Real-Life Experience of Continuously Infused Ceftolozane/Tazobactam in Patients with Bronchiectasis and Multidrug-Resistant Pseudomonas aeruginosa Infection in the Outpatient Setting
Abstract
1. Introduction
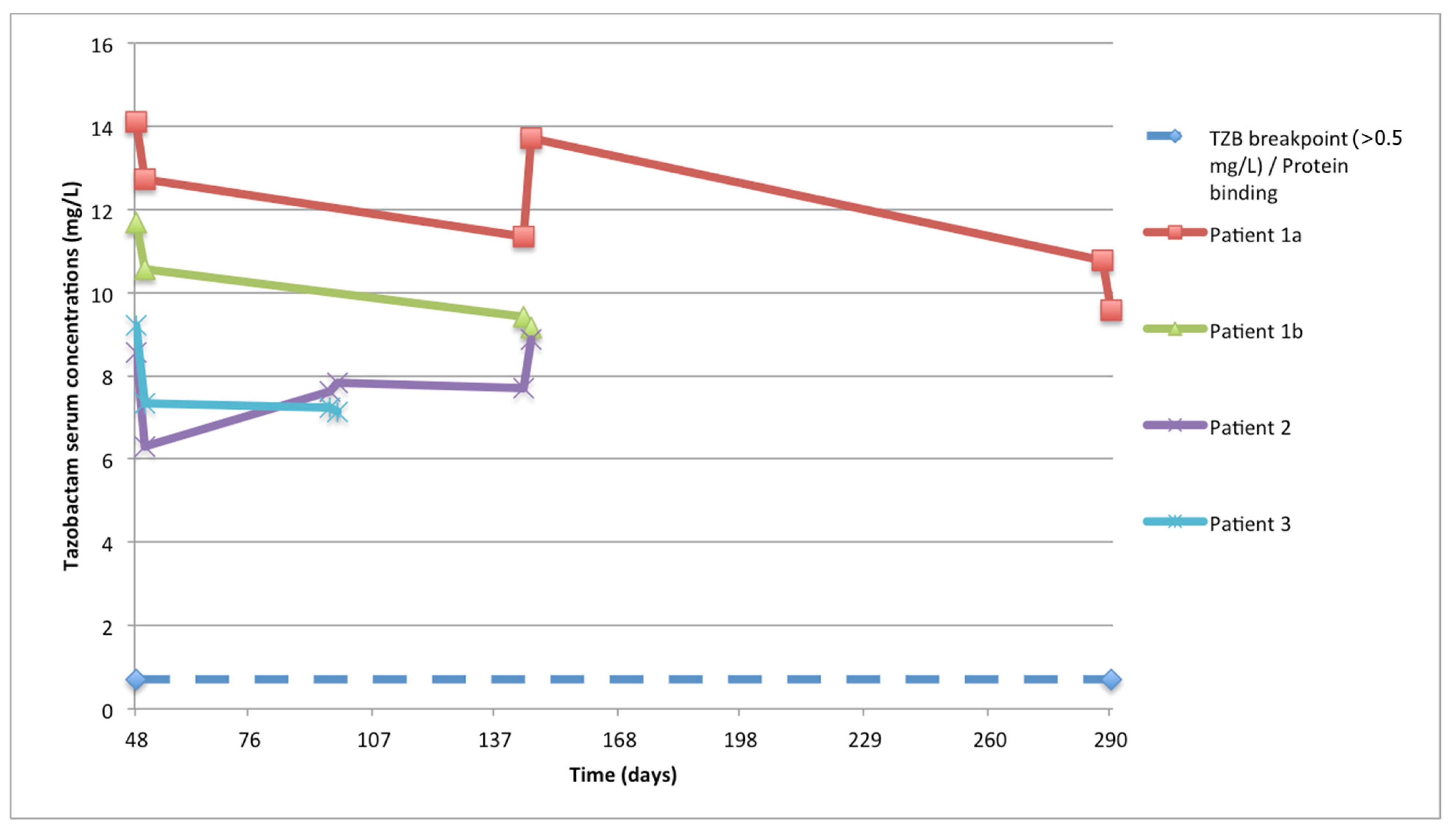
2. Results
Primary and Secondary Outcomes
3. Discussion
4. Materials and Methods
4.1. Participant Enrollment
4.2. Pharmacokinetic/Pharmacodynamic (PK/PD) Evaluation
4.3. Microbiology
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- van Duin, D.; Bonomo, R.A. Ceftazidime/Avibactam and Ceftolozane/Tazobactam: Second-generation β-Lactam/β-Lactamase Inhibitor Combinations. Clin. Infect. Dis. 2016, 63, 234–241. [Google Scholar] [CrossRef]
- Gallagher, J.C.; Satlin, M.J.; Elabor, A.; Saraiya, N.; McCreary, E.K.; Molnar, E.; El-Beyrouty, C.; Jones, B.M.; Dixit, D.; Heil, E.L.; et al. Ceftolozane-Tazobactam for the Treatment of Multidrug-Resistant Pseudomonas aeruginosa Infections: A Multicenter Study. Open Forum Infect. Dis. 2018, 5, ofy280. [Google Scholar] [CrossRef]
- Shortridge, D.; Castanheira, M.; Pfaller, M.A.; Flamm, R.K. Ceftolozane-Tazobactam Activity against Pseudomonas aeruginosa Clinical Isolates from U.S. Hospitals: Report from the PACTS Antimicrobial Surveillance Program, 2012 to 2015. Antimicrob. Agents Chemother. 2017, 61, e00465-17. [Google Scholar] [CrossRef]
- Zerbaxa® (Ceftolozane and Tazobactam). US Prescribing information. Merck Sharp & Dohme LLC. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/206829s011s012lbl.pdf (accessed on 10 May 2023).
- Zerbaxa® (Ceftolozane and Tazobactam). EMA Summary of Product Characteristics. Merck Sharp & Dohme B.V. Available online: https://www.ema.europa.eu/en/documents/product-information/zerbaxa-epar-product-information_en.pdf (accessed on 10 May 2023).
- Larson, K.B.; Patel, Y.T.; Willavize, S.; Bradley, J.S.; Rhee, E.G.; Caro, L.; Rizk, M.L. Ceftolozane-Tazobactam Population Pharmacokinetics and Dose Selection for Further Clinical Evaluation in Pediatric Patients with Complicated Urinary Tract or Complicated Intra-abdominal Infections. Antimicrob. Agents Chemother. 2019, 63, e02578-18. [Google Scholar] [CrossRef]
- Wise, R.; Logan, M.; Cooper, M.; Andrews, J.M. Pharmacokinetics and tissue penetration of tazobactam administered alone and with piperacillin. Antimicrob. Agents Chemother. 1991, 35, 1081–1084. [Google Scholar] [CrossRef]
- Craig, W.A. Pharmacokinetic/pharmacodynamic parameters: Rationale for antibacterial dosing of mice and men. Clin. Infect. Dis. 1998, 26, 1–10; quiz 11–12. [Google Scholar] [CrossRef]
