Antibacterial Activity of Dissolved Silver Fractions Released from Silver-Coated Titanium Dental Implant Abutments: A Study on Streptococcus mutans Biofilm Formation
Abstract
1. Introduction
2. Results
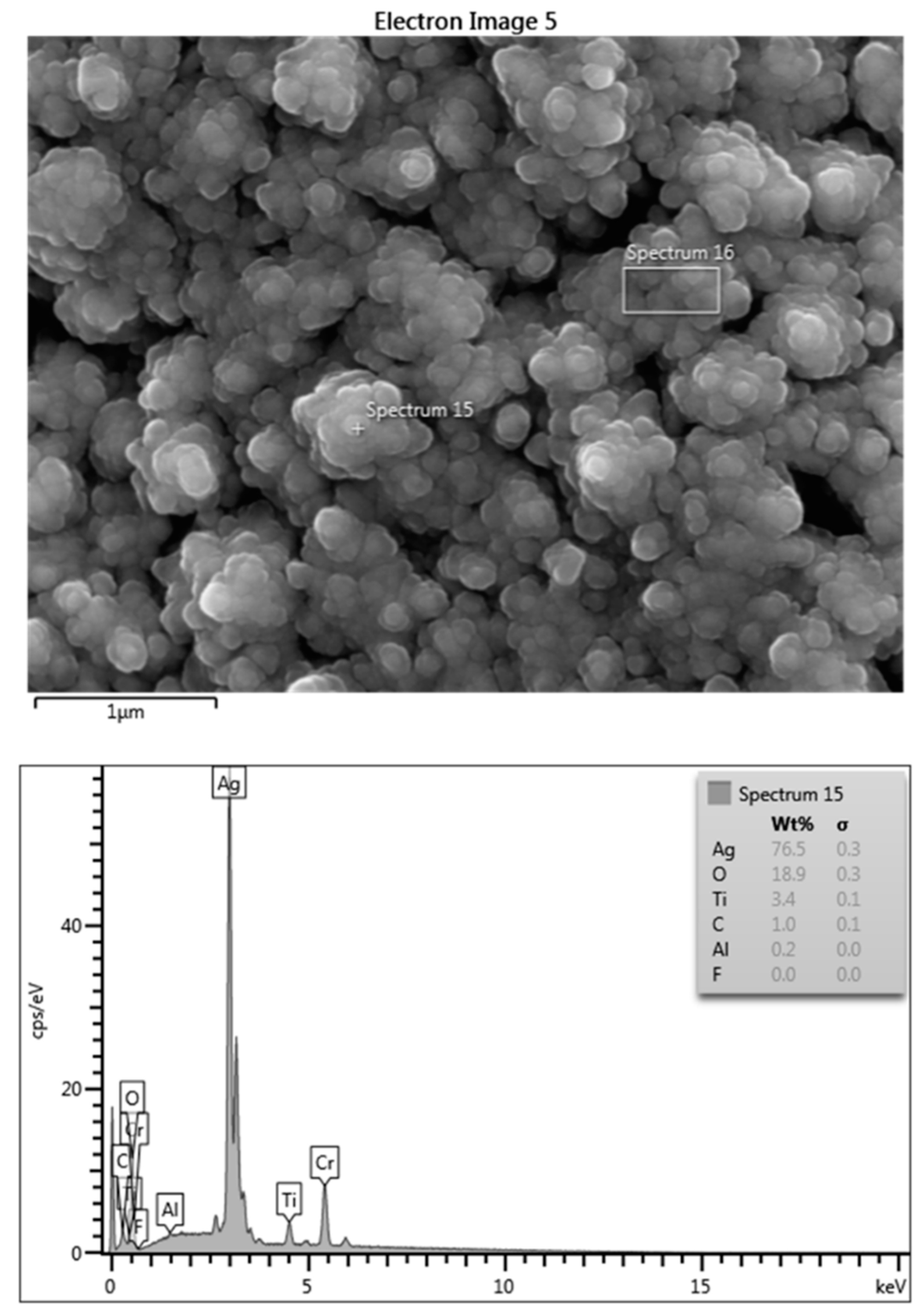
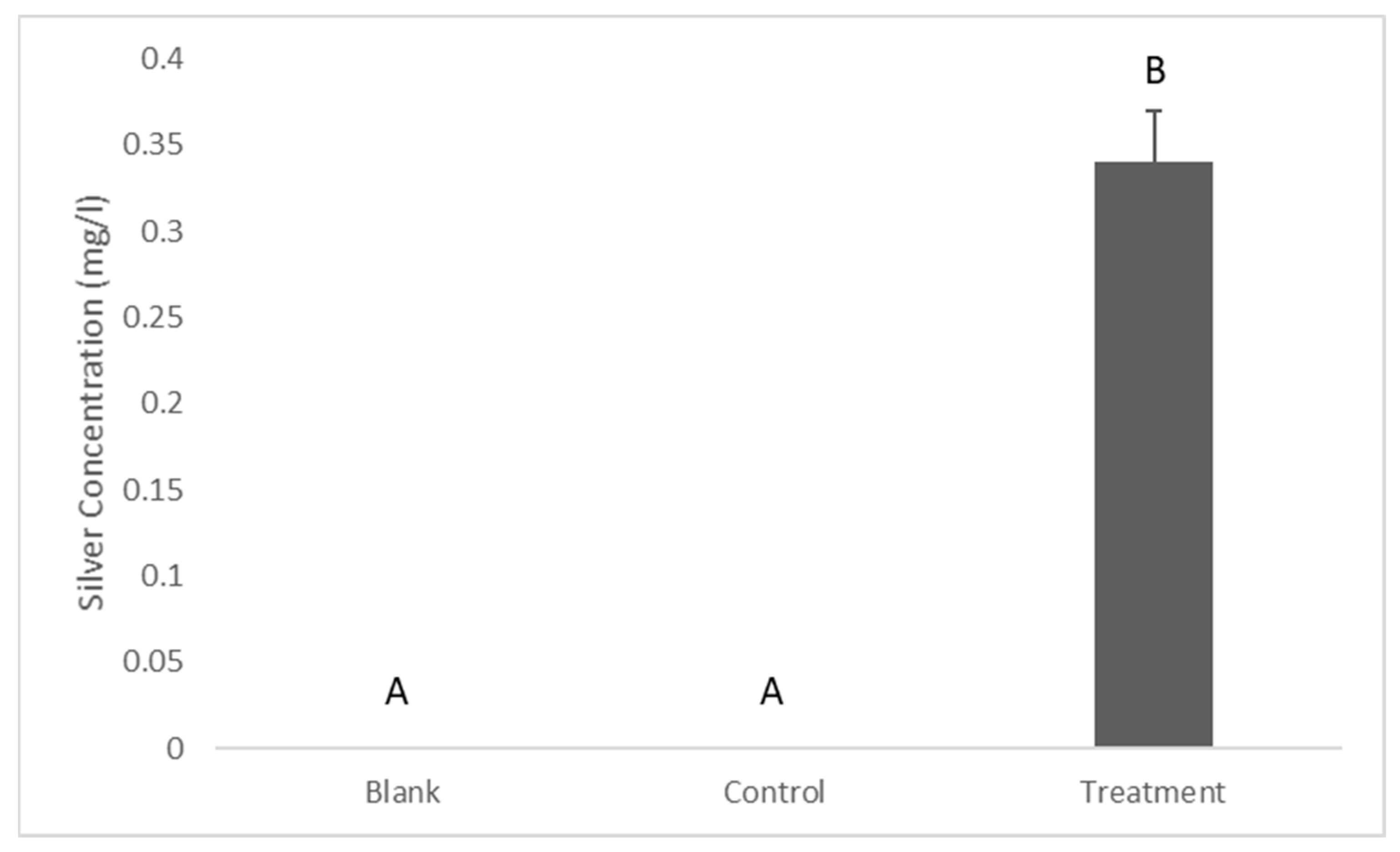
2.1. Release of Silver from the Coating
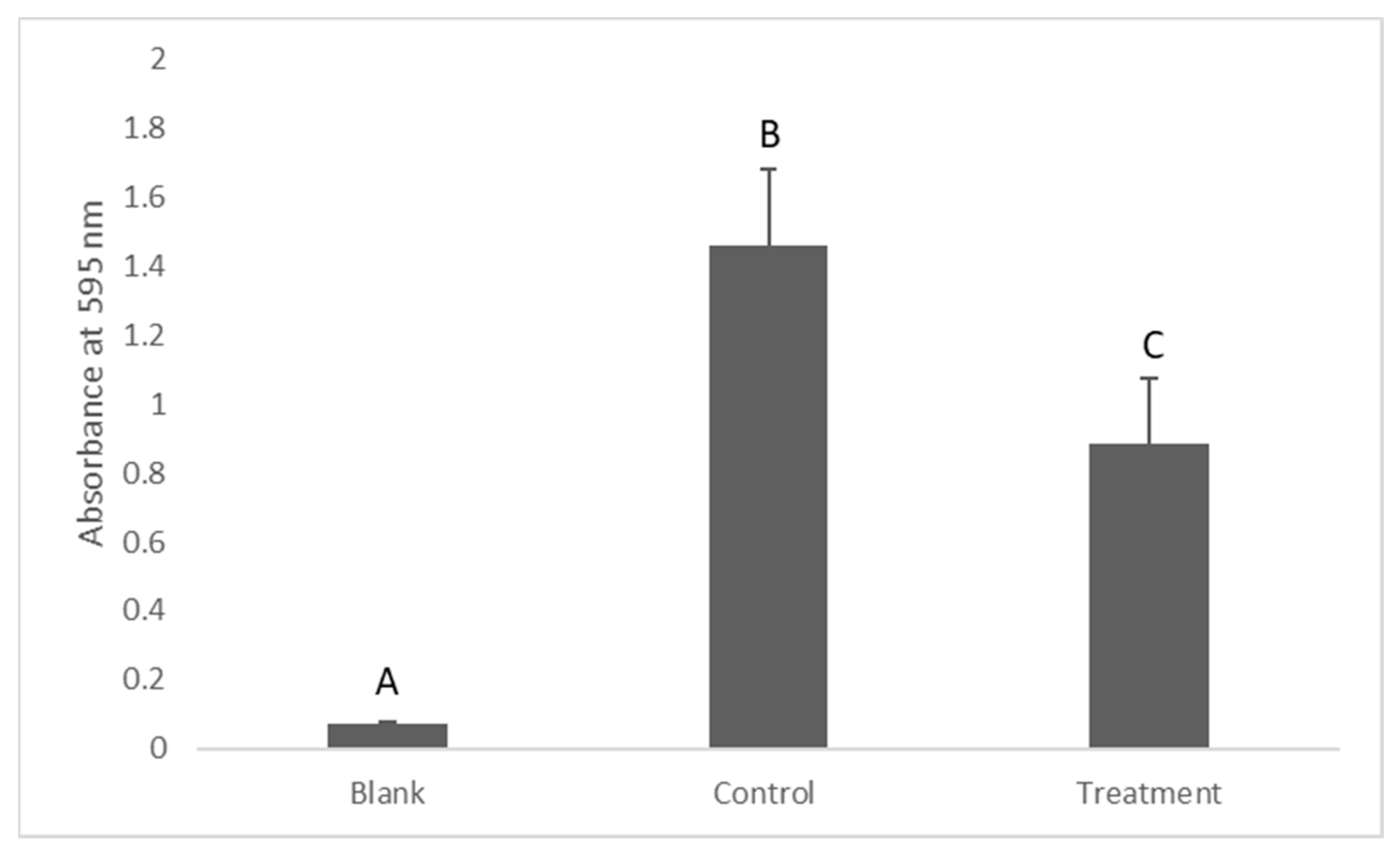
2.2. Assessment of Bacterial Viability in Suspension (Turbidity Measurement)
2.3. Antibiofilm Activity
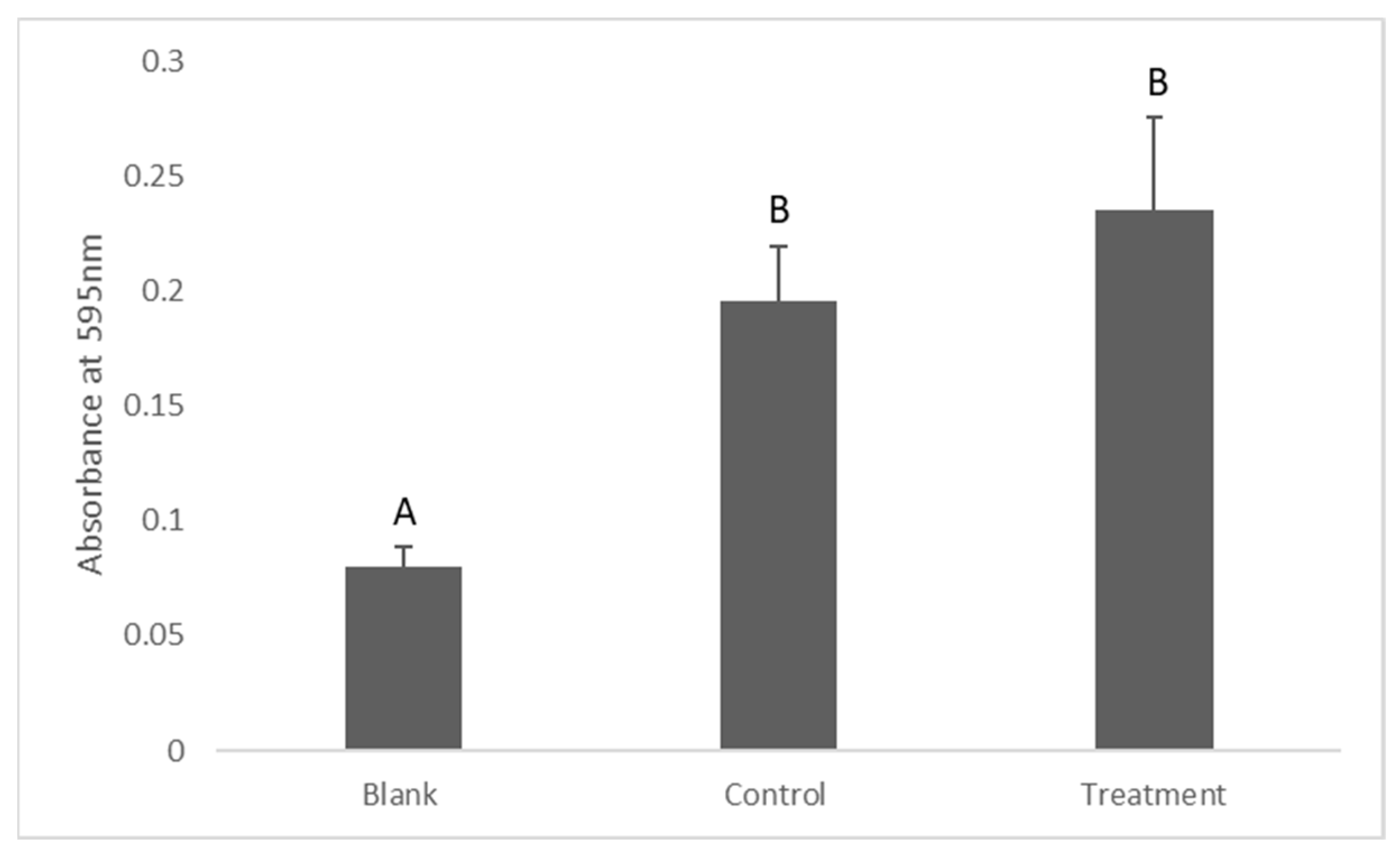
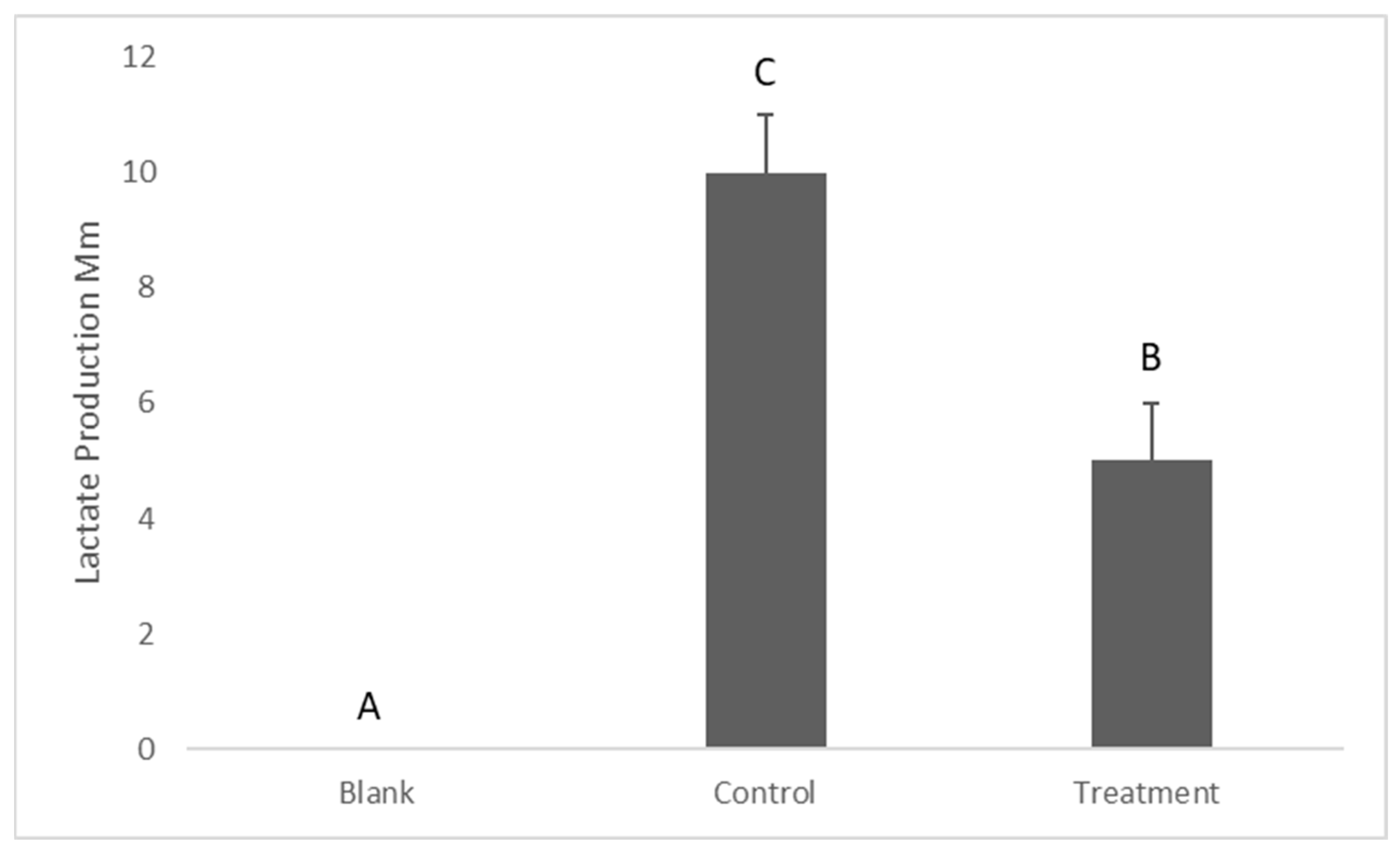
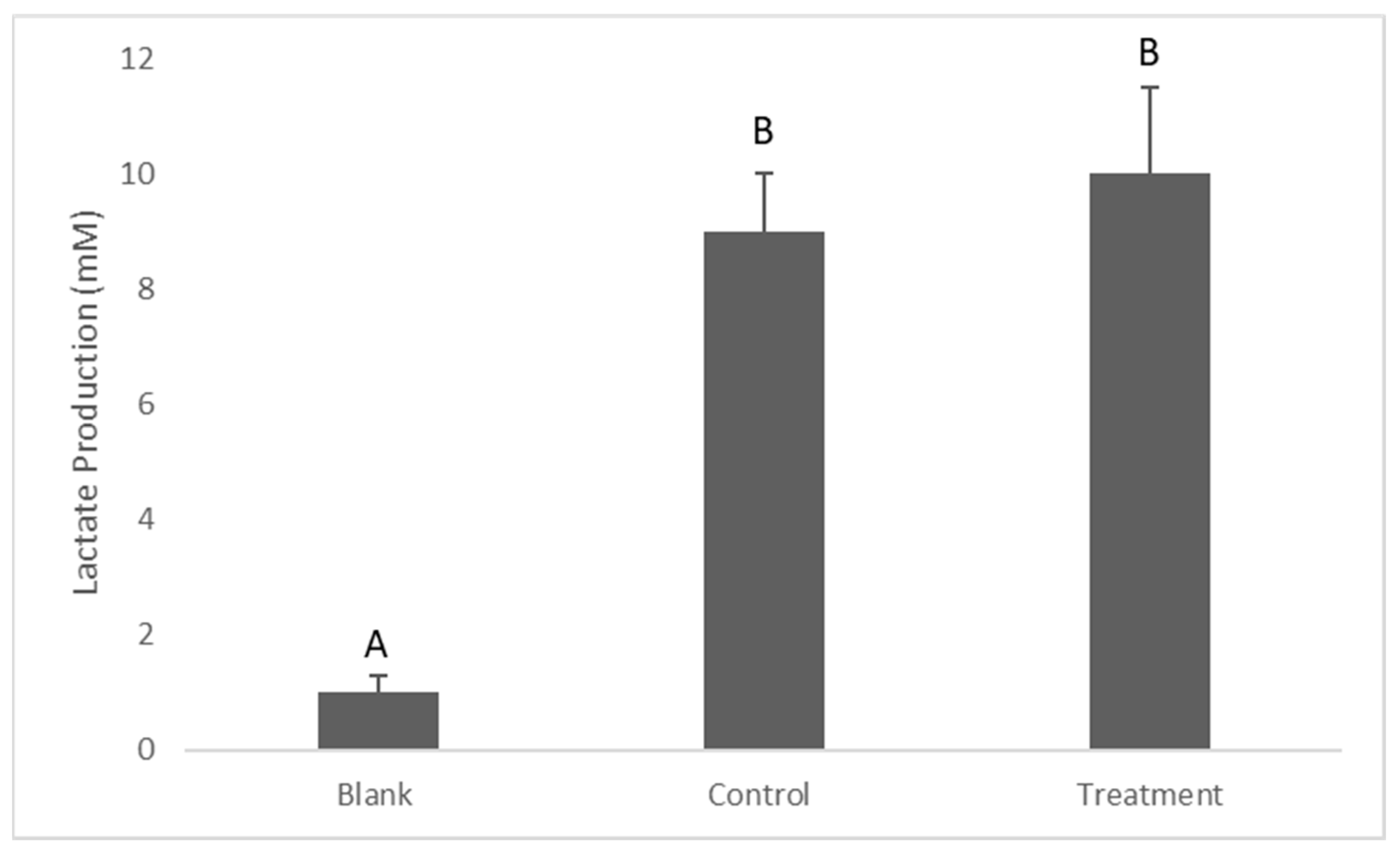
2.4. Determination of Lactate Production
3. Discussion
Assessment of the Antibacterial Activity of Dissolved Silver
4. Materials and Methods
4.1. Specimen Preparation
4.2. Surface Characterization of the Coating via EDS
4.3. Experimental Design
4.4. Silver Release
4.5. Isolation and Identification of S. mutans
4.6. Preparation of Bacterial Suspensions
4.7. Preparation of Treatment and Control Tubes
4.8. Assessing the Antibacterial Activity
4.9. Assessing the Antibiofilm Activity
4.10. Determination of the Lactate Production
4.11. Statistical Analysis
5. Conclusions
6. Limitations and Future Work
