The Use of Intravenous Fosfomycin in Clinical Practice: A 5-Year Retrospective Study in a Tertiary Hospital in Italy
Abstract
1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Candel, F.J.; David, M.M.; Barberán, J. New perspectives for reassessing fosfomycin: Applicability in current clinical practice. Rev. Esp. De Quimioter. 2019, 32, 1–7. [Google Scholar]
- Dijkmans, A.C.; Zacarías, N.V.O.; Burggraaf, J.; Mouton, J.W.; Wilms, E.B.; Van Nieuwkoop, C.; Touw, D.J.; Stevens, J.; Kamerling, I.M.C. Fosfomycin: Pharmacological, clinical and future perspectives. Antibiotics 2017, 6, 24. [Google Scholar] [CrossRef] [PubMed]
- Falagas, M.E.; Vouloumanou, E.K.; Samonis, G.; Vardakas, K.Z. Fosfomycin. Clin. Microbiol. Rev. 2016, 29, 321–347. [Google Scholar] [CrossRef] [PubMed]
- Fransen, F.; Hermans, K.; Melchers, M.J.B.; Lagarde, C.C.M.; Meletiadis, J.; Mouton, J.W. Pharmacodynamics of fosfomycin against ESBL- and/or carbapenemase-producing Enterobacteriaceae. J. Antimicrob. Chemother. 2017, 72, 3374–3381. [Google Scholar] [CrossRef] [PubMed]
- Louie, A.; Maynard, M.; Duncanson, B.; Nole, J.; Vicchiarelli, M.; Drusano, G.L. Determination of the Dynamically Linked Indices of Fosfomycin for Pseudomonas aeruginosa in the Hollow Fiber Infection Model. Antimicrob. Agents Chemother. 2018, 62, e02627-17. [Google Scholar] [CrossRef]
- Walsh, C.C.; McIntosh, M.P.; Peleg, A.Y.; Kirkpatrick, C.M.; Bergen, P.J. In vitro pharmacodynamics of fosfomycin against clinical isolates of Pseudomonas aeruginosa. J. Antimicrob. Chemother. 2015, 70, 3042–3050. [Google Scholar] [CrossRef]
- Kaase, M.; Szabados, F.; Anders, A.; Gatermann, S.G. Fosfomycin susceptibility in carbapenem-resistant enterobacteriaceae from Germany. J. Clin. Microbiol. 2014, 52, 1893–1897. [Google Scholar] [CrossRef]
- Falagas, M.E.; Maraki, S.; Karageorgopoulos, D.E.; Kastoris, A.C.; Mavromanolakis, E.; Samonis, G. Antimicrobial susceptibility of multidrug-resistant (MDR) and extensively drug-resistant (XDR) Enterobacteriaceae isolates to Fosfomycin. Int. J. Antimicrob. Agents 2010, 35, 240–243. [Google Scholar] [CrossRef]
