The Impact of COVID-19 Pandemic on ESBL-Producing Enterobacterales Infections: A Scoping Review
Abstract
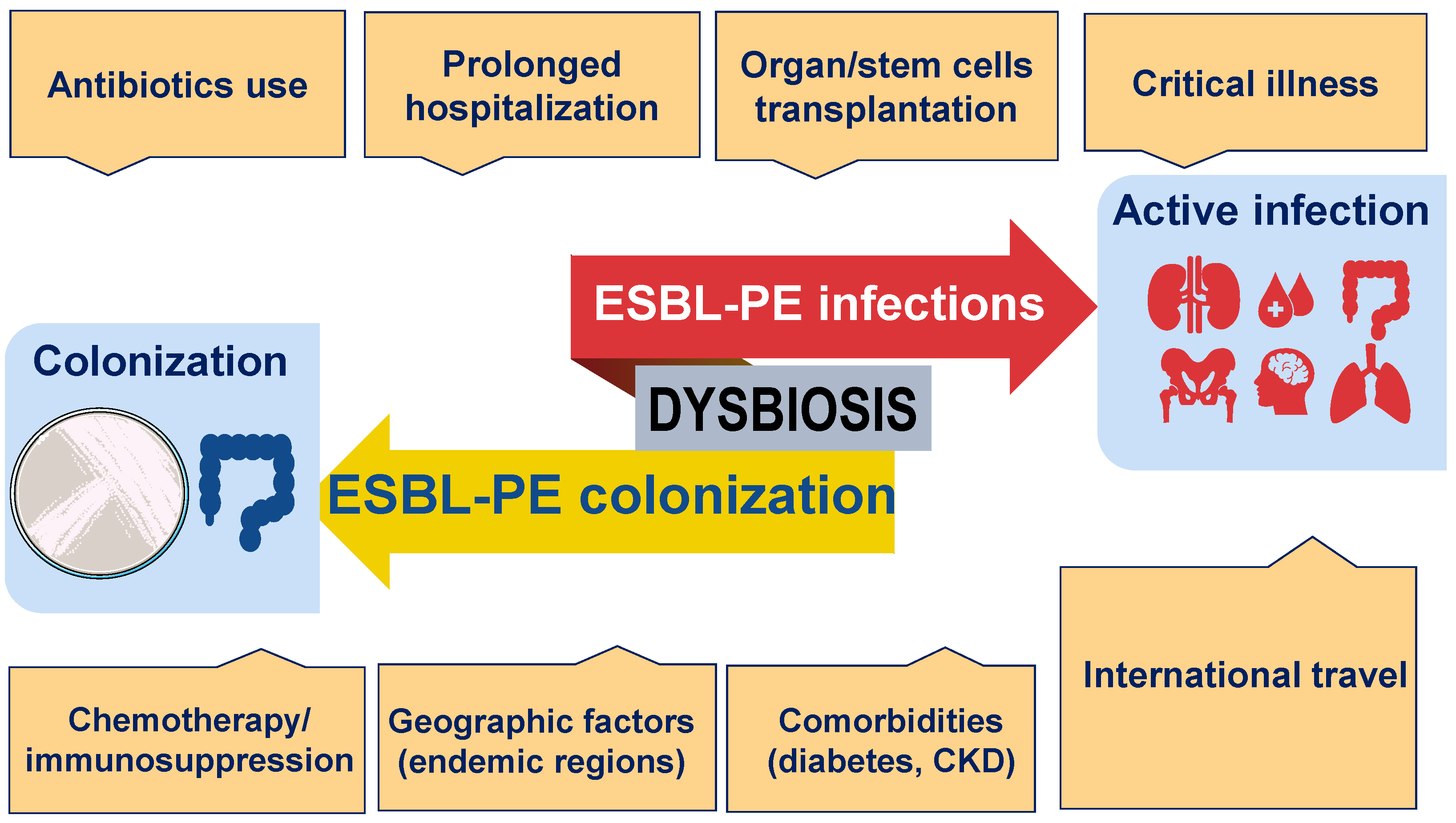
1. Introduction
2. The Epidemiology of ESBL-PE before the COVID-19 Pandemic
3. The Impact of the COVID-19 Pandemic on Multidrug-Resistant Infections
4. Did the COVID-19 Pandemic Affect the Epidemiology of ESBL-PE-Associated Infections?
5. Concluding Remarks
6. Materials and Methods
6.1. Eligibility Criteria
6.2. Study Selection and Extraction
6.3. Categorization and Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Urban-Chmiel, R.; Marek, A.; Stępień-Pyśniak, D.; Wieczorek, K.; Dec, M.; Nowaczek, A.; Osek, J. Antibiotic Resistance in Bacteria-A Review. Antibiotics 2022, 11, 1079. [Google Scholar] [CrossRef] [PubMed]
- Yadav, S.; Kapley, A. Antibiotic resistance: Global health crisis and metagenomics. Biotechnol. Rep. 2021, 29, e00604. [Google Scholar] [CrossRef] [PubMed]
- Rafailidis, P.I.; Kofteridis, D. Proposed amendments regarding the definitions of multidrug-resistant and extensively drug-resistant bacteria. Expert Rev. Anti Infect. Ther. 2022, 20, 139–146. [Google Scholar] [CrossRef] [PubMed]
- van Duin, D.; Paterson, D.L. Multidrug-Resistant Bacteria in the Community: An Update. Infect. Dis. Clin. N. Am. 2020, 34, 709–722. [Google Scholar] [CrossRef]
- Castanheira, M.; Simner, P.J.; Bradford, P.A. Extended-spectrum β-lactamases: An update on their characteristics, epidemiology and detection. JAC Antimicrob. Resist. 2021, 3, dlab092. [Google Scholar] [CrossRef]
- Wilson, H.; Török, M.E. Extended-spectrum β-lactamase-producing and carbapenemase-producing Enterobacteriaceae. Microb. Genom. 2018, 4, e000197. [Google Scholar] [CrossRef]
- Bassetti, M.; Peghin, M.; Pecori, D. The management of multidrug-resistant Enterobacteriaceae. Curr. Opin. Infect. Dis. 2016, 29, 583–594. [Google Scholar] [CrossRef]
- Rozenkiewicz, D.; Esteve-Palau, E.; Arenas-Miras, M.; Grau, S.; Duran, X.; Sorlí, L.; Montero, M.M.; Horcajada, J.P. Clinical and Economic Impact of Community-Onset Urinary Tract Infections Caused by ESBL-Producing. Antibiotics 2021, 10, 585. [Google Scholar] [CrossRef]
- Russo, A.; Berruti, M.; Giacobbe, D.R.; Vena, A.; Bassetti, M. Recent molecules in the treatment of severe infections caused by ESBL-producing bacteria. Expert Rev. Anti Infect. Ther. 2021, 19, 983–991. [Google Scholar] [CrossRef]
- Tal Jasper, R.; Coyle, J.R.; Katz, D.E.; Marchaim, D. The complex epidemiology of extended-spectrum β-lactamase-producing Enterobacteriaceae. Future Microbiol. 2015, 10, 819–839. [Google Scholar] [CrossRef]
