An In Vitro Study of Local Oxygen Therapy as Adjunctive Antimicrobial Therapeutic Option for Patients with Periodontitis
Abstract
1. Introduction
2. Results
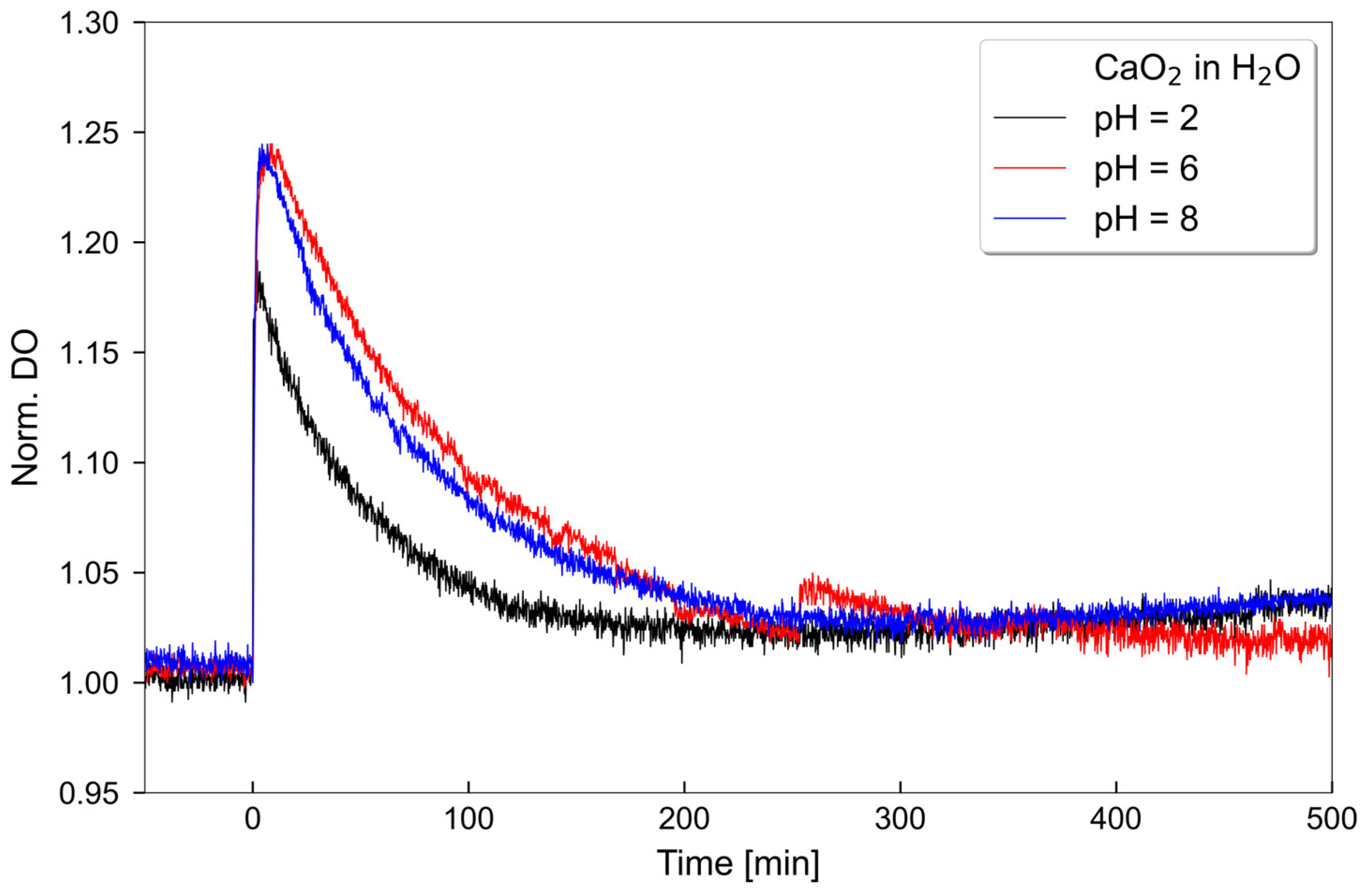
2.1. Oxygen Release Measurements
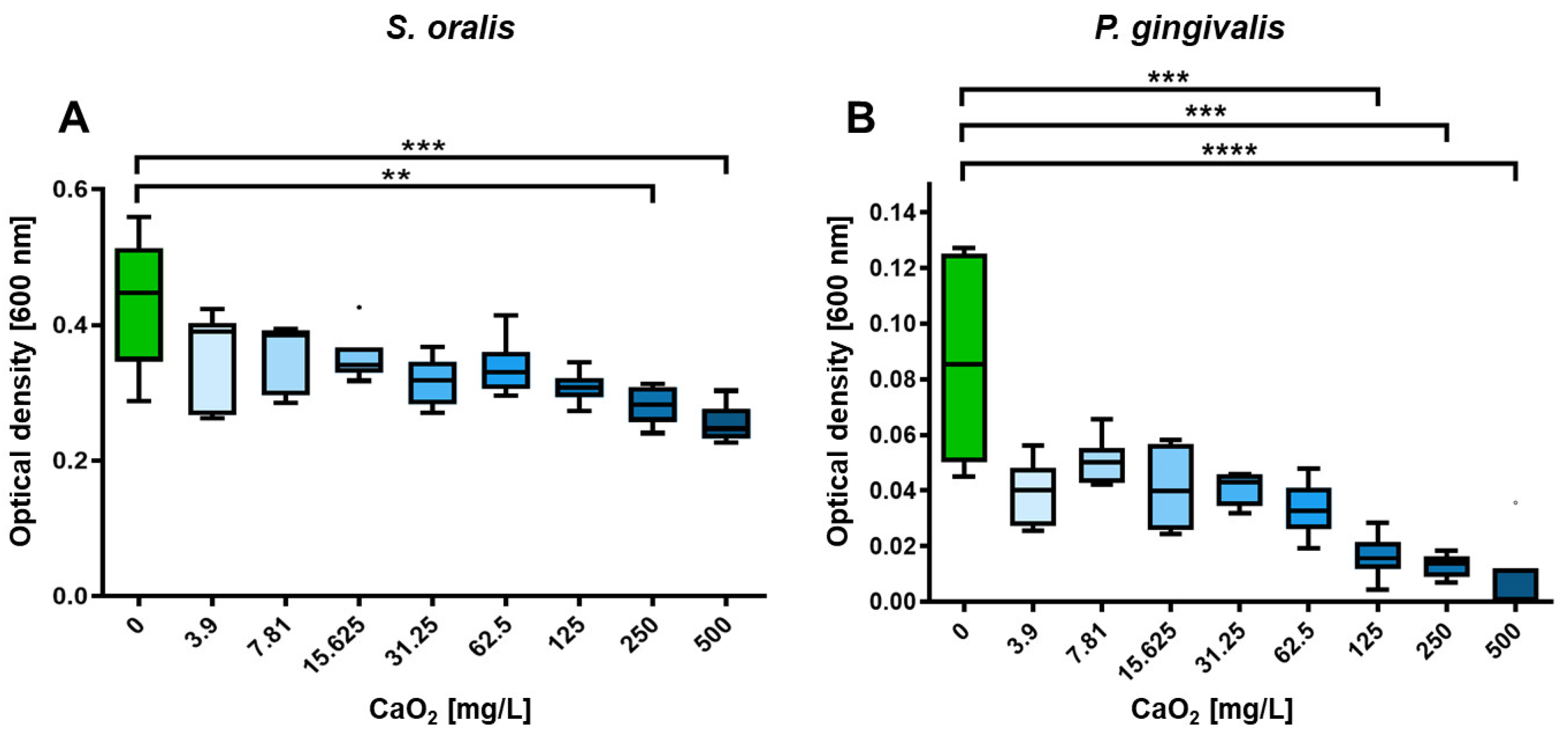
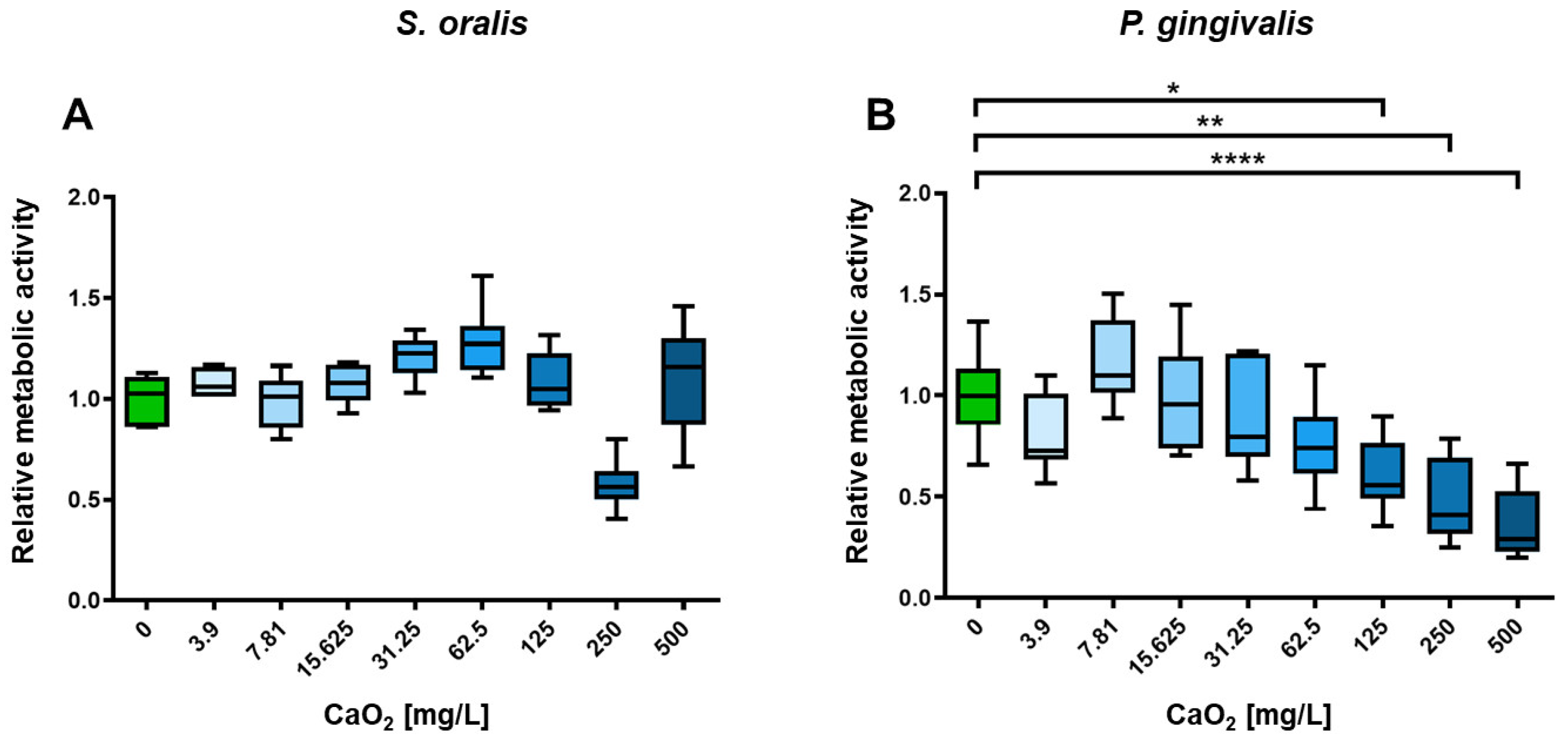
2.2. Selective Antimicrobial Effects of Oxygen on Planktonic S. oralis and P. gingivalis
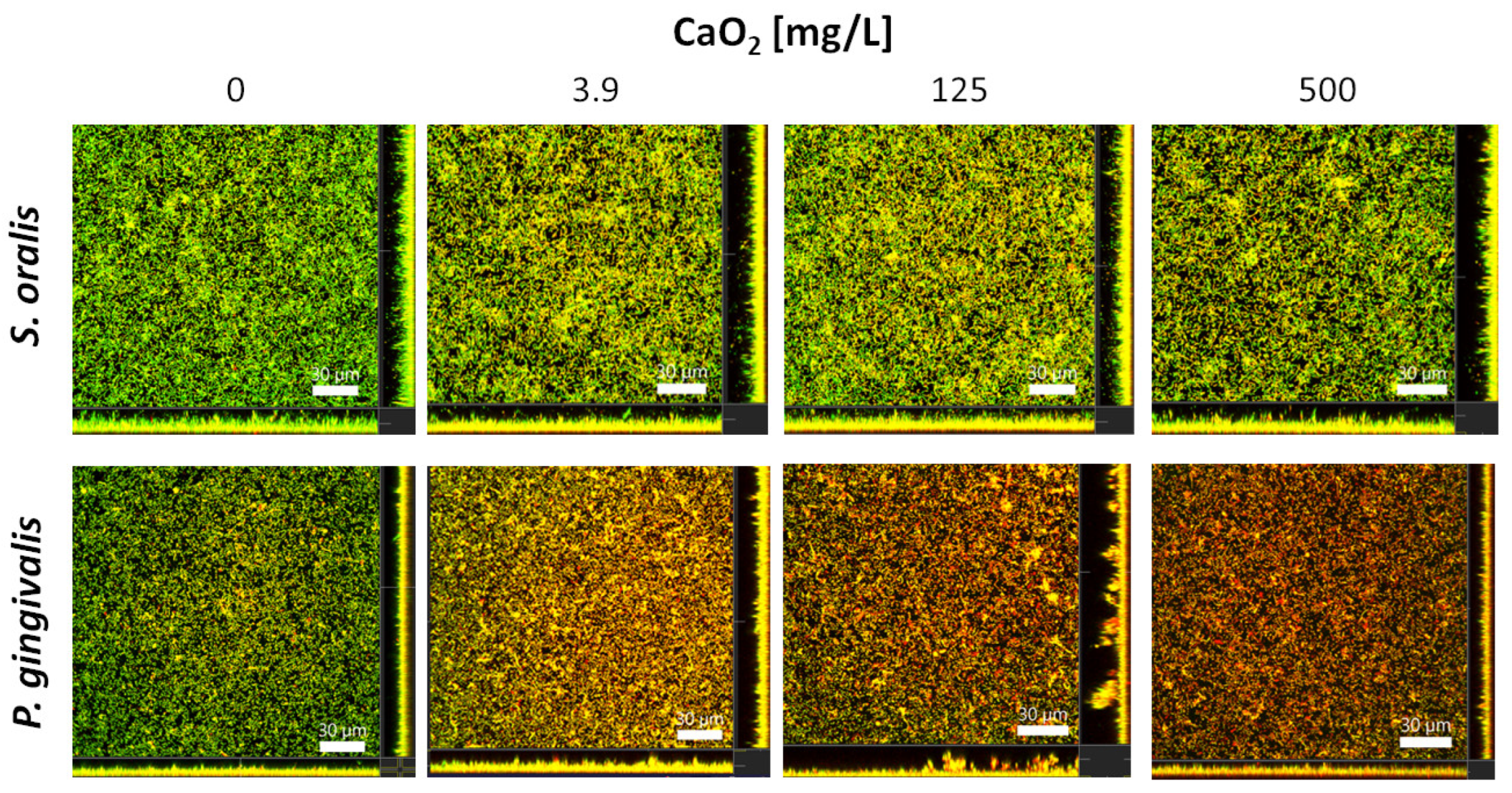
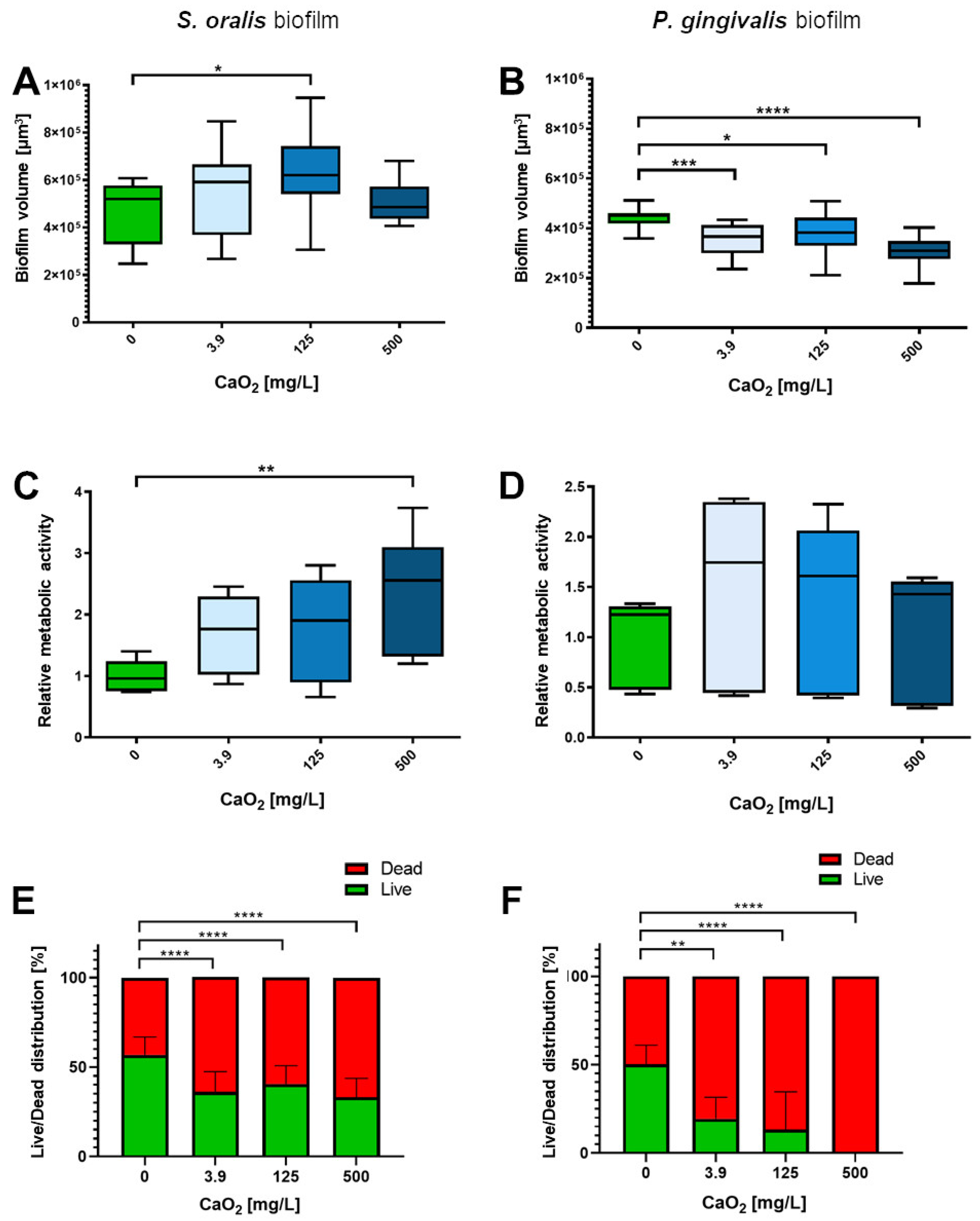
2.3. Selective Antibiofilm Activity of Oxygen on S. oralis and P. gingivalis
3. Discussion
4. Materials and Methods
4.1. Oxygen Release Measurements
4.2. Bacterial Strains and Culture Conditions
4.3. Preparation of Calcium Peroxide Solutions
4.4. In Vitro Investigation of CaO2 Effects on Planktonic Bacterial Growth and Metabolic Activity of S. oralis and P. gingivalis
4.5. In Vitro Investigation of CaO2 Effects on S. oralis and P. gingivalis Biofilms
4.6. Statistical Analysis
5. Conclusions
6. Patents
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Könönen, E.; Gursoy, M.; Gursoy, U.K. Periodontitis: A Multifaceted Disease of Tooth-Supporting Tissues. J. Clin. Med. 2019, 8, 1135. [Google Scholar] [CrossRef] [PubMed]
- Tonetti, M.S.; Jepsen, S.; Jin, L.; Otomo-Corgel, J. Impact of the global burden of periodontal diseases on health, nutrition and wellbeing of mankind: A call for global action. J. Clin. Periodontol. 2017, 44, 456–462. [Google Scholar] [CrossRef] [PubMed]
- Trindade, D.; Carvalho, R.; Machado, V.; Chambrone, L.; Mendes, J.J.; Botelho, J. Prevalence of periodontitis in dentate people between 2011 and 2020: A systematic review and meta-analysis of epidemiological studies. J. Clin. Periodontol. 2023, 50, 604–626. [Google Scholar] [CrossRef] [PubMed]
- Checchi, V.; Maravic, T.; Bellini, P.; Generali, L.; Consolo, U.; Breschi, L.; Mazzoni, A. The Role of Matrix Metalloproteinases in Periodontal Disease. Int. J. Environ. Res. Public. Health 2020, 17, 4923. [Google Scholar] [CrossRef]
- Celik, D.; Kantarci, A. Vascular Changes and Hypoxia in Periodontal Disease as a Link to Systemic Complications. Pathogens 2021, 10, 1280. [Google Scholar] [CrossRef]
