Relationship between COVID-19 and ICU-Acquired Bloodstream Infections Related to Multidrug-Resistant Bacteria
Abstract
1. Introduction
2. Patients and Methods
2.1. Population and Definitions
2.2. Data Collection
2.3. Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. Characteristics of Patients with ICU-Acquired BSI Related to MDRB According to COVID-19 Status
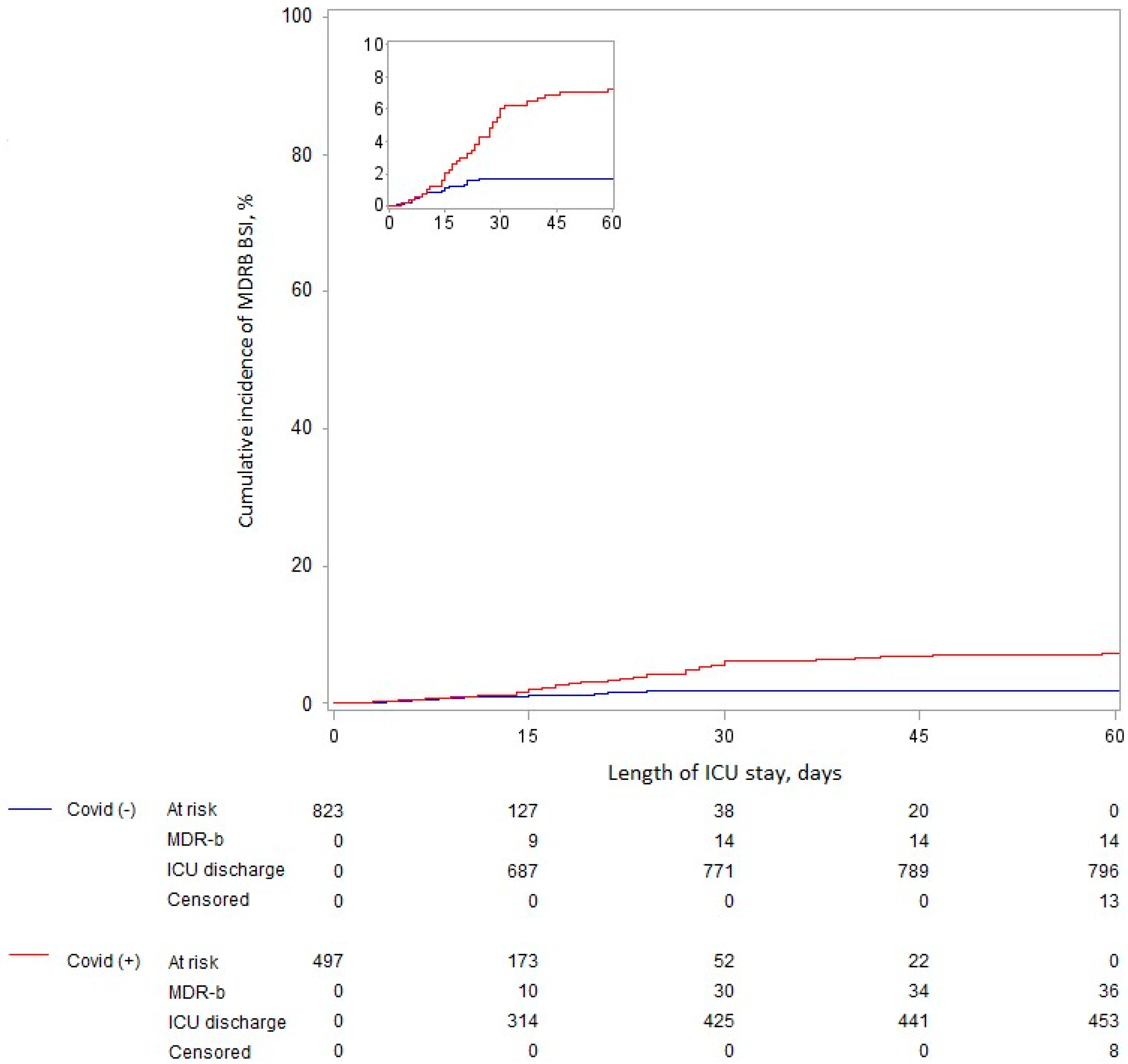
3.3. Relationship between COVID-19 and ICU-Acquired BSI Related to MDRB
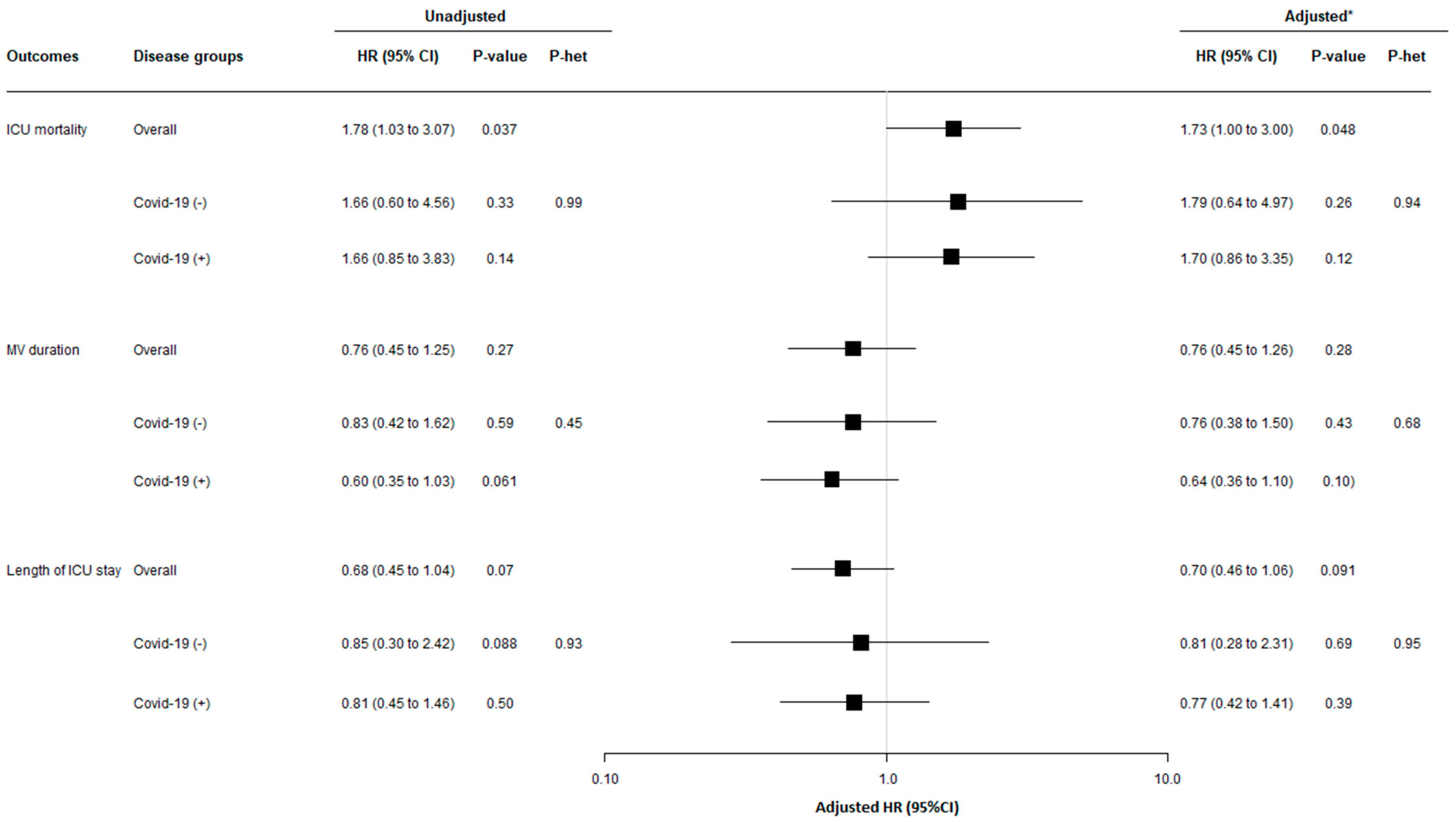
3.4. Relationship between ICU-Acquired BSI Related to MDRB and Mortality, Length of ICU Stay, and Duration of Mechanical Ventilation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kreitmann, L.; Vasseur, M.; Jermoumi, S.; Perche, J.; Richard, J.-C.; Wallet, F.; Chabani, M.; Nourry, E.; Garçon, P.; Zerbib, Y.; et al. Relationship between Immunosuppression and Intensive Care Unit-Acquired Colonization and Infection Related to Multidrug-Resistant Bacteria: A Prospective Multicenter Cohort Study. Intensive Care Med. 2023, 49, 154–165. [Google Scholar] [CrossRef] [PubMed]
