Prevalence of Enterococci and Vancomycin Resistance in the Throat of Non-Hospitalized Individuals Randomly Selected in Central Italy
Abstract
1. Introduction
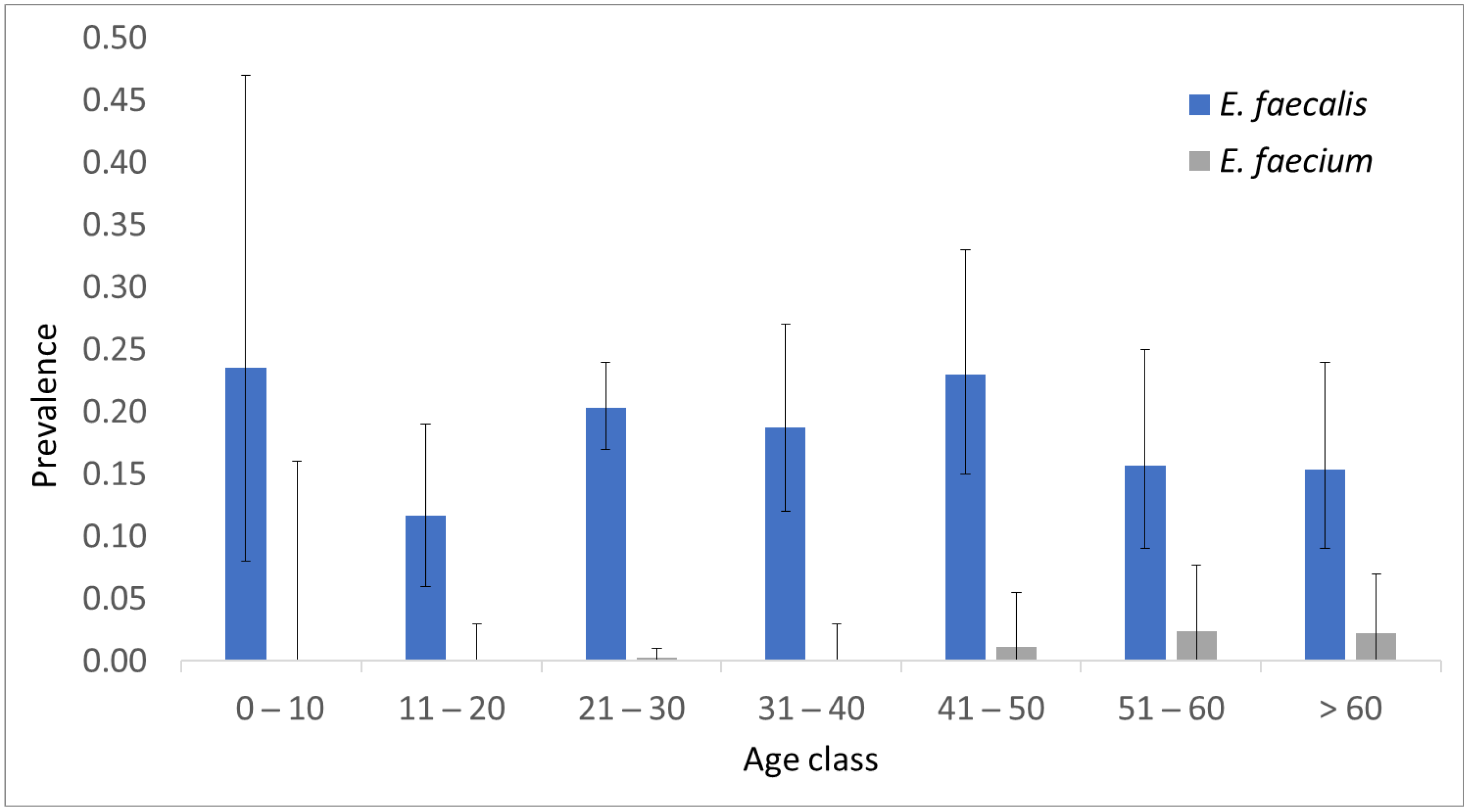
2. Results
3. Discussion
4. Materials and Methods
4.1. Sample Collection
4.2. Molecular Analysis
4.3. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sghir, A.; Gramet, G.; Suau, A.; Rochet, V.; Pochart, P.; Dore, J. Quantification of bacterial groups within human fecal flora by oligonucleotide probe hybridization. Appl. Environ. Microbiol. 2000, 66, 2263–2266. [Google Scholar] [CrossRef] [PubMed]
- Jepsen, K.; Falk, W.; Brune, F.; Cosgarea, R.; Fimmers, R.; Bekeredjian-Ding, I.; Jepsen, S. Prevalence and Antibiotic Susceptibility Trends of Selected Enterobacteriaceae, Enterococci, and Candida albicans in the Subgingival Microbiota of German Periodontitis Patients: A Retrospective Surveillance Study. Antibiotics 2022, 11, 385. [Google Scholar] [CrossRef] [PubMed]
- Kensara, A.; Saito, H.; Mongodin, E.F.; Masri, R. Microbiological profile of peri-implantitis: Analyses of microbiome within dental implants. J. Prosthodont. 2023, in press. [Google Scholar] [CrossRef] [PubMed]
- Zaman, S.B.; Hussain, M.A.; Nye, R.; Mehta, V.; Mamun, K.T.; Hossain, N. A Review on Antibiotic Resistance: Alarm Bells are Ringing. Cureus 2017, 9, e1403. [Google Scholar] [CrossRef] [PubMed]
- Davies, J.; Davies, D. Origins and evolution of antibiotic resistance. Microbiol. Mol. Biol. Rev. 2010, 74, 417–433. [Google Scholar] [CrossRef]
- Antimicrobial Resistance, C. Global burden of bacterial antimicrobial resistance in 2019: A systematic analysis. Lancet 2022, 399, 629–655. [Google Scholar] [CrossRef]
- Cong, Y.; Yang, S.; Rao, X. Vancomycin resistant Staphylococcus aureus infections: A review of case updating and clinical features. J. Adv. Res. 2020, 21, 169–176. [Google Scholar] [CrossRef]
- Cetinkaya, Y.; Falk, P.; Mayhall, C.G. Vancomycin-resistant enterococci. Clin. Microbiol. Rev. 2000, 13, 686–707. [Google Scholar] [CrossRef]
- Arthur, M.; Molinas, C.; Depardieu, F.; Courvalin, P. Characterization of Tn1546, a Tn3-related transposon conferring glycopeptide resistance by synthesis of depsipeptide peptidoglycan precursors in Enterococcus faecium BM4147. J. Bacteriol. 1993, 175, 117–127. [Google Scholar] [CrossRef]
