Systemic Antibiotic Therapy in Hidradenitis Suppurativa: A Review on Treatment Landscape and Current Issues
Abstract
1. Introduction
2. Materials and Methods
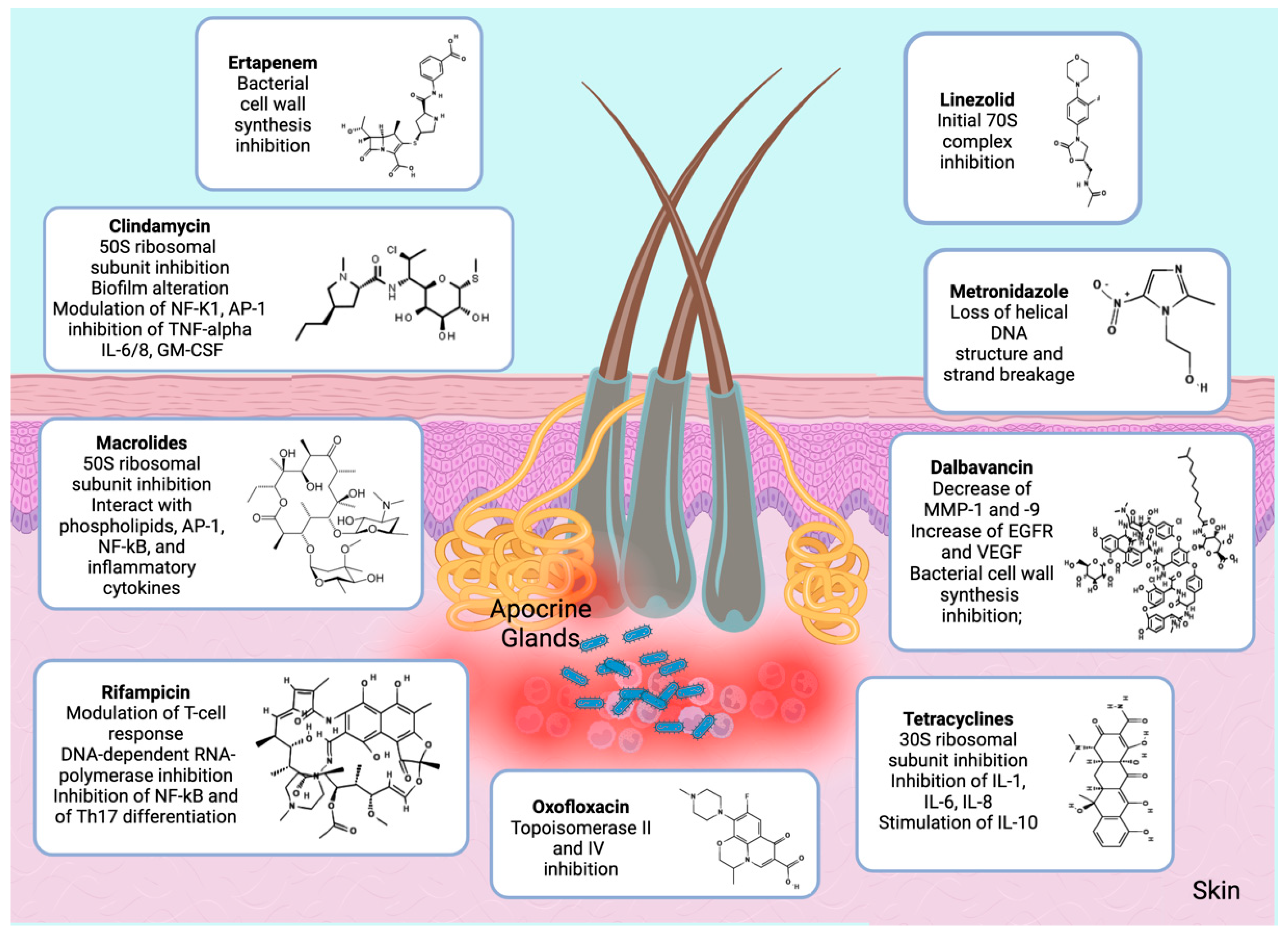
3. Current Antibiotic Therapy of HS
3.1. Lincosamidess
3.2. Tetracyclines
3.3. Beta Lactam Antibiotics
3.4. Macrolides
3.5. Metronidazole
| Treatment | Authors | Study | N | Patients’ Characteristics | Study Design Endpoints | Outcomes |
|---|---|---|---|---|---|---|
| CLINDAMYCIN (Monotherapy and combinations) | ||||||
| Clindamycin [23] | Clemmensen O.J. et al., 1983 | Double-blind trial | 30 | Hurley stage not specified | Clindamycin vs. placebo | Clindamycin significantly superior to placebo except for inflammatory nodules and abscesses at each monthly evaluation (p less than 0.01) |
| Clindamycin [24] | Jemec G.B.E. et al., 1998 | Double-blind placebo-controlled RCT | 46 | Hurley stage I or II HS | Tetraciclyne 1 g/die PO + topical placebo vs. placebo PO + topical clindamycin 1% for at least 3 mo | No significant differences between the two treatments in terms of VAS score Patients’ global assessment of disease was significantly worse than physician’s assessment in 3 of 5 evaluations (p = 0.0096 to 0.015), but the correlation between patients’ and physicians’ assessments was satisfactory after only one visit (rs = 0.761 to 0.895) |
| Clindamycin [25] | Molinelli E. et al., 2020 | Retrospective study | 124 | Hurley stage I or II HS |
| Patients treated with resorcinol 15% showed a significant improvement in HS clinical response, international HS severity core system and pain visual analogue scale score from baseline compared to patients treated with clindamycin; In group A (clindamycin 1%), clinical response (HiSCR) was obtained in 38 (52%) of 73 patients after 12 weeks (p < 0.01). In group B (resorcinol 15%), clinical response was achieved in 52 (85.3%) of 61 patients after 12 weeks (p < 0.001). At 12 weeks, the clinical response to resorcinol 15% was higher than the response to topical antibiotic, with statistically significance (p < 0.001). |
| Clindamycin [32] | Caposiena Caro R.D. et al., 2019 | Retrospective study | 60 | Hurley stage I or II HS |
| After 8 weeks of treatment the responses to antibiotics were similar in both groups. |
| Clindamycin [13] | An J.H. et al., 2021 | Retrospective study | 53 | Hurley stage II or III HS | Clindamycin monotherapy for 8 weeks | Improvement in rate of HS clinical response (Hi-SCR) achievers and comparing HS physician’s global assessment (HS-PGA) before (W0) and after (W8) the treatment. Twenty-one patients (61.76%) achieved Hi-SCR. The mean scoring of HS-PGA had significantly decreased from 3.24 to 2.15 (p = 0.001). |
| Clindamycin-rifampicin [28] | Gener G. et al., 2009 | Retrospective study | 116 | Hurley stage III HS | Clindamycin (300 mg PO BID) + rifampicin (600 mg PO daily) | Improvement of median Sartorius score at the end of treatment (29 vs. 14.5; p < 0.001), and of QoL score. |
| Clindamycin-rifampicin [30] | van der Zee H.H. et al., 2009 | Retrospective study | 34 | Hurley stage I, II, III HS | Clindamycin (300 mg PO BID) + rifampicin (600 mg PO daily) | Partial improvement: 82%; total remission: 47% (maximum effect of treatment within 10 weeks). Following total remission, 8 of 13 (61.5%) patients treated as mentioned above experienced a relapse after a mean period of 5.0 months. |
| Clindamycin-rifampicin [31] | Dessinioti C. et al., 2016 | Prospective study | 26 | Hurley stage I, II, III HS | Clindamycin (300 mg PO BID) + rifampicin (600 mg PO daily) for 12 weeks | 12-week clinical response rate: 73%; At the 1-year follow-up, there was sustained efficacy in 7 (41%) patients, while 10 (59%) had disease relapse after a mean time of 4.2 months. |
| Clidamycin-Oxofloxacin [34] | Delaunay J. et al., 2018 | Retrospective study | 65 | Hurley stage I, II, III HS | Clindamycin (600–1800 mg) + oflaxacin (200–400 mg) | Efficacy in disease control in HS. Thirty-eight patients (58.4%) reported improvement of disease activity under OC with complete response for 22/65 (33.8%) and partial remission in 16/65 (24.6%) patients. |
| TETRACYCLINES | ||||||
