Assessment of Antimicrobial Consumption in Multi-Field Hospitals with Pediatric Inpatients: Conventional vs. Novel Pediatric-Adjusted Methodologies
Abstract
1. Introduction
2. Results
2.1. Validation of the Proposed Pediatric-Adjusted Methodology
2.2. Comparative Evaluation of the Proposed Methodology and the Conventional One vs. the Objective Levels of AMC in Children 12 Years Old and Younger
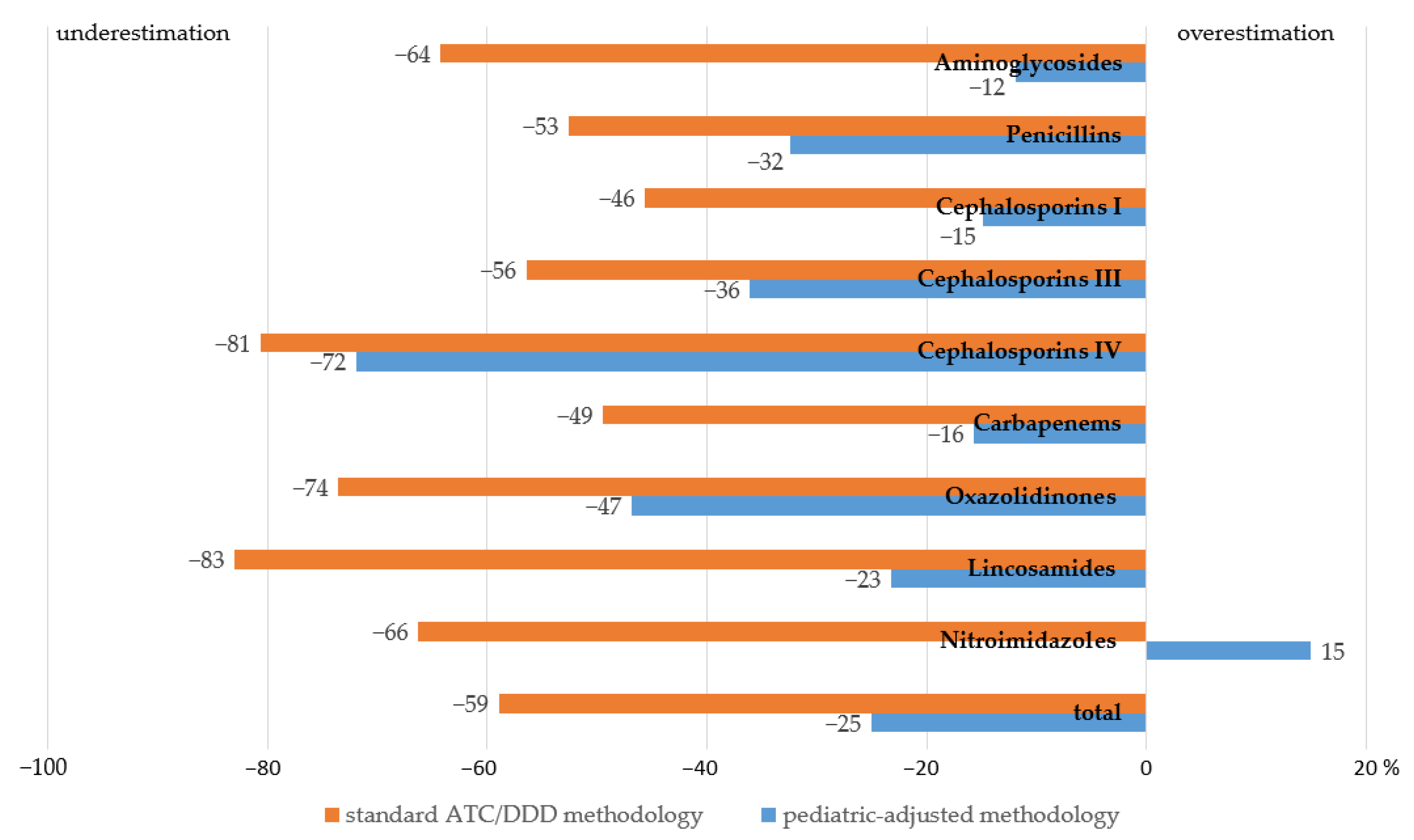
2.3. Assessment of Variances in AMC in Multi-Field Hospitals with Pediatric Inpatients Calculated by Means of the Proposed Methodology vs. the Conventional One
3. Discussion
4. Materials and Methods
4.1. Basic Concept and Validation of the Proposed Pediatric-Adjusted Methodology
- Based on the child’s body weight (dose per kg),
- Based on the child’s BSA (dose per m2),
- Based on the child’s age.