- Quintiliani, R., Jr.; Courvalin, P. Conjugal transfer of the vancomycin resistance determinant vanB between enterococci involves the movement of large genetic elements from chromosome to chromosome. FEMS Microbiol. Lett. 1994, 119, 359–363. [Google Scholar] [CrossRef]
- Lee, T.; Pang, S.; Abraham, S.; Coombs, G.W. Molecular characterization and evolution of the first outbreak of vancomycin-resistant Enterococcus faecium in Western Australia. Int. J. Antimicrob. Agents 2019, 53, 814–819. [Google Scholar] [CrossRef]
- Azzam, A.; Elkafas, H.; Khaled, H.; Ashraf, A.; Yousef, M.; Elkashef, A.A. Prevalence of Vancomycin-resistant enterococci (VRE) in Egypt (2010–2022): A systematic review and meta-analysis. J. Egypt. Public Health Assoc. 2023, 98, 8. [Google Scholar] [CrossRef]
- Werner, G.; Neumann, B.; Weber, R.E.; Kresken, M.; Wendt, C.; Bender, J.K.; The VRE Study Group. Thirty years of VRE in Germany—“Expect the unexpected”: The view from the National Reference Centre for Staphylococci and Enterococci. Drug Resist. Updat. 2020, 53, 100732. [Google Scholar] [CrossRef]
- Ammam, F.; Marvaud, J.C.; Lambert, T. Distribution of the vanG-like gene cluster in Clostridium difficile clinical isolates. Can. J. Microbiol. 2012, 58, 547–551. [Google Scholar] [CrossRef]
- Noble, W.C.; Virani, Z.; Cree, R.G. Co-transfer of vancomycin and other resistance genes from Enterococcus faecalis NCTC 12201 to Staphylococcus aureus. FEMS Microbiol. Lett. 1992, 72, 195–198. [Google Scholar] [CrossRef]
- Klare, I.; Heier, H.; Claus, H.; Bohme, G.; Marin, S.; Seltmann, G.; Hakenbeck, R.; Antanassova, V.; Witte, W. Enterococcus faecium strains with vanA-mediated high-level glycopeptide resistance isolated from animal foodstuffs and fecal samples of humans in the community. Microb. Drug Resist. 1995, 1, 265–272. [Google Scholar] [CrossRef]
- Devriese, L.A.; Ieven, M.; Goossens, H.; Vandamme, P.; Pot, B.; Hommez, J.; Haesebrouck, F. Presence of vancomycin-resistant enterococci in farm and pet animals. Antimicrob. Agents Chemother. 1996, 40, 2285–2287. [Google Scholar] [CrossRef]
- Wegener, H.C.; Aarestrup, F.M.; Jensen, L.B.; Hammerum, A.M.; Bager, F. Use of antimicrobial growth promoters in food animals and Enterococcus faecium resistance to therapeutic antimicrobial drugs in Europe. Emerg. Infect. Dis. 1999, 5, 329–335. [Google Scholar] [CrossRef]