- Mombelli, A. Microbial colonization of the periodontal pocket and its significance for periodontal therapy. Periodontology 2000 2018, 76, 85–96. [Google Scholar] [CrossRef]
- Hajishengallis, G.; Darveau, R.P.; Curtis, M.A. The keystone-pathogen hypothesis. Nat. Rev. Microbiol. 2012, 10, 717–725. [Google Scholar] [CrossRef]
- Olczak, T.; Simpson, W.; Liu, X.; Genco, C.A. Iron and heme utilization in Porphyromonas gingivalis. FEMS Microbiol. Rev. 2005, 29, 119–144. [Google Scholar] [CrossRef]
- Cobb, C.M.; Sottosanti, J.S. A Re-Evaluation of Scaling and Root Planing. J. Periodontol. 2021, 92, 1370–1378. [Google Scholar] [CrossRef]
- Suvan, J.; Leira, Y.; Moreno Sancho, F.M.; Graziani, F.; Derks, J.; Tomasi, C. Subgingival instrumentation for treatment of periodontitis. A systematic review. J. Clin. Periodontol. 2020, 47 (Suppl. S22), 155–175. [Google Scholar] [CrossRef]
- Smiley, C.J.; Tracy, S.L.; Abt, E.; Michalowicz, B.S.; John, M.T.; Gunsolley, J.; Cobb, C.M.; Rossmann, J.; Harrel, S.K.; Forrest, J.L.; et al. Systematic review and meta-analysis on the nonsurgical treatment of chronic periodontitis by means of scaling and root planing with or without adjuncts. J. Am. Dent. Assoc. 2015, 146, 508–524.e5. [Google Scholar] [CrossRef] [PubMed]
- Belstrøm, D.; Sembler-Møller, M.L.; Grande, M.A.; Kirkby, N.; Cotton, S.L.; Paster, B.J.; Holmstrup, P. Microbial profile comparisons of saliva, pooled and site-specific subgingival samples in periodontitis patients. PLoS ONE 2017, 12, e0182992. [Google Scholar] [CrossRef] [PubMed]
- Byrne, S.J.; Chang, D.; Adams, G.G.; Butler, C.A.; Reynolds, E.C.; Darby, I.B.; Dashper, S.G. Microbiome profiles of non-responding and responding paired periodontitis sites within the same participants following non-surgical treatment. J. Oral. Microbiol. 2022, 14, 2043595. [Google Scholar] [CrossRef] [PubMed]
- Butera, A.; Folini, E.; Cosola, S.; Russo, G.; Scribante, A.; Gallo, S.; Stablum, G.; Fabris, G.B.M.; Covani, U.; Genovesi, A. Evaluation of the Efficacy of Probiotics Domiciliary Protocols for the Management of Periodontal Disease, in Adjunction of Non-Surgical Periodontal Therapy (NSPT): A Systematic Literature Review. Appl. Sci. 2023, 13, 663. [Google Scholar] [CrossRef]