- Calculate the weighted mean cDDD of an AM for a population of patients 1 month to 12 years old over the given period of time by multiplying the weight of each age subgroup in the population by the cDDD value for this age subgroup and summing the results:where cDDDn is the cDDD value for a given age group, and xn is the share of this age subgroup in the population.
- Divide the total amount of AM used in grams over the given period of time by the weighted mean cDDD for this agent to estimate the number of cDDDs used.
- Calculate the level of AMC using the standard equation:where ncDDD is the number of weighted mean cDDDs used over a given period of time, and nb-d is the number of bed days for a population of patients aged 1 month to 12 years over a given period of time.
4.2. Comparative Evaluation of the Proposed Methodology and the Conventional One vs. the Objective Levels of AMC in Children Aged 12 Years and Younger
4.3. Assessment of Variances in AMC in Multi-Field Hospitals with Pediatric Inpatients Calculated by Means of the Proposed Methodology vs. the Conventional One
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Calbo, E.; Boix-Palop, L.; Garau, J. Clinical and economic impact of bacterial resistance: An approach to infection control and antimicrobial stewardship solutions. Curr. Opin. Infect. Dis. 2020, 33, 458–463. [Google Scholar] [CrossRef] [PubMed]
- Barriere, S.L. Clinical, economic and societal impact of antibiotic resistance. Expert Opin. Pharmacother. 2015, 16, 151–153. [Google Scholar] [CrossRef] [PubMed]
- Barreiro, C.; Barredo, J.L. Worldwide Clinical Demand for Antibiotics: Is It a Real Countdown? Methods Mol. Biol. 2021, 2296, 3–15. [Google Scholar] [CrossRef] [PubMed]
- Bell, B.G.; Schellevis, F.; Stobberingh, E.; Goossens, H.; Pringle, M. A systematic review and meta-analysis of the effects of antibiotic consumption on antibiotic resistance. BMC Infect. Dis. 2014, 14, 13. [Google Scholar] [CrossRef]
- Davey, P.; Marwick, C.A.; Scott, C.L.; Charani, E.; McNeil, K.; Brown, E.; Gould, I.M.; Ramsay, C.R.; Michie, S. Interventions to improve antibiotic prescribing practices for hospital inpatients. Cochrane Database Syst. Rev. 2017, 2, CD003543. [Google Scholar] [CrossRef]
- Chen, J.; Ekaney, I.; Shah, P.J. Comparison of antimicrobial utilization metrics: Food for thought for an antimicrobial stewardship programme. Int. J. Antimicrob. Agents 2022, 60, 106681. [Google Scholar] [CrossRef]
- WHO Collaborating Centre for Drug Statistics Methodology. Guidelines for ATC Classification and DDD Assignment 2023; WHO: Oslo, Norway, 2022. [Google Scholar]
- de With, K.; Bestehorn, H.; Steib-Bauert, M.; Kern, W.V. Comparison of defined versus recommended versus prescribed daily doses for measuring hospital antibiotic consumption. Infection 2009, 37, 349–352. [Google Scholar] [CrossRef]
- Liem, T.B.; Heerdink, E.R.; Egberts, A.C.; Rademaker, C.M. Quantifying antibiotic use in paediatrics: A proposal for neonatal DDDs. Eur. J. Clin. Microbiol. Infect. Dis. 2010, 29, 1301–1303. [Google Scholar] [CrossRef]
- Zhang, Z.C. The analysis of rationality of antibiotics in pediatric department. Anhui Med. Pharm. J. 2005, 9, 934–936. [Google Scholar]
- Han, L.; Zeng, L.N.; Guo, Y.C.; Lan, Y.; Wang, L.; Luo, C.; Zhang, L.L. Utilization analysis of drug efficacy of the 392 cases of Wenchuan earthquake women and children patients. Chin. J. Evid.-Based Med. 2009, 9, 265–272. [Google Scholar]
- Zhang, L.; Li, Y.; Zeng, L.; Wang, Z. Applying “children defined daily dose” to assess appropriate dose in pediatrics. J. Evid. Based Med. 2012, 5, 2–5. [Google Scholar] [CrossRef]