- Abdul-Aziz, M.H.; Portunato, F.; Roberts, J.A. Prolonged infusion of beta-lactam antibiotics for Gram-negative infections: Rationale and evidence base. Curr. Opin. Infect. Dis. 2020, 33, 501–510. [Google Scholar] [CrossRef]
- Gatti, M.; Pea, F. Pharmacokinetic/pharmacodynamic target attainment in critically ill renal patients on antimicrobial usage: Focus on novel beta-lactams and beta lactams/beta-lactamase inhibitors. Expert. Rev. Clin. Pharmacol. 2021, 14, 583–599. [Google Scholar] [CrossRef]
- Roberts, J.A.; Paul, S.K.; Akova, M.; Bassetti, M.; De Waele, J.J.; Dimopoulos, G.; Kaukonen, K.M.; Koulenti, D.; Martin, C.; Montravers, P.; et al. DALI: Defining antibiotic levels in intensive care unit patients: Are current β-lactam antibiotic doses sufficient for critically ill patients? Clin. Infect. Dis. 2014, 58, 1072–1083. [Google Scholar] [CrossRef]
- Guilhaumou, R.; Benaboud, S.; Bennis, Y.; Dahyot-Fizelier, C.; Dailly, E.; Gandia, P.; Goutelle, S.; Lefeuvre, S.; Mongardon, N.; Roger, C.; et al. Optimization of the treatment with beta-lactam antibiotics in critically ill patients-guidelines from the French Society of Pharmacology and Therapeutics (Société Française de Pharmacologie et Thérapeutique-SFPT) and the French Society of Anaesthesia and Intensive Care Medicine (Société Française d’Anesthésie et Réanimation-SFAR). Crit. Care. 2019, 23, 104. [Google Scholar] [CrossRef]
- Raby, E.; Naicker, S.; Sime, F.B.; Manning, L.; Wallis, S.C.; Pandey, S.; Roberts, J.A. Ceftolozane-tazobactam in an elastomeric infusion device for ambulatory care: An in vitro stability study. Eur. J. Hosp. Pharm. 2020, 27, e84–e86. [Google Scholar] [CrossRef]
- Dimitrova, M.; Gilchrist, M.; Seaton, R.A. Outpatient parenteral antimicrobial therapy (OPAT) versus inpatient care in the UK: A health economic assessment for six key diagnoses. BMJ Open. 2021, 11, e049733. [Google Scholar] [CrossRef]
- O’Donnell, A.E. Bronchiectasis—A Clinical Review. N. Engl. J. Med. 2022, 387, 533–545. [Google Scholar] [CrossRef]
- Fernández-Barat, L.; Alcaraz-Serrano, V.; Amaro, R.; Torres, A. Pseudomonas aeruginosa in Bronchiectasis. Semin. Respir. Crit. Care Med. 2021, 42, 587–594. [Google Scholar] [CrossRef]
- Seitz, A.E.; Olivier, K.N.; Steiner, C.A.; Montes de Oca, R.; Holland, S.M.; Prevots, D.R. Trends and burden of bronchiectasis-associated hospitalizations in the United States, 1993–2006. Chest 2010, 138, 944–949. [Google Scholar] [CrossRef]
- Finch, S.; McDonnell, M.J.; Abo-Leyah, H.; Aliberti, S.; Chalmers, J.D. A Comprehensive Analysis of the Impact of Pseudomonas aeruginosa Colonization on Prognosis in Adult Bronchiectasis. Ann. Am. Thorac. Soc. 2015, 12, 1602–1611. [Google Scholar] [CrossRef]