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Sykaras, N.; Iacopino, A.M.; Marker, V.A.; Triplett, R.G.; Woody, R.D. Implant materials, designs, and surface topographies: Their effect on osseointegration. A literature review. Int. J. Oral Maxillofac. Implant. 2000, 15, 675–690. [Google Scholar]
- Costa, R.C.; Nagay, B.E.; Bertolini, M.; Costa-Oliveira, B.E.; Sampaio, A.A.; Retamal-Valdes, B.; Shibli, J.A.; Feres, M.; Barao, V.A.; Souza, J.G.S. Fitting pieces into the puzzle: The impact of titanium-based dental implant surface modifications on bacterial accumulation and polymicrobial infections. Adv. Colloid Interface Sci. 2021, 298, 102551. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Chu, P.K.; Zhang, Y.; Wu, Z. Antibacterial coatings on titanium implants. J. Biomed. Mater. Res. Part B Appl. Biomater. 2009, 91, 470–480. [Google Scholar] [CrossRef] [PubMed]
- Souza, J.G.S.; Bertolini, M.M.; Costa, R.C.; Nagay, B.E.; Dongari-Bagtzoglou, A.; Barão, V.A.R. Targeting implant-associated infections: Titanium surface loaded with antimicrobial. IScience 2021, 24, 102008. [Google Scholar] [CrossRef]
- Hämmerle, C.H. Biofilm on dental implants: A review of the literature. Int. J. Oral Maxillofac Implant. 2009, 24, 616. [Google Scholar]
- Quirynen, M.; De Soete, M.; Van Steenberghe, D. Infectious risks for oral implants: A review of the literature. Clin. Oral Implant. Res. Rev. Artic. 2002, 13, 1–19. [Google Scholar] [CrossRef]