| Minocycline [37] | [37] K. et al., 2017 | Prospective study | 20 | Hurley stage I, II, III HS | Minocycline 100 mg PO + colchicine 0.5 mg BID for 6 months, followed by colchicine maintenance 0.5 mg BID for 3 months | Efficacy in disease control in HSPhysician global assessment (PGA) scale (PGA) shows good and excellent response in 95% of patients at 9 months. |
| Limecycline [20] | Caposiena Caro R.D. et al., 2021 | Retrospective study | 52 | Hurley stage I, II, III HS |
| Both treatments are effective in terms of IHS4, pain VAS scale and DLQI for patients with moderate-severe HS. Response rates at the end of the treatments were similar in both groups (p = 0.78): 57.7% in group A and 53.8% in group B met the primary outcome (HiSCR). |
| Tetracycline [36] | Jørgensen A.R. et al., 2021 | Prospective study | 143 | Hurley stage I, II, III HS |
| All treatments were effective and safe in HS patients. Tetracycline provided the greatest clinical improvement mean The mean HSS at baseline was 26.10 (SD 20.18) points, improving to 17.97 (SD 17.88) at follow-up, difference is 8.13 (95% CI 5.21–10.93), p < 0.0001 sured by HSS. |
| ERTAPENEM | ||||||
| Ertapenem [40] | Join-Lambert O. et al., 2016 | Retrospective study | 30 | Hurley stage III | Ertapenem 1 g die for 6 weeks + antibiotic consolidation treatments for 6 months (M6) in severe HS | Dramatic improvement of severe HS provided by ertapenem. The median (IQR) Sartorious score dropped from 49.5 (28–62) at baseline to 19.0 (12–28) after ertapenm (p < 10−4). |
| Ertapenem [3] | Braunberger T.L. et al., 2018 | Retrospective study | 36 | Hurley stage II, III HS | Ertapenem 1 g die | Clinical improvement in 97.2% of patients and an improvement in quality of life in 85.7% of patients. |
| METRONIDAZOLE (monotherapy and combinations) | ||||||
| Metronidazole [50] | Delage M. et al., 2023 | Prospective trial | 28 | Hurley stage I HS | Rifampicin + moxifloxacin + metronidazole | The primary endpoint was a Sartorius score of 0 (clinical remission) at week 12. The median Sartorius score dropped from 14 to 0 (p = 6 × 10−6) at week 12, with 75% of patients reaching clinical remission. |
| Ceftriaxone + Metronidazole [51] | Nassif A et al., 2012 | Case report | 4 | Hurley stage II HS | Ceftriaxone IV + metronidazole PO | Improvement of HS |
| DALBAVANCIN | ||||||
| Dalbavancin [6] | Molinelli E. et al., 2022 | Retrospective study | 8 | Hurley stage I, II or III HS | Dalbavancin 100 mg IV | Significant disease improvement at 12 weeks; Significant disease improvement was achieved at 12 weeks (T12) with average values of 7 for IHS4, 2 for pain VAS, and 8 for DLQI, and HiSCR was satisfied in six out of eight patients compared to baseline (T0) |
3.6. Dalbavancin
3.7. Linezolid
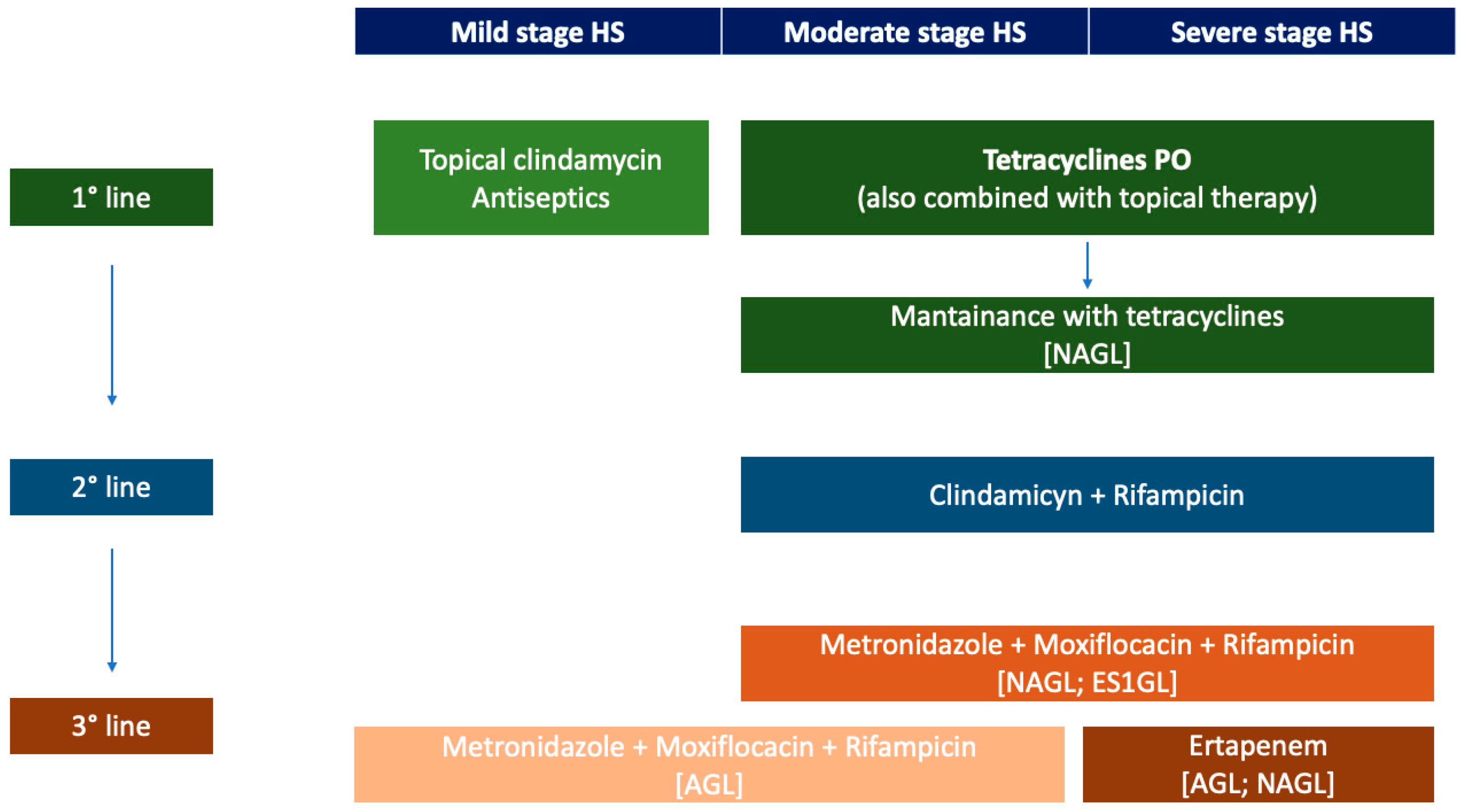
4. The Choice of Antibiotics Therapy in Clinical Practice
4.1. The Treatment of HS with Antibiotics across Guidelines
4.2. Limitations of Antibiotics in HS: Resistances and Toxicity
5. Current Issues and Future Challenges
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bianchi, L.; Caposiena Caro, R.D.; Ganzetti, G.; Molinelli, E.; Dini, V.; Oranges, T.; Romanelli, M.; Fabbrocini, G.; Monfrecola, G.; Napolitano, M.; et al. Sex-related Differences of Clinical Features in Hidradenitis Suppurativa: Analysis of an Italian-based Cohort. Clin. Exp. Dermatol. 2019, 44, e177–e180. [Google Scholar] [CrossRef] [PubMed]
- Vazquez, B.G.; Alikhan, A.; Weaver, A.L.; Wetter, D.A.; Davis, M.D. Incidence of Hidradenitis Suppurativa and Associated Factors: A Population-Based Study of Olmsted County, Minnesota. J. Investig. Dermatol. 2013, 133, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Braunberger, T.L.; Nartker, N.T.; Nicholson, C.L.; Nahhas, A.F.; Parks-Miller, A.; Hanna, Z.; Jayaprakash, R.; Ramesh, M.S.; Rambhatla, P.V.; Hamzavi, I.H. Ertapenem—A Potent Treatment for Clinical and Quality of Life Improvement in Patients with Hidradenitis Suppurativa. Int. J. Dermatol. 2018, 57, 1088–1093. [Google Scholar] [CrossRef] [PubMed]
- Marasca, C.; Tranchini, P.; Marino, V.; Annunziata, M.C.; Napolitano, M.; Fattore, D.; Fabbrocini, G. The Pharmacology of Antibiotic Therapy in Hidradenitis Suppurativa. Expert Rev. Clin. Pharmacol. 2020, 13, 521–530. [Google Scholar] [CrossRef]
