Comparison of Cefepime with Piperacillin/Tazobactam Treatment in Patients with Hospital-Acquired Pneumonia
Abstract
1. Introduction
2. Methods
2.1. Data Source
2.2. Study Design and Population
2.3. Data Collection and Clinical Outcomes
2.4. Statistical Analysis
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Magill, S.S.; Edwards, J.R.; Bamberg, W.; Beldavs, Z.G.; Dumyati, G.; Kainer, M.A.; Lynfield, R.; Maloney, M.; McAllister-Hollod, L.; Nadle, J.; et al. Multistate point-prevalence survey of health care-associated infections. N. Engl. J. Med. 2014, 370, 1198–1208. [Google Scholar] [CrossRef]
- Melsen, W.G.; Rovers, M.M.; Groenwold, R.H.; Bergmans, D.C.; Camus, C.; Bauer, T.T.; Hanisch, E.W.; Klarin, B.; Koeman, M.; Krueger, W.A.; et al. Attributable mortality of ventilator-associated pneumonia: A meta-analysis of individual patient data from randomised prevention studies. Lancet Infect. Dis. 2013, 13, 665–671. [Google Scholar] [CrossRef] [PubMed]
- Kalil, A.C.; Metersky, M.L.; Klompas, M.; Muscedere, J.; Sweeney, D.A.; Palmer, L.B.; Napolitano, L.M.; O’Grady, N.P.; Bartlett, J.G.; Carratalà, J.; et al. Management of Adults With Hospital-acquired and Ventilator-associated Pneumonia: 2016 Clinical Practice Guidelines by the Infectious Diseases Society of America and the American Thoracic Society. Clin. Infect. Dis. 2016, 63, e61–e111. [Google Scholar] [CrossRef]
- Torres, A.; Niederman, M.S.; Chastre, J.; Ewig, S.; Fernandez-Vandellos, P.; Hanberger, H.; Kollef, M.; Li Bassi, G.; Luna, C.M.; Martin-Loeches, I.; et al. International ERS/ESICM/ESCMID/ALAT guidelines for the management of hospital-acquired pneumonia and ventilator-associated pneumonia: Guidelines for the management of hospital-acquired pneumonia (HAP)/ventilator-associated pneumonia (VAP) of the European Respiratory Society (ERS), European Society of Intensive Care Medicine (ESICM), European Society of Clinical Microbiology and Infectious Diseases (ESCMID) and Asociación Latinoamericana del Tórax (ALAT). Eur. Respir. J. 2017, 50, 1700582. [Google Scholar]
- McLaughlin, M.; Advincula, M.R.; Malczynski, M.; Qi, C.; Bolon, M.; Scheetz, M.H. Correlations of antibiotic use and carbapenem resistance in enterobacteriaceae. Antimicrob. Agents Chemother. 2013, 57, 5131–5133. [Google Scholar] [CrossRef] [PubMed]