- Porta, A.; Hsia, Y.; Doerholt, K.; Spyridis, N.; Bielicki, J.; Menson, E.; Tsolia, M.; Esposito, S.; Wong, I.C.; Sharland, M. Comparing neonatal and paediatric antibiotic prescribing between hospitals: A new algorithm to help international benchmarking. J. Antimicrob. Chemother. 2012, 67, 1278–1286. [Google Scholar] [CrossRef] [PubMed]
- Raastad, R.; Tvete, I.F.; Abrahamsen, T.G.; Berild, D.; Leegaard, T.M.; Walberg, M.; Müller, F. A worrying trend in weight-adjusted paediatric antibiotic use in a Norwegian tertiary care hospital. Acta Paediatr. 2015, 104, 687–692. [Google Scholar] [CrossRef] [PubMed]
- Monnier, A.A.; Eisenstein, B.I.; Hulscher, M.E.; Gyssens, I.C.; DRIVE-AB WP1 group. Towards a global definition of responsible antibiotic use: Results of an international multidisciplinary consensus procedure. J. Antimicrob. Chemother. 2018, 1, vi3–vi16. [Google Scholar] [CrossRef] [PubMed]
- Dellit, T.H.; Owens, R.C.; McGowan, J.E., Jr.; Gerding, D.N.; Weinstein, R.A.; Burke, J.P.; Huskins, W.C.; Paterson, D.L.; Fishman, N.O.; Carpenter, C.F.; et al. Infectious Diseases Society of America; Society for Healthcare Epidemiology of America. Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America guidelines for developing an institutional program to enhance antimicrobial stewardship. Clin. Infect. Dis. 2007, 44, 159–177. [Google Scholar] [CrossRef] [PubMed]
- Hwang, S.; Kwon, K.T. Core Elements for Successful Implementation of Antimicrobial Stewardship Programs. Infect. Chemother. 2021, 53, 421–435. [Google Scholar] [CrossRef]
- Muller, A.; Monnet, D.L.; Talon, D.; Hénon, T.; Bertrand, X. Discrepancies between prescribed daily doses and WHO defined daily doses of antibacterials at a university hospital. Br. J. Clin. Pharmacol. 2006, 61, 585–591. [Google Scholar] [CrossRef]
- Shetka, M.; Pastor, J.; Phelps, P. Evaluation of the defined daily dose method for estimating anti-infective use in a university hospital. Am. J. Health Syst. Pharm. 2005, 62, 2288–2292. [Google Scholar] [CrossRef]
- Hsien, L.; Breddemann, A.; Frobel, A.K.; Heusch, A.; Schmidt, K.G.; Läer, S. Off-label drug use among hospitalised children: Identifying areas with the highest need for research. Pharm. World Sci. 2008, 30, 497–502. [Google Scholar] [CrossRef]
- Santos, D.B.; Clavenna, A.; Bonati, M.; Coelho, H.L. Off-label and unlicensed drug utilization in hospitalized children in Fortaleza, Brazil. Eur. J. Clin. Pharmacol. 2008, 64, 1111–1118. [Google Scholar] [CrossRef]
- CDC. Core Elements of Hospital Antibiotic Stewardship Programs; US Department of Health and Human Services, CDC: Atlanta, GA, USA, 2019. Available online: https://www.cdc.gov/antibiotic-use/core-elements/hospital.html (accessed on 1 June 2023).
- Gilbert, D.N.; Chambers, H.F.; Saag, M.S.; Pavia, A.; Boucher, H.W. The Sanford Guide to Antimicrobial Therapy 2023, 53rd ed.; Antimicrobial Therapy, Inc.: Sperryville, VA, USA, 2023; 339p. [Google Scholar]
- Cherry, J.; Demmler-Harrison, G.J.; Kaplan, S.L.; Steinbach, W.J.; Hotez, P.J. Feigin and Cherry’s Textbook of Pediatric Infectious Diseases, 8th ed.; Elsevier Health Sciences: Amsterdam, The Netherlands, 2017; 3000p. [Google Scholar]
- Jin, W. Antibiotic drug utilization research of the pediatric outpatients in our hospital. Guangdong Pharm. 2005, l15, 48–50. [Google Scholar]
- Russian State Register of Medicines. Available online: https://grls.rosminzdrav.ru/material/national/medicine-registry (accessed on 1 June 2023).