- Michalopoulos, A.; Virtzili, S.; Rafailidis, P.; Chalevelakis, G.; Damala, M.; Falagas, M.E. Intravenous fosfomycin for the treatment of nosocomial infections caused by carbapenem-resistant Klebsiella pneumoniae in critically ill patients: A prospective evaluation. Clin. Microbiol. Infect. 2010, 16, 184–186. [Google Scholar] [CrossRef]
- Grabein, B.; Graninger, W.; Baño, J.R.; Dinh, A.; Liesenfeld, D.B. Intravenous fosfomycin—Back to the future. Systematic review and meta-analysis of the clinical literature. Clin. Microbiol. Infect. 2017, 23, 363–372. [Google Scholar] [CrossRef]
- Docobo-Pérez, F.; Drusano, G.L.; Johnson, A.; Goodwin, J.; Whalley, S.; Ramos-Martín, V.; Ballestero-Tellez, M.; Rodriguez-Martinez, J.M.; Conejo, M.C.; van Guilder, M.; et al. Pharmacodynamics of fosfomycin: Insights into clinical use for antimicrobial resistance. Antimicrob. Agents Chemother. 2015, 59, 5602–5610. [Google Scholar] [CrossRef]
- Dinh, A.; Salomon, J.; Bru, J.P.; Bernard, L. Fosfomycin: Efficacy against infections caused by multidrug-resistant bacteria. Scand. J. Infect. Dis. 2012, 44, 182–189. [Google Scholar] [CrossRef] [PubMed]
- Pontikis, K.; Karaiskos, I.; Bastani, S.; Dimopoulos, G.; Kalogirou, M.; Katsiari, M.; Oikonomou, A.; Poulakou, G.; Roilides, E.; Giamarellou, H. Outcomes of critically ill intensive care unit patients treated with fosfomycin for infections due to pandrug-resistant and extensively drug-resistant carbapenemase-producing Gram-negative bacteria. Int. J. Antimicrob. Agents 2014, 43, 52–59. [Google Scholar] [CrossRef] [PubMed]
- Putensen, C.; Ellger, B.; Sakka, S.G.; Weyland, A.; Schmidt, K.; Zoller, M.; Weiler, N.; Kindgen-Milles, D.; Jaschinski, U.; Weile, J.; et al. Current clinical use of intravenous fosfomycin in ICU patients in two European countries. Infection 2019, 47, 827–836. [Google Scholar] [CrossRef] [PubMed]
- Mikhail, S.; Singh, N.B.; Kebriaei, R.; Rice, S.A.; Stamper, K.C.; Castanheira, M.; Rybak, M.J. Evaluation of the Synergy of Ceftazidime-Avibactam in Combination with Meropenem, Amikacin, Aztreonam, Colistin or Fosfomycin against well-characterized multidrug-resistant Klebsiella pneumoniae and Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 2019, 63, e00779–19. [Google Scholar] [CrossRef]