- Vincent, J.-L.; Sakr, Y.; Singer, M.; Martin-Loeches, I.; Machado, F.R.; Marshall, J.C.; Finfer, S.; Pelosi, P.; Brazzi, L.; Aditianingsih, D.; et al. Prevalence and Outcomes of Infection among Patients in Intensive Care Units in 2017. JAMA 2020, 323, 1478–1487. [Google Scholar] [CrossRef] [PubMed]
- Tabah, A.; Buetti, N.; Staiquly, Q.; Ruckly, S.; Akova, M.; Aslan, A.T.; Leone, M.; Conway Morris, A.; Bassetti, M.; Arvaniti, K.; et al. Epidemiology and Outcomes of Hospital-Acquired Bloodstream Infections in Intensive Care Unit Patients: The EUROBACT-2 International Cohort Study. Intensive Care Med. 2023, 49, 178–190. [Google Scholar] [CrossRef] [PubMed]
- Barbier, F.; Lisboa, T.; Nseir, S. Understanding Why Resistant Bacteria Are Associated with Higher Mortality in ICU Patients. Intensive Care Med. 2016, 42, 2066–2069. [Google Scholar] [CrossRef] [PubMed]
- Coronavirus COVID-19 (2019-NCoV). Available online: https://www.arcgis.com/apps/dashboards/bda7594740fd40299423467b48e9ecf6 (accessed on 17 February 2023).