- Saunte, D.M.; Boer, J.; Stratigos, A.; Szepietowski, J.C.; Hamzavi, I.; Kim, K.H.; Zarchi, K.; Antoniou, C.; Matusiak, L.; Lim, H.W.; et al. Diagnostic Delay in Hidradenitis Suppurativa Is a Global Problem. Br. J. Dermatol. 2015, 173, 1546–1549. [Google Scholar] [CrossRef]
- Molinelli, E.; Sapigni, C.; D’Agostino, G.M.; Brisigotti, V.; Rizzetto, G.; Bobyr, I.; Cirioni, O.; Giacometti, A.; Brescini, L.; Mazzanti, S.; et al. The Effect of Dalbavancin in Moderate to Severe Hidradenitis Suppurativa. Antibiotics 2022, 11, 1573. [Google Scholar] [CrossRef]
- Ofenloch, R.F. Health-Related Quality of Life in Hidradenitis Suppurativa. Br. J. Dermatol. 2017, 176, 861–862. [Google Scholar] [CrossRef]
- Napolitano, M.; Calzavara-Pinton, P.G.; Zanca, A.; Bianchi, L.; Caposiena Caro, R.D.; Offidani, A.M.; Ganzetti, G.; Molinelli, E.; Dini, V.; Oranges, T.; et al. Comparison of Clinical and Ultrasound Scores in Patients with Hidradenitis Suppurativa: Results from an Italian Ultrasound Working Group. J. Eur. Acad. Dermatol. Venereol. 2019, 33, e84–e87. [Google Scholar] [CrossRef]
- Lacarrubba, F.; Dini, V.; Napolitano, M.; Venturini, M.; Caposiena Caro, D.R.; Molinelli, E.; Passoni, E.; Monfrecola, G.; Argenziano, G.; Berti, E.; et al. Ultrasonography in the Pathway to an Optimal Standard of Care of Hidradenitis Suppurativa: The Italian Ultrasound Working Group Experience. J. Eur. Acad. Dermatol. Venereol. 2019, 33, 10–14. [Google Scholar] [CrossRef]
- Hendricks, A.J.; Hsiao, J.L.; Lowes, M.A.; Shi, V.Y. A Comparison of International Management Guidelines for Hidradenitis Suppurativa. Dermatology 2021, 237, 81–96. [Google Scholar] [CrossRef]
- Zouboulis, C.C.; Tzellos, T.; Kyrgidis, A.; Jemec, G.B.E.; Bechara, F.G.; Giamarellos-Bourboulis, E.J.; Ingram, J.R.; Kanni, T.; Karagiannidis, I.; Martorell, A.; et al. Development and Validation of the International Hidradenitis Suppurativa Severity Score System (IHS4), a Novel Dynamic Scoring System to Assess HS Severity. Br. J. Dermatol. 2017, 177, 1401–1409. [Google Scholar] [CrossRef]
- Gergely, L.H.; Gáspár, K.; Brodszky, V.; Kinyó, Á.; Szegedi, A.; Remenyik, É.; Kiss, N.F.; Bató, A.; Péntek, M.; Gulácsi, L.; et al. Validity of EQ-5D-5L, Skindex-16, DLQI and DLQI-R in Patients with Hidradenitis Suppurativa. J. Eur. Acad. Dermatol. Venereol. 2020, 34, 2584–2592. [Google Scholar] [CrossRef]
- An, J.H.; Moon, S.J.; Shin, J.U.; Kim, D.H.; Yoon, M.S.; Lee, H.J. Clindamycin Mono-Therapy of Hidradenitis Suppurativa Patients: A Single-Center Retrospective Study. Ann. Dermatol. 2021, 33, 515. [Google Scholar] [CrossRef] [PubMed]
- Napolitano, M.; Fabbrocini, G.; Marasca, C.; Monfrecola, G. Update on Pathogenesis of Hidradenitis Suppurativa. Ital. J. Dermatol. Venereol. 2018, 153, 3–7. [Google Scholar] [CrossRef]
- Biernat, M.M.; Wróbel, T. Bacterial Infection and Non-Hodgkin B-Cell Lymphoma: Interactions between Pathogen, Host and the Tumor Environment. Int. J. Mol. Sci. 2021, 22, 7372. [Google Scholar] [CrossRef]
- Goteri, G.; Ranaldi, R.; Simonetti, O.; Capretti, R.; Menzo, S.; Stramazzotti, D.; Morichetti, D.; Offidani, A.M.; Rupoli, S.; Leoni, P. Clinicopathological Features of Primary Cutaneous B-Cell Lymphomas from an Academic Regional Hospital in Central Italy: No Evidence of Borrelia burgdorferi Association. Leuk. Lymphoma 2007, 48, 2184–2188. [Google Scholar] [CrossRef] [PubMed]
- Ring, H.C.; Riis Mikkelsen, P.; Miller, I.M.; Jenssen, H.; Fuursted, K.; Saunte, D.M.; Jemec, G.B.E. The Bacteriology of Hidradenitis Suppurativa: A Systematic Review. Exp. Dermatol. 2015, 24, 727–731. [Google Scholar] [CrossRef]
- Ring, H.C.; Emtestam, L. The Microbiology of Hidradenitis Suppurativa. Dermatol. Clin. 2016, 34, 29–35. [Google Scholar] [CrossRef] [PubMed]
- Alikhan, A.; Sayed, C.; Alavi, A.; Alhusayen, R.; Brassard, A.; Burkhart, C.; Crowell, K.; Eisen, D.B.; Gottlieb, A.B.; Hamzavi, I.; et al. North American Clinical Management Guidelines for Hidradenitis Suppurativa: A Publication from the United States and Canadian Hidradenitis Suppurativa Foundations. J. Am. Acad. Dermatol. 2019, 81, 76–90. [Google Scholar] [CrossRef] [PubMed]