- Joseph, N.M.; Bhanupriya, B.; Shewade, D.G.; Harish, B.N. Relationship between Antimicrobial Consumption and the Incidence of Antimicrobial Resistance in Escherichia coli and Klebsiella pneumoniae Isolates. J. Clin. Diagn. Res. 2015, 9, DC08–DC12. [Google Scholar] [CrossRef]
- Yang, P.; Chen, Y.; Jiang, S.; Shen, P.; Lu, X.; Xiao, Y. Association between antibiotic consumption and the rate of carbapenem-resistant Gram-negative bacteria from China based on 153 tertiary hospitals data in 2014. Antimicrob. Agents Chemother. 2018, 7, 137. [Google Scholar] [CrossRef]
- Kadri, S.S.; Adjemian, J.; Lai, Y.L.; Spaulding, A.B.; Ricotta, E.; Prevots, D.R.; Palmore, T.N.; Rhee, C.; Klompas, M.; Dekker, J.P.; et al. Difficult-to-Treat Resistance in Gram-negative Bacteremia at 173 US Hospitals: Retrospective Cohort Analysis of Prevalence, Predictors, and Outcome of Resistance to All First-line Agents. Clin. Infect. Dis. 2018, 67, 1803–1814. [Google Scholar] [CrossRef]
- Alvarez-Marin, R.; Lopez-Cerero, L.; Guerrero-Sanchez, F.; Palop-Borras, B.; Rojo-Martin, M.D.; Ruiz-Sancho, A.; Herrero-Rodriguez, C.; Garcia, M.V.; Lazo-Torres, A.M.; Lopez, I.; et al. Do specific antimicrobial stewardship interventions have an impact on carbapenem resistance in Gram-negative bacilli? A multicentre quasi-experimental ecological study: Time-trend analysis and characterization of carbapenemases. J. Antimicrob. Chemother. 2021, 76, 1928–1936. [Google Scholar] [CrossRef]
- Kollef, M.H.; Morrow, L.E.; Niederman, M.S.; Leeper, K.V.; Anzueto, A.; Benz-Scott, L.; Rodino, F.J. Clinical characteristics and treatment patterns among patients with ventilator-associated pneumonia. Chest 2006, 129, 1210–1218. [Google Scholar] [CrossRef]
- Rello, J.; Ulldemolins, M.; Lisboa, T.; Koulenti, D.; Manez, R.; Martin-Loeches, I.; De Waele, J.J.; Putensen, C.; Guven, M.; Deja, M.; et al. Determinants of prescription and choice of empirical therapy for hospital-acquired and ventilator-associated pneumonia. Eur. Respir. J. 2011, 37, 1332–1339. [Google Scholar] [CrossRef]
- Ko, R.E.; Min, K.H.; Hong, S.B.; Baek, A.R.; Lee, H.K.; Cho, W.H.; Kim, C.; Chang, Y.; Lee, S.S.; Oh, J.Y.; et al. Characteristics, Management, and Clinical Outcomes of Patients with Hospital-Acquired and Ventilator-Associated Pneumonia: A Multicenter Cohort Study in Korea. Tuberc. Respir. Dis. 2021, 84, 317–325. [Google Scholar] [CrossRef] [PubMed]