- Albrektsson, T.; Brånemark, P.-I.; Hansson, H.-A.; Lindström, J. Osseointegrated titanium implants: Requirements for ensuring a long-lasting, direct bone-to-implant anchorage in man. Acta Orthop. Scand. 1981, 52, 155–170. [Google Scholar] [CrossRef]
- Coelho, P.G.; Granjeiro, J.M.; Romanos, G.E.; Suzuki, M.; Silva, N.R.; Cardaropoli, G.; Thompson, V.P.; Lemons, J.E. Basic research methods and current trends of dental implant surfaces. J. Biomed. Mater. Res. Part B Appl. Biomater. Off. J. Soc. Biomater. Jpn. Soc. Biomater. Aust. Soc. Biomater. Korean Soc. Biomater. 2009, 88, 579–596. [Google Scholar] [CrossRef]
- Salaie, R.N.; Besinis, A.; Le, H.; Tredwin, C.; Handy, R.D. The biocompatibility of silver and nanohydroxyapatite coatings on titanium dental implants with human primary osteoblast cells. Mater. Sci. Eng. C 2020, 107, 110210. [Google Scholar] [CrossRef]
- Haugen, H.J.; Makhtari, S.; Ahmadi, S.; Hussain, B. The Antibacterial and Cytotoxic Effects of Silver Nanoparticles Coated Titanium Implants: A Narrative Review. Materials 2022, 15, 5025. [Google Scholar] [CrossRef]
- Radin, S.; Campbell, J.T.; Ducheyne, P.; Cuckler, J.M. Calcium phosphate ceramic coatings as carriers of vancomycin. Biomaterials 1997, 18, 777–782. [Google Scholar] [CrossRef] [PubMed]
