Comparison of Disk Diffusion, E-Test, and Broth Microdilution Methods for Testing In Vitro Activity of Cefiderocol in Acinetobacter baumannii
Abstract
1. Introduction
2. Materials and Methods
2.1. Strain Collection
2.2. MALDI-TOF MS Identification
2.3. Β. Eta-Lactam AST
2.4. BMD for Cefiderocol AST
2.5. DD for Cefiderocol AST
2.6. MIC Test Strip for Cefiderocol AST
2.7. Data Analysis
2.8. Whole Genome Sequencing and Typing
2.9. Detection of β-Lactam Resistance Genes
3. Results
3.1. Antimicrobial Susceptibility and Genomic Analysis
3.2. Performances of Disk Diffusion to Assess Cefiderocol Susceptibility
3.3. Performances of E-Test to Assess Cefiderocol Susceptibility
3.4. Performances of ComASP to Assess Cefiderocol Susceptibility
3.5. Performances of UMIC to Assess Cefiderocol Susceptibility
3.6. Overall Performances of the Various Methods to Assess Cefiderocol Susceptibility
3.7. Synergy between Cefiderocol and Avibactam
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Giacobbe, D.R.; Ciacco, E.; Girmenia, C.; Pea, F.; Rossolini, G.M.; Sotgiu, G.; Tascini, C.; Tumbarello, M.; Viale, P.; Bassetti, M.; et al. Evaluating Cefiderocol in the Treatment of Multidrug-Resistant Gram-Negative Bacilli: A Review of the Emerging Data. Infect. Drug Resist. 2020, 13, 4697–4711. [Google Scholar] [CrossRef]
- Ayobami, O.; Willrich, N.; Harder, T.; Okeke, I.N.; Eckmanns, T.; Markwart, R. The incidence and prevalence of hospital-acquired (carbapenem-resistant) Acinetobacter baumannii in Europe, Eastern Mediterranean and Africa: A systematic review and meta-analysis. Emerg. Microbes Infect. 2019, 8, 1747–1759. [Google Scholar] [CrossRef]
- EUCAST (European Committee on Antimicrobial Susceptibility Testing). Breakpoint Tables for Interpretation of MICs and Zone Diameters Version 120. 2022. Available online: https://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/Breakpoint_tables/v_11.0_Breakpoint_Tables.pdf (accessed on 14 March 2023).
- CLSI (Clinical and Laboratory Standards Institute). Performance Standards for Antimicrobial Susceptibility Testing, 32nd ed.; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2022. [Google Scholar]
- FDA. Cefiderocol Injection; US Food and Drug Administration: Silver Spring, MD, USA, 2023. Available online: https://www.fda.gov/drugs/development-resources/cefiderocol-injection (accessed on 14 March 2023).
- Albano, M.; Karau, M.J.; Schuetz, A.N.; Patel, R. Comparison of Agar Dilution to Broth Microdilution for Testing In Vitro Activity of Cefiderocol against Gram-Negative Bacilli. J. Clin. Microbiol. 2020, 59, e00966-2. [Google Scholar] [CrossRef] [PubMed]
- Morris, C.P.; Bergman, Y.; Tekle, T.; Fissel, J.A.; Tamma, P.D.; Simner, P.J. Cefiderocol Antimicrobial Susceptibility Testing against Multidrug-Resistant Gram-Negative Bacilli: A Comparison of Disk Diffusion to Broth Microdilution. J. Clin. Microbiol. 2020, 59, 10–1128. [Google Scholar] [CrossRef]
- Kohira, N.; Hackel, M.A.; Ishioka, Y.; Kuroiwa, M.; Sahm, D.F.; Sato, T.; Maki, H.; Yamano, Y. Reduced susceptibility mechanism to cefiderocol, a siderophore cephalosporin, among clinical isolates from a global surveillance programme (SIDERO-WT-2014). J. Glob. Antimicrob. Resist. 2020, 22, 738–741. [Google Scholar] [CrossRef] [PubMed]
- Poirel, L.; Sadek, M.; Nordmann, P. Contribution of PER-Type and NDM-Type beta-Lactamases to Cefiderocol Resistance in Acinetobacter baumannii. Antimicrob. Agents Chemother. 2021, 65, e0087721. [Google Scholar] [CrossRef]
