Comments by Microbiologists for Interpreting Antimicrobial Susceptibility Testing and Improving the Appropriateness of Antibiotic Therapy in Community-Acquired Urinary Tract Infections: A Randomized Double-Blind Digital Case-Vignette Controlled Superiority Trial
Abstract
1. Introduction
2. Results
2.1. Attitudes toward Antimicrobial Susceptibility Testing
2.2. Empirical Antibiotic Therapy
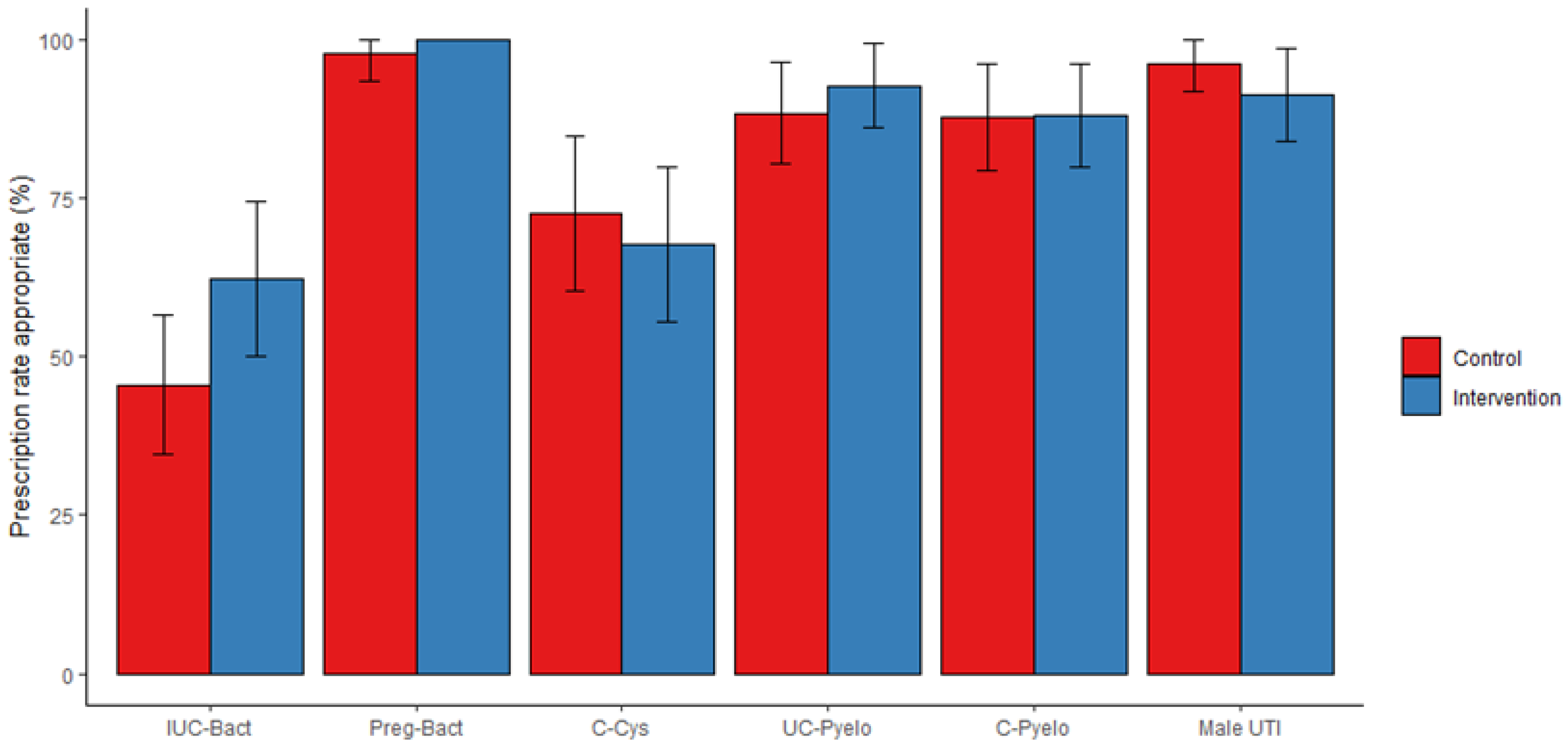
2.3. Targeted Antibiotic Therapy, Primary Outcome
2.4. Deviation from First-Line Antibiotic Regimen, Secondary Outcome
2.5. Use of Broad-Spectrum Antibiotics, Secondary Outcome
3. Discussion
4. Materials and Methods
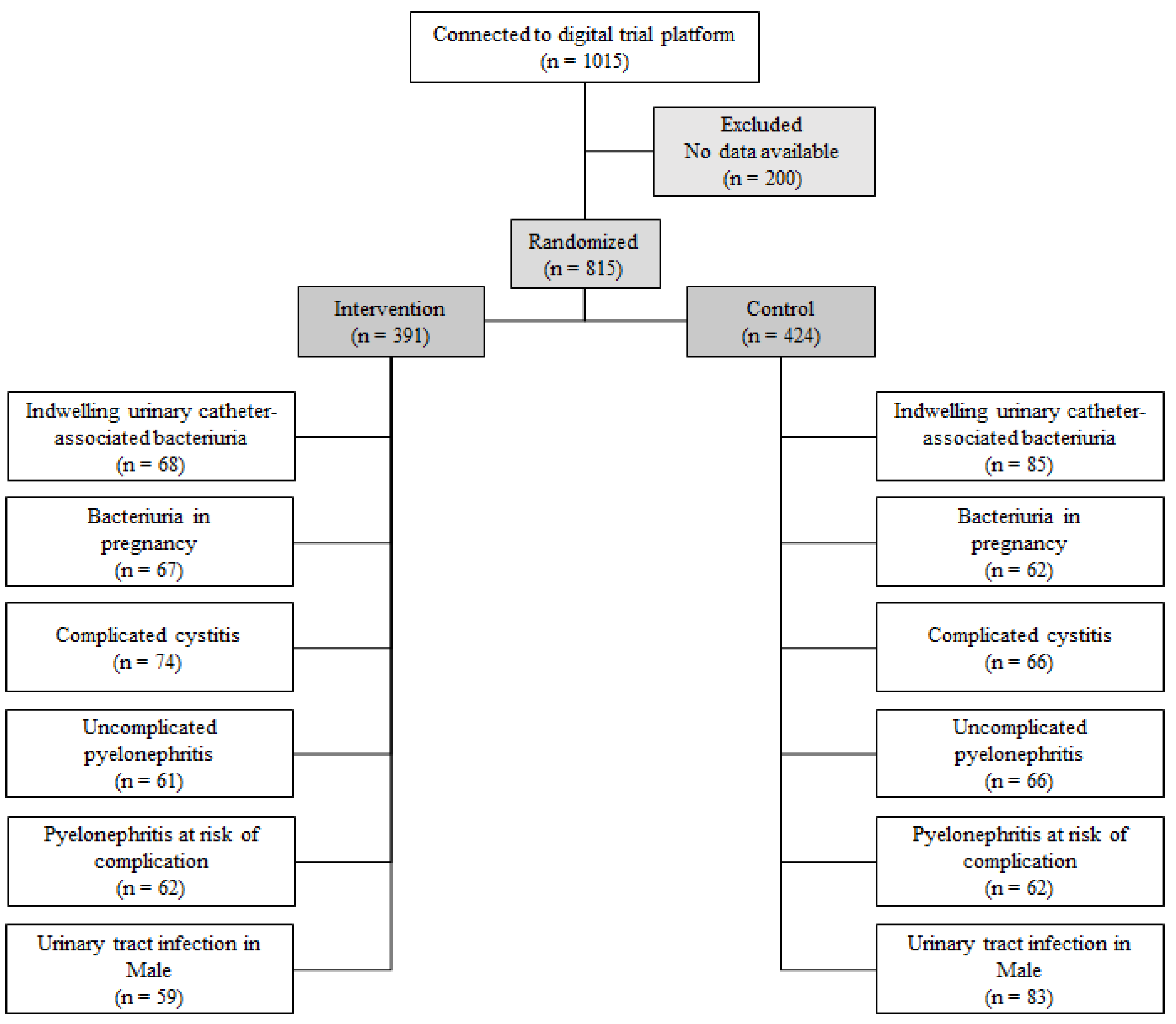
4.1. Design, Participants, and Data Collection
4.2. Self-Questionnaire
4.3. Comments, i.e., the Intervention
4.4. Outcomes
4.5. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- European Centre for Disease Prevention and Control. Antimicrobial Resistance in the EU/EEA (EARS-Net)-Annual Epidemiological Report for 2021; ECDC: Stockholm, Sweden, 2022. [Google Scholar]
- Antimicrobial Resistance Collaborators. Global Burden of Bacterial Antimicrobial Resistance in 2019: A Systematic Analysis. Lancet 2022, 399, 629–655. [Google Scholar] [CrossRef]
- Sands, K.; Carvalho, M.J.; Portal, E.; Thomson, K.; Dyer, C.; Akpulu, C.; Andrews, R.; Ferreira, A.; Gillespie, D.; Hender, T.; et al. Characterization of Antimicrobial-Resistant Gram-Negative Bacteria That Cause Neonatal Sepsis in Seven Low- and Middle-Income Countries. Nat. Microbiol. 2021, 6, 512–523. [Google Scholar] [CrossRef] [PubMed]
- Charani, E.; Mendelson, M.; Pallett, S.J.C.; Ahmad, R.; Mpundu, M.; Mbamalu, O.; Bonaconsa, C.; Nampoothiri, V.; Singh, S.; Peiffer-Smadja, N.; et al. An Analysis of Existing National Action Plans for Antimicrobial Resistance-Gaps and Opportunities in Strategies Optimising Antibiotic Use in Human Populations. Lancet Glob. Health 2023, 11, e466–e474. [Google Scholar] [CrossRef] [PubMed]