- Alt, V.; Bitschnau, A.; Österling, J.; Sewing, A.; Meyer, C.; Kraus, R.; Meissner, S.A.; Wenisch, S.; Domann, E.; Schnettler, R. The effects of combined gentamicin–hydroxyapatite coating for cementless joint prostheses on the reduction of infection rates in a rabbit infection prophylaxis model. Biomaterials 2006, 27, 4627–4634. [Google Scholar] [CrossRef] [PubMed]
- Kozlovsky, A.; Artzi, Z.; Moses, O.; Kamin-Belsky, N.; Greenstein, R.B.N. Interaction of chlorhexidine with smooth and rough types of titanium surfaces. J. Periodontol. 2006, 77, 1194–1200. [Google Scholar] [CrossRef]
- Lessa, F.C.R.; Aranha, A.M.F.; Nogueira, I.; Giro, E.M.A.; Hebling, J.; Costa, C.A.d.S. Toxicity of chlorhexidine on odontoblast-like cells. J. Appl. Oral Sci. 2010, 18, 50–58. [Google Scholar] [CrossRef]
- Tomsia, A.P.; Lee, J.S.; Wegst, U.G.; Saiz, E. Nanotechnology for dental implants. Oral Craniofacial Tissue Eng. 2012, 2, 915327. [Google Scholar] [CrossRef]
- Morones, J.R.; Elechiguerra, J.L.; Camacho, A.; Holt, K.; Kouri, J.B.; Ramírez, J.T.; Yacaman, M.J. The bactericidal effect of silver nanoparticles. Nanotechnology 2005, 16, 2346. [Google Scholar] [CrossRef] [PubMed]
- Besinis, A.; De Peralta, T.; Handy, R.D. The antibacterial effects of silver, titanium dioxide and silica dioxide nanoparticles compared to the dental disinfectant chlorhexidine on Streptococcus mutans using a suite of bioassays. Nanotoxicology 2014, 8, 1–16. [Google Scholar] [CrossRef]