- Kunhikannan, S.; Thomas, C.J.; Franks, A.E.; Mahadevaiah, S.; Kumar, S.; Petrovski, S. Environmental hotspots for antibiotic resistance genes. Microbiologyopen 2021, 10, e1197. [Google Scholar] [CrossRef]
- Nadgir, C.A.; Biswas, D.A. Antibiotic Resistance and Its Impact on Disease Management. Cureus 2023, 15, e38251. [Google Scholar] [CrossRef]
- Sedgley, C.M.; Nagel, A.C.; Shelburne, C.E.; Clewell, D.B.; Appelbe, O.; Molander, A. Quantitative real-time PCR detection of oral Enterococcus faecalis in humans. Arch. Oral Biol. 2005, 50, 575–583. [Google Scholar] [CrossRef] [PubMed]
- Roberts, A.P.; Mullany, P. Oral biofilms: A reservoir of transferable, bacterial, antimicrobial resistance. Expert. Rev. Anti Infect. Ther. 2010, 8, 1441–1450. [Google Scholar] [CrossRef] [PubMed]
- Scapoli, L.; Palmieri, A.; Pellati, A.; Carinci, F.; Lauritano, D.; Arcuri, C.; Baggi, L.; Gatto, R.; Martinelli, M. Prevalence of Staphylococcus aureus and mec-A Cassette in the Throat of Non-Hospitalized Individuals Randomly Selected in Central Italy. Antibiotics 2022, 11, 949. [Google Scholar] [CrossRef] [PubMed]
- Palmer, R.J., Jr. Composition and development of oral bacterial communities. Periodontol. 2000 2014, 64, 20–39. [Google Scholar] [CrossRef]
- Komiyama, E.Y.; Lepesqueur, L.S.; Yassuda, C.G.; Samaranayake, L.P.; Parahitiyawa, N.B.; Balducci, I.; Koga-Ito, C.Y. Enterococcus Species in the Oral Cavity: Prevalence, Virulence Factors and Antimicrobial Susceptibility. PLoS ONE 2016, 11, e0163001. [Google Scholar] [CrossRef]
- Sedgley, C.; Buck, G.; Appelbe, O. Prevalence of Enterococcus faecalis at multiple oral sites in endodontic patients using culture and PCR. J. Endod. 2006, 32, 104–109. [Google Scholar] [CrossRef]
- Chomicz, L.; Szubinska, D.; Piekarczyk, J.; Wojtowicz, A.; Piekarczyk, B.; Starosciak, B.; Fiedor, P. Occurrence of oral subclinical infections in insulin treated diabetics. Wiad. Parazytol. 2004, 50, 177–180. [Google Scholar]
- Bhardwaj, S.B.; Mehta, M.; Sood, S. Enterococci in the oral cavity of periodontitis patients from different urban socioeconomic groups. Dent. Res. J. 2020, 17, 147–151. [Google Scholar] [CrossRef]
- Garcia-Migura, L.; Liebana, E.; Jensen, L.B.; Barnes, S.; Pleydell, E. A longitudinal study to assess the persistence of vancomycin-resistant Enterococcus faecium (VREF) on an intensive broiler farm in the United Kingdom. FEMS Microbiol. Lett. 2007, 275, 319–325. [Google Scholar] [CrossRef]