- Ahn, E. Introducing big data analysis using data from National Health Insurance Service. Korean J. Anesthesiol. 2020, 73, 205–211. [Google Scholar] [CrossRef]
- Shin, D.W.; Cho, B.; Guallar, E. Korean National Health Insurance Database. JAMA Intern. Med. 2016, 176, 138. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.G.; Kang, M.; Lim, J.; Lee, J.; Kang, D.; Kim, M.; Kim, J.; Park, H.; Min, K.H.; Cho, J.; et al. Comprehensive risk assessment for hospital-acquired pneumonia: Sociodemographic, clinical, and hospital environmental factors associated with the incidence of hospital-acquired pneumonia. BMC Pulm. Med. 2022, 22, 21. [Google Scholar] [CrossRef] [PubMed]
- Korea Ministry of Health and Welfare. Medical Service Act. Act No. 14438. 20 December 2016. Available online: http://www.law.go.kr (accessed on 13 March 2022).
- Ellis, A.R.; Brookhart, M.A. Approaches to inverse-probability-of-treatment—Weighted estimation with concurrent treatments. J. Clin. Epidemiol. 2013, 66, S51–S56. [Google Scholar] [CrossRef] [PubMed]
- Mandell, L.A.; Niederman, M.S. Aspiration Pneumonia. N. Engl. J. Med. 2019, 380, 651–663. [Google Scholar] [CrossRef]
- Raveh, D.; Muallem-Zilcha, E.; Greenberg, A.; Wiener-Well, Y.; Schlesinger, Y.; Yinnon, A.M. Prospective drug utilization evaluation of three broad-spectrum antimicrobials: Cefepime, piperacillin-tazobactam and meropenem. QJM 2006, 99, 397–406. [Google Scholar] [CrossRef]
- Schoonover, L.L.; Occhipinti, D.J.; Rodvold, K.A.; Danziger, L.H. Piperacillin/tazobactam: A new beta-lactam/beta-lactamase inhibitor combination. Ann. Pharmacother. 1995, 29, 501–514. [Google Scholar] [CrossRef]
- Sanders, C.C. In vitro activity of fourth generation cephalosporins against enterobacteriaceae producing extended-spectrum beta-lactamases. J. Chemother. 1996, 8 (Suppl. 2), 57–62. [Google Scholar]
- Sanders, C.C. Cefepime: The next generation? Clin. Infect. Dis. 1993, 17, 369–379. [Google Scholar]
- Djordjevic, Z.M.; Folic, M.M.; Jankovic, S.M. Distribution and antibiotic susceptibility of pathogens isolated from adults with hospital-acquired and ventilator-associated pneumonia in intensive care unit. J. Infect. Public Health 2017, 10, 740–744. [Google Scholar] [CrossRef] [PubMed]
- Paul, M.; Yahav, D.; Fraser, A.; Leibovici, L. Empirical antibiotic monotherapy for febrile neutropenia: Systematic review and meta-analysis of randomized controlled trials. J. Antimicrob. Chemother. 2006, 57, 176–189. [Google Scholar] [CrossRef] [PubMed]