- Petersen, I.; Hayward, A.C. SACAR Surveillance Subgroup Antibacterial Prescribing in Primary Care. J. Antimicrob. Chemother. 2007, 60 (Suppl. 1), i43–i47. [Google Scholar] [CrossRef]
- Etienne, C.; Pulcini, C. Évaluation prospective des prescriptions antibiotiques d’un échantillon de médecins généralistes français. La Presse Médicale 2015, 44, e59–e66. [Google Scholar] [CrossRef]
- European Centre for Disease Prevention and Control. Antimicrobial Consumption in the EU/EEA (ESAC-Net)-Annual Epidemiological Report 2021; ECDC: Stockholm, Sweden, 2022. [Google Scholar]
- Delory, T. Time to Evaluate Decision Support Systems for Antimicrobial Prescribing Outside the Hospital. Lancet Infect. Dis. 2022, 22, 1408–1409. [Google Scholar] [CrossRef]
- Sutton, R.T.; Pincock, D.; Baumgart, D.C.; Sadowski, D.C.; Fedorak, R.N.; Kroeker, K.I. An Overview of Clinical Decision Support Systems: Benefits, Risks, and Strategies for Success. NPJ Digit. Med. 2020, 3, 17. [Google Scholar] [CrossRef]
- Rittmann, B.; Stevens, M.P. Clinical Decision Support Systems and Their Role in Antibiotic Stewardship: A Systematic Review. Curr. Infect. Dis. Rep. 2019, 21, 29. [Google Scholar] [CrossRef]
- Low, M.; Almog, R.; Balicer, R.D.; Liberman, N.; Raz, R.; Peretz, A.; Nitzan, O. Infectious Disease Burden and Antibiotic Prescribing in Primary Care in Israel. Ann. Clin. Microbiol. Antimicrob. 2018, 17, 26. [Google Scholar] [CrossRef] [PubMed]
- Tandogdu, Z.; Wagenlehner, F.M.E. Global Epidemiology of Urinary Tract Infections. Curr. Opin. Infect. Dis. 2016, 29, 73–79. [Google Scholar] [CrossRef]