- Malik, S.; Kaminski, M.; Landman, D.; Quale, J. Cefiderocol Resistance in Acinetobacter baumannii: Roles of Beta-Lactamases, Siderophore Receptors, and Penicillin Binding Protein 3. Antimicrob. Agents Chemother. 2020, 64, e01221-2. [Google Scholar] [CrossRef]
- Nordmann, P.; Shields, R.K.; Doi, Y.; Takemura, M.; Echols, R.; Matsunaga, Y.; Yamano, Y. Mechanisms of Reduced Susceptibility to Cefiderocol Among Isolates from the CREDIBLE-CR and APEKS-NP Clinical Trials. Microb. Drug Resist. 2022, 28, 398–407. [Google Scholar] [CrossRef]
- Karakonstantis, S.; Rousaki, M.; Kritsotakis, E.I. Cefiderocol: Systematic Review of Mechanisms of Resistance, Heteroresistance and In Vivo Emergence of Resistance. Antibiotics 2022, 11, 723. [Google Scholar] [CrossRef]
- Choby, J.E.; Ozturk, T.; Satola, S.W.; Jacob, J.T.; Weiss, D.S. Does cefiderocol heteroresistance explain the discrepancy between the APEKS-NP and CREDIBLE-CR clinical trial results? Lancet Microbe 2021, 2, e648–e649. [Google Scholar] [CrossRef] [PubMed]
- Stracquadanio, S.; Bonomo, C.; Marino, A.; Bongiorno, D.; Privitera, G.F.; Bivona, D.A.; Mirabile, A.; Bonacci, P.G.; Stefani, S. Acinetobacter baumannii and Cefiderocol, between Cidality and Adaptability. Microbiol. Spectr. 2022, 10, e0234722. [Google Scholar] [CrossRef] [PubMed]
- Choby, J.E.; Ozturk, T.; Satola, S.W.; Jacob, J.T.; Weiss, D.S. Widespread cefiderocol heteroresistance in carbapenem-resistant Gram-negative pathogens. Lancet Infect. Dis. 2021, 21, 597–598. [Google Scholar] [CrossRef] [PubMed]
- Balleste-Delpierre, C.; Ramirez, A.; Munoz, L.; Longshaw, C.; Roca, I.; Vila, J. Assessment of In Vitro Cefiderocol Susceptibility and Comparators against an Epidemiologically Diverse Collection of Acinetobacter baumannii Clinical Isolates. Antibiotics 2022, 11, 187. [Google Scholar] [CrossRef] [PubMed]
- Abdul-Mutakabbir, J.C.; Nguyen, L.; Maassen, P.T.; Stamper, K.C.; Kebriaei, R.; Kaye, K.S.; Castanheira, M.; Rybak, M.J. In Vitro Antibacterial Activity of Cefiderocol against Multidrug-Resistant Acinetobacter baumannii. Antimicrob. Agents Chemother. 2021, 65, e0264620. [Google Scholar] [CrossRef]
- Mezcord, V.; Wong, O.; Pasteran, F.; Corso, A.; Tolmasky, M.E.; Bonomo, R.A.; Ramirez, M.S. Role of beta-lactamase inhibitors on cefiderocol activity against carbapenem-resistant Acinetobacter species. Int. J. Antimicrob. Agents 2022, 61, 106700. [Google Scholar] [CrossRef] [PubMed]
- Schulthess, B.; Brodner, K.; Bloemberg, G.V.; Zbinden, R.; Bottger, E.C.; Hombach, M. Identification of Gram-positive cocci by use of matrix-assisted laser desorption ionization-time of flight mass spectrometry: Comparison of different preparation methods and implementation of a practical algorithm for routine diagnostics. J. Clin. Microbiol. 2013, 51, 1834–1840. [Google Scholar] [CrossRef] [PubMed]
- Hombach, M.; Zbinden, R.; Bottger, E.C. Standardisation of disk diffusion results for antibiotic susceptibility testing using the sirscan automated zone reader. BMC Microbiol. 2013, 13, 225. [Google Scholar] [CrossRef] [PubMed]
- EUCAST (European Committee on Antimicrobial Susceptibility Testing). Guidance Document on Broth Microdilution Testing of Cefiderocol. 2020. Available online: https://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/Guidance_documents/Cefiderocol_MIC_testing_EUCAST_guidance_document_201217.pdf (accessed on 14 March 2023).