- Petrilli, C.M.; Jones, S.A.; Yang, J.; Rajagopalan, H.; O’Donnell, L.; Chernyak, Y.; Tobin, K.A.; Cerfolio, R.J.; Francois, F.; Horwitz, L.I. Factors Associated with Hospital Admission and Critical Illness among 5279 People with Coronavirus Disease 2019 in New York City: Prospective Cohort Study. BMJ 2020, 369, m1966. [Google Scholar] [CrossRef]
- COVID-ICU Group on behalf of the REVA Network and the COVID-ICU Investigators Clinical Characteristics and Day-90 Outcomes of 4244 Critically Ill Adults with COVID-19: A Prospective Cohort Study. Intensive Care Med. 2021, 47, 60–73. [CrossRef]
- Lansbury, L.; Lim, B.; Baskaran, V.; Lim, W.S. Co-Infections in People with COVID-19: A Systematic Review and Meta-Analysis. J. Infect. 2020, 81, 266–275. [Google Scholar] [CrossRef]
- Langford, B.J.; So, M.; Raybardhan, S.; Leung, V.; Westwood, D.; MacFadden, D.R.; Soucy, J.-P.R.; Daneman, N. Bacterial Co-Infection and Secondary Infection in Patients with COVID-19: A Living Rapid Review and Meta-Analysis. Clin. Microbiol. Infect. Off. Publ. Eur. Soc. Clin. Microbiol. Infect. Dis. 2020, 26, 1622–1629. [Google Scholar] [CrossRef]
- Rouzé, A.; Martin-Loeches, I.; Povoa, P.; Metzelard, M.; Du Cheyron, D.; Lambiotte, F.; Tamion, F.; Labruyere, M.; Boulle Geronimi, C.; Nieszkowska, A.; et al. Early Bacterial Identification among Intubated Patients with COVID-19 or Influenza Pneumonia: A European Multicenter Comparative Clinical Trial. Am. J. Respir. Crit. Care Med. 2021, 204, 546–556. [Google Scholar] [CrossRef]
- Conway Morris, A.; Kohler, K.; De Corte, T.; Ercole, A.; De Grooth, H.-J.; Elbers, P.W.G.; Povoa, P.; Morais, R.; Koulenti, D.; Jog, S.; et al. Co-Infection and ICU-Acquired Infection in COVID-19 ICU Patients: A Secondary Analysis of the UNITE-COVID Data Set. Crit. Care 2022, 26, 236. [Google Scholar] [CrossRef]
- Maes, M.; Higginson, E.; Pereira-Dias, J.; Curran, M.D.; Parmar, S.; Khokhar, F.; Cuchet-Lourenço, D.; Lux, J.; Sharma-Hajela, S.; Ravenhill, B.; et al. Ventilator-Associated Pneumonia in Critically Ill Patients with COVID-19. Crit. Care Lond. Engl. 2021, 25, 25. [Google Scholar] [CrossRef]
- Pickens, C.O.; Gao, C.A.; Cuttica, M.J.; Smith, S.B.; Pesce, L.L.; Grant, R.A.; Kang, M.; Morales-Nebreda, L.; Bavishi, A.A.; Arnold, J.M.; et al. Bacterial Superinfection Pneumonia in Patients Mechanically Ventilated for COVID-19 Pneumonia. Am. J. Respir. Crit. Care Med. 2021, 204, 921–932. [Google Scholar] [CrossRef]
- Rouzé, A.; Martin-Loeches, I.; Povoa, P.; Makris, D.; Artigas, A.; Bouchereau, M.; Lambiotte, F.; Metzelard, M.; Cuchet, P.; Boulle Geronimi, C.; et al. Relationship between SARS-CoV-2 Infection and the Incidence of Ventilator-Associated Lower Respiratory Tract Infections: A European Multicenter Cohort Study. Intensive Care Med. 2021, 47, 188–198. [Google Scholar] [CrossRef]
- Lahmer, T.; Kriescher, S.; Herner, A.; Rothe, K.; Spinner, C.D.; Schneider, J.; Mayer, U.; Neuenhahn, M.; Hoffmann, D.; Geisler, F.; et al. Invasive Pulmonary Aspergillosis in Critically Ill Patients with Severe COVID-19 Pneumonia: Results from the Prospective AspCOVID-19 Study. PLoS ONE 2021, 16, e0238825. [Google Scholar] [CrossRef]