- Foxman, B. Urinary Tract Infection Syndromes: Occurrence, Recurrence, Bacteriology, Risk Factors, and Disease Burden. Infect. Dis. Clin. N. Am. 2014, 28, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Shallcross, L.J.; Davies, D.S.C. Antibiotic Overuse: A Key Driver of Antimicrobial Resistance. Br. J. Gen. Pract. 2014, 64, 604–605. [Google Scholar] [CrossRef] [PubMed]
- Llor, C.; Rabanaque, G.; López, A.; Cots, J.M. The Adherence of GPs to Guidelines for the Diagnosis and Treatment of Lower Urinary Tract Infections in Women Is Poor. Fam. Pract. 2011, 28, 294–299. [Google Scholar] [CrossRef] [PubMed]
- Neugebauer, M.; Ebert, M.; Vogelmann, R. A Clinical Decision Support System Improves Antibiotic Therapy for Upper Urinary Tract Infection in a Randomized Single-Blinded Study. BMC Health Serv. Res. 2020, 20, 185. [Google Scholar] [CrossRef]
- Denes, E.; Prouzergue, J.; Ducroix-Roubertou, S.; Aupetit, C.; Weinbreck, P. Antibiotic Prescription by General Practitioners for Urinary Tract Infections in Outpatients. Eur. J. Clin. Microbiol. Infect. Dis. 2012, 31, 3079–3083. [Google Scholar] [CrossRef]
- Plate, A.; Kronenberg, A.; Risch, M.; Mueller, Y.; Di Gangi, S.; Rosemann, T.; Senn, O. Treatment of Urinary Tract Infections in Swiss Primary Care: Quality and Determinants of Antibiotic Prescribing. BMC Fam. Pract. 2020, 21, 125. [Google Scholar] [CrossRef]
- Herter, W.E.; Khuc, J.; Cinà, G.; Knottnerus, B.J.; Numans, M.E.; Wiewel, M.A.; Bonten, T.N.; de Bruin, D.P.; van Esch, T.; Chavannes, N.H.; et al. Impact of a Machine Learning-Based Decision Support System for Urinary Tract Infections: Prospective Observational Study in 36 Primary Care Practices. JMIR Med. Inf. 2022, 10, e27795. [Google Scholar] [CrossRef]