- Gheisary, Z.; Mahmood, R.; Harri Shivanantham, A.; Liu, J.; Lieffers, J.R.L.; Papagerakis, P.; Papagerakis, S. The Clinical, Microbiological, and Immunological Effects of Probiotic Supplementation on Prevention and Treatment of Periodontal Diseases: A Systematic Review and Meta-Analysis. Nutrients 2022, 14, 1036. [Google Scholar] [CrossRef]
- Homayouni Rad, A.; Pourjafar, H.; Mirzakhani, E. A comprehensive review of the application of probiotics and postbiotics in oral health. Front. Cell. Infect. Microbiol. 2023, 13, 1120995. [Google Scholar] [CrossRef]
- Lu, Z.; Imlay, J.A. When anaerobes encounter oxygen: Mechanisms of oxygen toxicity, tolerance and defence. Nat. Rev. Microbiol. 2021, 19, 774–785. [Google Scholar] [CrossRef]
- Signoretto, C.; Bianchi, F.; Burlacchini, G.; Canepari, P. Microbiological evaluation of the effects of hyperbaric oxygen on periodontal disease. New. Microbiol. 2007, 30, 431–437. [Google Scholar]
- Burcea, A.; Mihai, L.L.; Bechir, A.; Suciu, M.; Bechir, E.S. Clinical Assessment of the Hyperbaric Oxygen Therapy Efficacy in Mild to Moderate Periodontal Affections: A Simple Randomised Trial. Medicina 2022, 58, 234. [Google Scholar] [CrossRef]
- Chen, T.L.; Xu, B.; Liu, J.C.; Li, S.G.; Li, D.Y.; Gong, G.C.; Wu, Z.F.; Lin, S.L.; Zhou, Y.J. Effects of hyperbaric oxygen on aggressive periodontitis and subgingival anaerobes in Chinese patients. J. Indian Soc. Periodontol. 2012, 16, 492–497. [Google Scholar] [CrossRef]
- Schlagenhauf, U.; Horlacher, V.; Netuschil, L.; Brecx, M. Repeated subgingival oxygen irrigations in untreated periodontal patients. J. Clin. Periodontol. 1994, 21, 48–50. [Google Scholar] [CrossRef] [PubMed]
- Evans, J.; Hannoodee, M.; Wittler, M. Amoxicillin Clavulanate. In StatPearls; StatPearls Publishing LLC.: Treasure Island, FL, USA, 2023. [Google Scholar]
- Hernández Ceruelos, A.; Romero-Quezada, L.C.; Ruvalcaba Ledezma, J.C.; López Contreras, L. Therapeutic uses of metronidazole and its side effects: An update. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 397–401. [Google Scholar] [CrossRef] [PubMed]
- Nibali, L.; Koidou, V.P.; Hamborg, T.; Donos, N. Empirical or microbiologically guided systemic antimicrobials as adjuncts to non-surgical periodontal therapy? A systematic review. J. Clin. Periodontol. 2019, 46, 999–1012. [Google Scholar] [CrossRef] [PubMed]
