Harmonization of Amoxicillin Dose, Duration, and Formulation for Acute Childhood Respiratory Infections
Abstract
1. Introduction
2. Respiratory Infections in Children
3. Amoxicillin
3.1. Clinical Pharmacology
3.2. Mechanism of Resistance
3.3. Clinical Indications
3.3.1. Acute Otitis Media and Acute Bacterial Sinusitis
3.3.2. Acute Pharyngitis/Pharyngotonsillitis
3.3.3. Community-Acquired Pneumonia
3.4. Standardization of Dosing Guidance for Multiple Infections
3.5. Variation in Oral Formulations of Amoxicillin
3.5.1. Available Strengths and Formulations of Amoxicillin
3.5.2. Cost
3.5.3. Weight Bands for Dosing
3.5.4. Administration of Higher Doses of Amoxicillin in ARI
3.5.5. Safety
4. Summary
Author Contributions
Funding
Conflicts of Interest
References
- World Health Organisation. The WHO AWaRe (Access, Watch, Reserve) Antibiotic Book; WHO: Geneva, Switzerland, 2022.
- Indian Council of Medical Research. Treatment Guidelines for Antimicrobial Use in Common Syndromes; Indian Council of Medical Research: New Delhi, India, 2023. [Google Scholar]
- Gastine, S.; Rashed, A.N.; Hsia, Y.; Jackson, C.; Barker, C.I.S.; Mathur, S.; Tomlin, S.; Lutsar, I.; Bielicki, J.; Standing, J.F.; et al. GAPPS (Grading and Assessment of Pharmacokinetic-Pharmacodynamic Studies) a Critical Appraisal System for Antimicrobial PKPD Studies-Development and Application in Pediatric Antibiotic Studies. Expert Rev. Clin. Pharmacol. 2019, 12, 1091–1098. [Google Scholar] [CrossRef] [PubMed]
- Falagas, M.E.; Vouloumanou, E.K.; Plessa, E.; Peppas, G.; Rafailidis, P.I. Inaccuracies in Dosing Drugs with Teaspoons and Tablespoons. Int. J. Clin. Pract. 2010, 64, 1185–1189. [Google Scholar] [CrossRef]
- Indian Academy of Pediatrics. IAP Consensus Guidelines on Rational Management of Respiratory Tract Infections in Children; Singh, V., Yewale, V., Eds.; Indian Academy of Pediatrics: Navi Mumbai, India, 2014. [Google Scholar]
- Rolinson, G.N. 6-APA and the Development of the β-Lactam Antibiotics. J. Antimicrob. Chemother. 1979, 5, 7–14. [Google Scholar] [CrossRef] [PubMed]
- Rolinson, G.N. Laboratory Evaluation of Amoxicillin. J. Infect. Dis. 1974, 129, S139–S145. [Google Scholar] [CrossRef] [PubMed]
- U.S. Food and Drug Administration. Microbiological Review. Application Number: NDA 50-542/S-005,010,011; U.S. Food and Drug Administration: Silver Spring, MD, USA, 1998.
- de Velde, F.; de Winter, B.C.M.; Koch, B.C.P.; van Gelder, T.; Mouton, J.W. Non-Linear Absorption Pharmacokinetics of Amoxicillin: Consequences for Dosing Regimens and Clinical Breakpoints. J. Antimicrob. Chemother. 2016, 71, 2909–2917. [Google Scholar] [CrossRef]
- EUCAST. Clinical Breakpoints and Dosing of Antibiotics. 2022. Available online: https://www.eucast.org/clinical_breakpoints/ (accessed on 25 December 2022).
- Lewis, J.S., II. M100 Performance Standards For Antimicrobial. Clinical and Laboratory Standards Institute Antimicrobial Susceptibility Testing Standards: Berwyn, PA, USA, 2022; ISBN 978-1-68440-134-5. Available online: https://clsi.org/standards/products/microbiology/documents/m100/ (accessed on 29 December 2022).