- Delory, T.; Jeanmougin, P.; Lariven, S.; Aubert, J.-P.; Peiffer-Smadja, N.; Boëlle, P.-Y.; Bouvet, E.; Lescure, F.-X.; Le Bel, J. A Computerized Decision Support System (CDSS) for Antibiotic Prescription in Primary Care—Antibioclic: Implementation, Adoption and Sustainable Use in the Era of Extended Antimicrobial Resistance. J. Antimicrob. Chemother. 2020, 75, 2353–2362. [Google Scholar] [CrossRef]
- Durand, C.; Alfandari, S.; Béraud, G.; Tsopra, R.; Lescure, F.-X.; Peiffer-Smadja, N. Clinical Decision Support Systems for Antibiotic Prescribing: An Inventory of Current French Language Tools. Antibiotics 2022, 11, 384. [Google Scholar] [CrossRef]
- Caron, F.; Galperine, T.; Flateau, C.; Azria, R.; Bonacorsi, S.; Bruyère, F.; Cariou, G.; Clouqueur, E.; Cohen, R.; Doco-Lecompte, T.; et al. Practice Guidelines for the Management of Adult Community-Acquired Urinary Tract Infections. Médecine Et Mal. Infect. 2018, 48, 327–358. [Google Scholar] [CrossRef]
- Bourdellon, L.; Thilly, N.; Fougnot, S.; Pulcini, C.; Henard, S. Impact of Selective Reporting of Antibiotic Susceptibility Test Results on the Appropriateness of Antibiotics Chosen by French General Practitioners in Urinary Tract Infections: A Randomised Controlled Case-Vignette Study. Int. J. Antimicrob. Agents 2017, 50, 258–262. [Google Scholar] [CrossRef] [PubMed]
- Morency-Potvin, P.; Schwartz, D.N.; Weinstein, R.A. Antimicrobial Stewardship: How the Microbiology Laboratory Can Right the Ship. Clin. Microbiol. Rev. 2017, 30, 381–407. [Google Scholar] [CrossRef] [PubMed]
- Australian Commission on Safety and Quality in Health Care. Antimicrobial Stewardship in Australian Health Care; ACSQHC: Sydney, Australia, 2022. [Google Scholar]
- M100. CLSI Performance Standards for Antimicrobial Susceptibility Testing, 33rd ed.; CLSI Supplement M100; Clinical and Laboratory Standards Institute (CLSI): Pittsburgh, PA, USA, 2023. [Google Scholar]
- Home. Available online: https://adoptaware.org/ (accessed on 16 May 2022).