- Ardila, C.-M.; Bedoya-García, J.-A.; Arrubla-Escobar, D.-E. Antibiotic resistance in periodontitis patients: A systematic scoping review of randomized clinical trials. Oral. Dis. 2022. early view. [Google Scholar] [CrossRef]
- Abe, F.C.; Kodaira, K.; Motta, C.C.B.; Barberato-Filho, S.; Silva, M.T.; Guimarães, C.C.; Martins, C.C.; Lopes, L.C. Antimicrobial resistance of microorganisms present in periodontal diseases: A systematic review and meta-analysis. Front. Microbiol. 2022, 13, 961986. [Google Scholar] [CrossRef]
- Galgut, P.N. The relevance of pH to gingivitis and periodontitis. J. Int. Acad. Periodontol. 2001, 3, 61–67. [Google Scholar]
- Xue, Y.; Rajic, L.; Chen, L.; Lyu, S.; Alshawabkeh, A.N. Electrolytic control of hydrogen peroxide release from calcium peroxide in aqueous solution. Electrochem. Commun 2018, 93, 81–85. [Google Scholar] [CrossRef]
- Wang, H.; Zhao, Y.; Li, T.; Chen, Z.; Wang, Y.; Qin, C. Properties of calcium peroxide for release of hydrogen peroxide and oxygen: A kinetics study. J. Chem. Eng. 2016, 303, 450–457. [Google Scholar] [CrossRef]
- Yadav, V.K.; Yadav, K.K.; Cabral-Pinto, M.M.S.; Choudhary, N.; Gnanamoorthy, G.; Tirth, V.; Prasad, S.; Khan, A.H.; Islam, S.; Khan, N.A. The processing of calcium rich agricultural and industrial waste for recovery of calcium carbonate and calcium oxide and their application for environmental cleanup: A review. Appl. Sci. 2021, 11, 4212. [Google Scholar] [CrossRef]
- Desai, S.; Chandler, N. Calcium hydroxide-based root canal sealers: A review. J. Endod. 2009, 35, 475–480. [Google Scholar] [CrossRef]
- Felippe, M.C.S.; Felippe, W.T.; Marques, M.M.; Antoniazzi, J.H. The effect of the renewal of calcium hydroxide paste on the apexification and periapical healing of teeth with incomplete root formation. Int. Endod. J. 2005, 38, 436–442. [Google Scholar] [CrossRef] [PubMed]
- Bryson, E.C.; Levin, L.; Banchs, F.; Abbott, P.V.; Trope, M. Effect of immediate intracanal placement of Ledermix Paste® on healing of replanted dog teeth after extended dry times. Dent. Traumatol. 2002, 18, 316–321. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.; Zhang, X.; Xue, Y. Application of calcium peroxide in water and soil treatment: A review. J. Hazard. Mater. 2017, 337, 163–177. [Google Scholar] [CrossRef] [PubMed]