- Vilarrasa, J.; Delgado, L.M.; Galofré, M.; Àlvarez, G.; Violant, D.; Manero, J.M.; Blanc, V.; Gil, F.J.; Nart, J. In vitro evaluation of a multispecies oral biofilm over antibacterial coated titanium surfaces. J. Mater. Sci. Mater. Med. 2018, 29, 1–10. [Google Scholar] [CrossRef]
- Pokrowiecki, R.; Zaręba, T.; Szaraniec, B.; Pałka, K.; Mielczarek, A.; Menaszek, E.; Tyski, S. In vitro studies of nanosilver-doped titanium implants for oral and maxillofacial surgery. Int. J. Nanomed. 2017, 12, 4285. [Google Scholar] [CrossRef]
- Reidy, B.; Haase, A.; Luch, A.; Dawson, K.A.; Lynch, I. Mechanisms of silver nanoparticle release, transformation and toxicity: A critical review of current knowledge and recommendations for future studies and applications. Materials 2013, 6, 2295–2350. [Google Scholar] [CrossRef]
- Greulich, C.; Braun, D.; Peetsch, A.; Diendorf, J.; Siebers, B.; Epple, M.; Köller, M. The toxic effect of silver ions and silver nanoparticles towards bacteria and human cells occurs in the same concentration range. RSC Adv. 2012, 2, 6981–6987. [Google Scholar] [CrossRef]
- Albers, C.E.; Hofstetter, W.; Siebenrock, K.A.; Landmann, R.; Klenke, F.M. In vitro cytotoxicity of silver nanoparticles on osteoblasts and osteoclasts at antibacterial concentrations. Nanotoxicology 2013, 7, 30–36. [Google Scholar] [CrossRef] [PubMed]
