Resistant Bacteria in Broiler Litter Used as Ruminant Feed: Effect of Biotic Treatment
Abstract
1. Introduction
2. Results
3. Discussion
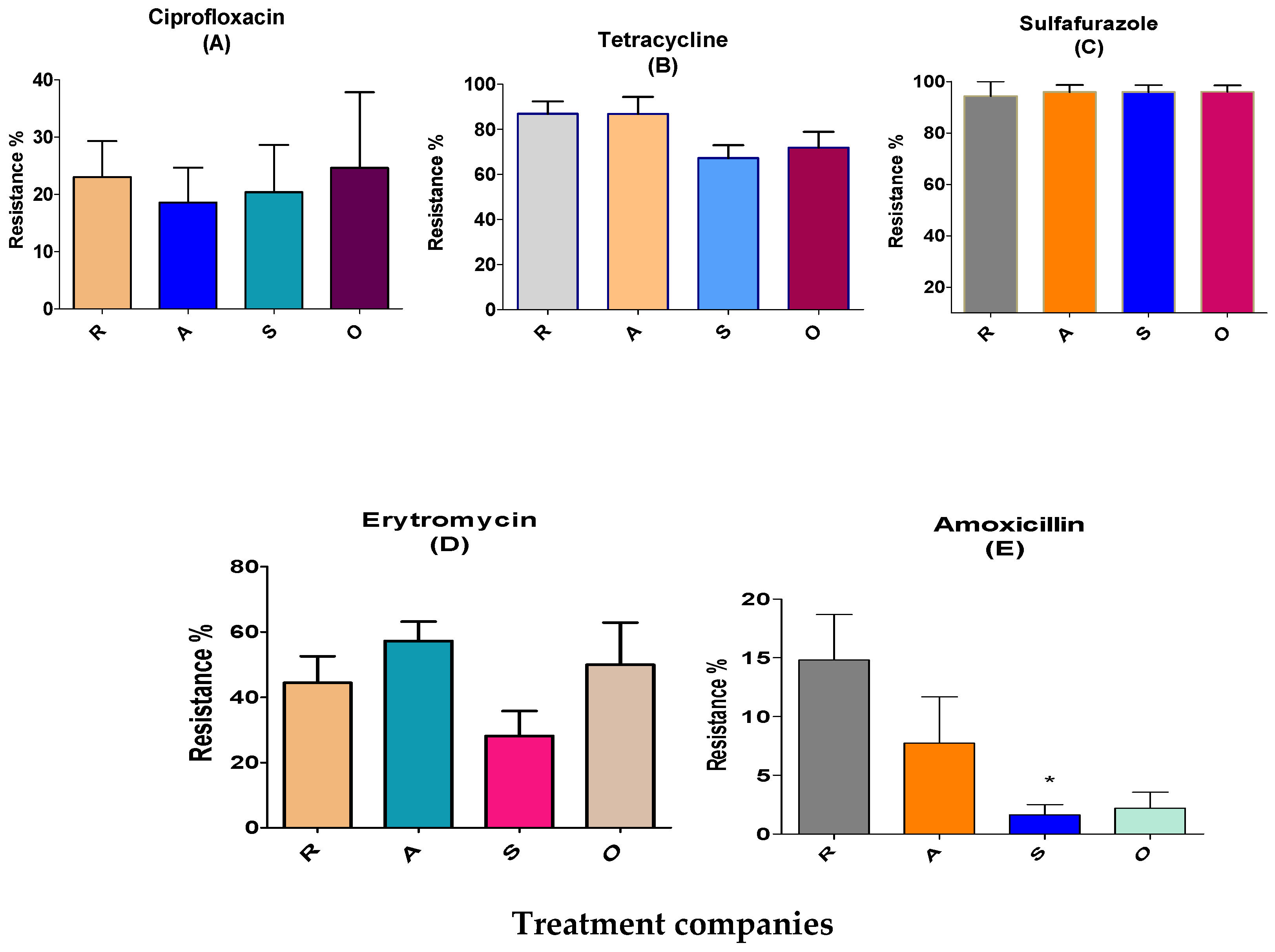
3.1. Evaluation of the Effects of Different Broiler Treatments on Antibiotic-Resistant Bacteria
3.2. Relationship between Resistant/Multi-Resistant Bacteria and Antimicrobial-Independent Therapy in Broiler Farms
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Sample Collection
4.3. Designing a Method for Aerobic, Anaerobic and Stacking BL Treatment in the Laboratory
4.4. Sample Preparation
4.5. Isolation, Identification and Confirmation
4.6. Kirby–Bauer Test for Antimicrobial Resistance
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hofmann, T.; Schmucker, S.S.; Bessei, W.; Grashorn, M.; Stefanski, V. Impact of Housing Environment on the Immune System in Chickens: A Review. Animals 2020, 10, 1138. [Google Scholar] [CrossRef] [PubMed]
- Hyun, S.H.; Sakaguchi, G. Implication of coprophagy in pathogenesis of chicken botulism. Nihon Juigaku Zasshi 1989, 51, 582–586. [Google Scholar] [CrossRef]
- McKeith, A.; Loper, M.; Tarrant, K.J. Research Note: Stocking density effects on production qualities of broilers raised without the use of antibiotics. Poult. Sci. 2020, 99, 698–701. [Google Scholar] [CrossRef]
- Tarakdjian, J.; Capello, K.; Pasqualin, D.; Cunial, G.; Lorenzetto, M.; Gavazzi, L.; Manca, G.; Di Martino, G. Antimicrobial use in broilers reared at different stocking densities: A retrospective study. Animals 2020, 10, 1751. [Google Scholar] [CrossRef]
- Neilson, Z. The case of the exploded Dutch chickens. Sustain. Food Trust 2016, 29, 2020–2029. [Google Scholar]
- Witte, W.; Klare, I.; Werner, G. Selective pressure by antibiotics as feed additives. Infection 1999, 27, 35–38. [Google Scholar] [CrossRef]
- Van Boeckel, T.P.; Brower, C.; Gilbert, M.; Grenfell, B.T.; Levin, S.A.; Robinson, T.P. Global trends in antimicrobial use in food animals. Proc. Natl. Acad. Sci. USA 2015, 112, 5649–5654. [Google Scholar] [CrossRef] [PubMed]
- Manyi-Loh, C.; Mamphweli, S.; Meyer, E.; Okoh, A. Antibiotic use in agriculture and its consequential resistance in environmental sources: Potential public health implications. Molecules 2018, 23, 795. [Google Scholar] [CrossRef] [PubMed]
- Keen, P.L.; Patrick, D.M. Tracking change: A look at the ecological foot-print of antibiotics and antimicrobial resistance. Antibiotics 2013, 2, 191–205. [Google Scholar] [CrossRef] [PubMed]
- Lai, C.C.; Wang, C.Y.; Chu, C.C.; Tan, C.K.; Lu, C.L.; Lee, Y.C.; Huang, Y.T.; Lee, P.I.; Hsueh, P.R. Correlation between antibiotic consumption and resistance of Gram-negative bacteria causing healthcare-associated infections at a university hospital in Taiwan from 2000 to 2009. J. Antimicrob. Chemother. 2011, 66, 1374–1382. [Google Scholar] [CrossRef]