- Demonchy, E.; Dufour, J.-C.; Gaudart, J.; Cervetti, E.; Michelet, P.; Poussard, N.; Levraut, J.; Pulcini, C. Impact of a Computerized Decision Support System on Compliance with Guidelines on Antibiotics Prescribed for Urinary Tract Infections in Emergency Departments: A Multicentre Prospective before-and-after Controlled Interventional Study. J. Antimicrob. Chemother. 2014, 69, 2857–2863. [Google Scholar] [CrossRef] [PubMed]
- DREES. La Démographie des Médecins (RPPS) au 1er Janvier 2022; Ministère de la Santé et de la Prévention: Paris, France, 2022.
- Arnaud, F. Atlas de La Démographie Médicale En France, Situation Au 1er Janvier 2022; Ordre National des Médecins: Paris, France, 2022. [Google Scholar]
- Cadieux, G.; Tamblyn, R.; Dauphinee, D.; Libman, M. Predictors of Inappropriate Antibiotic Prescribing among Primary Care Physicians. CMAJ 2007, 177, 877–883. [Google Scholar] [CrossRef]
- Coupat, C.; Pradier, C.; Degand, N.; Hofliger, P.; Pulcini, C. Selective Reporting of Antibiotic Susceptibility Data Improves the Appropriateness of Intended Antibiotic Prescriptions in Urinary Tract Infections: A Case-Vignette Randomised Study. Eur. J. Clin. Microbiol. Infect. Dis. 2013, 32, 627–636. [Google Scholar] [CrossRef]
- Delory, T.; Le Bel, J.; Lariven, S.; Peiffer-Smadja, N.; Lescure, F.-X.; Bouvet, E.; Jeanmougin, P.; Tubach, F.; Boëlle, P.-Y. Computerized Decision Support System (CDSS) Use for Surveillance of Antimicrobial Resistance in Urinary Tract Infections in Primary Care. J. Antimicrob. Chemother. 2022, 77, 524–530. [Google Scholar] [CrossRef]
- McNulty, C.A.M.; Lasseter, G.M.; Charlett, A.; Lovering, A.; Howell-Jones, R.; Macgowan, A.; Thomas, M. Does Laboratory Antibiotic Susceptibility Reporting Influence Primary Care Prescribing in Urinary Tract Infection and Other Infections? J. Antimicrob. Chemother. 2011, 66, 1396–1404. [Google Scholar] [CrossRef]
- Tan, T.Y.; McNulty, C.; Charlett, A.; Nessa, N.; Kelly, C.; Beswick, T. Laboratory Antibiotic Susceptibility Reporting and Antibiotic Prescribing in General Practice. J. Antimicrob. Chemother. 2003, 51, 379–384. [Google Scholar] [CrossRef]
- Binda, F.; Fougnot, S.; De Monchy, P.; Fagot-Campagna, A.; Pulcini, C.; Thilly, N. Impact of Selective Reporting of Antibiotic Susceptibility Test Results in Urinary Tract Infections in the Outpatient Setting: A Protocol for a Pragmatic, Prospective Quasi-Experimental Trial. BMJ Open 2019, 8, e025810. [Google Scholar] [CrossRef]
- Michelangeli, C.; Girard-Lamoulere, D.; Assi, A.; Della Guardia, M.; Roger, P.-M. Antibiotic Guidelines Coupled with Selective Reporting of Antibiograms. Infect. Dis. Now 2021, 51, 61–66. [Google Scholar] [CrossRef]
- Nicolle, L.E.; Gupta, K.; Bradley, S.F.; Colgan, R.; DeMuri, G.P.; Drekonja, D.; Eckert, L.O.; Geerlings, S.E.; Köves, B.; Hooton, T.M.; et al. Clinical Practice Guideline for the Management of Asymptomatic Bacteriuria: 2019 Update by the Infectious Diseases Society of America. Clin. Infect. Dis. 2019, 68, 1611–1615. [Google Scholar] [CrossRef] [PubMed]