- Gunputh, U.F.; Le, H.; Lawton, K.; Besinis, A.; Tredwin, C.; Handy, R.D. Antibacterial properties of silver nanoparticles grown in situ and anchored to titanium dioxide nanotubes on titanium implant against Staphylococcus aureus. Nanotoxicology 2020, 14, 97–110. [Google Scholar] [CrossRef]
- Loza, K.; Diendorf, J.; Sengstock, C.; Ruiz-Gonzalez, L.; Gonzalez-Calbet, J.; Vallet-Regi, M.; Köller, M.; Epple, M. The dissolution and biological effects of silver nanoparticles in biological media. J. Mater. Chem. B 2014, 2, 1634–1643. [Google Scholar] [CrossRef]
- Salaie, R. Nano Enhanced Surface Modification of Titanium Dental Implants for Improving Osseointegration and Biocompatibility; University of Plymouth: Plymouth, UK, 2018. [Google Scholar]
- Shi, J.; Karlsson, H.L.; Johansson, K.; Gogvadze, V.; Xiao, L.; Li, J.; Burks, T.; Garcia-Bennett, A.; Uheida, A.; Muhammed, M. Microsomal glutathione transferase 1 protects against toxicity induced by silica nanoparticles but not by zinc oxide nanoparticles. ACS Nano 2012, 6, 1925–1938. [Google Scholar] [CrossRef] [PubMed]
- Park, H.-J.; Kim, J.Y.; Kim, J.; Lee, J.-H.; Hahn, J.-S.; Gu, M.B.; Yoon, J. Silver-ion-mediated reactive oxygen species generation affecting bactericidal activity. Water Res. 2009, 43, 1027–1032. [Google Scholar] [CrossRef]