- Klaver, A.L.; Matthews, R.A. Effects of oxytetracycline on nitrification in a model aquatic system. Aquaculture 1994, 123, 237–247. [Google Scholar] [CrossRef]
- Dolliver, H.; Gupta, S.; Noll, S. Antibiotic degradation during manure composting. J. Environ. Qual. 2008, 37, 1245–1253. [Google Scholar] [CrossRef]
- Fleming, A. On the antibacterial action of cultures of a Penicillium with special reference to their use in the isolation of B. influenza. Br. J. Exp. Pathol. 1929, 10, 226–236. [Google Scholar] [CrossRef]
- Swann, M.M.; Baxter, K.L.; Field, H.I. Report of the Joint Committee on the Use of Antibiotics in Animal Husbandry and Veterinary Medicine; MHSO: London, UK, 1969. [Google Scholar]
- Smith, J.L.; Drum, D.J.V.; Dai, Y.; Kim, J.M.; Sanchez, S.; Maurer, J.J.; Hofacre, C.L.; Lee, M.D. Impact of antimicrobial usage on antimicrobial resistance in commensal Escherichia coli strains colonizing broiler chickens. Appl. Environ. Microbiol. 2007, 73, 1404–1414. [Google Scholar] [CrossRef]
- Wagenaar, J.A.; van der Giessen, A.W. Veegerelateerd MRSA: Epidemiologie in Dierlijke Productieketens, Tranmissie Naar de Mens en Karakteristieken van de Kloon; RIVM: Bilthoven, The Netherlands, 2009. [Google Scholar]
- Kawano, J.; Shimizu, A.; Saitoh, Y.; Yagi, M.; Saito, T.; Okamoto, R. Isolation of methicillin-resistant coagulase-negative staphylococci from chickens. J. Clin. Microbiol. 1996, 34, 2072–2077. [Google Scholar] [CrossRef] [PubMed]
- Munita, J.M.; Arias, C.A. Mechanisms of antibiotic resistance. Microbiol. Spectr. 2016, 4, 481–511. [Google Scholar] [CrossRef]
- Butler, M.S.; Buss, A.D. Natural products-the future scaffolds for novel antibiotics. Biochem. Pharmacol. 2006, 71, 919–929. [Google Scholar] [CrossRef]
- Subirats, J.; Murray, R.; Scott, A.; Lau, C.H.F.; Topp, E. Composting of chicken litter from commercial broiler farms reduces the abundance of viable enteric bacteria, Firmicutes, and selected antibiotic resistance genes. Sci. Total Environ. 2020, 746, 141113. [Google Scholar] [CrossRef]
- Ho, Y.B.; Zakaria, M.P.; Latif, P.A.; Saari, N. Degradation of veterinary antibiotics and hormone during broiler manure composting. Bioresour. Technol. 2013, 131, 476–484. [Google Scholar] [CrossRef]
- Kim, Y.J.; Park, J.H.; Seo, K.H. Comparison of the loads and antibiotic-resistance profiles of Enterococcus species from conventional and organic chick-en carcasses in South Korea. Poult. Sci. 2018, 97, 271–278. [Google Scholar] [CrossRef]
- Devriese, L.; Baele, M.; Butaye, P. The Genus Enterococcus. In The Prokaryotes; Springer: New York, NY, USA, 2006; pp. 163–174. [Google Scholar]