- Madar, R.; Ugon, A.; Ivanković, D.; Tsopra, R. A Web Interface for Antibiotic Prescription Recommendations in Primary Care: User-Centered Design Approach. J. Med. Internet Res. 2021, 23, e25741. [Google Scholar] [CrossRef] [PubMed]
- Peabody, J.W.; Luck, J.; Glassman, P.; Jain, S.; Hansen, J.; Spell, M.; Lee, M. Measuring the Quality of Physician Practice by Using Clinical Vignettes: A Prospective Validation Study. Ann. Intern. Med. 2004, 141, 771–780. [Google Scholar] [CrossRef]
- Lucet, J.-C.; Nicolas-Chanoine, M.-H.; Lefort, A.; Roy, C.; Diamantis, S.; Papy, E.; Riveros-Palacios, O.; Le Grand, J.; Rioux, C.; Fantin, B.; et al. Do Case Vignettes Accurately Reflect Antibiotic Prescription? Infect. Control. Hosp. Epidemiol. 2011, 32, 1003–1009. [Google Scholar] [CrossRef] [PubMed]
| Control (N = 424) | Intervention (N = 391) | Total (N = 815) | |
|---|---|---|---|
| Sex | N = 812 | ||
| Women | 280 (66.5%) | 245 (62.7%) | 525 (64.7%) |
| Men | 141 (33.5%) | 146 (37.3%) | 287 (35.3%) |
| Age | N = 815 | ||
| Mean ± SD | 37.1 ± 11.6 | 36.7 ± 11.2 | 36.9 ± 11.4 |
| Median (Q1–Q3) | 33.0 (29.0–42.0) | 33.0 (29.0–39.5) | 33.0 (29.0–41.0) |
| Min–max | 23.0–73.0 | 24.0–74.0 | 23.0–74.0 |
| Professional status of GPs * | N = 815 | ||
| Established | 258 (60.8%) | 237 (60.6%) | 495 (60.7%) |
| Replacing other GPs | 53 (12.5%) | 54 (13.8%) | 107 (13.1%) |
| Resident | 113 (26.7%) | 100 (25.6%) | 213 (26.2%) |
| Professional experience | N = 815 | ||
| Mean ± SD | 11.7 ± 11.8 | 10.6 ± 11.3 | 11.2 ± 11.6 |
| Median (Q1–Q3) | 7.00 (3.00–19.0) | 6.00 (3.00–16.0) | 6.00 (3.00–17.0) |
| Min–max | 0–48.0 | 0–46.0 | 0–48.0 |
| Working environment | N = 814 | ||
| Urban | 156 (36.9%) | 148 (37.9%) | 304 (37.3%) |
| Semi-rural | 208 (49.2%) | 187 (47.8%) | 395 (48.5%) |
| Rural | 59 (13.9%) | 56 (14.3%) | 115 (14.1%) |
| Main mode of practice | N = 815 | ||
| At hospital/healthcare facility | 41 (9.67%) | 32 (8.18%) | 73 (8.96%) |
| In group practices | 330 (77.8%) | 318 (81.3%) | 648 (79.5%) |
| Alone | 53 (12.5%) | 41 (10.5%) | 94 (11.5%) |
| Student mentoring | N = 815 | ||
| Yes | 104 (24.5%) | 82 (21.0%) | 186 (22.8%) |
| No | 320 (75.5%) | 309 (79.0%) | 629 (77.2%) |
| Control (N = 424) | Intervention (N = 391) | Total (N = 815) | |
|---|---|---|---|
| Attitude toward AST * interpretation | |||
| At ease to interpret; willing to be trained | 135 (31.8%) | 112 (28.7%) | 247 (30.3%) |
| At ease to interpret; not willing to be trained | 274 (64.6%) | 269 (68.9%) | 543 (66.6%) |
| Not at ease to interpret; willing to be trained | 12 (2.8%) | 9 (2.3%) | 21 (2.7%) |
| Not at ease to interpret; not willing to be trained | 3 (0.8%) | 1 (0.1%) | 4 (0.4%) |
| CDSS † use for prescribing antibiotics in UTIs ‡ | |||