- World Health Organisation. Global Antimicrobial Resistance Surveillance System (GLASS) Report: Early Implementation; WHO: Geneva, Switzerland, 2021.
- Mathur, S.; Jackson, C.; Urus, H.; Ziarko, I.; Goodbun, M.; Hsia, Y.; Ellis, S.; Sharland, M. A Comparison of Five Paediatric Dosing Guidelines for Antibiotics. Bull. World Health Organ. 2020, 98, 406–412F. [Google Scholar] [CrossRef] [PubMed]
- Cillóniz, C.; Garcia-Vidal, C.; Ceccato, A.; Torres, A. Antimicrobial Resistance Among Streptococcus Pneumoniae. In Antimicrobial Resistance in the 21st Century; Fong, I.W., Shlaes, D., Drlica, K., Eds.; Springer International Publishing: Cham, Switzerland, 2018; pp. 13–38. ISBN 978-3-319-78538-7. [Google Scholar]
- Delage, G.; Boucher, F.; Davies, H.D.; Embree, J.; Morin, C.; Speert, D.; Tan, B. High Dose Amoxicillin: Rationale for Use in Otitis Media Treatment Failures. Paediatr. Child Health 1999, 4, 321–323. [Google Scholar] [CrossRef]
- Cherazard, R.; Epstein, M.; Doan, T.-L.; Salim, T.; Bharti, S.; Smith, M.A. Antimicrobial Resistant Streptococcus Pneumoniae: Prevalence, Mechanisms, and Clinical Implications. Am. J. Ther. 2017, 24, e361–e369. [Google Scholar] [CrossRef] [PubMed]
- Nakao, A.; Hisata, K.; Fujimori, M.; Matsunaga, N.; Komatsu, M.; Shimizu, T. Amoxicillin Effect on Bacterial Load in Group a Streptococcal Pharyngitis: Comparison of Single and Multiple Daily Dosage Regimens. BMC Pediatr. 2019, 19, 205. [Google Scholar] [CrossRef] [PubMed]
- Danneels, P.; Postorino, M.C.; Strazzulla, A.; Belfeki, N.; Pitch, A.; Pourcine, F.; Jochmans, S.; Dubée, V.; Monchi, M.; Diamantis, S. A Retrospective Study on Amoxicillin Susceptibility in Severe Haemophilus Influenzae Pneumonia. Can. J. Infect. Dis. Med. Microbiol. 2020, 2020, 2093468. [Google Scholar] [CrossRef] [PubMed]
- Bielicki, J.A.; Stöhr, W.; Barratt, S.; Dunn, D.; Naufal, N.; Roland, D.; Sturgeon, K.; Finn, A.; Rodriguez-Ruiz, J.P.; Malhotra-Kumar, S.; et al. Effect of Amoxicillin Dose and Treatment Duration on the Need for Antibiotic Re-Treatment in Children with Community-Acquired Pneumonia: The CAP-IT Randomized Clinical Trial. JAMA 2021, 326, 1713–1724. [Google Scholar] [CrossRef] [PubMed]
- Jehan, F.; Nisar, I.; Kerai, S.; Balouch, B.; Brown, N.; Rahman, N.; Rizvi, A.; Shafiq, Y.; Zaidi, A.K.M. Randomized Trial of Amoxicillin for Pneumonia in Pakistan. N. Engl. J. Med. 2020, 383, 24–34. [Google Scholar] [CrossRef] [PubMed]