- Caposiena Caro, R.D.; Molinelli, E.; Brisigotti, V.; Offidani, A.; Bianchi, L. Lymecycline vs. Clindamycin plus Rifampicin in Hidradenitis Suppurativa Treatment: Clinical and Ultrasonography Evaluation. Clin. Exp. Dermatol. 2021, 46, 96–102. [Google Scholar] [CrossRef]
- Spížek, J.; Novotná, J.; Řezanka, T. Lincosamides: Chemical Structure, Biosynthesis, Mechanism of Action, Resistance, and Applications. Adv. Appl. Microbiol. 2004, 56, 121–154. [Google Scholar] [PubMed]
- Leigh, D.A. Antibacterial Activity and Pharmacokinetics of Clindamycin. J. Antimicrob. Chemother. 1981, 7, 3–9. [Google Scholar] [CrossRef]
- Clemmensen, O.J. Topical Treatment of Hidradenitis Suppurativa with Clindamycin. Int. J. Dermatol. 1983, 22, 325–328. [Google Scholar] [CrossRef]
- Jemec, G.B.E.; Wendelboe, P. Topical Clindamycin versus Systemic Tetracycline in the Treatment of Hidradenitis Suppurativa. J. Am. Acad. Dermatol. 1998, 39, 971–974. [Google Scholar] [CrossRef] [PubMed]
- Molinelli, E.; Brisigotti, V.; Simonetti, O.; Campanati, A.; Sapigni, C.; D’Agostino, G.M.; Giacchetti, A.; Cota, C.; Offidani, A. Efficacy and Safety of Topical Resorcinol 15% as Long-term Treatment of Mild-to-moderate Hidradenitis Suppurativa: A Valid Alternative to Clindamycin in the Panorama of Antibiotic Resistance. Br. J. Dermatol. 2020, 183, 1117–1119. [Google Scholar] [CrossRef] [PubMed]
- Molinelli, E.; Brisigotti, V.; Simonetti, O.; Sapigni, C.; D’Agostino, G.M.; Rizzetto, G.; Giacchetti, A.; Offidani, A. Efficacy and Safety of Topical Resorcinol 15% versus Topical Clindamycin 1% in the Management of Mild-to-moderate Hidradenitis Suppurativa: A Retrospective Study. Dermatol. Ther. 2022, 35, e15439. [Google Scholar] [CrossRef] [PubMed]
- Boer, J.; Jemec, G.B.E. Resorcinol Peels as a Possible Self-Treatment of Painful Nodules in Hidradenitis Suppurativa. Clin. Exp. Dermatol. 2010, 35, 36–40. [Google Scholar] [CrossRef]
- Gener, G.; Canoui-Poitrine, F.; Revuz, J.E.; Faye, O.; Poli, F.; Gabison, G.; Pouget, F.; Viallette, C.; Wolkenstein, P.; Bastuji-Garin, S. Combination Therapy with Clindamycin and Rifampicin for Hidradenitis Suppurativa: A Series of 116 Consecutive Patients. Dermatology 2009, 219, 148–154. [Google Scholar] [CrossRef]
- Mendonça, C.O.; Griffiths, C.E.M. Clindamycin and Rifampicin Combination Therapy for Hidradenitis Suppurativa. Br. J. Dermatol. 2006, 154, 977–978. [Google Scholar] [CrossRef]
- van der Zee, H.H.; Boer, J.; Prens, E.P.; Jemec, G.B.E. The Effect of Combined Treatment with Oral Clindamycin and Oral Rifampicin in Patients with Hidradenitis Suppurativa. Dermatology 2009, 219, 143–147. [Google Scholar] [CrossRef]
- Dessinioti, C.; Zisimou, C.; Tzanetakou, V.; Stratigos, A.; Antoniou, C. Oral Clindamycin and Rifampicin Combination Therapy for Hidradenitis Suppurativa: A Prospective Study and 1-Year Follow-Up. Clin. Exp. Dermatol. 2016, 41, 852–857. [Google Scholar] [CrossRef] [PubMed]
- Caposiena Caro, R.D.; Bianchi, L. Clindamycin Alone May Be Enough. Is It Time to Abandon Rifampicin for Hidradenitis Suppurativa? Br. J. Dermatol. 2019, 180, 1262. [Google Scholar] [CrossRef] [PubMed]
- Caposiena Caro, R.D.; Cannizzaro, M.V.; Botti, E.; Di Raimondo, C.; Di Matteo, E.; Gaziano, R.; Bianchi, L. Clindamycin versus Clindamycin plus Rifampicin in Hidradenitis Suppurativa Treatment: Clinical and Ultrasound Observations. J. Am. Acad. Dermatol. 2019, 80, 1314–1321. [Google Scholar] [CrossRef] [PubMed]
- Delaunay, J.; Villani, A.P.; Guillem, P.; Tristan, A.; Boibieux, A.; Jullien, D. Oral Ofloxacin and Clindamycin as an Alternative to the Classic Rifampicin–Clindamycin in Hidradenitis Suppurativa: Retrospective Analysis of 65 Patients. Br. J. Dermatol. 2018, 178, e15–e16. [Google Scholar] [CrossRef] [PubMed]
- Vural, S.; Gündoğdu, M.; Akay, B.N.; Boyvat, A.; Erdem, C.; Koçyiğit, P.; Bostancı, S.; Sanli, H.; Kundakci, N. Hidradenitis Suppurativa: Clinical Characteristics and Determinants of Treatment Efficacy. Dermatol. Ther. 2019, 32, e13003. [Google Scholar] [CrossRef]
- Jørgensen, A.R.; Yao, Y.; Thomsen, S.F.; Ring, H.C. Treatment of Hidradenitis Suppurativa with Tetracycline, Doxycycline, or Lymecycline: A Prospective Study. Int. J. Dermatol. 2021, 60, 785–791. [Google Scholar] [CrossRef]