- Macareño-Castro, J.; Solano-Salazar, A.; Dong, L.T.; Mohiuddin, M.; Espinoza, J.L. Fecal microbiota transplantation for Carbapenem-Resistant Enterobacteriaceae: A systematic review. J. Infect. 2022, 84, 749–759. [Google Scholar] [CrossRef] [PubMed]
- Dong, L.T.; Espinoza, H.V.; Espinoza, J.L. Emerging superbugs: The threat of Carbapenem Resistant Enterobacteriaceae. AIMS Microbiol. 2020, 6, 176–182. [Google Scholar] [CrossRef] [PubMed]
- Hagel, S.; Makarewicz, O.; Hartung, A.; Weiß, D.; Stein, C.; Brandt, C.; Schumacher, U.; Ehricht, R.; Patchev, V.; Pletz, M.W. ESBL colonization and acquisition in a hospital population: The molecular epidemiology and transmission of resistance genes. PLoS ONE 2019, 14, e0208505. [Google Scholar] [CrossRef] [PubMed]
- Titelman, E.; Hasan, C.M.; Iversen, A.; Nauclér, P.; Kais, M.; Kalin, M.; Giske, C.G. Faecal carriage of extended-spectrum β-lactamase-producing Enterobacteriaceae is common 12 months after infection and is related to strain factors. Clin. Microbiol. Infect. 2014, 20, O508–O515. [Google Scholar] [CrossRef]
- Abubakar, U.; Al-Anazi, M.; Alanazi, Z.; Rodríguez-Baño, J. Impact of COVID-19 pandemic on multidrug resistant gram positive and gram negative pathogens: A systematic review. J. Infect. Public Health 2023, 16, 320–331. [Google Scholar] [CrossRef] [PubMed]
- Sun Jin, L.; Fisher, D. MDRO transmission in acute hospitals during the COVID-19 pandemic. Curr. Opin. Infect. Dis. 2021, 34, 365–371. [Google Scholar] [CrossRef]
- Giannitsioti, E.; Louka, C.; Mamali, V.; Kousouli, E.; Velentza, L.; Papadouli, V.; Loizos, G.; Mavroudis, P.; Kranidiotis, G.; Rekleiti, N.; et al. Bloodstream Infections in a COVID-19 Non-ICU Department: Microbial Epidemiology, Resistance Profiles and Comparative Analysis of Risk Factors and Patients’ Outcome. Microorganisms 2022, 10, 1314. [Google Scholar] [CrossRef]
- Thoma, R.; Seneghini, M.; Seiffert, S.N.; Vuichard Gysin, D.; Scanferla, G.; Haller, S.; Flury, D.; Boggian, K.; Kleger, G.R.; Filipovic, M.; et al. The challenge of preventing and containing outbreaks of multidrug-resistant organisms and Candida auris during the coronavirus disease 2019 pandemic: Report of a carbapenem-resistant Acinetobacter baumannii outbreak and a systematic review of the literature. Antimicrob. Resist. Infect. Control 2022, 11, 12. [Google Scholar] [CrossRef]
- Shah, P.M.; Stille, W. Escherichia coli and Klebsiella pneumoniae strains more susceptible to cefoxitin than to third generation cephalosporins. J. Antimicrob. Chemother. 1983, 11, 597–598. [Google Scholar] [CrossRef]