- CLSI (Clinical and Laboratory Standards Institute). Performance Standards for Antimicrobial Susceptibility Testing—Thirty-First Informational Supplement: M100; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2021. [Google Scholar]
- EUCAST (European Committee on Antimicrobial Susceptibility Testing). EUCAST Disk Diffusion Method for Antimicrobial Susceptibility Testing, Version 80; EUCAST: Växjö, Sweden, 2020; Available online: http://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/Disk_test_documents/2020_manuals/Manual_v_8.0_EUCAST_Disk_Test_2020.pdf (accessed on 14 March 2023).
- ISO 20776-2:2007; Clinical Laboratory Testing and In Vitro Diagnostic Test Systems—Susceptibility Testing of Infectious Agents and Evaluation of Performance of Antimicrobial Susceptibility Test Devices—Part 2: Evaluation of Performance of Antimicrobial Susceptibility Test Devices. International Organization for Standardization: Geneva, Switzerland, 2007.
- Bolger, A.M.; Lohse, M.; Usadel, B. Trimmomatic: A flexible trimmer for Illumina sequence data. Bioinformatics 2014, 30, 2114–2120. [Google Scholar] [CrossRef]
- Wick, R.R.; Judd, L.M.; Gorrie, C.L.; Holt, K.E. Unicycler: Resolving bacterial genome assemblies from short and long sequencing reads. PLoS Comput. Biol. 2017, 13, e1005595. [Google Scholar] [CrossRef]
- Higgins, P.G.; Prior, K.; Harmsen, D.; Seifert, H. Development and evaluation of a core genome multilocus typing scheme for whole-genome sequence-based typing of Acinetobacter baumannii. PLoS ONE 2017, 12, e0179228. [Google Scholar] [CrossRef]
- Feldgarden, M.; Brover, V.; Haft, D.H.; Prasad, A.B.; Slotta, D.J.; Tolstoy, I.; Tyson, G.H.; Zhao, S.; Hsu, C.H.; McDermott, P.F.; et al. Validating the AMRFinder Tool and Resistance Gene Database by Using Antimicrobial Resistance Genotype-Phenotype Correlations in a Collection of Isolates. Antimicrob. Agents Chemother. 2019, 63, e00483-19. [Google Scholar] [CrossRef] [PubMed]