- Talebi, M.; Sadeghi, J.; Rahimi, F.; Pourshafie, M.R. Isolation and Biochemical Fingerprinting of Vancomycin-Resistant Enterococcus faecium From Meat, Chicken and Cheese. Jundishapur J. Microbiol. 2015, 8, e15815. [Google Scholar] [CrossRef]
- Nilsson, O.; Alm, E.; Greko, C.; Bengtsson, B. The rise and fall of a vancomycin-resistant clone of Enterococcus faecium among broilers in Sweden. J. Glob. Antimicrob. Resist. 2019, 17, 233–235. [Google Scholar] [CrossRef]
- Van der Auwera, P.; Pensart, N.; Korten, V.; Murray, B.E.; Leclercq, R. Influence of oral glycopeptides on the fecal flora of human volunteers: Selection of highly glycopeptide-resistant enterococci. J. Infect. Dis. 1996, 173, 1129–1136. [Google Scholar] [CrossRef]
- Wang, J.T.; Chang, S.C.; Wang, H.Y.; Chen, P.C.; Shiau, Y.R.; Lauderdale, T.L.; Hospitals, T. High rates of multidrug resistance in Enterococcus faecalis and E. faecium isolated from inpatients and outpatients in Taiwan. Diagn. Microbiol. Infect. Dis. 2013, 75, 406–411. [Google Scholar] [CrossRef]
- Hannaoui, I.; Barguigua, A.; Serray, B.; El Mdaghri, N.; Timinouni, M.; Ait Chaoui, A.; El Azhari, M. Intestinal carriage of vancomycin-resistant enterococci in a community setting in Casablanca, Morocco. J. Glob. Antimicrob. Resist. 2016, 6, 84–87. [Google Scholar] [CrossRef]
- Jannati, E.; Amirmozaffari, N.; Saadatmand, S.; Arzanlou, M. Faecal carriage of high-level aminoglycoside-resistant and ampicillin-resistant Enterococcus species in healthy Iranian children. J. Glob. Antimicrob. Resist. 2020, 20, 135–144. [Google Scholar] [CrossRef]
- Volgenant, C.M.C.; Hoogenkamp, M.A.; Dahlen, G.; Kalfas, S.; Petti, S.; De Soet, J.J. Low prevalence of multi-resistant bacteria in undergraduate dental students; an observational case-control multi-centre study in Europe. J. Oral. Microbiol. 2021, 13, 1889898. [Google Scholar] [CrossRef]
- Kim, S.M.; Kim, H.C.; Lee, S.W. Characterization of antibiotic resistance determinants in oral biofilms. J. Microbiol. 2011, 49, 595–602. [Google Scholar] [CrossRef]
- Ye, J.; Coulouris, G.; Zaretskaya, I.; Cutcutache, I.; Rozen, S.; Madden, T.L. Primer-BLAST: A tool to design target-specific primers for polymerase chain reaction. BMC Bioinform. 2012, 13, 134. [Google Scholar] [CrossRef]
| E. faecalis | p Value | OR (95% C.I.) 1 | |||
|---|---|---|---|---|---|
| (−) | (+) | ||||
| sex | male | 281 | 53 | 0.10 | 1.35 (0.94–1.95) |
| female | 420 | 107 | |||