- Bartoletti, M.; Pascale, R.; Cricca, M.; Rinaldi, M.; Maccaro, A.; Bussini, L.; Fornaro, G.; Tonetti, T.; Pizzilli, G.; Francalanci, E.; et al. Epidemiology of Invasive Pulmonary Aspergillosis among COVID-19 Intubated Patients: A Prospective Study. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2020, 73, ciaa1065. [Google Scholar] [CrossRef]
- Buetti, N.; Ruckly, S.; de Montmollin, E.; Reignier, J.; Terzi, N.; Cohen, Y.; Siami, S.; Dupuis, C.; Timsit, J.-F. COVID-19 Increased the Risk of ICU-Acquired Bloodstream Infections: A Case-Cohort Study from the Multicentric OUTCOMEREA Network. Intensive Care Med. 2021, 47, 180–187. [Google Scholar] [CrossRef]
- Buetti, N.; Tabah, A.; Loiodice, A.; Ruckly, S.; Aslan, A.T.; Montrucchio, G.; Cortegiani, A.; Saltoglu, N.; Kayaaslan, B.; Aksoy, F.; et al. Different Epidemiology of Bloodstream Infections in COVID-19 Compared to Non-COVID-19 Critically Ill Patients: A Descriptive Analysis of the Eurobact II Study. Crit. Care 2022, 26, 319. [Google Scholar] [CrossRef]
- Lai, C.-C.; Wang, C.-Y.; Hsueh, P.-R. Co-Infections among Patients with COVID-19: The Need for Combination Therapy with Non-Anti-SARS-CoV-2 Agents? J. Microbiol. Immunol. Infect. 2020, 53, 505–512. [Google Scholar] [CrossRef]
- Giacobbe, D.R.; Battaglini, D.; Ball, L.; Brunetti, I.; Bruzzone, B.; Codda, G.; Crea, F.; De Maria, A.; Dentone, C.; Di Biagio, A.; et al. Bloodstream Infections in Critically Ill Patients with COVID-19. Eur. J. Clin. Invest. 2020, 50, e13319. [Google Scholar] [CrossRef]
- Søgaard, K.K.; Baettig, V.; Osthoff, M.; Marsch, S.; Leuzinger, K.; Schweitzer, M.; Meier, J.; Bassetti, S.; Bingisser, R.; Nickel, C.H.; et al. Community-Acquired and Hospital-Acquired Respiratory Tract Infection and Bloodstream Infection in Patients Hospitalized with COVID-19 Pneumonia. J. Intensive Care 2021, 9, 10. [Google Scholar] [CrossRef]
- Bongiovanni, M.; Barda, B. Pseudomonas Aeruginosa Bloodstream Infections in SARS-CoV-2 Infected Patients: A Systematic Review. J. Clin. Med. 2023, 12, 2252. [Google Scholar] [CrossRef] [PubMed]
- Nori, P.; Szymczak, W.; Puius, Y.; Sharma, A.; Cowman, K.; Gialanella, P.; Fleischner, Z.; Corpuz, M.; Torres-Isasiga, J.; Bartash, R.; et al. Emerging Co-Pathogens: New Delhi Metallo-Beta-Lactamase Producing Enterobacterales Infections in New York City COVID-19 Patients. Int. J. Antimicrob. Agents 2020, 56, 106179. [Google Scholar] [CrossRef] [PubMed]
- Contou, D.; Claudinon, A.; Pajot, O.; Micaëlo, M.; Longuet Flandre, P.; Dubert, M.; Cally, R.; Logre, E.; Fraissé, M.; Mentec, H.; et al. Bacterial and Viral Co-Infections in Patients with Severe SARS-CoV-2 Pneumonia Admitted to a French ICU. Ann. Intensive Care 2020, 10, 119. [Google Scholar] [CrossRef] [PubMed]
- Tiri, B.; Sensi, E.; Marsiliani, V.; Cantarini, M.; Priante, G.; Vernelli, C.; Martella, L.A.; Costantini, M.; Mariottini, A.; Andreani, P.; et al. Antimicrobial Stewardship Program, COVID-19, and Infection Control: Spread of Carbapenem-Resistant Klebsiella Pneumoniae Colonization in ICU COVID-19 Patients. What Did Not Work? J. Clin. Med. 2020, 9, 2744. [Google Scholar] [CrossRef] [PubMed]
- Le Gall, J.-R.; Lemeshow, S.; Saulnier, F. A New Simplified Acute Physiology Score (SAPS II) Based on a European/North American Multicenter Study. JAMA 1993, 270, 2957–2963. [Google Scholar] [CrossRef]