- Bortolaia, V.; Kaas, R.S.; Ruppe, E.; Roberts, M.C.; Schwarz, S.; Cattoir, V.; Philippon, A.; Allesoe, R.L.; Rebelo, A.R.; Florensa, A.F.; et al. ResFinder 4.0 for predictions of phenotypes from genotypes. J. Antimicrob. Chemother. 2020, 75, 3491–3500. [Google Scholar] [CrossRef] [PubMed]
- Wallace, L.; Daugherty, S.C.; Nagaraj, S.; Johnson, J.K.; Harris, A.D.; Rasko, D.A. Use of Comparative Genomics To Characterize the Diversity of Acinetobacter baumannii Surveillance Isolates in a Health Care Institution. Antimicrob. Agents Chemother. 2016, 60, 5933–5941. [Google Scholar] [CrossRef] [PubMed]

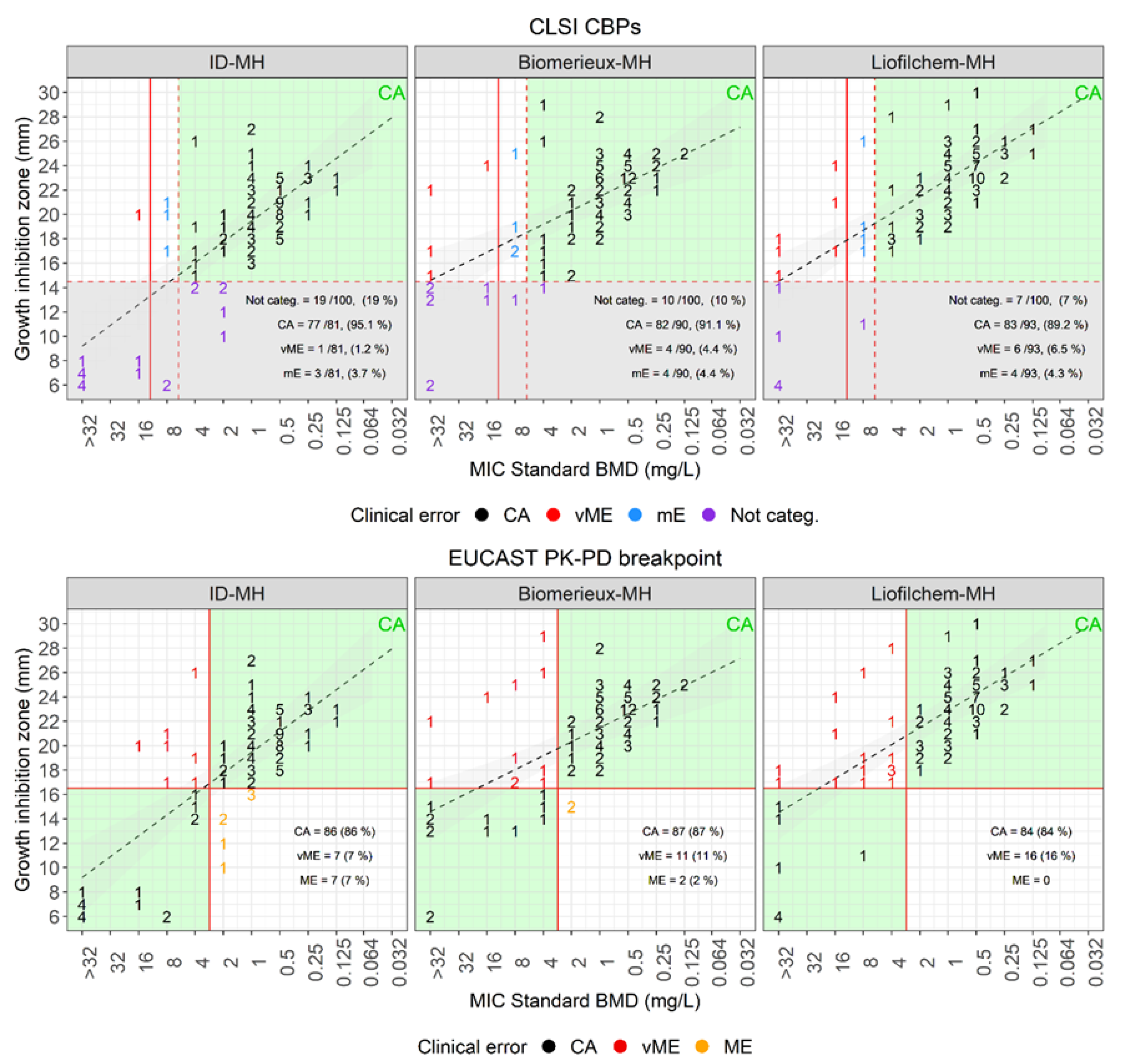

| Disk Diffusion Versus Standard BMD | |||||||||
| Plate | Breakpoint (mm) | Categorized, ≥ 15 mm (%) | Not Categorized (%) | CA (%) | mE (%) | vME (%) | |||
| S≥ | R< | Source | |||||||
| MH-BioMérieux | 15 | CLSI | 90 (90) | 10 (10) | 82/90 (91.1) | 4 (4.4) | 4 (4.4) | ||
| MH-Liofilchem | 93 (93) | 7 (67) | 83/93 (89.2) | 4 (4.3) | 6 (6.5) | ||||
| ID-MH-homemade | 81 (81) | 19 (19) | 77/81 (95.1) | 3 (3.7) | 1 (1.2) | ||||
| Plate | Breakpoint (mm) | CA (%) | ME (%) | vME (%) | |||||
| S≥ | R< | Source | |||||||
| MH-BioMérieux | 17 | 17 | EUCAST PK-PD | 87 (87) | 2 (2) | 11 (11) | |||
| MH-Liofilchem | 84 (84) | 0 (0) | 16 (16) | ||||||
| ID-MH-homemade | 86 (86) | 7 (7) | 7 (7) | ||||||
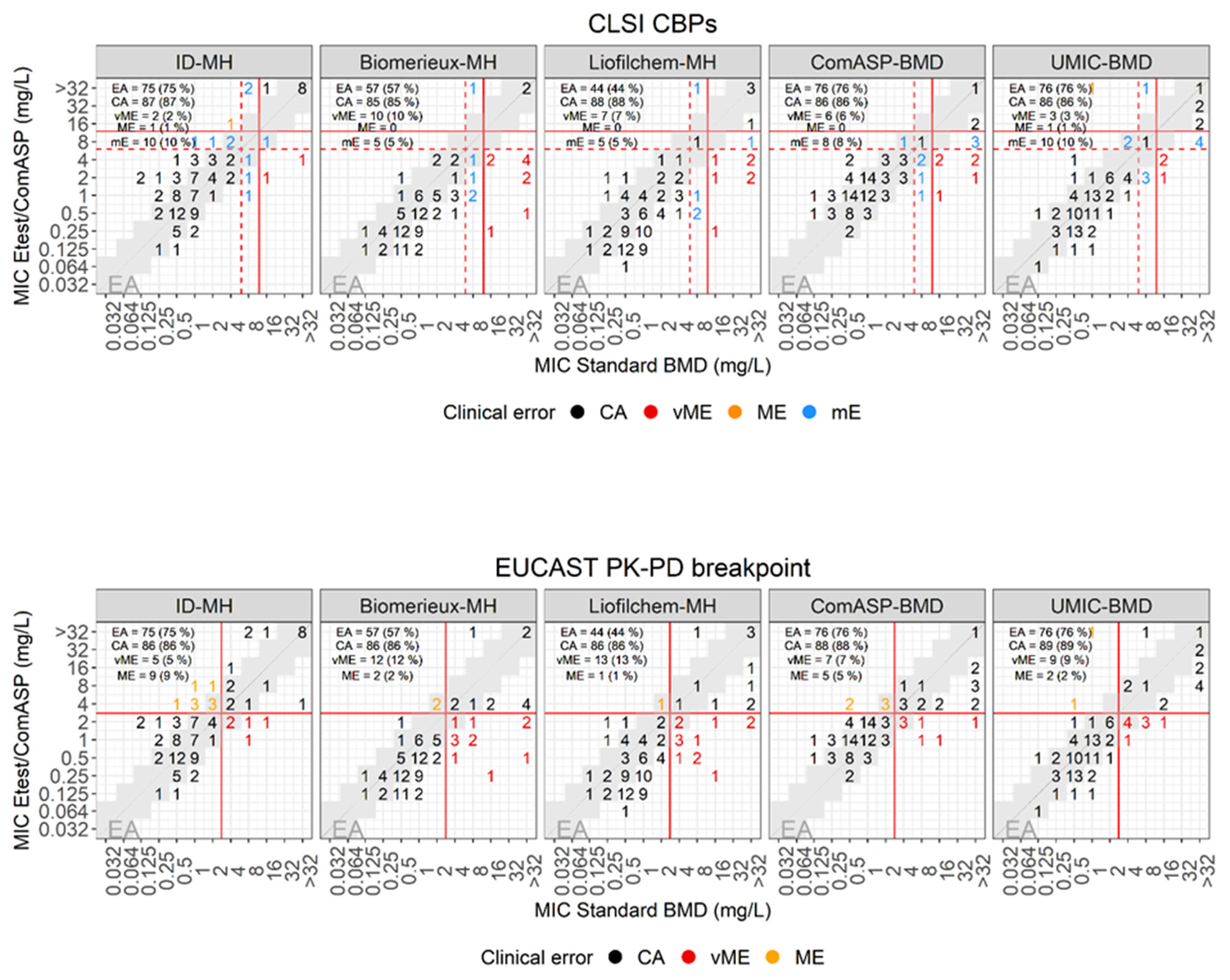
| E-test versus standard BMD | |||||||||
| Plate | Breakpoint (mm) | EA (%) | CA (%) | mE (%) | ME (%) | vME (%) | |||
| S≤ | I = | R> | Source | ||||||