- Bradford, P.A. Extended-spectrum beta-lactamases in the 21st century: Characterization, epidemiology, and detection of this important resistance threat. Clin. Microbiol. Rev. 2001, 14, 933–951. [Google Scholar] [CrossRef]
- Turner, P.J. Extended-spectrum beta-lactamases. Clin. Infect. Dis. 2005, 41 (Suppl. 4), S273–S275. [Google Scholar] [CrossRef] [PubMed]
- Cantón, R.; Coque, T.M. The CTX-M beta-lactamase pandemic. Curr. Opin. Microbiol. 2006, 9, 466–475. [Google Scholar] [CrossRef] [PubMed]
- Drieux, L.; Brossier, F.; Sougakoff, W.; Jarlier, V. Phenotypic detection of extended-spectrum beta-lactamase production in Enterobacteriaceae: Review and bench guide. Clin. Microbiol. Infect. 2008, 14 (Suppl. 1), 90–103. [Google Scholar] [CrossRef]
- Doi, Y.; Iovleva, A.; Bonomo, R.A. The ecology of extended-spectrum β-lactamases (ESBLs) in the developed world. J. Travel Med. 2017, 24, S44–S51. [Google Scholar] [CrossRef]
- Chao, C.M.; Lai, C.C.; Yu, W.L. Epidemiology of extended-spectrum β-lactamases in. Front. Microbiol. 2022, 13, 1060050. [Google Scholar] [CrossRef] [PubMed]
- Tamma, P.D.; Smith, T.T.; Adebayo, A.; Karaba, S.M.; Jacobs, E.; Wakefield, T.; Nguyen, K.; Whitfield, N.N.; Simner, P.J. Prevalence of bla CTX-M genes in Gram-negative bloodstream isolates across 66 hospitals in the United States. J. Clin. Microbiol. 2021, 59, e00127-21. [Google Scholar] [CrossRef]
- Singh, S.R.; Teo, A.K.J.; Prem, K.; Ong, R.T.; Ashley, E.A.; van Doorn, H.R.; Limmathurotsakul, D.; Turner, P.; Hsu, L.Y. Epidemiology of Extended-Spectrum Beta-Lactamase and Carbapenemase-Producing Enterobacterales in the Greater Mekong Subregion: A Systematic-Review and Meta-Analysis of Risk Factors Associated With Extended-Spectrum Beta-Lactamase and Carbapenemase Isolation. Front. Microbiol. 2021, 12, 695027. [Google Scholar] [CrossRef]
- Karanika, S.; Karantanos, T.; Arvanitis, M.; Grigoras, C.; Mylonakis, E. Fecal Colonization With Extended-spectrum Beta-lactamase-Producing Enterobacteriaceae and Risk Factors Among Healthy Individuals: A Systematic Review and Metaanalysis. Clin. Infect. Dis. 2016, 63, 310–318. [Google Scholar] [CrossRef]
- Siriphap, A.; Kitti, T.; Khuekankaew, A.; Boonlao, C.; Thephinlap, C.; Thepmalee, C.; Suwannasom, N.; Khoothiam, K. High prevalence of extended-spectrum beta-lactamase-producing. Front. Cell. Infect. Microbiol. 2022, 12, 955774. [Google Scholar] [CrossRef]
- Armand-Lefèvre, L.; Andremont, A.; Ruppé, E. Travel and acquisition of multidrug-resistant Enterobacteriaceae. Med. Mal. Infect. 2018, 48, 431–441. [Google Scholar] [CrossRef]