- Ross, R.C.; Rosen, A.N.; Tran, K.K.; Smith, K.L.; Franck, A.J. A Comparison Between Cefepime and Piperacillin-Tazobactam in the Management of Septic Shock. Cureus 2021, 13, e18742. [Google Scholar] [CrossRef] [PubMed]
- Luther, M.K.; Timbrook, T.T.; Caffrey, A.R.; Dosa, D.; Lodise, T.P.; LaPlante, K.L. Vancomycin Plus Piperacillin-Tazobactam and Acute Kidney Injury in Adults: A Systematic Review and Meta-Analysis. Crit. Care Med. 2018, 46, 12–20. [Google Scholar] [CrossRef]
- Gomes, G.F.; Pisani, J.C.; Macedo, E.D.; Campos, A.C. The nasogastric feeding tube as a risk factor for aspiration and aspiration pneumonia. Curr. Opin. Clin. Nutr. Metab. Care 2003, 6, 327–333. [Google Scholar] [CrossRef]
- Brook, I.; Wexler, H.M.; Goldstein, E.J. Antianaerobic antimicrobials: Spectrum and susceptibility testing. Clin. Microbiol. Rev. 2013, 26, 526–546. [Google Scholar] [CrossRef]
- Kim, M.K.; Han, K.; Lee, S.H. Current Trends of Big Data Research Using the Korean National Health Information Database. Diabetes Metab. J. 2022, 46, 552–563. [Google Scholar] [CrossRef]
- Kollef, M.H.; Juang, P.; Micek, S.T. Vancomycin/Piperacillin-tazobactam Nephrotoxicity in the Critically Ill. Clin. Infect. Dis. 2020, 70, 1520–1521. [Google Scholar] [CrossRef]
- Mullins, B.P.; Kramer, C.J.; Bartel, B.J.; Catlin, J.S.; Gilder, R.E. Comparison of the Nephrotoxicity of Vancomycin in Combination With Cefepime, Meropenem, or Piperacillin/Tazobactam: A Prospective, Multicenter Study. Ann. Pharmacother. 2018, 52, 639–644. [Google Scholar] [CrossRef]
- Khan, A.; DeMott, J.M.; Varughese, C.; Hammond, D.A. Effect of Cefepime on Neurotoxicity Development in Critically Ill Adults With Renal Dysfunction. Chest 2020, 158, 157–163. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, D.D.; Lai, S. Prolonged Cefepime-Induced Neurotoxicity in a Patient with End-Stage Renal Disease. Am. J. Case Rep. 2022, 23, e934083. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.D.; Heintz, B.H.; Mosher, H.J.; Livorsi, D.J.; Egge, J.A.; Lund, B.C. Risk of Acute Kidney Injury and Clostridioides difficile Infection With Piperacillin/Tazobactam, Cefepime, and Meropenem With or Without Vancomycin. Clin. Infect. Dis. 2021, 73, e1579–e1586. [Google Scholar] [CrossRef] [PubMed]
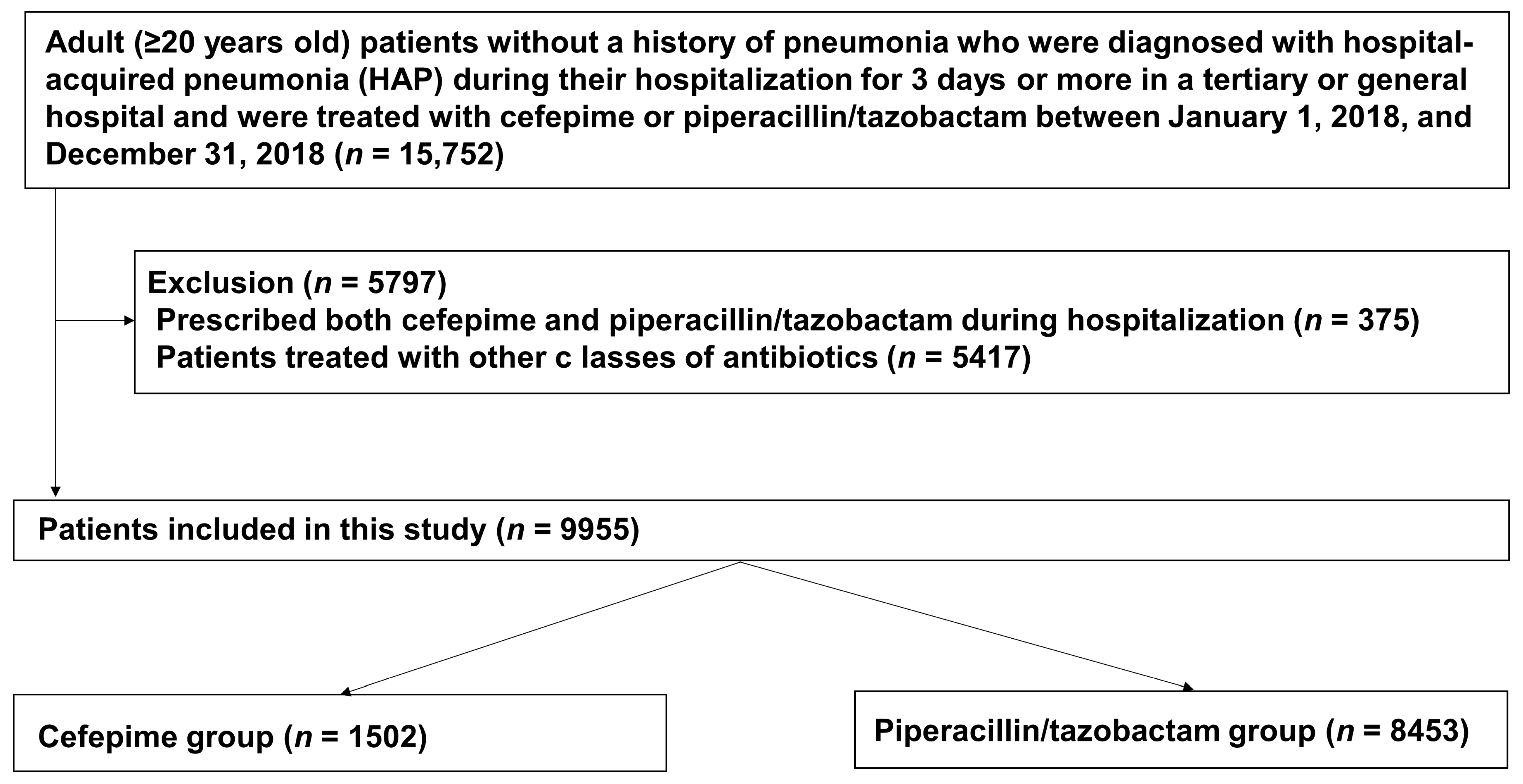