- Ali, A.; Imran, M.; Sial, S.; Khan, A. Effective antibiotic dosing in the presence of resistant strains. PLoS ONE 2022, 10, e0275762. [Google Scholar] [CrossRef] [PubMed]
- Abdul-Aziz, M.H.; Lipman, J.; Roberts, J.A. Antibiotic dosing for multidrug-resistant pathogen pneumonia. Curr. Opin. Infect. Dis. 2017, 30, 231–239. [Google Scholar] [CrossRef] [PubMed]
- WHO. Child Growth Standards. Weight-for-Age (0–5 Years). Available online: https://www.who.int/tools/child-growth-standards/standards/weight-for-age (accessed on 1 June 2023).
- WHO. Growth Reference Data For 5–19 Years. Weight-for-Age (5–10 Years). Available online: https://www.who.int/tools/growth-reference-data-for-5to19-years/indicators/weight-for-age-5to10-years (accessed on 1 June 2023).
- WHO. Growth Reference Data For 5–19 Years. Height-for-Age (5–19 Years). Available online: https://www.who.int/tools/growth-reference-data-for-5to19-years/indicators/height-for-age (accessed on 1 June 2023).
- WHO. Growth Reference Data For 5–19 Years. BMI-for-Age (5–19 Years). Available online: https://www.who.int/tools/growth-reference-data-for-5to19-years/indicators/bmi-for-age (accessed on 1 June 2023).
- Mosteller, R.D. Simplified calculation of body-surface area. N. Engl. J. Med. 1987, 317, 1098. [Google Scholar] [CrossRef]
- Wan, X.; Wang, W.; Liu, J.; Tong, T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med. Res. Methodol. 2014, 14, 135. [Google Scholar] [CrossRef]
- Drummond, M.; Sculpher, M.J.; Claxton, K.; Stoddart, G.L.; Torrance, G.W. Methods for the Economic Evaluation of Health Care Programmes; Oxford University Press: Oxford, UK, 2002; 379p. [Google Scholar]