- Armyra, K.; Kouris, A.; Markantoni, V.; Katsambas, A.; Kontochristopoulos, G. Hidradenitis Suppurativa Treated with Tetracycline in Combination with Colchicine: A Prospective Series of 20 Patients. Int. J. Dermatol. 2017, 56, 346–350. [Google Scholar] [CrossRef]
- Alfei, S.; Zuccari, G. Recommendations to Synthetize Old and New β-Lactamases Inhibitors: A Review to Encourage Further Production. Pharmaceuticals 2022, 15, 384. [Google Scholar] [CrossRef] [PubMed]
- Zhanel, G.G.; Johanson, C.; Embil, J.M.; Noreddin, A.; Gin, A.; Vercaigne, L.; Hoban, D.J. Ertapenem: Review of a New Carbapenem. Expert Rev. Anti Infect. Ther. 2005, 3, 23–39. [Google Scholar] [CrossRef]
- Join-Lambert, O.; Coignard-Biehler, H.; Jais, J.-P.; Delage, M.; Guet-Revillet, H.; Poirée, S.; Duchatelet, S.; Jullien, V.; Hovnanian, A.; Lortholary, O.; et al. Efficacy of Ertapenem in Severe Hidradenitis Suppurativa: A Pilot Study in a Cohort of 30 Consecutive Patients. J. Antimicrob. Chemother. 2016, 71, 513–520. [Google Scholar] [CrossRef]
- Teppler, H. Safety and Tolerability of Ertapenem. J. Antimicrob. Chemother. 2004, 53, ii75–ii81. [Google Scholar] [CrossRef] [PubMed]
- Patel, P.H.; Hashmi, M.F. Macrolides. 2022 Aug 18. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar] [PubMed]
- Molinelli, E.; Brisigotti, V.; Campanati, A.; Sapigni, C.; Giacchetti, A.; Cota, C.; Offidani, A. Efficacy of Oral Zinc and Nicotinamide as Maintenance Therapy for Mild/Moderate Hidradenitis Suppurativa: A Controlled Retrospective Clinical Study. J. Am. Acad. Dermatol. 2020, 83, 665–667. [Google Scholar] [CrossRef] [PubMed]
- Offidani, A.; Molinelli, E.; Sechi, A.; Brisigotti, V.; Campanati, A.; Raone, B.; Neri, I.; Patrizi, A. Hidradenitis Suppurativa in a Prepubertal Case Series: A Call for Specific Guidelines. J. Eur. Acad. Dermatol. Venereol. 2019, 33, 28–31. [Google Scholar] [CrossRef]
- Simonetti, O.; Lucarini, G.; Orlando, F.; Pierpaoli, E.; Ghiselli, R.; Provinciali, M.; Castelli, P.; Guerrieri, M.; Di Primio, R.; Offidani, A.; et al. Role of Daptomycin on Burn Wound Healing in an Animal Methicillin-Resistant Staphylococcus Aureus Infection Model. Antimicrob. Agents Chemother. 2017, 61, e00606-17. [Google Scholar] [CrossRef]
- Gierek, M.; Ochała-Gierek, G.; Kitala, D.; Łabuś, W.; Bergler-Czop, B. Hidradenitis Suppurativa: Bacteriological Study in Surgical Treatment. Adv. Dermatol. Allergol. 2022, 39, 1101–1105. [Google Scholar] [CrossRef]
- Molinelli, E.; Sapigni, C.; Simonetti, O.; D’Agostino, G.M.; Brisigotti, V.; Rizzetto, G.; Offidani, A. Acitretin plus Macrolides and Acitretin Monotherapy in the Management of Hidradenitis Suppurativa. J. Eur. Acad. Dermatol. Venereol. 2023, 37, e392–e394. [Google Scholar] [CrossRef]
- Dingsdag, S.A.; Hunter, N. Metronidazole: An Update on Metabolism, Structure–Cytotoxicity and Resistance Mechanisms. J. Antimicrob. Chemother. 2018, 73, 265–279. [Google Scholar] [CrossRef]
- Join-Lambert, O.; Coignard, H.; Jais, J.-P.; Guet-Revillet, H.; Poirée, S.; Fraitag, S.; Jullien, V.; Ribadeau-Dumas, F.; Thèze, J.; Le Guern, A.-S.; et al. Efficacy of Rifampin-Moxifloxacin-Metronidazole Combination Therapy in Hidradenitis Suppurativa. Dermatology 2011, 222, 49–58. [Google Scholar] [CrossRef]
- Delage, M.; Jais, J.-P.; Lam, T.; Guet-Revillet, H.; Ungeheuer, M.-N.; Consigny, P.-H.; Nassif, A.; Join-Lambert, O. Rifampin-Moxifloxacin-Metronidazole Combination Therapy for Severe Hurley Stage 1 Hidradenitis Suppurativa: Prospective Short-Term Trial and 1-Year Follow-up in 28 Consecutive Patients. J. Am. Acad. Dermatol. 2023, 88, 94–100. [Google Scholar] [CrossRef]
- Complete Remission of Severe Hidradenitis Suppurativa Obtained in 4 Patients Using Wide-Spectrum Antimicrobial Treatment. J. Am. Acad. Dermatol. 2012, 66, AB46. [CrossRef]
- Van Bambeke, F.; Van Laethem, Y.; Courvalin, P.; Tulkens, P.M. Glycopeptide Antibiotics. Drugs 2004, 64, 913–936. [Google Scholar] [CrossRef] [PubMed]