- Meran, Z.; Besinis, A.; De Peralta, T.; Handy, R.D. Antifungal properties and biocompatibility of silver nanoparticle coatings on silicone maxillofacial prostheses in vitro. J. Biomed. Mater. Res. Part B Appl. Biomater. 2018, 106, 1038–1051. [Google Scholar] [CrossRef]
- Costa, R.C.; Bertolini, M.; Costa Oliveira, B.E.; Nagay, B.E.; Dini, C.; Benso, B.; Klein, M.I.; Barāo, V.A.; Souza, J.G.S. Polymicrobial biofilms related to dental implant diseases: Unravelling the critical role of extracellular biofilm matrix. Crit. Rev. Microbiol. 2023, 49, 370–390. [Google Scholar] [CrossRef] [PubMed]
- Hellstrand, E.L.I.; Andersson, A.; Drakenberg, T.; Dahlbäck, B.; Dawson, K.A.; Linse, S.; Cedervall, T. Complete high-density lipoproteins in nanoparticle corona. FEBS J. 2009, 276, 3372–3381. [Google Scholar] [CrossRef] [PubMed]
- Besinis, A.; De Peralta, T.; Handy, R.D. Inhibition of biofilm formation and antibacterial properties of a silver nano-coating on human dentine. Nanotoxicology 2014, 8, 745–754. [Google Scholar] [CrossRef]
- Christensen, G.D.; Simpson, W.A.; Bisno, A.L.; Beachey, E.H. Adherence of slime-producing strains of Staphylococcus epidermidis to smooth surfaces. Infect. Immun. 1982, 37, 318–326. [Google Scholar] [CrossRef] [PubMed]