- World Health Organisation. Child Growth Standards. Available online: https://www.who.int/tools/child-growth-standards (accessed on 1 June 2023).
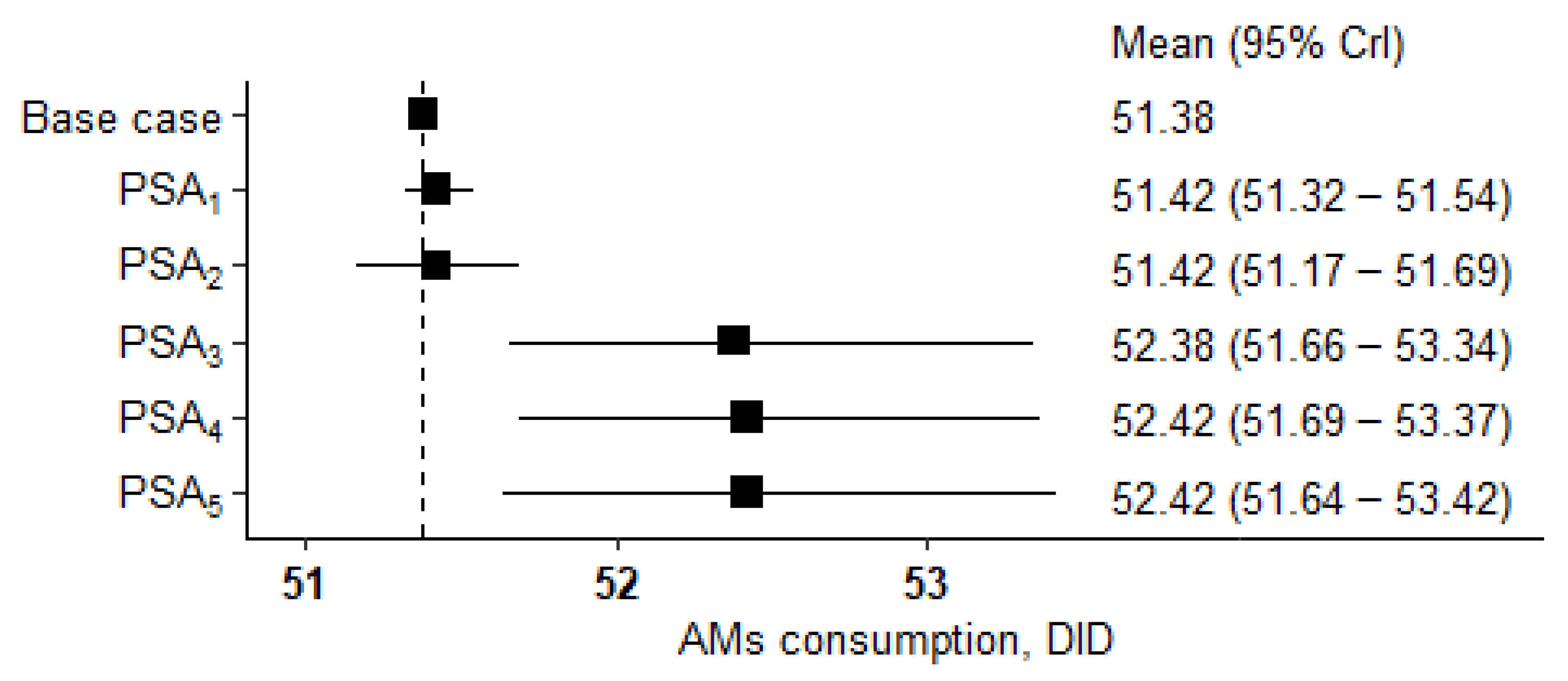

| PSA # | PSA Description | Mean (SD) | 95% CrI * | Difference from the Main Result (51.38 DID), % |
|---|---|---|---|---|
| 1 | Fixed AM doses. Weights and BSA values are randomly assigned for each AM in the structure. | 51.42 (0.05) | 51.32–51.54 | 0.1 |
| 2 | Fixed AM doses. Weights, and BSA values are randomly assigned once to be the same for all AMs in the structure. | 51.42 (0.13) | 51.17–51.69 | 0.1 |
| 3 | Doses are randomly assigned for each AM. Fixed mean weight and BSA values. | 52.38 (0.50) | 51.66–53.34 | 1.9 |
| 4 | Doses are randomly assigned for each AM. Weight and BSA values are randomly assigned for each AM in the structure. | 52.42 (0.49) | 51.69–53.37 | 2 |
| 5 | Doses are randomly assigned for each AM. Weight and BSA values are randomly assigned once to be the same for all AMs in the structure. | 52.42 (0.51) | 51.64–53.42 | 2 |
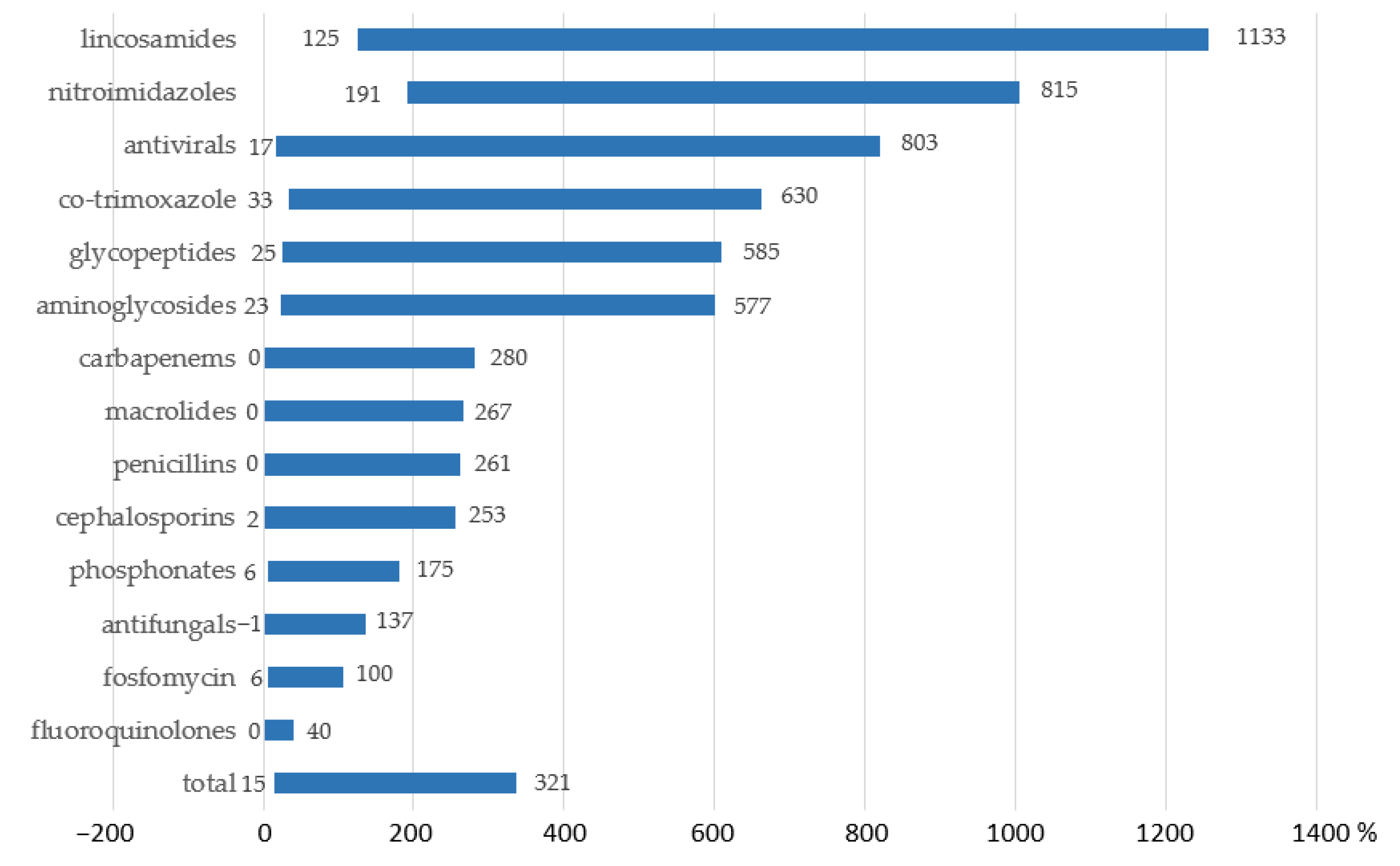
| AM Class | Consumption, g | Consumption, DID | ||
|---|---|---|---|---|
| ATC/DDD Methodology | Proposed Methodology | Based on PDDs | ||
| Aminoglycosides | 73.8 | 2.42 | 5.97 | 6.78 |
| Penicillins | 27.9 | 0.37 | 0.53 | 0.78 |
| Cephalosporins I | 9.6 | 0.08 | 0.12 | 0.14 |
| Cephalosporins III | 1377.7 | 12.95 | 19.01 | 29.74 |
| Cephalosporins IV | 6.9 | 0.04 | 0.06 | 0.21 |
| Carbapenems | 69.2 | 0.78 | 1.31 | 1.55 |
| Oxazolidinones | 5.3 | 0.11 | 0.21 | 0.40 |
| Lincosamides | 2.7 | 0.04 | 0.16 | 0.21 |
| Nitroimidazoles | 135.7 | 2.08 | 7.11 | 6.18 |