- Arcilla, M.S.; van Hattem, J.M.; Haverkate, M.R.; Bootsma, M.C.J.; van Genderen, P.J.J.; Goorhuis, A.; Grobusch, M.P.; Lashof, A.M.O.; Molhoek, N.; Schultsz, C.; et al. Import and spread of extended-spectrum β-lactamase-producing Enterobacteriaceae by international travellers (COMBAT study): A prospective, multicentre cohort study. Lancet Infect. Dis. 2017, 17, 78–85. [Google Scholar] [CrossRef] [PubMed]
- Leo, S.; Lazarevic, V.; Gaïa, N.; Estellat, C.; Girard, M.; Matheron, S.; Armand-Lefèvre, L.; Andremont, A.; Schrenzel, J.; Ruppé, E. The intestinal microbiota predisposes to traveler’s diarrhea and to the carriage of multidrug-resistant Enterobacteriaceae after traveling to tropical regions. Gut Microbes 2019, 10, 631–641. [Google Scholar] [CrossRef] [PubMed]
- Langelier, C.; Graves, M.; Kalantar, K.; Caldera, S.; Durrant, R.; Fisher, M.; Backman, R.; Tanner, W.; DeRisi, J.L.; Leung, D.T. Microbiome and Antimicrobial Resistance Gene Dynamics in International Travelers. Emerg. Infect. Dis. 2019, 25, 1380–1383. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Liang, S.; Poonsuk, K.; On, H.; Li, S.W.; Maurin, M.M.P.; Chan, C.H.; Chan, C.L.; Sin, Z.Y.; Tun, H.M. Role of gut microbiota in travel-related acquisition of extended spectrum β-lactamase-producing Enterobacteriaceae. J. Travel Med. 2021, 28, taab022. [Google Scholar] [CrossRef]
- Lai, C.C.; Chen, S.Y.; Ko, W.C.; Hsueh, P.R. Increased antimicrobial resistance during the COVID-19 pandemic. Int. J. Antimicrob. Agents 2021, 57, 106324. [Google Scholar] [CrossRef]
- Cole, J.; Barnard, E. The impact of the COVID-19 pandemic on healthcare acquired infections with multidrug resistant organisms. Am. J. Infect. Control 2021, 49, 653–654. [Google Scholar] [CrossRef]
- Bhargava, A.; Riederer, K.; Sharma, M.; Fukushima, E.A.; Johnson, L.; Saravolatz, L. High rate of Multidrug-Resistant Organisms (MDROs) among COVID-19 patients presenting with bacteremia upon hospital admission. Am. J. Infect. Control 2021, 49, 1441–1442. [Google Scholar] [CrossRef]
- Sulayyim, H.J.A.; Ismail, R.; Hamid, A.A.; Ghafar, N.A. Antibiotic Resistance during COVID-19: A Systematic Review. Int. J. Environ. Res. Public. Health 2022, 19, 11931. [Google Scholar] [CrossRef] [PubMed]
- Lontsi Ngoula, G.; Houcke, S.; Matheus, S.; Nkontcho, F.; Pujo, J.M.; Higel, N.; Ba, A.; Cook, F.; Gourjault, C.; Mounier, R.; et al. Impact of Antibiotic Consumption on the Acquisition of Extended-Spectrum β-Lactamase Producing. Antibiotics 2022, 12, 58. [Google Scholar] [CrossRef]