| MH-BioMérieux | 4 | 8 | 8 | CLSI | 57 (57) | 85 (85) | 5 (5) | 10 (10) | |
| MH-Liofilchem | 44 (44) | 88 (88) | 5 (5) | 7 (7) | |||||
| ID-MH-homemade | 75 (75) | 87 (87) | 10 (10) | 1 (1) | 2 (2) | ||||
| ComASP | 76 (76) | 86 (86) | 8 (8) | 6 (6) | |||||
| UMIC | 76 (76) | 86 (86) | 10 (10) | 1 (1) | 3 (3) | ||||
| Plate | Breakpoint (mm) | EA (%) | CA (%) | mE (%) | ME (%) | vME (%) | |||
| S≤ | I = | R> | Source | ||||||
| MH-BioMérieux | 2 | 2 | EUCAST PK-PD | 57 (57) | 86 (86) | 2 (2) | 12 (12) | ||
| MH-Liofilchem | 44 (44) | 86 (86) | 1 (1) | 13 (13) | |||||
| ID-MH-homemade | 75 (75) | 86 (86) | 9 (9) | 5 (5) | |||||
| ComASP | 76 (76) | 88 (88) | 5 (5) | 7 (7) | |||||
| UMIC | 76 (76) | 89 (89) | 2 (2) | 9 (9) | |||||
| Method | Standard BMD, MIC (μg/mL) | E-Test on ID-MH-Agar, MIC (μg/mL) | Double Disk Diffusion on ID-MH-Agar, Inhibition Zone (mm) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Isolate n. | Major Plasmidic β-Lactamase(s) | CFD | CFD + AVI 1 | Fold Difference | CZA | CFD | CFD + CZA | Fold Difference | CFD | CZA14 | CZA50 |
| 9 | OXA-72 | >32 | 2 | >4 | 32 | >256 | 0.5 | >9 | 6 | 6 | 14 |
| 22 | OXA-23/PER-1 | 8 | 0.5 | 4 | 32 | 1 | 0.125 | 3 | 19 | 10 | 16 |
| 25 | OXA-23/PER-1 | >32 | 0.5 | >6 | 16 | >256 | 0.125 | >11 | 12 | 12 | 17 |
| 30 | OXA-58 | 8 | 4 | 1 | >256 | 6 | 2 | 1.5 | 14 | 6 | 11 |
| 45 | OXA-72/PER-1 | >32 | 1 | >5 | 32 | >256 | 1 | >8 | 6 | 11 | 19 |
| 56 | OXA-23/PER-7 | >32 | 1 | >5 | 16 | >256 | 0.19 | >10 | 6 | 10 | 15 |
| 57 | OXA-23/PER-7 | >32 | 1 | >5 | 96 | >256 | 2 | >7 | 6 | 8 | 14 |
| 69 | OXA-23/PER-7 | >32 | 1 | >5 | 16 | 12 | 1 | 3.5 | 10 | 13 | 18 |
| 73 | OXA-23 | 16 | 2 | 3 | 48 | 0.75 | 0.38 | 1 | 23 | 8 | 15 |
| 78 | OXA-23/PER-7 | >32 | 1 | >5 | 24 | 16 | 0.125 | 7 | 8 | 12 | 17 |