- Hanchi, H.; Mottawea, W.; Sebei, K.; Hammami, R. The genus Enterococcus: Between probiotic potential and safety concerns-an update. Front. Microbiol. 2018, 9, 1791. [Google Scholar] [CrossRef] [PubMed]
- García-Solache, M.; Rice, L.B. The enterococcus: A model of adaptability to its environment. Clin. Microbiol. Rev. 2019, 32, e00058-18. [Google Scholar] [CrossRef]
- Shiadeh, S.M.J.; Pormohammad, A.; Hashemi, A.; Lak, P. Global prevalence of antibiotic resistance in blood-isolated Enterococcus faecalis and Enterococcus faecium: A systematic review and meta-analysis. Infect. Drug Resist. 2019, 12, 2713–2725. [Google Scholar] [CrossRef] [PubMed]
- Esperón, F.; Albero, B.; Ugarte-ruíz, M.; Domínguez, L.; Carballo, M.; Tadeo, J.L.; Delgado, M.; Moreno, M.Á.; De Torre, A. Assessing the benefits of composting poultry manure in reducing antimicrobial residues, pathogenic bacteria, and antimicrobial resistance genes : A field-scale study. Environ. Sci. Pollut. Res. 2020, 27, 27738–27749. [Google Scholar] [CrossRef] [PubMed]
- Gurtler, J.B.; Doyle, M.P.; Erickson, M.C.; Jiang, X.; Millner, P.; Sharma, M. Composting to inactivate foodborne pathogens for crop soil application. J. Food Prot. 2018, 81, 1821–1837. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Diao, J.; Shepherd, M.W.; Singh, R.; Heringa, S.D.; Gong, C.; Jiang, X. Validating thermal inactivation of Salmonella spp. in fresh and aged chicken litter. Appl. Environ. Microbiol. 2012, 78, 1302–1307. [Google Scholar] [CrossRef]
- Wilkinson, K.G.; Tee, E.; Tomkins, R.B.; Hepworth, G.; Premier, R. Effect of Heating and Aging of Poultry Litter on the Persistence of Enteric Bacteria. Poult. Sci. 2011, 90, 10–18. [Google Scholar] [CrossRef]
- Gurmessa, B.; Pedretti, E.F.; Cocco, S.; Cardelli, V.; Corti, G. Manure anaerobic digestion effects and the role of pre- and post-treatments on veterinary anti-biotics and antibiotic resistance genes removal efficiency. Sci. Total Environ. 2020, 721, 137532. [Google Scholar] [CrossRef]
- Youngquist, C.P.; Mitchell, S.M.; Cogger, C.G. Fate of Antibiotics and Anti-biotic Resistance during Digestion and Composting: A Review. Environment 2016, 45, 537–545. [Google Scholar] [CrossRef]
- Chander, Y.; Gupta, S.C.; Kumar, K.; Goyal, S.M.; Murray, H. Antibiotic use and the prevalence of antibiotic resistant bacteria on turkey farms. J. Sci. Food Agric. 2008, 88, 714–719. [Google Scholar] [CrossRef]
- Thibodeau, A.; Quessy, S.; Guévremont, E.; Houde, A.; Topp, E.; Diarra, M.S.; Letellier, A. Antibiotic resistance in Escherichia coli and Enterococcus spp. isolates from commercial broiler chickens receiving growth-promoting doses of bacitracin or virginiamycin. Can. J. Vet. Res. 2008, 72, 129–136. [Google Scholar] [PubMed]