| Variables | Piperacillin/Tazobactam (n = 8453) | Cefepime (n = 1502) | p Value |
|---|---|---|---|
| Mean age, years (SD) | 74.26 (13.21) | 72.14 (13.11) | <0.001 |
| Sex | 0.13 | ||
| Male | 5230 (61.87%) | 960 (63.91%) | |
| Female | 3223 (38.13%) | 542 (36.09%) | |
| Mean Charlson comorbidity index (SD) | 6.21 (3.63) | 6.25 (3.44) | 0.71 |
| Comorbidity | |||
| Cancer | 2370 (28.04%) | 526 (35.02%) | <0.001 |
| Asthma | 3728 (44.1%) | 758 (50.47%) | <0.001 |
| COPD | 2269 (26.84%) | 503 (33.49%) | <0.001 |
| Other chronic lower respiratory disease | 4325 (51.17%) | 785 (52.26%) | 0.43 |
| CKD | 1213 (14.35%) | 186 (12.38%) | 0.04 |
| ESRD | 1216 (14.39%) | 188 (12.52%) | 0.06 |
| Anemia | 2527 (29.89%) | 385 (25.63%) | <0.001 |
| History of hospitalization | 4041 (47.81%) | 759 (50.53%) | 0.05 |
| Location of hospital | <0.001 | ||
| Metropolitan | 5889 (69.67%) | 1154 (76.83%) | |
| Rural | 2564 (30.33%) | 348 (23.17%) | |
| Type of hospital | <0.001 | ||
| Tertiary | 2341 (27.69%) | 577 (38.42%) | |
| General | 6112 (72.31%) | 925 (61.58%) | |
| Tube feeding | 2456 (29.05%) | 356 (23.7%) | <0.001 |
| Suctioning | 2329 (27.55%) | 328 (21.84%) | <0.001 |
| Requiring positioning care | 3013 (35.64%) | 395 (26.3%) | <0.001 |
| Mechanical ventilation | 904 (10.69%) | 152 (10.12%) | 0.50 |
| ICU admission | 2748 (32.51%) | 403 (26.83%) | <0.001 |
| Co-medication with quinolones | 5821 (68.86%) | 1015 (67.58%) | 0.32 |
| Population | Piperacillin/Tazobactam | Cefepime |
|---|---|---|
| Overall | ||
| Crude | Reference | 1.05 (0.87–1.27) |
| Model 1 | Reference | 1.02 (0.85–1.23) |
| Model 2 | Reference | 1.07 (0.88–1 29) |
| IPTW analysis | Reference | 1.14 (0.91–1.44) |
| Patients without tube feeding | ||
| Crude | Reference | 0.95 (0.75–1.21) |
| Model 1 | Reference | 0.91 (0.72–1.15) |
| Model 2 | Reference | 0.92 (0.72–1.18) |
| IPTW analysis | Reference | 1.00 (0.78–1.28) |
| Patients with tube feeding | ||
| Crude | Reference | 1.37 (1.01–1.87) |
| Model 1 | Reference | 1.39 (1.01–1.91) |
| Model 2 | Reference | 1.43 (1.04–1.97) |
| IPTW analysis | Reference | 1.49 (1.05–2.11) |
| Population | Piperacillin/Tazobactam | Cefepime |
|---|---|---|
| Overall | ||
| Crude | Reference | 1.00 (0.59–1.70) |
| Model 1 | Reference | 1.06 (0.63–1.80) |
| Model 2 | Reference | 1.05 (0.62–1.78) |
| IPTW analysis | Reference | 1.13 (0.66–1.95) |
| Patients without tube feeding | ||