- Enhanced Management of Pneumonia in Community (EMPIC) Study; Nisar, Y.B. Community-Based Amoxicillin Treatment for Fast Breathing Pneumonia in Young Infants 7–59 Days Old: A Cluster Randomised Trial in Rural Bangladesh, Ethiopia, India and Malawi. BMJ Glob. Health 2021, 6, e006578. [Google Scholar] [CrossRef] [PubMed]
- Mane, S.S.; Sathe, V.; Pustake, M.V.; Walhekar, S.; Ramakrishnan, S. Efficacy of Oral Amoxicillin versus Parenteral Ceftriaxone in Treatment of Uncomplicated Community Acquired Pneumonia (CAP): A Prospective, Single Blinded, Parallel Design, Randomized Controlled Trial. Pediatric. Oncall 2021, 18, 1–6. [Google Scholar] [CrossRef]
- Sadruddin, S.; Khan, I.U.H.; Fox, M.P.; Bari, A.; Khan, A.; Thea, D.M.; Khan, A.; Khan, I.; Ahmad, I.; Qazi, S.A. Comparison of 3 Days Amoxicillin Versus 5 Days Co-Trimoxazole for Treatment of Fast-Breathing Pneumonia by Community Health Workers in Children Aged 2-59 Months in Pakistan: A Cluster-Randomized Trial. Clin. Infect. Dis. 2019, 69, 397–404. [Google Scholar] [CrossRef] [PubMed]
- Ginsburg, A.-S.; Mvalo, T.; Nkwopara, E.; McCollum, E.D.; Phiri, M.; Schmicker, R.; Hwang, J.; Ndamala, C.B.; Phiri, A.; Lufesi, N.; et al. Amoxicillin for 3 or 5 Days for Chest-Indrawing Pneumonia in Malawian Children. N. Engl. J. Med. 2020, 383, 13–23. [Google Scholar] [CrossRef] [PubMed]
- World Health Organisation. Model List of Essential Medicines; WHO: Geneva, Switzerland, 2021.
- TATA 1 mg. Medicines. Available online: https://www.1mg.com/drugs/mox-drops-redimix-328033 (accessed on 20 October 2022).
- Angwa, L.M.; Ouma, C.; Okoth, P.; Nyamai, R.; Kamau, N.G.; Mutai, K.; Onono, M.A. Acceptability, Adherence, and Clinical Outcomes, of Amoxicillin Dispersible Tablets versus Oral Suspension in Treatment of Children Aged 2–59 Months with Pneumonia, Kenya: A Cluster Randomized Controlled Trial. Heliyon 2020, 6, e03786. [Google Scholar] [CrossRef] [PubMed]
- UNICEF. Amoxicillin Dispersible Tablets: Market and Supply Update. May 2018. UNICEF. Available online: https://www.unicef.org/supply/media/511/file/amoxicillin-dispersible-tablets-market-and-supply-update (accessed on 22 November 2022).
- Medplusmart. Online Pharmacy Store in India. Available online: https://www.medplusmart.com/ (accessed on 12 November 2022).
- Bielicki, J.A.; Barker, C.I.S.; Saxena, S.; Wong, I.C.K.; Long, P.F.; Sharland, M. Not Too Little, Not Too Much: Problems of Selecting Oral Antibiotic Dose for Children. BMJ 2015, 351, h5447. [Google Scholar] [CrossRef] [PubMed]
- World Health Organsiation. Pocket Book of Hospital Care for Children, 2nd ed.; WHO: Geneva, Switzerland, 2013. Available online: https://www.who.int/publications/i/item/978-92-4-154837-3 (accessed on 10 November 2022).