- Lemenand, O.; Coeffic, T.; Thibaut, S.; Colomb Cotinat, M.; Caillon, J.; Birgand, G.; Clinical Laboratories of PRIMO Network. Nantes, France. Decreasing proportion of extended-spectrum beta-lactamase among E. coli infections during the COVID-19 pandemic in France. J. Infect. 2021, 83, 664–670. [Google Scholar] [CrossRef]
- Bentivegna, E.; Luciani, M.; Arcari, L.; Santino, I.; Simmaco, M.; Martelletti, P. Reduction of Multidrug-Resistant (MDR) Bacterial Infections during the COVID-19 Pandemic: A Retrospective Study. Int. J. Environ. Res. Public Health 2021, 18, 1003. [Google Scholar] [CrossRef] [PubMed]
- Wardoyo, E.H.; Suardana, I.W.; Yasa, I.W.P.S.; Sukrama, I.D.M. Antibiotics susceptibility of. Iran. J. Microbiol. 2021, 13, 156–160. [Google Scholar] [CrossRef] [PubMed]
- Mena Lora, A.J.; Sorondo, C.; Billini, B.; Gonzalez, P.; Bleasdale, S.C. Antimicrobial resistance in Escherichia coli and Pseudomonas aeruginosa before and after the coronavirus disease 2019 (COVID-19) pandemic in the Dominican Republic. Antimicrob. Steward. Healthc. Epidemiol. 2022, 2, e191. [Google Scholar] [CrossRef] [PubMed]
- Santoso, P.; Sung, M.; Hartantri, Y.; Andriyoko, B.; Sugianli, A.K.; Alisjahbana, B.; Tjiam, J.S.L.; Debora, J.; Kusumawati, D.; Soeroto, A.Y. MDR Pathogens Organisms as Risk Factor of Mortality in Secondary Pulmonary Bacterial Infections Among COVID-19 Patients: Observational Studies in Two Referral Hospitals in West Java, Indonesia. Int. J. Gen. Med. 2022, 15, 4741–4751. [Google Scholar] [CrossRef] [PubMed]
- Karataş, M.; Yaşar-Duman, M.; Tünger, A.; Çilli, F.; Aydemir, Ş.; Özenci, V. Secondary bacterial infections and antimicrobial resistance in COVID-19: Comparative evaluation of pre-pandemic and pandemic-era, a retrospective single center study. Ann. Clin. Microbiol. Antimicrob. 2021, 20, 51. [Google Scholar] [CrossRef]
- Chang, H.C.; Chen, Y.C.; Lin, M.C.; Liu, S.F.; Chung, Y.H.; Su, M.C.; Fang, W.F.; Tseng, C.C.; Lie, C.H.; Huang, K.T.; et al. Mortality risk factors in patients with Acinetobacter baumannii ventilator: Associated pneumonia. J. Ther. Med. Assoc. 2011, 110, 564–571. [Google Scholar] [CrossRef]
- Alrahmany, D.; Omar, A.F.; Alreesi, A.; Harb, G.; Ghazi, I.M. Infection-Related Mortality in Hospitalized Patients: Risk Factors and Potential Targets for Clinical and Antimicrobial Stewardship Interventions. Antibiotics 2022, 11, 1086. [Google Scholar] [CrossRef]
- Hasan, M.R.; Vincent, Y.M.; Leto, D.; Almohri, H. Trends in the Rates of Extended-Spectrum-β-Lactamase-Producing. Microbiol. Spectr. 2023, 11, e0312422. [Google Scholar] [CrossRef]