- Choate, R.; Aksamit, T.R.; Mannino, D.; Addrizzo-Harris, D.; Barker, A.; Basavaraj, A.; Daley, C.L.; Daniels, M.L.; Eden, E.; DiMango, A.; et al. Pseudomonas aeruginosa associated with severity of non-cystic fibrosis bronchiectasis measured by the modified bronchiectasis severity score (BSI) and the FACED: The US bronchiectasis and NTM Research Registry (BRR) study. Respir. Med. 2020, 177, 106285. [Google Scholar] [CrossRef]
- Aksamit, T.R.; O’Donnell, A.E.; Barker, A.; Olivier, K.N.; Winthrop, K.L.; Daniels, M.L.; Johnson, M.; Eden, E.; Griffith, D.; Knowles, M.; et al. Adult Patients With Bronchiectasis: A First Look at the US Bronchiectasis Research Registry. Chest 2017, 151, 982–992. [Google Scholar] [CrossRef]
- Lonni, S.; Chalmers, J.D.; Goeminne, P.C.; McDonnell, M.J.; Dimakou, K.; De Soyza, A.; Polverino, E.; Van de Kerkhove, C.; Rutherford, R.; Davison, J.; et al. Etiology of Non-Cystic Fibrosis Bronchiectasis in Adults and Its Correlation to Disease Severity. Ann. Am. Thorac. Soc. 2015, 12, 1764–1770. [Google Scholar] [CrossRef]
- Guan, W.J.; Gao, Y.H.; Xu, G.; Lin, Z.Y.; Tang, Y.; Li, H.M.; Lin, Z.M.; Zheng, J.P.; Chen, R.C.; Zhong, N.S. Sputum bacteriology in steady-state bronchiectasis in Guangzhou, China. Int. J. Tuberc. Lung Dis. 2015, 19, 610–619. [Google Scholar] [CrossRef]
- Cabrera, R.; Fernández-Barat, L.; Vázquez, N.; Alcaraz-Serrano, V.; Bueno-Freire, L.; Amaro, R.; López-Aladid, R.; Oscanoa, P.; Muñoz, L.; Vila, J.; et al. Resistance mechanisms and molecular epidemiology of Pseudomonas aeruginosa strains from patients with bronchiectasis. J. Antimicrob. Chemother. 2022, 77, 1600–1610. [Google Scholar] [CrossRef]
- Polverino, E.; Goeminne, P.C.; McDonnell, M.J.; Aliberti, S.; Marshall, S.E.; Loebinger, M.R.; Murris, M.; Cantón, R.; Torres, A.; Dimakou, K.; et al. European Respiratory Society guidelines for the management of adult bronchiectasis. Eur. Respir. J. 2017, 50, 1700629. [Google Scholar] [CrossRef]
- Venuti, F.; Trunfio, M.; Martson, A.G.; Lipani, F.; Audagnotto, S.; Di Perri, G.; Calcagno, A. Extended and Continuous Infusion of Novel Protected β-Lactam Antibiotics: A Narrative Review. Drugs 2023, 83, 967–983. [Google Scholar] [CrossRef]
- Allwood, M.C.; Stonkute, D.; Wallace, A.; Wilkinson, A.S.; Hills, T.; Jamieson, C. Assessment of the stability of citrate-buffered flucloxacillin for injection when stored in two commercially available ambulatory elastomeric devices: INfusor LV (Baxter) and Accufuser (Woo Young Medical): A study compliant with the NHS Yellow Cover Document (YCD) requirements. Eur. J. Hosp. Pharm. 2020, 27, 90–94. [Google Scholar] [CrossRef]