- Hum, S.W.; Shaikh, K.J.; Musa, S.S.; Shaikh, N. Adverse Events of Antibiotics Used to Treat Acute Otitis Media in Children: A Systematic Meta-Analysis. J. Pediatr. 2019, 215, 139–143.e7. [Google Scholar] [CrossRef] [PubMed]
| Infection | Leading Bacterial Respiratory Pathogens |
|---|---|
| Acute pharyngitis/pharyngotonsillitis | Group A β-hemolytic streptocococci |
| Others: | |
| Group C and G streptococci, Mycoplasma pneumoniae, | |
| Neisseria gonorrhoeae, Corynebacterium | |
| diphtheriae. | |
| Acute Otitis media | Streptococcus pneumoniae, |
| Moraxella catarrhalis | |
| Non-typable Haemophilus influenzae | |
| Rarely | |
| Streptococci spp., Staphylococci aureus, Pseudomonas | |
| Acute sinusitis | Streptococcus pneumoniae, |
| Moraxella catarrhalis | |
| Non-typable Haemophilus influenzae | |
| Streptococcus pyogenes | |
| Community Acquired pneumonia | 0–2 months |
| Gram-negative organisms | |
| Group B Streptococcus | |
| Streptococcus pyogenes | |
| Chlamydia | |
| 2 months–5 years | |
| Streptococcus pneumoniae | |
| Haemophilus influenzae | |
| Staphylococcus aureus | |
| Streptococcus pyogenes | |
| Mycoplasma pneumonia | |
| Above 5 years | |
| Streptococcus pneumoniae | |
| Staphylococcus aureus | |
| Streptococcus pyogenes | |
| Haemophilus influenzae | |
| Mycoplasma pneumoniae | |
| Chlamydia pneumoniae |
| EUCAST 2022 | CLSI 2022 | ||||
|---|---|---|---|---|---|
| Species | MIC | Interpretation | MIC | Interpretation | Comment |
| Staphylococcus spp.* | ≤0.125 >0.125 | S R | ≤0.12 ≥0.25 | S R | |
| Streptococcus pneumoniae | ≤0.5 >1 | S R | ≤2 4 ≥8 | S I R | Applicable for non meningial isolates for amoxicillin regimen of 500 mg administered every 8 h or 875 mg administered every 12 h |
| Group A Streptococcus | ≤0.25 >0.25 | S R | ≤0.12 0.25–2 ≥4 | S I R | |
| Haemophilus influenzae | ≤0.001 >2 | S R | ≤1 2 ≥4 | S I R | |
| Authors | Study | Age Group | Doses Given | Setting | Conclusions |
|---|---|---|---|---|---|
| [20] | Randomized control trial of amoxicillin versus placebo for management of pneumonia diagnosed by WHO criteria of tachypnea | 2 to 59 months | According to WHO weight bands 40–50 mg/kg/dose q 12 hourly | Karachi | Difference in the treatment failure did not confirm the inferiority of placebo. A total of 44 children needed to be treated to prevent one treatment failure, indicating opportunities for improving antibiotic stewardship. |