| smoking | (−) | 466 | 102 | 0.51 | 1.13 (0.78–1.61) |
| (+) | 235 | 58 | |||
| alchool | (−) | 433 | 104 | 0.43 | 0.87 (0.60–1.24) |
| (+) | 269 | 56 | |||
| E. faecium | p Value | OR (95% C.I.) 1 | |||
|---|---|---|---|---|---|
| (−) | (+) | ||||
| sex | male | 332 | 2 | 0.78 | 1.27 (0.22–9.96) |
| female | 523 | 4 | |||
| smoking | (−) | 563 | 5 | 0.37 | 0.39 (0.02–2.80) |
| (+) | 292 | 1 | |||
| alchool | (−) | 533 | 4 | 0.82 | 0.82 (0.11–4.68) |
| (+) | 323 | 2 | |||
| Target | Primer (5′–3′) | Probe (5′–3′) |
|---|---|---|
| E. faecalis | F-GGCATAAGAGTGAAAGGCGC | JOE-TTTCGTGTCGCTGATGGATGGACCCG-BHQ1 |
| R-CATCGTGGCCTTGGTGAG | ||
| E. faecium | F-ACATGCAAGTCGAACGCTTC | 6_FAM-TGCTCCACCGGAAAAAGAGGAGTGGCGA-BMN_Q535 |
| R-TACCCACGTGTTACTCACCC | ||
| vanA | F-TTCATCAGGAAGTCGAGCCG | 6_FAM-CCCGCAGACCTTTCAGCAGAGGAGCGA-BMN_Q535 |
| R-TGCCGTTTCCTGTATCCGTC | ||
| vanB | F-ATTGAGCAAGCGATTTCGGG | CY5-TGTGAGGTCGGCTGCGCGGTCATGGGA-BMN_Q620 |
| R-TCCACTTCGCCGACAATCAA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Palmieri, A.; Martinelli, M.; Pellati, A.; Carinci, F.; Lauritano, D.; Arcuri, C.; Baggi, L.; Gatto, R.; Scapoli, L. Prevalence of Enterococci and Vancomycin Resistance in the Throat of Non-Hospitalized Individuals Randomly Selected in Central Italy. Antibiotics 2023, 12, 1161. https://doi.org/10.3390/antibiotics12071161
Palmieri A, Martinelli M, Pellati A, Carinci F, Lauritano D, Arcuri C, Baggi L, Gatto R, Scapoli L. Prevalence of Enterococci and Vancomycin Resistance in the Throat of Non-Hospitalized Individuals Randomly Selected in Central Italy. Antibiotics. 2023; 12(7):1161. https://doi.org/10.3390/antibiotics12071161
Chicago/Turabian StylePalmieri, Annalisa, Marcella Martinelli, Agnese Pellati, Francesco Carinci, Dorina Lauritano, Claudio Arcuri, Luigi Baggi, Roberto Gatto, and Luca Scapoli. 2023. "Prevalence of Enterococci and Vancomycin Resistance in the Throat of Non-Hospitalized Individuals Randomly Selected in Central Italy" Antibiotics 12, no. 7: 1161. https://doi.org/10.3390/antibiotics12071161
APA StylePalmieri, A., Martinelli, M., Pellati, A., Carinci, F., Lauritano, D., Arcuri, C., Baggi, L., Gatto, R., & Scapoli, L. (2023). Prevalence of Enterococci and Vancomycin Resistance in the Throat of Non-Hospitalized Individuals Randomly Selected in Central Italy. Antibiotics, 12(7), 1161. https://doi.org/10.3390/antibiotics12071161