| Yes | 383 (90.3%) | 351 (89.8%) | 734 (90.1%) |
| No | 41 (9.67%) | 40 (10.2%) | 81 (9.94%) |
| Seek advice from an infectious disease specialist for management of UTIs ‡ | |||
| Frequently | 5 (1.18%) | 4 (1.02%) | 9 (1.10%) |
| Occasionally | 87 (20.5%) | 69 (17.6%) | 156 (19.1%) |
| Rarely | 247 (58.3%) | 225 (57.5%) | 472 (57.9%) |
| Never | 85 (20.0%) | 93 (23.8%) | 178 (21.8%) |
| Variable | N | OR * | 95% CI | p-Value |
|---|---|---|---|---|
| Sex | ||||
| Women | 452 | Ref. | ||
| Man | 258 | 1.12 | 0.71–1.78 | 0.600 |
| Age (categories) | ||||
| 23 to 34 years-old | 425 | Ref. | ||
| 35 to 74 years-old | 285 | 0.91 | 0.42–1.91 | 0.800 |
| Main mode of practice | ||||
| In primary care | 646 | Ref. | ||
| In healthcare facilities | 64 | 2.38 | 1.02–6.16 | 0.046 |
| Professional experience | ||||
| >5 years | 319 | Ref. | ||
| ≤5 years | 200 | 0.71 | 0.32–1.53 | 0.400 |
| Resident | 191 | 0.82 | 0.34–1.92 | 0.600 |
| Student mentoring | ||||
| Yes | 167 | Ref. | ||
| No | 543 | 1.08 | 0.63–1.84 | 0.800 |
| CDSS † use for antibiotic prescribing | ||||
| Yes | 643 | Ref. | ||
| No | 37 | 0.76 | 0.36–1.69 | 0.500 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Piet, E.; N’Diaye, Y.; Marzani, J.; Pires, L.; Petitprez, H.; Delory, T. Comments by Microbiologists for Interpreting Antimicrobial Susceptibility Testing and Improving the Appropriateness of Antibiotic Therapy in Community-Acquired Urinary Tract Infections: A Randomized Double-Blind Digital Case-Vignette Controlled Superiority Trial. Antibiotics 2023, 12, 1272. https://doi.org/10.3390/antibiotics12081272
Piet E, N’Diaye Y, Marzani J, Pires L, Petitprez H, Delory T. Comments by Microbiologists for Interpreting Antimicrobial Susceptibility Testing and Improving the Appropriateness of Antibiotic Therapy in Community-Acquired Urinary Tract Infections: A Randomized Double-Blind Digital Case-Vignette Controlled Superiority Trial. Antibiotics. 2023; 12(8):1272. https://doi.org/10.3390/antibiotics12081272
Chicago/Turabian StylePiet, Emilie, Youssoupha N’Diaye, Johann Marzani, Lucas Pires, Hélène Petitprez, and Tristan Delory. 2023. "Comments by Microbiologists for Interpreting Antimicrobial Susceptibility Testing and Improving the Appropriateness of Antibiotic Therapy in Community-Acquired Urinary Tract Infections: A Randomized Double-Blind Digital Case-Vignette Controlled Superiority Trial" Antibiotics 12, no. 8: 1272. https://doi.org/10.3390/antibiotics12081272
APA StylePiet, E., N’Diaye, Y., Marzani, J., Pires, L., Petitprez, H., & Delory, T. (2023). Comments by Microbiologists for Interpreting Antimicrobial Susceptibility Testing and Improving the Appropriateness of Antibiotic Therapy in Community-Acquired Urinary Tract Infections: A Randomized Double-Blind Digital Case-Vignette Controlled Superiority Trial. Antibiotics, 12(8), 1272. https://doi.org/10.3390/antibiotics12081272