| [21] | Cluster randomised trial for vommunity-based amoxicillin treatment for fast breathing pneumonia. | 7–59 days | WHO weight bands: 40–50 mg/kg/dose q 12 hourly (125 mg two times per day for <4 kg body weight and 250 mg two times per day for >4 kg body weight.) | rural Bangladesh, Ethiopia, India and Malawi | A 7-day amoxicillin treatment for 7–59 days old non-hypoxaemic infants with fast breathing pneumonia by community level health workers was non-inferior to the currently recommended referral strategy. |
| [22] | Prospective, single blinded, parallel design, randomized controlled trial, efficacy of oral amoxicillin versus parenteral ceftriaxone in treatment of uncomplicated community-acquired pneumonia. | 6 months to 12 years | 100 mg/kg/day in three divided doses for 7 days. | tertiary centre, Mumbai, India | Use of oral amoxicillin for uncomplicated community-acquired pneumonia in children had a similar outcome as compared to parenteral ceftriaxone. 4% of children required step up to high dose amoxicillin. |
| [23] | Unblinded, cluster-randomized, controlled-equivalency trial for comparison of 3 days amoxicillin versus 5 days co-trimoxazole for treatment of fast-breathing pneumonia by community health workers. | 2–59 months | 50 mg/kg/day for 3 days | Haripur District, Pakistan | A 3-day course of oral amoxicillin was effective and safe treatment for fast-breathing pneumonia |
| [24] | Randomized control trial of Amoxicillin for 3 or 5 days for chest-indrawing pneumonia. | 2–59 months | 40 mg/kg/dose twice a day for 3 days versus 5 days | Outpatient departments in Malawi, Africa | Treatment with amoxicillin for chest-indrawing pneumonia for 3 days was noninferior to treatment for 5 days. |
| Clinical Conditions | WHO | Indian National Guidelines (ICMR) 2019 | Indian Academy of Pediatrics 2014 |
|---|---|---|---|
| Acute Otitis media | Amoxicillin 40–50 mg/kg/dose oral 12 hourly Duration 5 days | Amoxicillin 20 mg/kg/dose oral 12 hourly Or Co-amoxiclav 15–20 mg/kg/dose oral of amoxicilin component 12 hourly. Duration: 5–7 days In severe cases/children less than 2 years–10 days | Amoxicillin 20 mg/kg/dose oral 12 hourly Or Co-amoxiclav 45 mg/kg/day dose of amoxicllin component in two divided doses. Duration: 10 days |
| Group A streptococcal pharyngitis/tonsillitis | Amoxicillin 40–50 mg/kg/dose 12 hourly Duration 5 days (10 days in areas with high risk of Rheumatic fever) | Amoxicillin 15–20 mg/kg twice daily oral Duration 10 days | Amoxicillin Dose 50 mg/kg /dose 12 hourly Duration 10 days |