- Wielders, C.L.; Vriens, S.; Brisse, L.A.; de Graaf-Miltenburg, A.; Troelstra, A.; Fleer, F.J.; Schmitz, J.; Verhoef, A.C. In-vivo transfer of mecA to Staphylococcus aureus. Lancet 2001, 357, 1674–1675. [Google Scholar] [CrossRef] [PubMed]
- Palmer, K.L.; Kos, V.N.; Gilmore, M.S. Horizontal gene transfer and the ge-nomics of enterococcal antibiotic resistance. Curr. Opin. Microbiol. 2010, 13, 632–639. [Google Scholar] [CrossRef] [PubMed]
- Hayes, J.R.; English, L.E.; Carr, D.D.; Wagner, S.W. Multiple-antibiotic resistance of Enterococcus spp. isolated from commercial poultry pro-duction environments. Appl. Environ. Microbiol. 2004, 70, 6005–6011. [Google Scholar] [CrossRef]
- Schulz, J.; Friese, A.; Klees, S.; Tenhagen, B.A.; Fetsch, A.; Rösler, U.; Hartung, J. Longitudinal study of the contamination of air and of soil surfaces in the vicinity of pig barns by livestock-associated methicillin-resistant Staphylococcus aureus. Appl. Environ. Microbiol. 2012, 78, 5666–5671. [Google Scholar] [CrossRef]
- Kayser, F.H. Safety aspects of enterococci from the medical point of view. Int. J. Food Microbiol. 2003, 88, 255–262. [Google Scholar] [CrossRef]
- Shorr, A.F. Epidemiolog y of staphylococcal resistance. Clin. Infect. Dis. 2007, 45, 171–176. [Google Scholar] [CrossRef]
- Magill, S.S.; Edwards, J.R.; Bamberg, W.; Beldavs, Z.G.; Dumyati, G.; Kainer, M.A.; Lynfield, R.; Maloney, M.; McAllister-Hollod, L.; Nadle, J.; et al. Multistate Point-Prevalence Survey of Health Care-Associated Infections for the Emerging Infections Program Healthcare-Associated Infections and Antimicrobial Use Prevalence Survey Team Centers for Disease Control and Prevention. N. Engl. J. Med. 2014, 370, 1198–1208. [Google Scholar] [CrossRef]
- Khurana, C.M.; Wojack, B.R. Prevalence of ciprofloxacin resistance in multiresistant Gram-negative intensive care unit isolates. Infection 1994, 22, S99–S104. [Google Scholar] [CrossRef]
- Furtula, V.; Jackson, C.R.; Farrell, E.G.; Barrett, J.B.; Hiott, L.M.; Chambers, P.A. Antimicrobial resistance in Enterococcus spp. isolated from environmental samples in an area of intensive poultry production. Int. J. Environ. Res. Public Health 2013, 10, 1020–1036. [Google Scholar] [CrossRef]
- Singhal, N.; Kumar, M.; Kanaujia, P.K.; Virdi, J.S. MALDI-TOF mass spectrometry: An emerging technology for microbial identification and diagnosis. Front. Microbiol. 2015, 6, 791. [Google Scholar] [CrossRef] [PubMed]
- Peplow, M.O.; Correa-Prisant, M.; Stebbins, M.E.; Jones, F.; Davies, P. Sensitivity, specificity and predictive values of three Salmonella rapid detection kits using fresh and frozen poultry environmental samples versus those of standard plating. Appl. Environ. Microbiol. 1999, 65, 1055–1060. [Google Scholar] [CrossRef] [PubMed]
- Roberts, M.C.; Meschke, J.S.; Soge, O.O.; Reynolds, K.A. Comment on MRSA studies in high school wrestling and athletic training facilities. Environ. Health 2010, 72, 48–49. [Google Scholar]