- Gawehn, K. D-(-)-Lactate determination with lactate dehydrogenase and NAD. Methods Enzym. Anal. 1974, 3, 1492–1495. [Google Scholar]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salaie, R.N.; Hassan, P.A.; Meran, Z.D.; Hamad, S.A. Antibacterial Activity of Dissolved Silver Fractions Released from Silver-Coated Titanium Dental Implant Abutments: A Study on Streptococcus mutans Biofilm Formation. Antibiotics 2023, 12, 1097. https://doi.org/10.3390/antibiotics12071097
Salaie RN, Hassan PA, Meran ZD, Hamad SA. Antibacterial Activity of Dissolved Silver Fractions Released from Silver-Coated Titanium Dental Implant Abutments: A Study on Streptococcus mutans Biofilm Formation. Antibiotics. 2023; 12(7):1097. https://doi.org/10.3390/antibiotics12071097
Chicago/Turabian StyleSalaie, Ranj Nadhim, Pakhshan A. Hassan, Zhala Dara Meran, and Shehab Ahmed Hamad. 2023. "Antibacterial Activity of Dissolved Silver Fractions Released from Silver-Coated Titanium Dental Implant Abutments: A Study on Streptococcus mutans Biofilm Formation" Antibiotics 12, no. 7: 1097. https://doi.org/10.3390/antibiotics12071097
APA StyleSalaie, R. N., Hassan, P. A., Meran, Z. D., & Hamad, S. A. (2023). Antibacterial Activity of Dissolved Silver Fractions Released from Silver-Coated Titanium Dental Implant Abutments: A Study on Streptococcus mutans Biofilm Formation. Antibiotics, 12(7), 1097. https://doi.org/10.3390/antibiotics12071097





