar] [CrossRef] [PubMed]
- Gunasekaran, S.; Charles, J. Spectral Measurements and Qualitative Analysis of Ceftriaxone and Cefotaxime. Asian J. Chem. 2008, 20, 1343–1356. [Google Scholar]
- AbouElleef, E.M.; Mahrouka, M.M.; Salem, S.E. A Physical-Chemical Study of the Interference of Ceftriaxone Antibiotic with Copper Chloride Salt. Bioinorg. Chem. Appl. 2021, 2021, 4018843. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Li, X.; Liu, X.; Ji, X.; Ma, X.; Chen, J.; Bao, Y.; Zhang, Y.; Xu, L.; Yang, L.; et al. The usnic acid derivative peziculone targets cell walls of Gram-positive bacteria revealed by high-throughput CRISPRi-seq analysis. Int. J. Antimicrob. Agents 2023, 62, 106876. [Google Scholar] [CrossRef] [PubMed]
- Marchesi, J.R.; Owen, S.A.; White, G.F.; House, W.A.; Russell, N.J. SDS-degrading bacteria attach to riverine sediment in response to the surfactant or its primary biodegradation product dodecan-1-01. Microbiology 1994, 140, 2999–3006. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Klotz, S.A.; Drutz, D.S.; Zajic, J.E. Factors governing adherence of Candida species to plastic surfaces. Infect. Immun. 1985, 50, 97–101. [Google Scholar] [CrossRef]
- Rosenberg, E.; Gottlieb, A.; Rosenberg, M. Inhibition of bacterial adherence to hydrocarbons and epithelial cells by emulsan. Infect. Immun. 1983, 39, 1024–1028. [Google Scholar] [CrossRef]
- Izano, E.A.; Wang, H.; Ragunath, C.; Ramasubbu, N.; Kaplan, J.B. Detachment and Killing of Aggregatibacter actinomycetemcomitans Biofilms by Dispersin B and SDS. J. Dent. Res. 2007, 86, 618–622. [Google Scholar] [CrossRef]
- Dubey, G.P.; Ben-Yehuda, S. Intercellular nanotubes mediate bacterial communication. Cell 2011, 144, 590–600. [Google Scholar] [CrossRef] [PubMed]
- Achtman, M.; Morelli, G.; Schwuchow, S. Cell-cell interactions in conjugating Escherichia coli: Role of F pili and fate of mating aggregates. J. Bacteriol. 1978, 135, 1053–1061. [Google Scholar] [CrossRef] [PubMed]
- Ghigo, J.M. Natural conjugative plasmids induce bacterial biofilm development. Nature 2001, 412, 442–445. [Google Scholar] [CrossRef] [PubMed]
- Dıaz De Rienzo, M.A.; Stevenson, P.; Marchant, R.; Banat, I.M. Antibacterial properties of biosurfactants against selected Gram-positive and -negative bacteria. FEMS Microbiol. Lett. 2016, 363, fnv224. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Molin, S.; Yang, L.; Ndoni, S. Sodium Dodecyl Sulfate (SDS)-Loaded Nanoporous Polymer as Anti-Biofilm Surface Coating Material. Int. J. Mol. Sci. 2013, 14, 3050–3064. [Google Scholar] [CrossRef]







Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pirușcă, I.A.; Balaure, P.C.; Grumezescu, V.; Irimiciuc, S.-A.; Oprea, O.-C.; Bîrcă, A.C.; Vasile, B.; Holban, A.M.; Voinea, I.C.; Stan, M.S.; et al. New Fe3O4-Based Coatings with Enhanced Anti-Biofilm Activity for Medical Devices. Antibiotics 2024, 13, 631. https://doi.org/10.3390/antibiotics13070631
Pirușcă IA, Balaure PC, Grumezescu V, Irimiciuc S-A, Oprea O-C, Bîrcă AC, Vasile B, Holban AM, Voinea IC, Stan MS, et al. New Fe3O4-Based Coatings with Enhanced Anti-Biofilm Activity for Medical Devices. Antibiotics. 2024; 13(7):631. https://doi.org/10.3390/antibiotics13070631
Chicago/Turabian StylePirușcă, Ioana Adelina, Paul Cătălin Balaure, Valentina Grumezescu, Stefan-Andrei Irimiciuc, Ovidiu-Cristian Oprea, Alexandra Cătălina Bîrcă, Bogdan Vasile, Alina Maria Holban, Ionela C. Voinea, Miruna S. Stan, and et al. 2024. "New Fe3O4-Based Coatings with Enhanced Anti-Biofilm Activity for Medical Devices" Antibiotics 13, no. 7: 631. https://doi.org/10.3390/antibiotics13070631
APA StylePirușcă, I. A., Balaure, P. C., Grumezescu, V., Irimiciuc, S.-A., Oprea, O.-C., Bîrcă, A. C., Vasile, B., Holban, A. M., Voinea, I. C., Stan, M. S., Trușcă, R., Grumezescu, A. M., & Croitoru, G.-A. (2024). New Fe3O4-Based Coatings with Enhanced Anti-Biofilm Activity for Medical Devices. Antibiotics, 13(7), 631. https://doi.org/10.3390/antibiotics13070631