- McLain, J.E.T.; Rock, C.M.; Lohse, K.; Walworth, J. False positive identification of Escherichia coli in treated municipal wastewater and wastewater-irrigated soils. Can. J. Microbiol. 2011, 57, 775–784. [Google Scholar] [CrossRef]
- Aminov, R.I.; Mackie, R.I. Evolution and ecology of antibiotic resistance genes. FEMS Microbiol. Lett. 2007, 27, 147–161. [Google Scholar] [CrossRef] [PubMed]


| Treatment Companies | South (R) | North West (A) | ||||||
|---|---|---|---|---|---|---|---|---|
| Species | Enteroccocus | E. coli | Salmonella | Staph aureus | Enteroccocus | E. coli | Salmonella | Staph aureus |
| N (n), C | 15 (15), 283 | 15 (4), 39 | 15 (0) | 15 (0) | 11 (11), 153 | 11 (1), 34 | 11 (0) | 11 (0) |
| Treatment companies | North East (S; antimicrobial-independent) | Others (O) | ||||||
| Species | Enteroccocus | E. coli | Salmonella | Staph aureus | Enteroccocus | E. coli | Salmonella | Staph aureus |
| N (n), C | 11 (11), 189 | 11 (0) | 11 (0) | 11 (0) | 5 (5), 88 | 5 (0) | 5 (0) | 5 (0) |
| Enteroccocus | E. coli | Staph aureus and Salmonella | ||||
|---|---|---|---|---|---|---|
| Treatment | Treatment | Treatment | ||||
| before | after | before | after | before | after | |
| Prevalence | 42/42 | 3/42 | 5/42 | ND/42 | ND/42 | ND/42 |
| Isolated colonies | 713 | 42 | 144 | ND | ND | ND |
| Sulfafurazole resistance, % | 96 | 19 | 10 | ND | ND | ND |
| Tetracycline resistance, % | 78 | 16 | 10 | ND | ND | ND |
| Erythromycin resistance, % | 45 | 11 | NS | NS | ND | NS |
| Ciprofloxacin resistance, % | 22 | 7 | 5 | ND | ND | ND |
| Amoxicillin resistance, % | 7 | 1 | 7 | ND | ND | ND |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Efriem, S.; Sabastian, C.; Blum, S.; Fleker, M.; Mabjeesh, S.J.; Britzi, M. Resistant Bacteria in Broiler Litter Used as Ruminant Feed: Effect of Biotic Treatment. Antibiotics 2023, 12, 1093. https://doi.org/10.3390/antibiotics12071093
Efriem S, Sabastian C, Blum S, Fleker M, Mabjeesh SJ, Britzi M. Resistant Bacteria in Broiler Litter Used as Ruminant Feed: Effect of Biotic Treatment. Antibiotics. 2023; 12(7):1093. https://doi.org/10.3390/antibiotics12071093
Chicago/Turabian StyleEfriem, Solomon, Chris Sabastian, Shlomo Blum, Marcelo Fleker, Sameer J. Mabjeesh, and Malka Britzi. 2023. "Resistant Bacteria in Broiler Litter Used as Ruminant Feed: Effect of Biotic Treatment" Antibiotics 12, no. 7: 1093. https://doi.org/10.3390/antibiotics12071093
APA StyleEfriem, S., Sabastian, C., Blum, S., Fleker, M., Mabjeesh, S. J., & Britzi, M. (2023). Resistant Bacteria in Broiler Litter Used as Ruminant Feed: Effect of Biotic Treatment. Antibiotics, 12(7), 1093. https://doi.org/10.3390/antibiotics12071093