- Vincent, J.L.; Moreno, R.; Takala, J.; Willatts, S.; De Mendonça, A.; Bruining, H.; Reinhart, C.K.; Suter, P.M.; Thijs, L.G. The SOFA (Sepsis-Related Organ Failure Assessment) Score to Describe Organ Dysfunction/Failure. On Behalf of the Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine. Intensive Care Med. 1996, 22, 707–710. [Google Scholar] [CrossRef]
- Prentice, R.L.; Kalbfleisch, J.D.; Peterson, A.V.; Flournoy, N.; Farewell, V.T.; Breslow, N.E. The Analysis of Failure Times in the Presence of Competing Risks. Biometrics 1978, 34, 541–554. [Google Scholar] [CrossRef]
- Andersen, P.K.; Geskus, R.B.; de Witte, T.; Putter, H. Competing Risks in Epidemiology: Possibilities and Pitfalls. Int. J. Epidemiol. 2012, 41, 861–870. [Google Scholar] [CrossRef]
- Gouel-Cheron, A.; Swihart, B.J.; Warner, S.; Mathew, L.; Strich, J.R.; Mancera, A.; Follmann, D.; Kadri, S.S. Epidemiology of ICU-Onset Bloodstream Infection: Prevalence, Pathogens, and Risk Factors Among 150,948 ICU Patients at 85 U.S. Hospitals. Crit. Care Med. 2022, 50, 1725. [Google Scholar] [CrossRef]
- Ritter, L.A.; Britton, N.; Heil, E.L.; Teeter, W.A.; Murthi, S.B.; Chow, J.H.; Ricotta, E.; Chertow, D.S.; Grazioli, A.; Levine, A.R. The Impact of Corticosteroids on Secondary Infection and Mortality in Critically Ill COVID-19 Patients. J. Intensive Care Med. 2021, 36, 1201–1208. [Google Scholar] [CrossRef]
- Tomazini, B.M.; Maia, I.S.; Cavalcanti, A.B.; Berwanger, O.; Rosa, R.G.; Veiga, V.C.; Avezum, A.; Lopes, R.D.; Bueno, F.R.; Silva, M.V.A.O.; et al. Effect of Dexamethasone on Days Alive and Ventilator-Free in Patients With Moderate or Severe Acute Respiratory Distress Syndrome and COVID-19: The CoDEX Randomized Clinical Trial. JAMA 2020, 324, 1307–1316. [Google Scholar] [CrossRef]
- Gragueb-Chatti, I.; Lopez, A.; Hamidi, D.; Guervilly, C.; Loundou, A.; Daviet, F.; Cassir, N.; Papazian, L.; Forel, J.-M.; Leone, M.; et al. Impact of Dexamethasone on the Incidence of Ventilator-Associated Pneumonia and Blood Stream Infections in COVID-19 Patients Requiring Invasive Mechanical Ventilation: A Multicenter Retrospective Study. Ann. Intensive Care 2021, 11, 87. [Google Scholar] [CrossRef]
- Meynaar, I.A.; van Rijn, S.; Ottens, T.H.; van Burgel, N.D.; van Nieuwkoop, C. Increased Risk of Central Line-Associated Bloodstream Infection in COVID-19 Patients Associated with Dexamethasone but Not with Interleukin Antagonists. Intensive Care Med. 2022, 48, 954–957. [Google Scholar] [CrossRef]
- Bellinvia, S.; Edwards, C.J.; Schisano, M.; Banfi, P.; Fallico, M.; Murabito, P. The Unleashing of the Immune System in COVID-19 and Sepsis: The Calm before the Storm? Inflamm. Res. 2020, 69, 757–763. [Google Scholar] [CrossRef]
- López-Collazo, E.; Avendaño-Ortiz, J.; Martín-Quirós, A.; Aguirre, L.A. Immune Response and COVID-19: A Mirror Image of Sepsis. Int. J. Biol. Sci. 2020, 16, 2479–2489. [Google Scholar] [CrossRef]
- Giamarellos-Bourboulis, E.J.; Netea, M.G.; Rovina, N.; Akinosoglou, K.; Antoniadou, A.; Antonakos, N.; Damoraki, G.; Gkavogianni, T.; Adami, M.-E.; Katsaounou, P.; et al. Complex Immune Dysregulation in COVID-19 Patients with Severe Respiratory Failure. Cell Host Microbe 2020, 27, 992–1000.e3. [Google Scholar] [CrossRef]
- Tappe, B.; Lauruschkat, C.D.; Strobel, L.; Pantaleón García, J.; Kurzai, O.; Rebhan, S.; Kraus, S.; Pfeuffer-Jovic, E.; Bussemer, L.; Possler, L.; et al. COVID-19 Patients Share Common, Corticosteroid-Independent Features of Impaired Host Immunity to Pathogenic Molds. Front. Immunol. 2022, 13, 954985. [Google Scholar] [CrossRef]