- Tharavichitkul, P.B.M.; Khantawa, B.; Bousoung, V. Activity of fosfomycin against extended-spectrum-b-lactamase-producing Klebsiella pneumoniae and Escherichia coli in Maharaj Nakorn Chiang Mai Hospital. J. Infect. Dis. Antimicrob. Agents 2005, 121, 126. [Google Scholar]
- Vardakas, K.Z.; Legakis, N.J.; Triarides, N.; Falagas, M.E. Susceptibility of contemporary isolates to fosfomycin: A systematic review of the literature. Int. J. Antimicrob. Agents 2016, 47, 269–285. [Google Scholar] [CrossRef] [PubMed]
- Oliva, A.; Volpicelli, L.; Di Bari, S.; Curtolo, A.; Borrazzo, C.; Cogliati Dezza, F.; Cona, A.; Agrenzano, S.; Mularoni, A.; Trancassini, M.; et al. Effect of ceftazidime/avibactam plus fosfomycin combination on 30 day mortality in patients with bloodstream infections caused by KPC-producing Klebsiella pneumoniae: Results from a multicentre retrospective study. JAC Antimicrob. Resist. 2022, 4, dlac121. [Google Scholar] [CrossRef]
- Zheng, G.; Zhang, J.; Wang, B.; Cai, J.; Wang, L.; Hou, K.; Zhang, Y.; Zhang, L.; Yang, Z.; He, J.; et al. Ceftazidime-Avibactam in Combination with In Vitro Non-susceptible Antimicrobials Versus Ceftazidime-Avibactam in Monotherapy in Critically Ill Patients with Carbapenem-Resistant Klebsiella Pneumoniae Infection: A Retrospective Cohort Study. Infect. Dis. Ther. 2021, 10, 1699–1713, Erratum in: Infect. Dis. Ther. 2022, 11, 935–936. [Google Scholar] [CrossRef] [PubMed]
- Burastero, G.J.; Orlando, G.; Santoro, A.; Menozzi, M.; Franceschini, E.; Bedini, A.; Cervo, A.; Faltoni, M.; Bacca, E.; Biagioni, E.; et al. Ceftazidime/Avibactam in Ventilator-Associated Pneumonia Due to Difficult-to-Treat Non-Fermenter Gram-Negative Bacteria in COVID-19 Patients: A Case Series and Review of the Literature. Antibiotics 2022, 11, 1007. [Google Scholar] [CrossRef]
- Zhanel, G.; Baxter, M.; Wong, M.; Mirzanejad, Y.; Lee, A.; Dhami, R.; Kosar, J.; Werry, D.; Irfan, N.; Tessier, J.-F.; et al. Real-life experience with IV fosfomycin in Canada: Results from the Canadian LEadership on Antimicrobial Real-life usage (CLEAR) registry. J. Glob. Antimicrob. Resist. 2023, 33, 171–176. [Google Scholar] [CrossRef]