- Ljungquist, O.; Kampmann, C.; Resman, F.; Riesbeck, K.; Tham, J. Probiotics for intestinal decolonization of ESBL-producing Enterobacteriaceae: A randomized, placebo-controlled clinical trial. Clin. Microbiol. Infect. 2020, 26, 456–462. [Google Scholar] [CrossRef]
- Kuwelker, K.; Langeland, N.; Löhr, I.H.; Gidion, J.; Manyahi, J.; Moyo, S.J.; Blomberg, B.; Klingenberg, C. Use of probiotics to reduce infections and death and prevent colonization with extended-spectrum beta-lactamase (ESBL)-producing bacteria among newborn infants in Tanzania (ProRIDE Trial): Study protocol for a randomized controlled clinical trial. Trials 2021, 22, 312. [Google Scholar] [CrossRef]
- Catho, G.; Huttner, B.D. Strategies for the eradication of extended-spectrum beta-lactamase or carbapenemase-producing Enterobacteriaceae intestinal carriage. Expert Rev. Anti Infect. Ther. 2019, 17, 557–569. [Google Scholar] [CrossRef] [PubMed]
- Borgmann, S.; Rieß, B.; Meintrup, D.; Klare, I.; Werner, G. Long-Lasting Decrease of the Acquisition of. Int. J. Environ. Res. Public Health 2020, 17, 6100. [Google Scholar] [CrossRef] [PubMed]
- Ooijevaar, R.E.; van Beurden, Y.H.; Terveer, E.M.; Goorhuis, A.; Bauer, M.P.; Keller, J.J.; Mulder, C.J.J.; Kuijper, E.J. Update of treatment algorithms for Clostridium difficile infection. Clin. Microbiol. Infect. 2018, 24, 452–462. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; de Groot, P.F.; Geerlings, S.E.; Hodiamont, C.J.; Belzer, C.; Berge, I.J.M.T.; de Vos, W.M.; Bemelman, F.J.; Nieuwdorp, M. Fecal microbiota transplantation against intestinal colonization by extended spectrum beta-lactamase producing Enterobacteriaceae: A proof of principle study. BMC Res. Notes 2018, 11, 190. [Google Scholar] [CrossRef]
- Yau, Y.K.; Mak, W.Y.J.; Lui, N.S.R.; Ng, W.Y.R.; Cheung, C.Y.K.; Li, Y.L.A.; Ching, Y.L.J.; Chin, M.L.; Lau, H.S.L.; Chan, K.L.F.; et al. High prevalence of extended-spectrum beta-lactamase organisms and the COVID-19 pandemic impact on donor recruitment for fecal microbiota transplantation in Hong Kong. United Eur. Gastroenterol. J. 2021, 9, 1027–1038. [Google Scholar] [CrossRef]
- Tricco, A.C.; Lillie, E.; Zarin, W.; O’Brien, K.K.; Colquhoun, H.; Levac, D.; Moher, D.; Peters, M.D.J.; Horsley, T.; Weeks, L.; et al. PRISMA Extension for Scoping Reviews (PRISMA-ScR): Checklist and Explanation. Ann. Intern. Med. 2018, 169, 467–473. [Google Scholar] [CrossRef]


| Reference Author/Country/ Journal | No of Subjects/Specimens | Type of Study | Bacterial Strains | Main Findings |