- Voumard, R.; Van Neyghem, N.; Cochet, C.; Gardiol, C.; Decosterd, L.; Buclin, T.; de Valliere, S. Antibiotic stability related to temperature variations in elastomeric pumps used for outpatient parenteral antimicrobial therapy (OPAT). J. Antimicrob. Chemother. 2017, 72, 1462–1465. [Google Scholar] [CrossRef]
- Manning, L.; Wright, C.; Ingram, P.R.; Whitmore, T.J.; Heath, C.H.; Manson, I.; Page-Sharp, M.; Salman, S.; Dyer, J.; Davis, T.M. Continuous infusions of meropenem in ambulatory care: Clinical efficacy, safety and stability. PLoS ONE 2014, 9, e102023. [Google Scholar] [CrossRef]
- Terracciano, J.; Rhee, E.G.; Walsh, J. Chemical Stability of Ceftolozane/Tazobactam in Polyvinylchloride Bags and Elastomeric Pumps. Curr. Ther. Res. Clin. Exp. 2017, 84, 22–25. [Google Scholar] [CrossRef]
- Jones, B.M.; Huelfer, K.; Bland, C.M. Clinical and Safety Evaluation of Continuously Infused Ceftolozane/Tazobactam in the Outpatient Setting. Open Forum Infect. Dis. 2020, 7, ofaa014. [Google Scholar] [CrossRef]
- Sheffield, M.; Nelson, D.; O’Neal, M.; Gould, A.P.; Bouchard, J.; Nicolau, D.; Justo, J.A.; Hucks, J.; Bookstaver, P.B. Use of continuous-infusion ceftolozane/tazobactam for resistant Gram-negative bacterial infections: A retrospective analysis and brief review of the literature. Int. J. Antimicrob. Agents. 2020, 56, 106158. [Google Scholar] [CrossRef]
- Van Anglen, L.J.; Schroeder, C.P.; Couch, K.A. A Real-world Multicenter Outpatient Experience of Ceftolozane/Tazobactam. Open Forum Infect. Dis. 2023, 10, ofad173. [Google Scholar] [CrossRef]
- Hill, A.T.; Sullivan, A.L.; Chalmers, J.D.; De Soyza, A.; Elborn, J.S.; Floto, R.A.; Grillo, L.; Gruffydd-Jones, K.; Harvey, A.; Haworth, C.S.; et al. British Thoracic Society Guideline for bronchiectasis in adults. Thorax 2019, 74 (Suppl. 1), 1–69. [Google Scholar] [CrossRef]
- Lee, R.A.; Centor, R.M.; Humphrey, L.L.; Jokela, J.A.; Andrews, R.; Qaseem, A.; Scientific Medical Policy Committee of the American College of Physicians. Appropriate Use of Short-Course Antibiotics in Common Infections: Best Practice Advice from the American College of Physicians. Ann. Intern. Med. 2021, 174, 822–827. [Google Scholar] [CrossRef]
- Kalaria, S.N.; Gopalakrishnan, M.; Heil, E.L. A Population Pharmacokinetics and Pharmacodynamic Approach To Optimize Tazobactam Activity in Critically Ill Patients. Antimicrob. Agents Chemother. 2020, 64, e02093-19. [Google Scholar] [CrossRef]