- Maraki, S.; Samonis, G.; Rafailidis, P.I.; Vouloumanou, E.K.; Mavromanolakis, E.; Falagas, M.E. Susceptibility of urinary tract bacteria to Fosfomycin. Antimicrob. Agents Chemother. 2009, 53, 4508–4510. [Google Scholar] [CrossRef] [PubMed]
- Bassetti, M.; Graziano, E.; Berruti, M.; Giacobbe, D.R. The role of fosfomycin for multidrug-resistant gram-negative infections. Curr. Opin. Infect. Dis. 2019, 32, 617–625. [Google Scholar] [CrossRef] [PubMed]
- Abdallah, T.A.K.; Elajez, R.; Ibrahim, T.B.; Alimam, A.B.; Omrani, A.S. Efficacy and safety of intravenous fosfomycin for the treatment of difficult-to-treat Gram-negative bacterial infections. J. Infect. Public Health 2021, 14, 1620–1622. [Google Scholar] [CrossRef] [PubMed]
- Falagas, M.E.; Kastoris, A.C.; Karageorgopoulos, D.; Rafailidis, P.I. Fosfomycin for the treatment of infections caused by multidrugresistant non-fermenting Gram-negative bacilli: A systematic review of microbiological, animal and clinical studies. Int. J. Antimicrob. Agents 2009, 34, 111–120. [Google Scholar] [CrossRef]
- Sirijatuphat, R.; Thamlikitkul, V. Preliminary study of colistin versus colistin plus fosfomycin for treatment of carbapenem-resistant Acinetobacter baumannii infections. Antimicrob. Agents Chemother. 2014, 58, 5598–5601. [Google Scholar] [CrossRef]
- Russo, A.; Bassetti, M.; Bellelli, V.; Bianchi, L.; Cattaneo, F.M.; Mazzocchetti, S.; Paciacconi, E.; Cottini, F.; Schiattarella, A.; Tufaro, G.; et al. Efficacy of a Fosfomycin-Containing Regimen for Treatment of Severe Pneumonia Caused by Multidrug-Resistant Acinetobacter baumannii: A Prospective, Observational Study. Infect. Dis. Ther. 2021, 10, 187–200. [Google Scholar] [CrossRef]
- Fournier, D.; Chirouze, C.; Leroy, J.; Cholley, P.; Talon, D.; Plésiat, P.; Bertrand, X. Alternatives to carbapenems in ESBL-producing Escherichia coli infections. Méd. Mal. Infect. 2013, 43, 62–66. [Google Scholar] [CrossRef]
- Bouxom, H.; Fournier, D.; Bouiller, K.; Hocquet, D.; Bertrand, X. Which non-carbapenem antibiotics are active against extended-spectrum β-lactamase-producing Enterobacteriaceae? Int. J. Antimicrob. Agents 2018, 52, 100–103. [Google Scholar] [CrossRef]