- Moser, D.; Biere, K.; Han, B.; Hoerl, M.; Schelling, G.; Choukér, A.; Woehrle, T. COVID-19 Impairs Immune Response to Candida Albicans. Front. Immunol. 2021, 12, 640644. [Google Scholar] [CrossRef]
- Nomani, M.; Varahram, M.; Tabarsi, P.; Hashemian, S.M.; Jamaati, H.; Malekmohammad, M.; Ghazi, M.; Adcock, I.M.; Mortaz, E. Decreased Neutrophil-mediated Bacterial Killing in COVID-19 Patients. Scand. J. Immunol. 2021, 94, e13083. [Google Scholar] [CrossRef]
- García-García, J.; Diez-Echave, P.; Yuste, M.E.; Chueca, N.; García, F.; Cabeza-Barrera, J.; Fernández-Varón, E.; Gálvez, J.; Colmenero, M.; Rodríguez-Cabezas, M.E.; et al. Gut Microbiota Composition Can Predict Colonization by Multidrug-Resistant Bacteria in SARS-CoV-2 Patients in Intensive Care Unit: A Pilot Study. Antibiotics 2023, 12, 498. [Google Scholar] [CrossRef]
- Kreitmann, L.; Jermoumi, S.; Vasseur, M.; Chabani, M.; Nourry, E.; Richard, J.; Wallet, F.; Garcon, P.; Kachmar, S.; Zerbib, Y.; et al. Relationship between COVID-19 and ICU-acquired colonization and infection related to multidrug-resistant bacteria: A prospective multicenter before-after study. Intensive Care Med. 2023; online ahead of print. [Google Scholar] [CrossRef]
- Martin-Loeches, I.; Torres, A.; Rinaudo, M.; Terraneo, S.; de Rosa, F.; Ramirez, P.; Diaz, E.; Fernández-Barat, L.; Li Bassi, G.L.; Ferrer, M. Resistance Patterns and Outcomes in Intensive Care Unit (ICU)-Acquired Pneumonia. Validation of European Centre for Disease Prevention and Control (ECDC) and the Centers for Disease Control and Prevention (CDC) Classification of Multidrug Resistant Organisms. J. Infect. 2015, 70, 213–222. [Google Scholar] [CrossRef] [PubMed]
- Adrie, C.; Garrouste-Orgeas, M.; Ibn Essaied, W.; Schwebel, C.; Darmon, M.; Mourvillier, B.; Ruckly, S.; Dumenil, A.-S.; Kallel, H.; Argaud, L.; et al. Attributable Mortality of ICU-Acquired Bloodstream Infections: Impact of the Source, Causative Micro-Organism, Resistance Profile and Antimicrobial Therapy. J. Infect. 2017, 74, 131–141. [Google Scholar] [CrossRef] [PubMed]
- Grasselli, G.; Scaravilli, V.; Mangioni, D.; Scudeller, L.; Alagna, L.; Bartoletti, M.; Bellani, G.; Biagioni, E.; Bonfanti, P.; Bottino, N.; et al. Hospital-Acquired Infections in Critically Ill Patients With COVID-19. Chest 2021, 160, 454–465. [Google Scholar] [CrossRef] [PubMed]
- Bonazzetti, C.; Morena, V.; Giacomelli, A.; Oreni, L.; Casalini, G.; Galimberti, L.R.; Bolis, M.; Rimoldi, M.; Ballone, E.; Colombo, R.; et al. Unexpectedly High Frequency of Enterococcal Bloodstream Infections in Coronavirus Disease 2019 Patients Admitted to an Italian ICU: An Observational Study. Crit. Care Med. 2021, 49, e31–e40. [Google Scholar] [CrossRef]
| Overall | COVID-19 | |||
|---|---|---|---|---|
n = 1320 | Yes n = 497 | No n = 823 | Standardized Difference % | |
| Age (years) | 62.0 (50.0–70.0) | 65.0 (56.0–73.0) | 60.0 (47.0–69.0) | 36.6 |
| Male gender | 884 (67.0) | 364 (73.2) | 520 (63.2) | 21.7 |
| BMI (kg/m2) a | 27.4 (23.7–32.6) | 29.4 (25.7–34.6) | 26.1 (22.4–31.2) | 53.4 |
| SAPS II b | 39.0 (29.0–55.0) | 38.0 (30.0–51.0) | 40.0 (29.0–57.0) | −8.9 |
| SOFA c | 4 (2–8) | 4 (3–8) | 4 (2–7) | 4.5 |
| Comorbidities | ||||
| Current smokers | 367 (27.8) | 77 (15.5) | 290 (35.2) | −46.6 |