- Simonetti, O.; Lucarini, G.; Morroni, G.; Orlando, F.; Lazzarini, R.; Zizzi, A.; Brescini, L.; Provinciali, M.; Giacometti, A.; Offidani, A.; et al. New Evidence and Insights on Dalbavancin and Wound Healing in a Mouse Model of Skin Infection. Antimicrob. Agents Chemother. 2020, 64, e02062-19. [Google Scholar] [CrossRef] [PubMed]
- Simonetti, O.; Lucarini, G.; Campanati, A.; Goteri, G.; Zizzi, A.; Marconi, B.; Ganzetti, G.; Minardi, D.; Di Primio, R.; Offidani, A. VEGF, Survivin and NOS Overexpression in Psoriatic Skin: Critical Role of Nitric Oxide Synthases. J. Dermatol. Sci. 2009, 54, 205–208. [Google Scholar] [CrossRef]
- Sazdanovic, P.; Jankovic, S.M.; Kostic, M.; Dimitrijevic, A.; Stefanovic, S. Pharmacokinetics of Linezolid in Critically Ill Patients. Expert Opin. Drug Metab. Toxicol. 2016, 12, 595–600. [Google Scholar] [CrossRef]
- Hashemian, S.M.; Farhadi, T.; Ganjparvar, M. Linezolid: A Review of Its Properties, Function, and Use in Critical Care. Drug Des. Devel. Ther. 2018, 12, 1759–1767. [Google Scholar] [CrossRef]
- Scheinfeld, N. Extensive Hidradenitis Suppurativa (HS) Hurly Stage III Disease Treated with Intravenous (IV) Linezolid and Meropenem with Rapid Remission. Dermatol. Online J. 2015, 21, 7. [Google Scholar] [CrossRef]
- Bettoli, V.; Manfredini, M.; Massoli, L.; Carillo, C.; Barozzi, A.; Amendolagine, G.; Ruina, G.; Musmeci, D.; Libanore, M.; Curtolo, A.; et al. Rates of Antibiotic Resistance/Sensitivity in Bacterial Cultures of Hidradenitis Suppurativa Patients. J. Eur. Acad. Dermatol. Venereol. 2019, 33, 930–936. [Google Scholar] [CrossRef] [PubMed]
- Bettoli, V.; Join-Lambert, O.; Nassif, A. Antibiotic Treatment of Hidradenitis Suppurativa. Dermatol. Clin. 2016, 34, 81–89. [Google Scholar] [CrossRef]
- Ingram, J.R.; Collier, F.; Brown, D.; Burton, T.; Burton, J.; Chin, M.F.; Desai, N.; Goodacre, T.E.E.; Piguet, V.; Pink, A.E.; et al. British Association of Dermatologists Guidelines for the Management of Hidradenitis Suppurativa (Acne Inversa) 2018. Br. J. Dermatol. 2019, 180, 1009–1017. [Google Scholar] [CrossRef]
- Zouboulis, C.C.; Bechara, F.G.; Dickinson-Blok, J.L.; Gulliver, W.; Horváth, B.; Hughes, R.; Kimball, A.B.; Kirby, B.; Martorell, A.; Podda, M.; et al. Hidradenitis Suppurativa/Acne Inversa: A Practical Framework for Treatment Optimization—Systematic Review and Recommendations from the HS ALLIANCE Working Group. J. Eur. Acad. Dermatol. Venereol. 2019, 33, 19–31. [Google Scholar] [CrossRef]
- Magalhães, R.F.; Rivitti-Machado, M.C.; Duarte, G.V.; Souto, R.; Nunes, D.H.; Chaves, M.; Hirata, S.H.; Ramos, A.M.C. Consensus on the Treatment of Hidradenitis Suppurativa—Brazilian Society of Dermatology. An. Bras. Dermatol. 2019, 94, 7–19. [Google Scholar] [CrossRef] [PubMed]
- Hunger, R.E.; Laffitte, E.; Läuchli, S.; Mainetti, C.; Mühlstädt, M.; Schiller, P.; Lapointe, A.-K.; Meschberger, P.; Navarini, A.A. Swiss Practice Recommendations for the Management of Hidradenitis Suppurativa/Acne Inversa. Dermatology 2017, 233, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Gulliver, W.; Zouboulis, C.C.; Prens, E.; Jemec, G.B.E.; Tzellos, T. Evidence-Based Approach to the Treatment of Hidradenitis Suppurativa/Acne Inversa, Based on the European Guidelines for Hidradenitis Suppurativa. Rev. Endocr. Metab. Disord. 2016, 17, 343–351. [Google Scholar] [CrossRef] [PubMed]
- Albrecht, J.; Baine, P.A.; Ladizinski, B.; Jemec, G.B.; Bigby, M. Long-term Clinical Safety of Clindamycin and Rifampicin Combination for the Treatment of Hidradenitis Suppurativa. A Critically Appraised Topic. Br. J. Dermatol. 2019, 180, 749–755. [Google Scholar] [CrossRef]
- Hambly, R.; Kirby, B. Prolonged Clindamycin and Rifampicin for Hidradenitis Suppurativa: Resist to Prevent Resistance. Br. J. Dermatol. 2019, 180, 702–703. [Google Scholar] [CrossRef]
- Zouboulis, C.C.; del Marmol, V.; Mrowietz, U.; Prens, E.P.; Tzellos, T.; Jemec, G.B.E. Hidradenitis Suppurativa/Acne Inversa: Criteria for Diagnosis, Severity Assessment, Classification and Disease Evaluation. Dermatology 2015, 231, 184–190. [Google Scholar] [CrossRef]
- Zouboulis, C.C.; Desai, N.; Emtestam, L.; Hunger, R.E.; Ioannides, D.; Juhász, I.; Lapins, J.; Matusiak, L.; Prens, E.P.; Revuz, J.; et al. European S1 Guideline for the Treatment of Hidradenitis Suppurativa/Acne Inversa. J. Eur. Acad. Dermatol. Venereol. 2015, 29, 619–644. [Google Scholar] [CrossRef]