- Apisarnthanarak, A.; Mundy, L.M. Carbapenem-resistant Pseudomonas aeruginosa pneumonia with intermediate minimum inhibitory concentrations to doripenem: Combination therapy with high-dose, 4-h infusion of doripenem plus fosfomycin versus intravenous colistin plus fosfomycin. Int. J. Antimicrob. Agents 2012, 39, 271–272. [Google Scholar] [CrossRef]
- Navarro-San Francisco, C.; Mora-Rillo, M.; Romero-Gómez, M.P.; Moreno-Ramos, F.; Rico-Nieto, A.; Ruiz-Carrascoso, G.; Gómez-Gil, R.; Arribas-López, J.R.; Mingorance, J.; Paño-Pardo, J.R. Bacteraemia due to OXA-48-carbapenemase-producing Enterobacteriaceae: A major clinical challenge. Clin. Microbiol. Infect. 2013, 19, E72–E79. [Google Scholar] [CrossRef]
- Thampithak A, Chaisiri K, Siangsuebchart O, Phengjaturat K, Aonjumras W, Hemapanpairoa J. Prescription Pattern of Intravenous Fosfomycin in a Provincial Hospital in Thailand. Infect Chemother. 2022, 54, 699–710. [CrossRef]
- Iarikov, D.; Wassel, R.; Farley, J.; Nambiar, S. Adverse Events Associated with Fosfomycin Use: Review of the Literature and Analyses of the FDA Adverse Event Reporting System Database. Infect. Dis. Ther. 2015, 4, 433–458. [Google Scholar] [CrossRef] [PubMed]
- Zirpe, K.G.; Mehta, Y.; Pandit, R.; Pande, R.; Deshmukh, A.M.; Patil, S.; Bhagat, S.; Barkate, H. A real-world study on prescription pattern of fosfomycin in critical care patients. Indian J. Crit. Care Med. 2021, 25, 1055–1058. [Google Scholar] [PubMed]
- Gatti, M.; Giannella, M.; Rinaldi, M.; Gaibani, P.; Viale, P.; Pea, F. Pharmacokinetic/Pharmacodynamic Analysis of Continuous-Infusion Fosfomycin in Combination with Extended-Infusion Cefiderocol or Continuous-Infusion Ceftazidime-Avibactam in a Case Series of Difficult-to-Treat Resistant Pseudomonas aeruginosa Bloodstream Infections and/or Hospital-Acquired Pneumonia. Antibiotics 2022, 11, 1739. [Google Scholar] [PubMed]
- Assimakopoulos, S.F.; Karamouzos, V.; Eleftheriotis, G.; Lagadinou, M.; Bartzavali, C.; Kolonitsiou, F.; Paliogianni, F.; Fligou, F.; Marangos, M. Efficacy of Fosfomycin-Containing Regimens for Treatment of Bacteremia due to Pan-Drug Resistant Acinetobacter baumannii in Critically Ill Patients: A Case Series Study. Pathogens 2023, 12, 286. [Google Scholar] [CrossRef] [PubMed]