| 85 | OXA-23 | 8 | 1 | 3 | >256 | 3 | 0.38 | 3 | 18 | 6 | 14 |
| 90 | OXA-23 | 8 | 0.0625 | 7 | 192 | >256 | 1.5 | >7 | 6 | 6 | 12 |
| 92 | OXA-23/OXA-72 | ≥32 | ≥32 | 0 | >256 | >256 | 32 | >3 | 6 | 6 | 8 |
| 95 | OXA-23 | 8 | 0.125 | 6 | 64 | 3 | 0.5 | 3 | 18 | 8 | 14 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kolesnik-Goldmann, N.; Seth-Smith, H.M.B.; Haldimann, K.; Imkamp, F.; Roloff, T.; Zbinden, R.; Hobbie, S.N.; Egli, A.; Mancini, S. Comparison of Disk Diffusion, E-Test, and Broth Microdilution Methods for Testing In Vitro Activity of Cefiderocol in Acinetobacter baumannii. Antibiotics 2023, 12, 1212. https://doi.org/10.3390/antibiotics12071212
Kolesnik-Goldmann N, Seth-Smith HMB, Haldimann K, Imkamp F, Roloff T, Zbinden R, Hobbie SN, Egli A, Mancini S. Comparison of Disk Diffusion, E-Test, and Broth Microdilution Methods for Testing In Vitro Activity of Cefiderocol in Acinetobacter baumannii. Antibiotics. 2023; 12(7):1212. https://doi.org/10.3390/antibiotics12071212
Chicago/Turabian StyleKolesnik-Goldmann, Natalia, Helena M. B. Seth-Smith, Klara Haldimann, Frank Imkamp, Tim Roloff, Reinhard Zbinden, Sven N. Hobbie, Adrian Egli, and Stefano Mancini. 2023. "Comparison of Disk Diffusion, E-Test, and Broth Microdilution Methods for Testing In Vitro Activity of Cefiderocol in Acinetobacter baumannii" Antibiotics 12, no. 7: 1212. https://doi.org/10.3390/antibiotics12071212
APA StyleKolesnik-Goldmann, N., Seth-Smith, H. M. B., Haldimann, K., Imkamp, F., Roloff, T., Zbinden, R., Hobbie, S. N., Egli, A., & Mancini, S. (2023). Comparison of Disk Diffusion, E-Test, and Broth Microdilution Methods for Testing In Vitro Activity of Cefiderocol in Acinetobacter baumannii. Antibiotics, 12(7), 1212. https://doi.org/10.3390/antibiotics12071212