- Simonart, T. Hidradenitis Suppurativa and Smoking. J. Am. Acad. Dermatol. 2010, 62, 149–150. [Google Scholar] [CrossRef]
- Fischer, A.H.; Haskin, A.; Okoye, G.A. Patterns of Antimicrobial Resistance in Lesions of Hidradenitis Suppurativa. J. Am. Acad. Dermatol. 2017, 76, 309–313.e2. [Google Scholar] [CrossRef]
- Mendes-Bastos, P.; Macedo, R.; Duarte, R. Treatment of Hidradenitis Suppurativa with Rifampicin: Have We Forgotten Tuberculosis? Br. J. Dermatol. 2017, 177, e150–e151. [Google Scholar] [CrossRef]
- Marasca, C.; Masarà, A.; Annunziata, M.C.; Bettoli, V.; Luciano, M.A.; Fabbrocini, G. Long-term Clinical Safety of Clindamycin and Rifampicin Combination for the Treatment of Hidradenitis Suppurativa: A Strategy to Reduce Side-effects, Improving Patients’ Compliance. Br. J. Dermatol. 2019, 180, 949. [Google Scholar] [CrossRef] [PubMed]
- Cross, R.; Ling, C.; Day, N.P.J.; McGready, R.; Paris, D.H. Revisiting Doxycycline in Pregnancy and Early Childhood—Time to Rebuild Its Reputation? Expert Opin. Drug Saf. 2016, 15, 367–382. [Google Scholar] [CrossRef] [PubMed]
- Matusiak, Ł.; Bieniek, A.; Szepietowski, J. Bacteriology of Hidradenitis Suppurativa—Which Antibiotics Are the Treatment of Choice? Acta Derm. Venereol. 2014, 94, 699–702. [Google Scholar] [CrossRef]
- Silvestri, C.; Cirioni, O.; Arzeni, D.; Ghiselli, R.; Simonetti, O.; Orlando, F.; Ganzetti, G.; Staffolani, S.; Brescini, L.; Provinciali, M.; et al. In Vitro Activity and in Vivo Efficacy of Tigecycline Alone and in Combination with Daptomycin and Rifampin against Gram-Positive Cocci Isolated from Surgical Wound Infection. Eur. J. Clin. Microbiol. Infect. Dis. 2012, 31, 1759–1764. [Google Scholar] [CrossRef] [PubMed]
- Kitts, S.; Govea, R.; Maczuga, S.; Kirby, J. Long-term Antibiotic Use for the Treatment of Hidradenitis Suppurativa Consistent with Guideline Recommendations. Clin. Exp. Dermatol. 2021, 46, 582–583. [Google Scholar] [CrossRef]
- Ring, H.C.; Bay, L.; Nilsson, M.; Kallenbach, K.; Miller, I.M.; Saunte, D.M.; Bjarnsholt, T.; Tolker-Nielsen, T.; Jemec, G.B. Bacterial Biofilm in Chronic Lesions of Hidradenitis Suppurativa. Br. J. Dermatol. 2017, 176, 993–1000. [Google Scholar] [CrossRef]
- Ardon, C.B.; Prens, E.P.; Fuursted, K.; Ejaz, R.N.; Shailes, J.; Jenssen, H.; Jemec, G.B.E. Biofilm Production and Antibiotic Susceptibility of Staphylococcus epidermidis Strains from Hidradenitis Suppurativa Lesions. J. Eur. Acad. Dermatol. Venereol. 2019, 33, 170–177. [Google Scholar] [CrossRef]
- Simonetti, O.; Morroni, G.; Ghiselli, R.; Orlando, F.; Brenciani, A.; Xhuvelaj, L.; Provinciali, M.; Offidani, A.; Guerrieri, M.; Giacometti, A.; et al. In Vitro and in Vivo Activity of Fosfomycin Alone and in Combination with Rifampin and Tigecycline against Gram-Positive Cocci Isolated from Surgical Wound Infections. J. Med. Microbiol. 2018, 67, 139–143. [Google Scholar] [CrossRef]
- Simonetti, O.; Rizzetto, G.; Molinelli, E.; Cirioni, O.; Offidani, A. Review: A Safety Profile of Dalbavancin for On- and Off-Label Utilization. Ther. Clin. Risk Manag. 2021, 17, 223–232. [Google Scholar] [CrossRef]
- Mikkelsen, P.R.; Dufour, D.N.; Zarchi, K.; Jemec, G.B.E. Recurrence Rate and Patient Satisfaction of CO2 Laser Evaporation of Lesions in Patients with Hidradenitis Suppurativa. Dermatol. Surg. 2015, 41, 255–260. [Google Scholar] [CrossRef]
- Manfredini, M.; Garbarino, F.; Bigi, L.; Pellacani, G.; Magnoni, C. Surgical and Postsurgical Wound Care in Hidradenitis Suppurativa. Dermatol. Ther. 2020, 33, e13282. [Google Scholar] [CrossRef]
- Molinelli, E.; Sapigni, C.; Simonetti, O.; Brisigotti, V.; Giuliodori, K.; Offidani, A. Alexandrite Laser as an Adjuvant Therapy in the Management of Mild to Moderate Hidradenitis Suppurativa: A Controlled Prospective Clinical Study. J. Am. Acad. Dermatol. 2022, 87, 674–675. [Google Scholar] [CrossRef] [PubMed]
- Ellis, L.Z. Hidradenitis Suppurativa: Surgical and Other Management Techniques. Dermatol. Surg. 2012, 38, 517–536. [Google Scholar] [CrossRef] [PubMed]
- Recruiting Clinical Trials with Antibiotics in HS. Available online: https://clinicaltrials.gov/ (accessed on 30 March 2023).