- Krizanova, O.; Penesova, A.; Sokol, J.; Hokynkova, A.; Samadian, A.; Babula, P. Signaling pathways in cutaneous wound healing. Front. Physiol. 2022, 13, 851. [Google Scholar] [CrossRef]
- Subramaniam, T.; Fauzi, M.B.; Lokanathan, Y.; Law, J.X. The Role of Calcium in Wound Healing. Int. J. Mol. Sci. 2021, 22, 6486. [Google Scholar] [CrossRef]
- Liu, T.; Li, Z.; Zhao, L.; Chen, Z.; Lin, Z.; Li, B.; Feng, Z.; Jin, P.; Zhang, J.; Wu, Z.; et al. Customized design 3D printed PLGA/calcium sulfate scaffold enhances mechanical and biological properties for bone regeneration. Front. Bioeng. Biotechnol. 2022, 10, 874931. [Google Scholar] [CrossRef]
- Wang, T.; Gu, Q.; Zhao, J.; Mei, J.; Shao, M.; Pan, Y.; Zhang, J.; Wu, H.; Zhang, Z.; Liu, F. Calcium alginate enhances wound healing by up-regulating the ratio of collagen types I/III in diabetic rats. Int. J. Clin. Exp. Pathol. 2015, 8, 6636–6645. [Google Scholar]
- Jeong, J.; Kim, J.H.; Shim, J.H.; Hwang, N.S.; Heo, C.Y. Bioactive calcium phosphate materials and applications in bone regeneration. Biomater. Res. 2019, 23, 4. [Google Scholar] [CrossRef]
- Afjoul, H.; Shamloo, A.; Kamali, A. Freeze-gelled alginate/gelatin scaffolds for wound healing applications: An in vitro, in vivo study. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 113, 110957. [Google Scholar] [CrossRef]
- Yang, B.; Pignatello, J.; Qu, D.; Xing, B. Activation of hydrogen peroxide and solid peroxide reagents by phosphate ion in alkaline solution. Environ. Eng. Sci. 2016, 33, 193–199. [Google Scholar] [CrossRef]
- Ma, Y.; Zhang, B.-T.; Zhao, L.; Guo, G.; Lin, J.-M. Study on the generation mechanism of reactive oxygen species on calcium peroxide by chemiluminescence and UV-visible spectra. Luminescence 2007, 22, 575–580. [Google Scholar] [CrossRef]
- Goi, A.; Trapido, M. Chlorophenols contaminated soil remediation by peroxidation. J. Adv. Oxid. Technol. 2010, 13, 50–58. [Google Scholar] [CrossRef]
- Komine, C.; Uchibori, S.; Tsudukibashi, O.; Tsujimoto, Y. Application of reactive oxygen species in dental treatment. J. Pers. Med. 2022, 12, 1531. [Google Scholar] [CrossRef]