- Zhanel, G.G.; Chung, P.; Adam, H.; Zelenitsky, S.; Denisuik, A.; Schweizer, F.; Lagacé-Wiens, P.R.; Rubinstein, E.; Gin, A.S.; Walkty, A.; et al. Ceftolozane/tazobactam: A novel cephalosporin/β-lactamase inhibitor combination with activity against multidrug-resistant gram-negative bacilli. Drugs 2014, 74, 31–51. [Google Scholar] [CrossRef]
- Gilchrist, M.; Barr, D.; Drummond, F.; Muir, A.; Williams, J.; Scriven, J.; Snape, S.; Hemsley, C.; Durojaiye, C.O.; Patel, S.; et al. Outpatient parenteral antimicrobial therapy (OPAT) in the UK: Findings from the BSAC National Outcomes Registry (2015–19). J. Antimicrob. Chemother. 2022, 77, 1481–1490. [Google Scholar] [CrossRef]
- Psaltikidis, E.M.; Silva, E.N.D.; Bustorff-Silva, J.M.; Moretti, M.L.; Resende, M.R. Economic evaluation of outpatient parenteral antimicrobial therapy: A systematic review. Expert. Rev. Pharmacoecon. Outcomes Res. 2017, 17, 355–375. [Google Scholar] [CrossRef]
- Heintz, B.H.; Halilovic, J.; Christensen, C.L. Impact of a multidisciplinary team review of potential outpatient parenteral antimicrobial therapy prior to discharge from an academic medical center. Ann. Pharmacother. 2011, 45, 1329–1337. [Google Scholar] [CrossRef]
- Madaline, T.; Nori, P.; Mowrey, W.; Zukowski, E.; Gohil, S.; Sarwar, U.; Weston, G.; Urrely, R.; Palombelli, M.; Pierino, V.F.; et al. Bundle in the Bronx: Impact of a Transition-of-Care Outpatient Parenteral Antibiotic Therapy Bundle on All-Cause 30-Day Hospital Readmissions. Open Forum Infect. Dis. 2017, 4, ofx097. [Google Scholar] [CrossRef]

| Patient ID | Age (Years) | P. aeruginosa MIC (mg/L) | Other Pathogens | Duration (Days) | Concomitant Antibiotics | Clinical Outcome | Microbiological Outcome (EOT) | AEs |
|---|---|---|---|---|---|---|---|---|
| Pt 1 | 75 | 1 | MSSA | 14 | Clindamycin 600 mg q8h | Symptom resolution | Clearance | None |
| 1 | MSSA | 7 | Aerosol amikacin 500 mg q12h | Symptom resolution | Clearance | Headache after inhaled amikacin | ||
| Pt 2 | 65 | 0.125 | MAC | 11 | NA | Clinical failure | NA | Catheter thrombosis |
| Pt 3 | 69 | 0.5 | Not present | 14 | NA | Symptom resolution | Clearance | Catheter thrombosis |
| PK/PD Parameters for Ceftolozane | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pt ID | Cmax (mg/L) | Cmin (mg/L) | Conc. Variability (CV%) | AUC24h (mg/L·h) | AUC8h (mg/L·h) | CLss (L/h) | MIC (mg/L) | Breakpoint MIC (mg/L) | ƒCmin (mg/L) | ƒCmin/MIC (Observed) | ƒCmin/4 × MIC (Observed) | ƒCmin/4 × MIC (Breakpoint) |
| Pt 1 | 89.8 | 84.2 | 5.8% | 2120.1 | 706.7 | 2.9 | 1 | 4 | 66.5 | 66.5 | 16.6 | 4.2 |
| Pt 1 (2° treatment) | 76.2 | 55.1 | 7.6% | 1375.5 | 458.5 | 3.9 | 1 | 4 | 43.5 | 43.5 | 10.9 | 2.7 |