- Diotallevi, F.; Campanati, A.; Radi, G.; Molinelli, E.; Offidani, A. Ixekizumab for Treatment of Moderate to Severe Plaque Psoriasis: Real World Clinical Experience. G. Ital. Dermatol. Venereol. 2021, 155, 739–743. [Google Scholar] [CrossRef] [PubMed]
- Campanati, A.; Paolinelli, M.; Diotallevi, F.; Martina, E.; Molinelli, E.; Offidani, A. Pharmacodynamics OF TNF α Inhibitors for the Treatment of Psoriasis. Expert Opin. Drug Metab. Toxicol. 2019, 15, 913–925. [Google Scholar] [CrossRef] [PubMed]
- Brownstone, N.; Hong, J.; Mosca, M.; Hadeler, E.; Liao, W.; Bhutani, T.; Koo, J. Biologic Treatments of Psoriasis: An Update for the Clinician. Biologics 2021, 15, 39–51. [Google Scholar] [CrossRef]
- Deleanu, D.; Nedelea, I. Biological Therapies for Atopic Dermatitis: An Update (Review). Exp. Ther. Med. 2018, 17, 1061–1067. [Google Scholar] [CrossRef]
- Aarts, P.; Dudink, K.; Vossen, A.R.J.V.; van Straalen, K.R.; Ardon, C.B.; Prens, E.P.; van der Zee, H.H. Clinical Implementation of Biologics and Small Molecules in the Treatment of Hidradenitis Suppurativa. Drugs 2021, 81, 1397–1410. [Google Scholar] [CrossRef]
- Marzano, A.V.; Genovese, G.; Casazza, G.; Moltrasio, C.; Dapavo, P.; Micali, G.; Sirna, R.; Gisondi, P.; Patrizi, A.; Dini, V.; et al. Evidence for a ‘Window of Opportunity’ in Hidradenitis Suppurativa Treated with Adalimumab: A Retrospective, Real-life Multicentre Cohort Study*. Br. J. Dermatol. 2021, 184, 133–140. [Google Scholar] [CrossRef]
- Molinelli, E.; Sapigni, C.; Campanati, A.; Brisigotti, V.; Offidani, A. Metabolic, Pharmacokinetic, and Toxicological Issues of Biologic Therapies Currently Used in the Treatment of Hidradenitis Suppurativa. Expert Opin. Drug Metab. Toxicol. 2020, 16, 1019–1037. [Google Scholar] [CrossRef]
| Clinical and Research Settings | |||
|---|---|---|---|
| International Hidradenitis Suppurativa Severity Score System (IHS4) | |||
IHS4 (point) =
| |||
| |||
| Advantages | Limitation | ||
|
| ||
| Hurley Staging System | |||
| Stage | I | II | III |
| Abscess | Single or multiple | Single or multiple, widely separated, recurrent | Diffuse or near-diffuse involvement |
| Sinus tract | – | + | Multiple interconnected |
| Cicatrization | – | + | + |
| Area | Entire area | ||
| Advantages | Limitation | ||
|
| ||
| Hidradenitis Suppurativa Clinical Response—HiSCR | |||
HiSCR is defined by the status of three types of criteria lesions, considering abscesses (fluctuant, with or without drainage, tender or painful), inflammatory nodules (tender, erythematous, pyogenic granuloma lesion), and draining fistulas (sinus tracts, with communications to skin surface, draining purulent fluid). The proposed definition of responders to treatment (HiSCR achievers) is:
| |||
| Advantages | Limitation | ||
|
| ||
| Hidradenitis Suppurativa Physician’s Global Assessment (HS-PGA) | |||
| HS-PGA | |||
| Clear (score = 0) | 0 abscesses, 0 draining fistulas, 0 inflammatory nodules, and 0 non-inflammatory nodules | ||
| Minimal (score = 1) | 0 abscesses, 0 draining fistulas, 0 inflammatory nodules, and presence of non-inflammatory nodules | ||
| Mild (score = 2) | 0 abscesses, 0 draining fistulas, and 1–4 inflammatory nodules; or 1 abscess or draining fistula and 0 inflammatory nodules | ||
| Moderate (score = 3) | 0 abscesses, 0 draining fistulas, and 1 ≥ 5 inflammatory nodules; or 1 abscess or draining fistula and ≥1 inflammatory nodule; or 2–5 abscesses or draining fistulas and <10 inflammatory nodules | ||
| Severe (score = 4) | 2–5 abscesses or draining fistulas, and ≥10 inflammatory nodules; | ||
| Advantages | Limitation | ||
|
| ||
| Patient-Reported Outcomes | |||
| Dermatology Life Quality Index (DLQI Score) | |||
| DLQI Total score | |||
| 0–1 | No effect on patient’s life | ||
| 2–5 | Small effect on patient’s life | ||
| 6–10 | Moderate effect on patient’s life | ||
| 11–20 | Very large effect on patient’s life | ||
| 21–30 | Extremely large effect on patient’s life | ||
| Advantages | Limitation | ||
|
| ||
| Pain Visual Analog Scale (Pain VAS) and Numeric Rating Scale (NRAS) for Pain | |||
| The pain VAS is a continuous scale comprised of a horizontal (HVAS) or vertical (VVAS) line, usually 10 cm in length, anchored by 2 verbal descriptors, one for each symptom extreme. The NRAS for pain is a single 11-point numeric scale, which can be administered verbally or graphically. Response options/scale. For pain intensity, both scales are most commonly anchored by “no pain” (score of 0) and “pain as bad as it could be” or “worst imaginable pain” (score of 10). | |||
 |  | ||
| Advantages | Limitation | ||
|
| ||
| Types of Toxicity | Antibiotic Drugs |
|---|---|
| Liver toxicity |
|
| Renal toxicity |
|
| Gastrointestinal toxicity |
|
| Nervous system toxicity |
|
| Hemolymphopoietic toxicity |
|
| Vascular toxicity |
|
| Cardiac toxicity |
|
| Cutaneous and subcutaneous toxicity |
|
| Infections |
|
| Ototoxicity |
|
| Musculoskeletal toxicity |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Molinelli, E.; De Simoni, E.; Candelora, M.; Sapigni, C.; Brisigotti, V.; Rizzetto, G.; Offidani, A.; Simonetti, O. Systemic Antibiotic Therapy in Hidradenitis Suppurativa: A Review on Treatment Landscape and Current Issues. Antibiotics 2023, 12, 978. https://doi.org/10.3390/antibiotics12060978
Molinelli E, De Simoni E, Candelora M, Sapigni C, Brisigotti V, Rizzetto G, Offidani A, Simonetti O. Systemic Antibiotic Therapy in Hidradenitis Suppurativa: A Review on Treatment Landscape and Current Issues. Antibiotics. 2023; 12(6):978. https://doi.org/10.3390/antibiotics12060978
Chicago/Turabian StyleMolinelli, Elisa, Edoardo De Simoni, Matteo Candelora, Claudia Sapigni, Valerio Brisigotti, Giulio Rizzetto, Annamaria Offidani, and Oriana Simonetti. 2023. "Systemic Antibiotic Therapy in Hidradenitis Suppurativa: A Review on Treatment Landscape and Current Issues" Antibiotics 12, no. 6: 978. https://doi.org/10.3390/antibiotics12060978
APA StyleMolinelli, E., De Simoni, E., Candelora, M., Sapigni, C., Brisigotti, V., Rizzetto, G., Offidani, A., & Simonetti, O. (2023). Systemic Antibiotic Therapy in Hidradenitis Suppurativa: A Review on Treatment Landscape and Current Issues. Antibiotics, 12(6), 978. https://doi.org/10.3390/antibiotics12060978