- Jha, N.; Ryu, J.J.; Choi, E.H.; Kaushik, N.K. Generation and role of reactive oxygen and nitrogen species induced by plasma, lasers, chemical agents, and other systems in dentistry. Oxid. Med. Cell. Longev. 2017, 2017, 7542540. [Google Scholar] [CrossRef]
- Liu, F.; Hong, T.; Xie, J.; Zhan, X.; Wang, Y. Application of Reactive Oxygen Species-Based Nanomaterials in Dentistry: A Review. Crystals 2021, 11, 266. [Google Scholar] [CrossRef]
- Zhai, J.; Jiang, C. Synthesis of calcium peroxide microparticles and its application in glyphosate wastewater pretreatment. Adv. Mat. Res. 2014, 881–883, 1139–1143. [Google Scholar] [CrossRef]
- Gao, D.M.; Zhang, M.S.; Hong, B. The quality control of eutrophic culture sediment by immobilized cells and conditions manipulation. Appl. Mech. Mater. 2014, 644–650, 5169–5173. [Google Scholar] [CrossRef]
- Cassidy, D.P.; Irvine, R.L. Use of calcium peroxide to provide oxygen for contaminant biodegradation in a saturated soil. J. Hazard. Mater. 1999, 69, 25–39. [Google Scholar] [CrossRef] [PubMed]
- Walawska, B.; Gluzińska, J.; Miksch, K.; Turek-Szytow, J. Solid inorganic peroxy compounds in environmental protection. Pol. J. Chem. Technol. 2007, 9, 68–72. [Google Scholar] [CrossRef]
- Müller-Heupt, L.K.; Wiesmann-Imilowski, N.; Schröder, S.; Groß, J.; Ziskoven, P.C.; Bani, P.; Kämmerer, P.W.; Schiegnitz, E.; Eckelt, A.; Eckelt, J.; et al. Oxygen-releasing hyaluronic acid-based dispersion with controlled oxygen delivery for enhanced periodontal tissue engineering. Int. J. Mol. Sci. 2023, 24, 5936. [Google Scholar] [CrossRef]
- Hansen, M.C.; Palmer, R.J.; White, D.C. Flowcell culture of Porphyromonas gingivalis biofilms under anaerobic conditions. J. Microbiol. Methods 2000, 40, 233–239. [Google Scholar] [CrossRef] [PubMed]
- Hamanaka, R.B.; Chandel, N.S. Mitochondrial reactive oxygen species regulate hypoxic signaling. Curr. Opin. Cell. Biol. 2009, 21, 894–899. [Google Scholar] [CrossRef] [PubMed]
- Markkanen, E. Not breathing is not an option: How to deal with oxidative DNA damage. DNA Repair 2017, 59, 82–105. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, G.T.; Green, E.R.; Mecsas, J. Neutrophils to the ROScue: Mechanisms of NADPH oxidase activation and bacterial resistance. Front. Cell. Infect. Microbiol. 2017, 7, 373. [Google Scholar] [CrossRef]