| Cases N | Recovery N (%) | Relapse N (%) | Death N (%) | Missing Data | |
|---|---|---|---|---|---|
| Total | 343 | 226 (65.8) | 17 (4.9) | 90 (26.2) | 10 (2.9) |
| Sex | |||||
| Male | 216 (62.9) | 148 (68.5) | 9 (4.2) | 54 (25) | 5 (2.3) |
| Female | 127 (37.1) | 78 (61.4) | 8 (6.2) | 36 (28.3) | 5 (3.9) |
| Age (mean ± DS) 68 ± 13.9 65.3 ± 13.9 64.1 ± 15 65.3 ± 14 | |||||
| 18–45 years | 34 | 25 (73.5) | 2 (5.9) | 6 (17.6) | 1 |
| 46–65 years | 113 | 75 (66.4) | 6 (5.3) | 26 (23) | 6 |
| 66–95 years | 195 | 126 (64.6) | 9 (4.6) | 57 (29.2) | 3 |
| Comorbidity | |||||
| Solid neoplasm Hematological diseases Cardiovascular diseases Diabetes mellitus Kidney failure Lung diseases HIV/AIDS SARS-CoV-2 Other | 31 (9.1) 29 (8.4) 57 (16.6) 48 (13.9) 29 (8.4) 57 (16.6) 11 (3.2) 18 (5.2) 59 (17.2) | 21 (67.7) 18 (62.1) 35 (61.4) 37 (77.1) 20 (68.9) 29 (50.9) 9 (81.8) 9 (50) 38 (64.4) | 3 (9.6) 1 (3.4) 3 (5.2) 1 (2.1) 2 (6.8) 4 (7) 0 2 (11.1) 3 (5.1) | 4 (12.9) 9 (31) 16 (28.1) 9 (18.8) 7 (24.1) 23 (40.4) 2 (18.2) 7 (38.9) 17 (28.8) | 3 1 3 1 - 1 - - 1 |
| Cases N | Recovery N (%) | Relapse N (%) | Death N (%) | |
|---|---|---|---|---|
| Type of infection and subgroups in the ICU (intensive care unit) | ||||
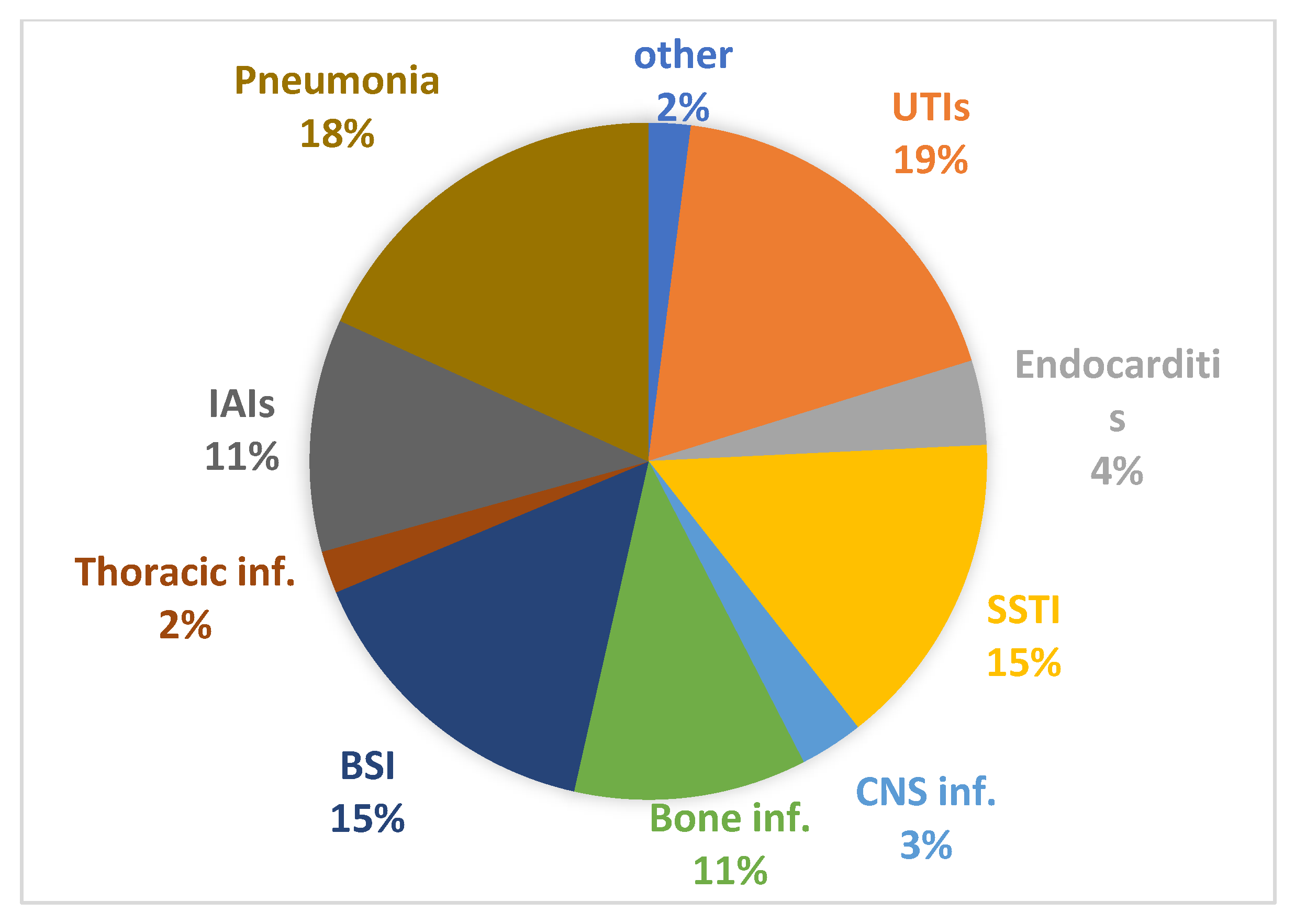
| UTI/Pyelonephritis UTI/Pyelonephritis in the ICU Endocarditis Endocarditis in the ICU Skin and soft tissue infections (SSTI) SSTI in the ICU Central nervous system (CNS) infections CNS infections in the ICU Osteomyelitis Bacteremia/sepsis Bacteremia/sepsis in the ICU Intrathoracic infections Intrathoracic infections in the ICU Intra-abdominal infections Intra-abdominal infections in the ICU Pneumonia Pneumonia in the ICU Other infections | 69 6 13 4 49 4 10 4 37 52 12 6 2 37 9 63 16 7 | 55 (79.7) 2 (40) 6 (46.1) - 33 (67.3) - 6 (60) - 31 (83.8) 32 (61.5) 1 (8.3) 3 (50) - 24 (64.9) - 31 (49.2) 3 (18.7) 6 (85.7) | 3 (4.3) - 2 (15.4) - 3 (6.1) - - - 2 (5.4) 1 (1.9) - 1 (10) - 1 (2.7) - 4 (6.3) - 0 | 11 (15.9) 4 (60) 5 (38.5) 3 (75) 10 (20.4) 3 (75) 3 (30) 3 (75) 2 (5.4) 18 (34.6) 11 (91.7) 2 (40) 2 (100) 12 (32.4) 9 (100) 27 (42.9) 13 (81.3) 1 (14.3) |