| Crude | Reference | 0.90 (0.49–1.64) |
| Model 1 | Reference | 0.95 (0.52–1.72) |
| Model 2 | Reference | 0.94 (0.52–1.71) |
| IPTW analysis | Reference | 1.07 (0.58–1.94) |
| Patients with tube feeding | ||
| Crude | Reference | 1.36 (0.52–3.55) |
| Model 1 | Reference | 1.44 (0.55–3.78) |
| Model 2 | Reference | 1.42 (0.54–3.74) |
| IPTW analysis | Reference | 1.34 (0.50–3.63) |
| Population | Piperacillin/Tazobactam | Cefepime |
|---|---|---|
| Overall | ||
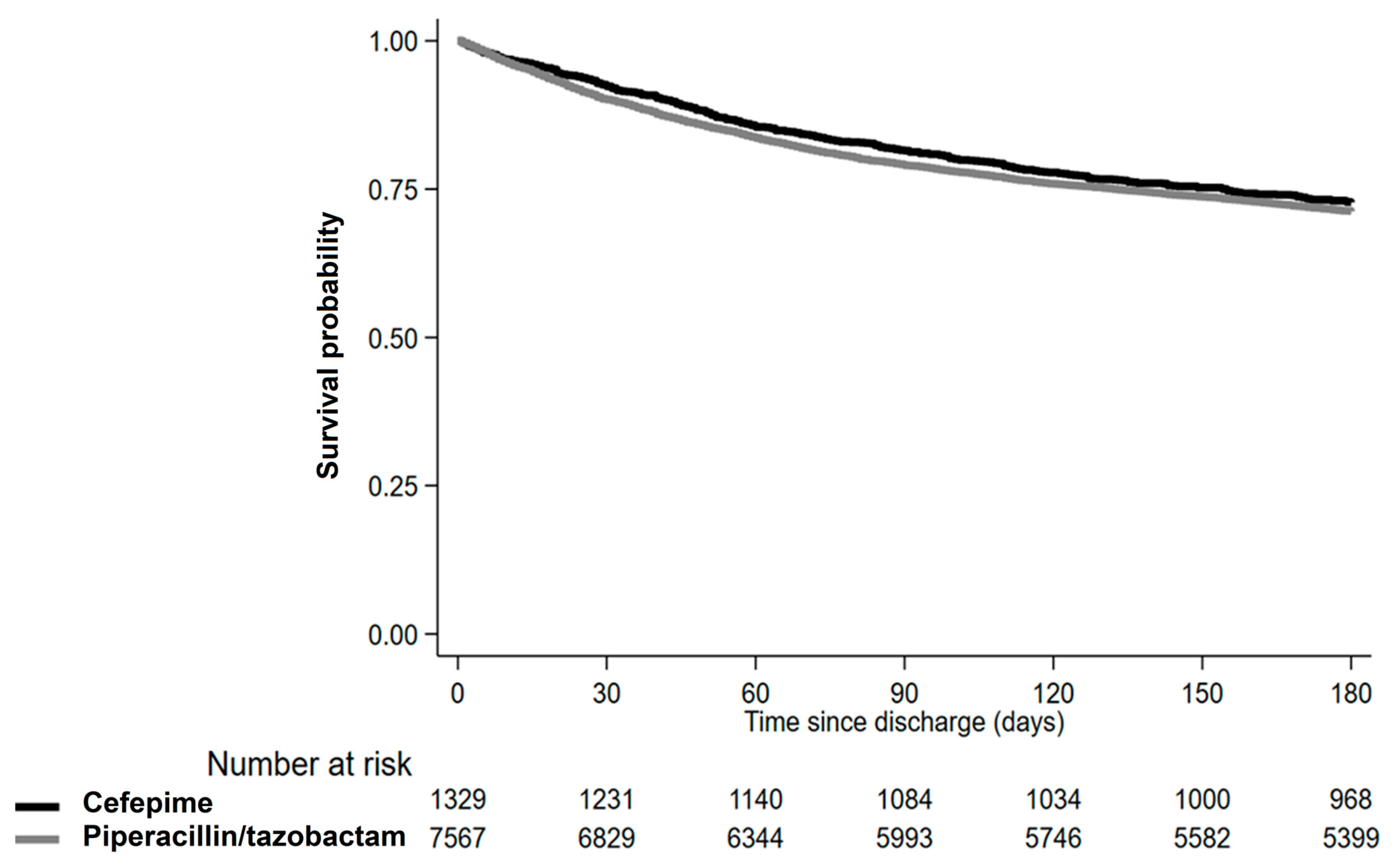
| Number of deaths | 2176 | 362 |
| Incidence per 100 person-years | 0.20 | 0.18 |
| Crude | Reference | 0.92 (0.82–1.05) |
| Model 1 | Reference | 0.92 (0.81–1.05) |
| Model 2 | Reference | 0.95 (0.84–1.08) |
| IPTW analysis | Reference | 0.98 (0.86–1.11) |
| Patients without tube feeding | ||
| Number of deaths | 1351 | 242 |
| Incidence per 100 person-years | 0.16 | 0.15 |
| Crude | Reference | 0.93 (0.80–1.07) |
| Model 1 | Reference | 0.91 (0.79–1.06) |
| Model 2 | Reference | 0.92 (0.80–1.07) |
| IPTW | Reference | 0.95 (0.81–1.10) |
| Patients with tube feeding | ||
| Number of deaths | 825 | 120 |
| Incidence per 100 person-years | 0.29 | 0.32 |
| Crude | Reference | 1.11 (0.91–1.37) |
| Model 1 | Reference | 1.14 (0.93–1.40) |
| Model 2 | Reference | 1.15 (0.93–1.42) |
| IPTW analysis | Reference | 1.18 (0.97–1.44) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, B.-G.; Kang, D.; Min, K.H.; Cho, J.; Jeon, K. Comparison of Cefepime with Piperacillin/Tazobactam Treatment in Patients with Hospital-Acquired Pneumonia. Antibiotics 2023, 12, 984. https://doi.org/10.3390/antibiotics12060984
Kim B-G, Kang D, Min KH, Cho J, Jeon K. Comparison of Cefepime with Piperacillin/Tazobactam Treatment in Patients with Hospital-Acquired Pneumonia. Antibiotics. 2023; 12(6):984. https://doi.org/10.3390/antibiotics12060984
Chicago/Turabian StyleKim, Bo-Guen, Danbee Kang, Kyung Hoon Min, Juhee Cho, and Kyeongman Jeon. 2023. "Comparison of Cefepime with Piperacillin/Tazobactam Treatment in Patients with Hospital-Acquired Pneumonia" Antibiotics 12, no. 6: 984. https://doi.org/10.3390/antibiotics12060984
APA StyleKim, B.-G., Kang, D., Min, K. H., Cho, J., & Jeon, K. (2023). Comparison of Cefepime with Piperacillin/Tazobactam Treatment in Patients with Hospital-Acquired Pneumonia. Antibiotics, 12(6), 984. https://doi.org/10.3390/antibiotics12060984