- Seixas, A.F.; Quendera, A.P.; Sousa, J.P.; Silva, A.F.Q.; Arraiano, C.M.; Andrade, J.M. Bacterial response to oxidative stress and RNA cxidation. Front. Genet. 2021, 12, 821535. [Google Scholar] [CrossRef]
- Mittal, M.; Siddiqui, M.R.; Tran, K.; Reddy, S.P.; Malik, A.B. Reactive oxygen species in inflammation and tissue injury. Antioxid. Redox Signal. 2014, 20, 1126–1167. [Google Scholar] [CrossRef]
- Gholipourmalekabadi, M.; Zhao, S.; Harrison, B.S.; Mozafari, M.; Seifalian, A.M. Oxygen-Generating Biomaterials: A New, Viable Paradigm for Tissue Engineering? Trends Biotechnol. 2016, 34, 1010–1021. [Google Scholar] [CrossRef]
- Mollajavadi, M.Y.; Saadatmand, M.; Ghobadi, F. Effect of calcium peroxide particles as oxygen-releasing materials on cell growth and mechanical properties of scaffolds for tissue engineering. Iran. Polym. J. 2023, 32, 599–608. [Google Scholar] [CrossRef]
- Pitt Ford, T.R.; Rowe, A.H.R. A new root canal sealer based on calcium hydroxide. J. Endod. 1989, 15, 286–289. [Google Scholar] [CrossRef]
- Mohammadi, Z.; Dummer, P.M. Properties and applications of calcium hydroxide in endodontics and dental traumatology. Int. Endod. J. 2011, 44, 697–730. [Google Scholar] [CrossRef]
- Jamshidi, D.; Ansari, M.; Gheibi, N. Cytotoxicity and Genotoxicity of Calcium Hydroxide and Two Antibiotic Pastes on Human Stem Cells of The Apical Papilla. Eur. Endod. J. 2021, 6, 303–308. [Google Scholar] [CrossRef] [PubMed]
- Duran-Pinedo, A.E.; Frias-Lopez, J. Beyond microbial community composition: Functional activities of the oral microbiome in health and disease. Microbes Infect. 2015, 17, 505–516. [Google Scholar] [CrossRef] [PubMed]
- Scapoli, L.; Girardi, A.; Palmieri, A.; Testori, T.; Zuffetti, F.; Monguzzi, R.; Lauritano, D.; Carinci, F. Microflora and periodontal disease. Dent. Res. J. 2012, 9, S202-206. [Google Scholar]
- Borriello, G.; Werner, E.; Roe, F.; Kim, A.M.; Ehrlich, G.D.; Stewart, P.S. Oxygen limitation contributes to antibiotic tolerance of Pseudomonas aeruginosa in biofilms. Antimicrob. Agents Chemother. 2004, 48, 2659–2664. [Google Scholar] [CrossRef]