| Main microbiological isolates | ||||
| Klebsiella pneumoniae | 193 | 129 (66.8) | 5 (2.6) | 57 (29.5) |
| Pseudomonas aeruginosa | 42 | 28 (66.7) | 1 (2.4) | 7 (16.7) |
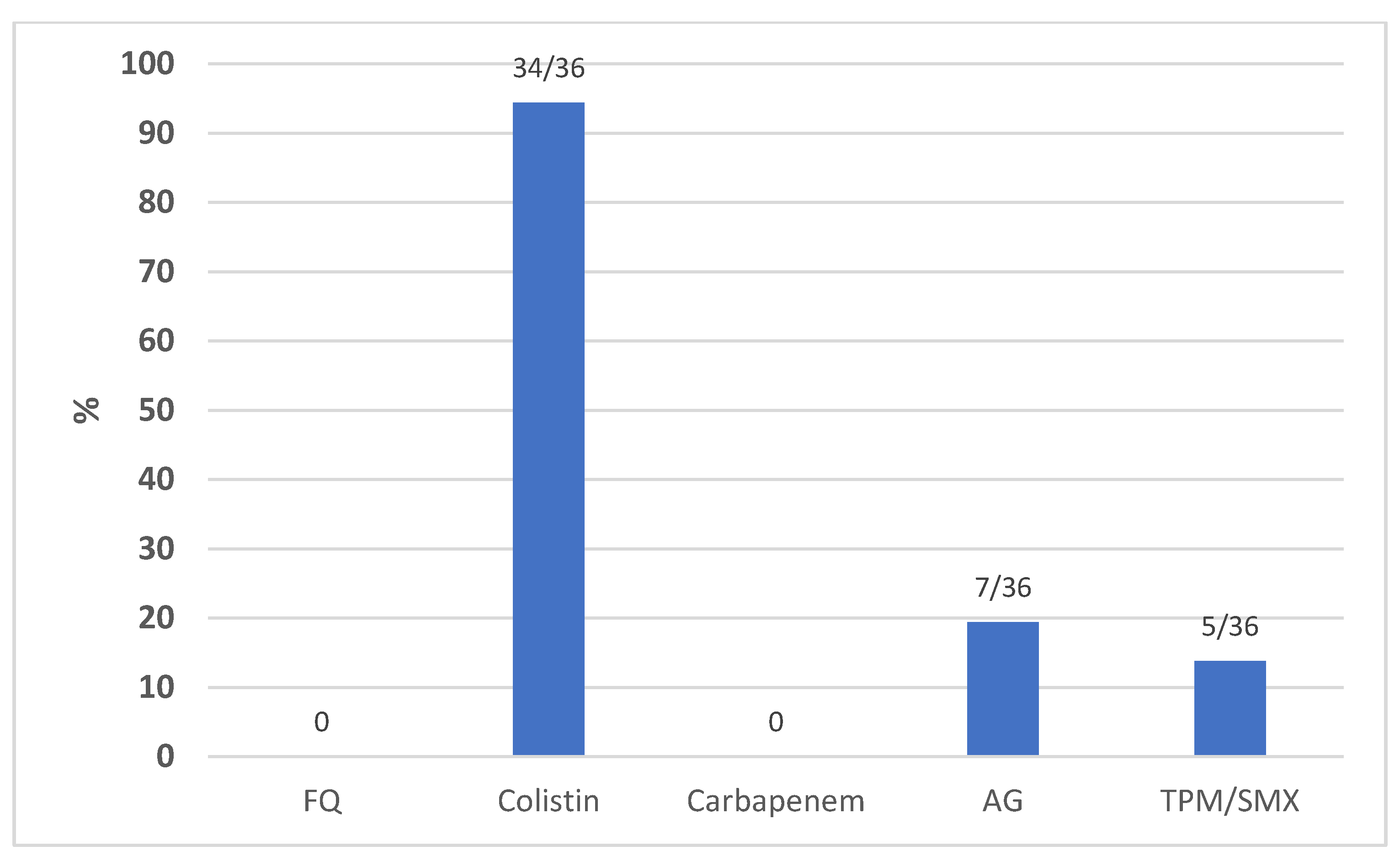
| Acinetobacter baumannii | 36 | 17 (47.2) | 2 (5.5) | 16 (44.4) |
| Staphylococcus aureus | 16 | 12 (75) | 1 (6.3) | 2 (12.5) |
| Enterococcus spp. | 28 | 17 (60.7) | 2 (7.1) | 9 (32.1) |
| Cases N | Recovery N (%) | Relapse N (%) | Death N (%) | |
|---|---|---|---|---|
| Main combination therapies and subgroups with specific isolates | ||||
MEROPENEM
| 57 21 3 5 21 | 35 (61.4) 13 (61.9) 3 (100) 3 (60) 13 (61.9) | 1 (1.8) - - - - | 18 (31.5) 8 (38.1) - 2 (40) 8 (38.1) |
CEFTAZIDIME–AVIBACTAM
| 122 99 6 5 7 | 73 (59.8) 61 (61.6) - - 5 (71.4) | 3 (2.5) 2 (2) - - - | 42 (34.4) 35 (35.5) 6 (100) 5 (100) 2 (29.6) |
COLISTIN
| 49 23 6 17 3 | 25 (51.1) 13 (56.2) 4 (66.7) 8 (47.1) 1 (33.3) | 2 (4.1) - - 2 (11.8) - | 15 (30.6) 9 (39.1) - 7 (41.2) 2 (66.7) |
DAPTOMYCIN
| 39 4 3 21 | 30 (76.9) 4 (100) 2 (66.7) 14 (66.7) | 3 (7.7) - - 3 (14.3) | 6 (15.4) - 1 (33.1) 4 (19) |
VANCOMYCIN
| 28 2 3 9 | 20 (71.4) 1 (50) 1 (33.3) 7 (77.8) | - - - - | 6 (21.4) - 1 (33.3) 2 (22.2) |
| ADVERSE EVENT | N (%) |
|---|---|
| Absence of side effects Side effects
| 323 (94.2) 20 (5.8) 7 (2) 4 (1.2) 4 (1.2) 3 (0.9) 1 (0.3) 1 (0.3) |
| Combination Therapy with Intravenous Fosfomycin and Ceftazidime/Avibactam | ||||
|---|---|---|---|---|
| Author, year | Study | No. of cases | Pathogens | Clinical cure |
| Our study | Retrospective | 122 | K.pneumoniae (81.2 %) and other | 73/122 (59.8%) |
| Oliva et al., 2022 [18] | Retrospective, two-center | 61 | KPC-producing K.pneumoniae | 45/61 (75.4%) |
| Zheng et al., 2021 [19] | Retrospective | 6 | Carbapenem-Resistant K.pneumoniae | 4/6 (66.7%) |
| Burastero et al., 2022 [20] | Retrospective | 9 | P.aeruginosa | 6/9 (66.7%) ¹ |
| Zhanel et al., 2023 [21] | Retrospective | 16 | K.pneumoniae | 11/16 (68.7%) |
| Gatti et al., 2022 [35] | Retrospective | 2 | P.aeruginosa | 1/2 (50%) ¹ |