| Sinusitis | Amoxicillin 40–50 mg/kg/dose oral 12 hourly Second choice: Co-amoxiclav 40–50 mg/kg/dose (amoxicillin component) 12 hourly or 30 mg/kg/dose 8 hourly Duration 5 days | Amoxicillin 15–20 mg/kg twice daily Coamoxiclav 15–20 mg/kg/dose oral of amoxicilin component 12 hourly. Duration 10–14days | Amoxicillin 40 mg/kg/day in two divided doses If no improvement in 72 h or severe, Co-amoxiclav Duration 10 days |
| Mild to Moderate Community acquired pneumonia | Amoxicillin 40–50 mg/kg/dose 12 hourly Duration 5 days | Amoxicillin 15–20 mg/kg twice daily oral Co-amoxiclav 15–20 mg/kg of amoxicillin twice daily oral Duration 5 days | Amoxicillin 90 mg/kg/day (since penicillin resistant isolates are less than 10 percent, 50 mg/kg/day is sufficient) Or Coamoxclav Duration 5–7days |
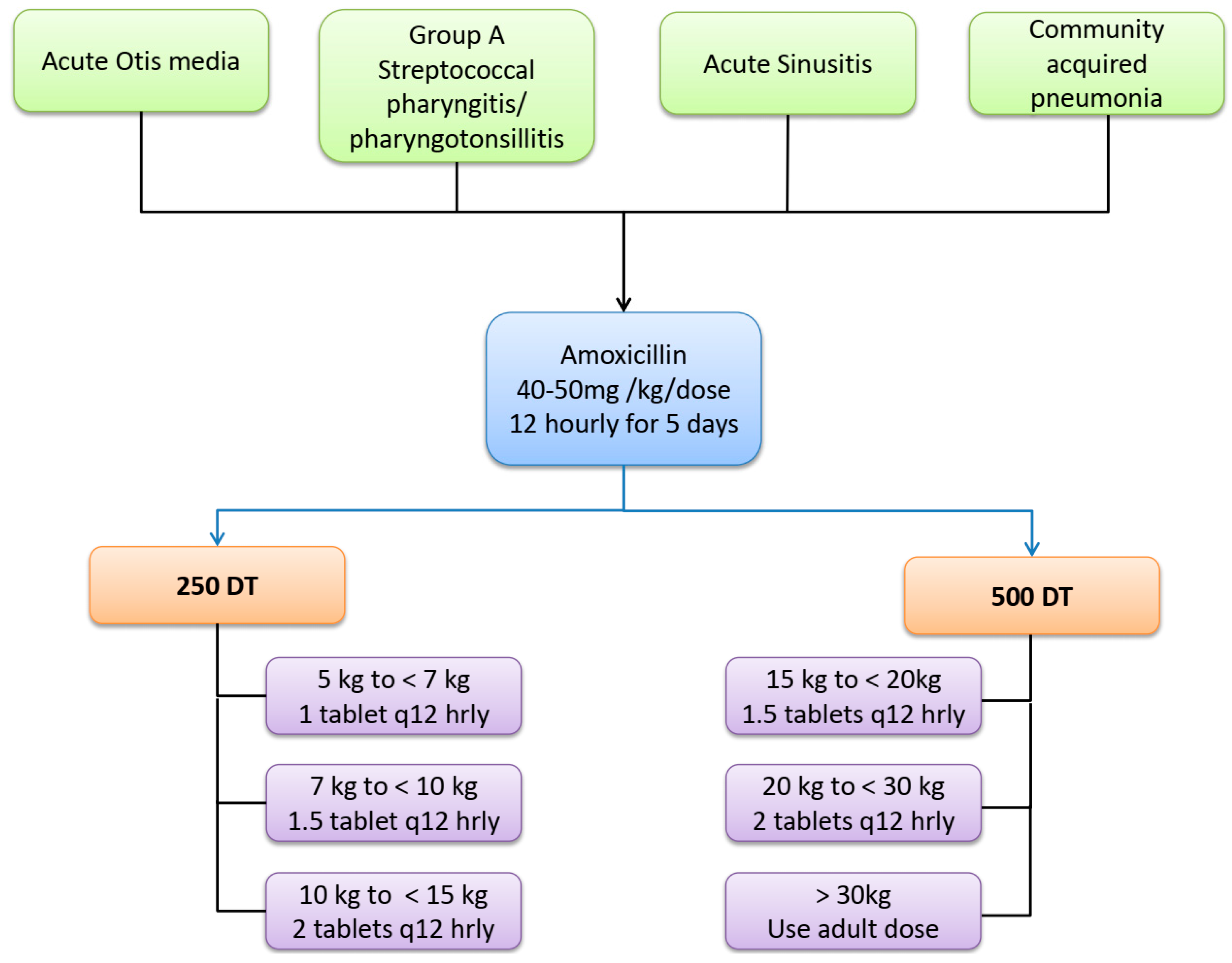
| Weight Bands | Dose | Amoxicillin Formulation | Prescription |
|---|---|---|---|
| 5 to <7 kg | 250 mg q 12 hourly | 250 DT | One tablet q 12 hourly |
| 7 to <10 kg | 375 mg q 12 hourly | 250 DT | 1.5 tablets q 12 hourly |
| 10 to <15 kg | 500 mg q 12 hourly | 250 DT | 2 tablets q 12 hourly |
| 15 to <20 kg | 750 mg q 12 hourly | 500 DT | 1.5 tablets q 12 hourly |
| 20 to <30 kg | 1000 mg q 12 hourly | 500 DT | 2 tablets q 12 hourly |
| 30 kg and above | Use adult dose | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dharmapalan, D.; Bielicki, J.; Sharland, M. Harmonization of Amoxicillin Dose, Duration, and Formulation for Acute Childhood Respiratory Infections. Antibiotics 2023, 12, 1138. https://doi.org/10.3390/antibiotics12071138
Dharmapalan D, Bielicki J, Sharland M. Harmonization of Amoxicillin Dose, Duration, and Formulation for Acute Childhood Respiratory Infections. Antibiotics. 2023; 12(7):1138. https://doi.org/10.3390/antibiotics12071138
Chicago/Turabian StyleDharmapalan, Dhanya, Julia Bielicki, and Mike Sharland. 2023. "Harmonization of Amoxicillin Dose, Duration, and Formulation for Acute Childhood Respiratory Infections" Antibiotics 12, no. 7: 1138. https://doi.org/10.3390/antibiotics12071138
APA StyleDharmapalan, D., Bielicki, J., & Sharland, M. (2023). Harmonization of Amoxicillin Dose, Duration, and Formulation for Acute Childhood Respiratory Infections. Antibiotics, 12(7), 1138. https://doi.org/10.3390/antibiotics12071138