| Alcohol consumption | 216 (16.4) | 39 (7.8) | 177 (21.5) | −39.3 |
| Diabetes mellitus | 392 (29.7) | 158 (31.8) | 234 (28.4) | 7.3 |
| Hypertension | 646 (48.9) | 260 (52.3) | 386 (46.9) | 10.8 |
| Ischemic heart disease | 188 (14.2) | 76 (15.3) | 112 (13.6) | 4.8 |
| Heart failure | 154 (11.7) | 39 (7.8) | 115 (14.0) | −19.8 |
| Thromboembolic disease | 86 (6.5) | 27 (5.4) | 59 (7.2) | −7.2 |
| Lung disease | 250 (18.9) | 48 (9.7) | 202 (24.5) | −40.3 |
| Chronic obstructive pulmonary disease | 177 (13.4) | 48 (9.7) | 129 (15.7) | −18.2 |
| Hemodialysis | 128 (9.7) | 39 (7.8) | 89 (10.8) | −10.2 |
| Liver cirrhosis | 56 (4.2) | 6 (1.2) | 50 (6.1) | −26.2 |
| Hematological malignancy | 88 (6.7) | 22 (4.4) | 66 (8.0) | −14.9 |
| Solid organ transplantation | 33 (2.5) | 5 (1.0) | 28 (3.4) | −16.4 |
| Immunosuppressive treatment | 146 (11.1) | 31 (6.2) | 115 (14.0) | −25.9 |
| Corticosteroid use | 108 (8.2) | 30 (6.0) | 78 (9.5) | −12.9 |
| Solid neoplasia | 136 (10.3) | 31 (6.2) | 105 (12.8) | −22.4 |
| Metastatic neoplasia | 53 (4.0) | 11 (2.2) | 42 (5.1) | −15.4 |
| HIV | 4 (0.3) | 1 (0.2) | 3 (0.4) | −3.1 |
| Hospitalization < 3 months | 352 (26.7) | 87 (17.5) | 265 (32.2) | −34.5 |
| Surgery < 3 months | 111 (8.4) | 18 (3.6) | 93 (11.3) | −29.5 |
| Recent antibiotic treatment < 3 months | 327 (24.8) | 114 (22.9) | 213 (25.9) | −6.9 |
| MDRB colonization < 3 months | 149 (11.3) | 48 (9.7) | 101 (12.3) | −8.4 |
| Location before ICU admission d | ||||
| Home | 736 (57.5) | 253/488 (51.8) | 483/792 (61.0) | 34.2 |
| Hospital ward | 368 (28.8) | 131/488 (26.8) | 237/792 (29.9) | |
| Another ICU | 176 (13.8) | 104/488 (21.3) | 72/71,322 (9.1) | |
| Type of admission | 48.3 | |||
| Medical | 1234 (93.5) | 497 (100.0) | 737 (89.6) | |
| Surgical | 86 (6.5) | 0 (0) | 86 (10.4) | |
| Cause of admission | ||||
| Acute respiratory failure | 796 (60.3) | 475 (95.6) | 321 (39.0) | 151.1 |
| ARDS | 360 (27.3) | 263 (52.9) | 97 (11.8) | 97.9 |
| Congestive heart failure | 35 (2.7) | 1 (0.2) | 34 (4.1) | −27.3 |
| Septic shock | 142 (10.8) | 10 (2.0) | 132 (16.0) | −50.5 |
| Shock other than septic | 72 (5.5) | 2 (0.4) | 70 (8.5) | −40.1 |
| Sepsis | 385 (29.2) | 55 (11.1) | 330 (40.1) | −70.6 |
| Community-acquired pneumonia | 130 (9.8) | 38 (7.6) | 92 (11.2) | −12.1 |
| Healthcare-associated pneumonia | 85 (6.4) | 9 (1.8) | 76 (9.2) | −32.9 |
| Intra-abdominal infection | 44 (3.3) | 2 (0.4) | 42 (5.1) | −29.0 |
| Skin and soft tissue infection | 65 (4.9) | 4 (0.8) | 61 (7.4) | −33.8 |
| Neurological deficiency e | 164 (12.4) | 8 (1.6) | 156 (19.0) | −10.7 |
| Acute kidney injury | 91 (6.9) | 9 (1.8) | 82 (9.9) | 2.2 |
| Acute liver failure | 17 (1.3) | 0 (0) | 17 (2.1) | 7.2 |
| Drug poisoning | 99 (7.5) | 28 (5.6) | 71 (8.6) | −11.7 |
| Hanging | 28 (2.1) | 0 (0) | 28 (3.4) | −15.7 |
| Cardiorespiratory arrest | 62 (4.7) | 2 (0.4) | 60 (7.2) | −3.6 |
| Other causes | 111 (8.4) | 12 (2.4) | 98 (11.9) | −36.4 |
| Overall | COVID-19 | |||
|---|---|---|---|---|
n = 1320 | Yes n = 497 | No n = 823 | Standardized Difference, % | |
| Invasive devices and procedures | ||||
| Central venous catheters a | 807 61.5) | 297 (59.9) | 510 (62.4) | −5.2 |
| Arterial catheters b | 952 (73.2) | 430 (87.0) | 522 (64.7) | 54.1 |
| Dialysis catheters | 140 (10.6) | 59 (11.9) | 81 (9.8) | 6.5 |