| Combination therapy with intravenous fosfomycin and colistin | ||||
| Author, year | Study | No. of cases | Pathogens | Clinical cure |
| Our study | Retrospective | 49 | K.pneumoniae, P.aeruginosa, A.baumannii | 25/49 (51.1%) |
| Apisarnthanarak et al., 2011 [30] | Retrospective | 24 | P.aeruginosa | 14 (58%) |
| Sirijatuphat et al., 2014 [26] | Randomized controlled | 47 | A.baumannii | 28 (59.6%) |
| Navarro et al., 2012 [31] | Prospective observational | 4 | K.penumoniae | 3 (75%) ² |
| Assimakopoulos et al., 2023 [36] | Retrospective | 7 | A.baumannii | 6/7 (85.7%) ¹ |
| Russo et al., 2020 [27] | Prospective, observational | 18 | A.baumannii | 16/18 (88.9%) ² |
| Thampithak et al., 2022 [32] | Retrospective | 82 | A.baumannii, P.aeruginosa, Enterobacterales | 36/82 (43.9%) ² |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Anastasia, A.; Bonura, S.; Rubino, R.; Giammanco, G.M.; Miccichè, I.; Di Pace, M.R.; Colomba, C.; Cascio, A. The Use of Intravenous Fosfomycin in Clinical Practice: A 5-Year Retrospective Study in a Tertiary Hospital in Italy. Antibiotics 2023, 12, 971. https://doi.org/10.3390/antibiotics12060971
Anastasia A, Bonura S, Rubino R, Giammanco GM, Miccichè I, Di Pace MR, Colomba C, Cascio A. The Use of Intravenous Fosfomycin in Clinical Practice: A 5-Year Retrospective Study in a Tertiary Hospital in Italy. Antibiotics. 2023; 12(6):971. https://doi.org/10.3390/antibiotics12060971
Chicago/Turabian StyleAnastasia, Antonio, Silvia Bonura, Raffaella Rubino, Giovanni Maurizio Giammanco, Irene Miccichè, Maria Rita Di Pace, Claudia Colomba, and Antonio Cascio. 2023. "The Use of Intravenous Fosfomycin in Clinical Practice: A 5-Year Retrospective Study in a Tertiary Hospital in Italy" Antibiotics 12, no. 6: 971. https://doi.org/10.3390/antibiotics12060971
APA StyleAnastasia, A., Bonura, S., Rubino, R., Giammanco, G. M., Miccichè, I., Di Pace, M. R., Colomba, C., & Cascio, A. (2023). The Use of Intravenous Fosfomycin in Clinical Practice: A 5-Year Retrospective Study in a Tertiary Hospital in Italy. Antibiotics, 12(6), 971. https://doi.org/10.3390/antibiotics12060971