| Total | 1708.8 | 18.9 | 34.5 | 46 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rachina, S.; Belkova, Y.; Kozlov, R.; Mladov, V.; Mishchenko, V.; Andreeva, A.; Domanskaya, O.; Portnjagina, U.; Dushina, A.; Zainalabidova, K. Assessment of Antimicrobial Consumption in Multi-Field Hospitals with Pediatric Inpatients: Conventional vs. Novel Pediatric-Adjusted Methodologies. Antibiotics 2023, 12, 1162. https://doi.org/10.3390/antibiotics12071162
Rachina S, Belkova Y, Kozlov R, Mladov V, Mishchenko V, Andreeva A, Domanskaya O, Portnjagina U, Dushina A, Zainalabidova K. Assessment of Antimicrobial Consumption in Multi-Field Hospitals with Pediatric Inpatients: Conventional vs. Novel Pediatric-Adjusted Methodologies. Antibiotics. 2023; 12(7):1162. https://doi.org/10.3390/antibiotics12071162
Chicago/Turabian StyleRachina, Svetlana, Yuliya Belkova, Roman Kozlov, Vladimir Mladov, Vladimir Mishchenko, Alla Andreeva, Olga Domanskaya, Ulyana Portnjagina, Anastasiia Dushina, and Khadizhat Zainalabidova. 2023. "Assessment of Antimicrobial Consumption in Multi-Field Hospitals with Pediatric Inpatients: Conventional vs. Novel Pediatric-Adjusted Methodologies" Antibiotics 12, no. 7: 1162. https://doi.org/10.3390/antibiotics12071162
APA StyleRachina, S., Belkova, Y., Kozlov, R., Mladov, V., Mishchenko, V., Andreeva, A., Domanskaya, O., Portnjagina, U., Dushina, A., & Zainalabidova, K. (2023). Assessment of Antimicrobial Consumption in Multi-Field Hospitals with Pediatric Inpatients: Conventional vs. Novel Pediatric-Adjusted Methodologies. Antibiotics, 12(7), 1162. https://doi.org/10.3390/antibiotics12071162