| Pt 2 | 68.9 | 53.2 | 7.3% | 1461.1 | 487.0 | 4.4 | 0.125 | 4 | 42.0 | 336.0 | 84.0 | 2.6 |
| Pt 3 | 55.3 | 53.6 | 11.5% | 1348.1 | 449.4 | 4.6 | 0.5 | 4 | 42.3 | 84.7 | 21.2 | 2.6 |
| PK/PD Parameters for Tazobactam | ||||||||||||
| Pt ID | Cmax (mg/L) | Cmin (mg/L) | Conc. Variability (CV%) | AUC24h (mg/L·h) | AUC8h (mg/L·h) | CLss (L/h) | - | Target conc. (mg/L) | ƒCmin (mg/L) | - | ƒCmin/Ctarget | - |
| Pt 1 | 14.1 | 12.7 | 9.8% | 293.4 | 97.8 | 9.6 | - | 0.5 | 8.89 | - | 17.8 | - |
| Pt 1 (2° treatment) | 9.2 | 7.2 | 9.7% | 174.1 | 58.0 | 15.9 | - | 0.5 | 5.04 | - | 10.1 | - |
| Pt 2 | 8.6 | 6.3 | 13.5% | 174.8 | 58.3 | 17.2 | - | 0.5 | 4.41 | - | 8.8 | - |
| Pt 3 | 9.4 | 8.5 | 11.2% | 207.3 | 69.1 | 10.8 | - | 0.5 | 5.95 | - | 11.9 | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Venuti, F.; Gaviraghi, A.; De Nicolò, A.; Stroffolini, G.; Longo, B.M.; Di Vincenzo, A.; Ranzani, F.A.; Quaranta, M.; Romano, F.; Catellani, E.; et al. Real-Life Experience of Continuously Infused Ceftolozane/Tazobactam in Patients with Bronchiectasis and Multidrug-Resistant Pseudomonas aeruginosa Infection in the Outpatient Setting. Antibiotics 2023, 12, 1214. https://doi.org/10.3390/antibiotics12071214
Venuti F, Gaviraghi A, De Nicolò A, Stroffolini G, Longo BM, Di Vincenzo A, Ranzani FA, Quaranta M, Romano F, Catellani E, et al. Real-Life Experience of Continuously Infused Ceftolozane/Tazobactam in Patients with Bronchiectasis and Multidrug-Resistant Pseudomonas aeruginosa Infection in the Outpatient Setting. Antibiotics. 2023; 12(7):1214. https://doi.org/10.3390/antibiotics12071214
Chicago/Turabian StyleVenuti, Francesco, Alberto Gaviraghi, Amedeo De Nicolò, Giacomo Stroffolini, Bianca Maria Longo, Alessia Di Vincenzo, Fabio Antonino Ranzani, Matilde Quaranta, Francesca Romano, Eleonora Catellani, and et al. 2023. "Real-Life Experience of Continuously Infused Ceftolozane/Tazobactam in Patients with Bronchiectasis and Multidrug-Resistant Pseudomonas aeruginosa Infection in the Outpatient Setting" Antibiotics 12, no. 7: 1214. https://doi.org/10.3390/antibiotics12071214
APA StyleVenuti, F., Gaviraghi, A., De Nicolò, A., Stroffolini, G., Longo, B. M., Di Vincenzo, A., Ranzani, F. A., Quaranta, M., Romano, F., Catellani, E., Marchiaro, C., Cinnirella, G., D’Avolio, A., Bonora, S., & Calcagno, A. (2023). Real-Life Experience of Continuously Infused Ceftolozane/Tazobactam in Patients with Bronchiectasis and Multidrug-Resistant Pseudomonas aeruginosa Infection in the Outpatient Setting. Antibiotics, 12(7), 1214. https://doi.org/10.3390/antibiotics12071214