| Invasive mechanical ventilation | 672 (50.9) | 269 (54.1) | 403 (49.0) | 10.3 |
| Duration (days) c | 8.0 (4.0–17.0) | 14.0 (8.0–24.0) | 6.0 (3.0–11.0) | 91.3 |
| Tracheostomy | 80 (6.1) | 51 (10.3) | 29 (3.5) | 26.8 |
| Prone positioning | 220 (16.7) | 185 (37.2) | 35 (4.3) | 89.0 |
| ECMO/ECLS | 61 (4.6) | 49 (9.9) | 12 (1.5) | 37.0 |
| Treatments | ||||
| Parenteral nutrition | 111 (8.4) | 66 (13.3) | 45 (5.5) | 27.1 |
| Transfusion | 326 (24.7) | 123 (24.7) | 203 (24.7) | 0.2 |
| Antibiotics | 1134 (85.9) | 467 (93.9) | 667 (81.0) | 39.8 |
| Steroids | 619 (46.9) | 376 (75.7) | 243 (29.5) | 104.2 |
| Tocilizumab | 15 (1.1) | 11 (2.2) | 4 (0.5) | 15.0 |
| Other immunosuppressive treatments | 34 (2.6) | 15 (3.0) | 19 (2.3) | 4.4 |
| Overall | COVID-19 | ||
|---|---|---|---|
n = 50 | Yes n = 36 | No n = 14 | |
| Type of MDRB BSI | |||
| ESBL Enterobacteriaceae | 23 (46.0) | 12 (33.3) | 11 (71.4) |
| CR-Enterobacteriaceae | 9 (18.0) | 8 (22.2) | 1 (7.1) |
| CR-Acinetobacter baumannii | 15 (30.0) | 13 (36.1) | 2 (14.2) |
| MDR Pseudomonas aeruginosa | 2 (4.0)) | 2 (5.5) | 0 (0) |
| MRSA | 1 (2.0) | 1 (2.7) | 0 (0) |
| Source of MDRB BSI | |||
| Catheter-related | 12 (24.0) | 10 (27.8) | 2 (14.3) |
| Respiratory tract | 26 (52.0) | 20 (55.6) | 6 (42.9) |
| Unknown | 9 (18.0) | 4 (11.1) | 5 (35.7) |
| Intra-abdominal | 1 (2.0) | 1 (2.8) | 0 (0) |
| Urinary tract | 1 (2.0) | 1 (2.8) | 0 (0) |
| Multiple | 1 (2.0) | 0 (0) | 1 (7.1) |
| COVID-19 | Unadjusted Analysis | Adjusted Analysis c | ||||
|---|---|---|---|---|---|---|
| No (n = 823) | Yes (n = 497) | cHR (95%CI) | p-Value | cHR (95%CI) | p-Value | |
| Overall period a | 14/823 (1.7) | 36/497 (7.2) | 2.22 (1.18 to 4.13) | 0.012 | 2.65 (1.25 to 5.59) | 0.011 |
| 0 to 14 days b | 8/823 (1.0) | 8/497 (1.6) | 1.11 (0.41 to 2.97) | 0.84 | 1.40 (0.49 to 3.99) | 0.53 |
| 15 to 60 days b | 6/137 (4.2) | 28/190 (14.8) | 3.52 (1.45 to 8.52) | 0.005 | 4.35 (1.58 to 11.90) | 0.004 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Piantoni, A.; Houard, M.; Piga, G.; Zebian, G.; Ruffier des Aimes, S.; Holik, B.; Wallet, F.; Rouzé, A.; Kreitmann, L.; Loiez, C.; et al. Relationship between COVID-19 and ICU-Acquired Bloodstream Infections Related to Multidrug-Resistant Bacteria. Antibiotics 2023, 12, 1105. https://doi.org/10.3390/antibiotics12071105
Piantoni A, Houard M, Piga G, Zebian G, Ruffier des Aimes S, Holik B, Wallet F, Rouzé A, Kreitmann L, Loiez C, et al. Relationship between COVID-19 and ICU-Acquired Bloodstream Infections Related to Multidrug-Resistant Bacteria. Antibiotics. 2023; 12(7):1105. https://doi.org/10.3390/antibiotics12071105
Chicago/Turabian StylePiantoni, Antoine, Marion Houard, Gaetan Piga, Ghadi Zebian, Sarah Ruffier des Aimes, Bérénice Holik, Frédéric Wallet, Anahita Rouzé, Louis Kreitmann, Caroline Loiez, and et al. 2023. "Relationship between COVID-19 and ICU-Acquired Bloodstream Infections Related to Multidrug-Resistant Bacteria" Antibiotics 12, no. 7: 1105. https://doi.org/10.3390/antibiotics12071105
APA StylePiantoni, A., Houard, M., Piga, G., Zebian, G., Ruffier des Aimes, S., Holik, B., Wallet, F., Rouzé, A., Kreitmann, L., Loiez, C., Labreuche, J., & Nseir, S. (2023). Relationship between COVID-19 and ICU-Acquired Bloodstream Infections Related to Multidrug-Resistant Bacteria. Antibiotics, 12(7), 1105. https://doi.org/10.3390/antibiotics12071105