- Koshikawa, T.; Abe, M.; Nagi, M.; Miyazaki, Y.; Takemura, H. Biofilm-formation capability depends on environmental oxygen concentrations in Candida species. J. Infect. Chemother. 2022, 28, 643–650. [Google Scholar] [CrossRef]
- Schlagenhauf, U.; Jockel-Schneider, Y. Probiotics in the Management of Gingivitis and Periodontitis. A Review. Front. Dent. Med. 2021, 2, 708666. [Google Scholar] [CrossRef]
- Kommerein, N.; Vierengel, N.; Groß, J.; Opatz, T.; Al-Nawas, B.; Müller-Heupt, L.K. Antiplanktonic and antibiofilm activity of Rheum palmatum against Streptococcus oralis and Porphyromonas gingivalis. Microorganisms 2022, 10, 965. [Google Scholar] [CrossRef]
- Elashiry, M.; Morandini, A.C.; Cornelius Timothius, C.J.; Ghaly, M.; Cutler, C.W. Selective Antimicrobial Therapies for Periodontitis: Win the “Battle and the War”. Int. J. Mol. Sci. 2021, 22, 6459. [Google Scholar] [CrossRef]
- Lamont, R.J.; Koo, H.; Hajishengallis, G. The oral microbiota: Dynamic communities and host interactions. Nat. Rev. Microbiol. 2018, 16, 745–759. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Müller-Heupt, L.K.; Eckelt, A.; Eckelt, J.; Groß, J.; Opatz, T.; Kommerein, N. An In Vitro Study of Local Oxygen Therapy as Adjunctive Antimicrobial Therapeutic Option for Patients with Periodontitis. Antibiotics 2023, 12, 990. https://doi.org/10.3390/antibiotics12060990
Müller-Heupt LK, Eckelt A, Eckelt J, Groß J, Opatz T, Kommerein N. An In Vitro Study of Local Oxygen Therapy as Adjunctive Antimicrobial Therapeutic Option for Patients with Periodontitis. Antibiotics. 2023; 12(6):990. https://doi.org/10.3390/antibiotics12060990
Chicago/Turabian StyleMüller-Heupt, Lena Katharina, Anja Eckelt, John Eckelt, Jonathan Groß, Till Opatz, and Nadine Kommerein. 2023. "An In Vitro Study of Local Oxygen Therapy as Adjunctive Antimicrobial Therapeutic Option for Patients with Periodontitis" Antibiotics 12, no. 6: 990. https://doi.org/10.3390/antibiotics12060990
APA StyleMüller-Heupt, L. K., Eckelt, A., Eckelt, J., Groß, J., Opatz, T., & Kommerein, N. (2023). An In Vitro Study of Local Oxygen Therapy as Adjunctive Antimicrobial Therapeutic Option for Patients with Periodontitis. Antibiotics, 12(6), 990. https://doi.org/10.3390/antibiotics12060990