|---|---|---|---|---|
| G. Ngoula, 2023, French Guiana. Antibiotics [39] | 311 patients | Observational study | K. pneumonieae E. Coli E. cloacae K. aerogenes | 22.8% of ICU patients had ESBL-PE. Risk of ESBL-PE carriage among patients with severe COVID-19 was higher when they were exposed to cefotaxime. |
| O. Lemenand, 2021, France. J Infection [40] | 793,954 E. coli isolates from 1022 clinical laboratories | Retrospective multicenter study | E.coli | In general practice, Eco-ESBL decreased lightly during the pandemic (3.1% before vs. 2.9% during the pandemic). In nursing homes, the Eco-ESBL rate decreased from 9.3% to 8.3%. |
| E. Bentivegna, 2021, Italy, Int J Environ Res Public Health. [41]. | 1617 patients | Case-control study | S. aureus, K. pneumoniae, C. difficile, and A. baumannii. | Significant higher incidence of MDRB infections in COVID-19 departments than in other medical departments (29% vs. 19%); Kp-ESBL was the pathogen with the highest increase. |
| E. Wardoyo, 2021, Indonesia. Iran J Microbiol [42]. | 210 E. coli isolates | Retrospective single center study | E. coli | Among E. coli specimens isolated before the pandemic, 50% were Eco-ESBL and 21% of those collected during the pandemic were Eco-ESBL. |
| A. Mena, 2022, the Dominican Republic. Antimicrob Steward Health Epidemiol [43]. | 27,718 urine cultures and 2111 body fluid cultures | Retrospective study | E. coli P. aeruginosa | The frequency of Eco-ESBL was 25.63% before and 24.75% after the COVID-19 pandemic. |
| P. Santoso, 2022, Indonesia. Int J Gen Med [44]. | 182 patients | Observational study in two hospitals | A. baumanii, P. aeruginosa K. pneumoniae | 45.9% of COVID-19 isolates were MDRB, including CR- A. baumannii (84%) and Kp-ESBL (61%). |
| M. Karataş, 2021, Turkey. Ann Clin Microbiol Antimicrob [45]. | Total N = 4859 isolates. Pre-pandemic: 3034 isolates. Pandemic non-COVID: 1702 isolates. COVID-19 patients: 123 isolates. | Retrospective single-center study | E. coli K. pneumonieae A. baumannii S. aureus | ESBL-PE infections were less common in isolates from COVID-19 patients (8.94%) compared to pre-pandemic samples (20.7%) and samples from non-COVID-19 patients collected during the pandemic (20.7%). Among COVID-19 patients, E. coli was rarely detected, but A. baumannii was more commonly found than in controls. |
| M.R. Hasan, 2023, Canada, Microbiol Spectrum [48]. | 8,652,381 urine cultures | Retrospective, observational study | Eco-ESBL Kp-ESBL | The rate of ESBL isolation was higher during the pandemic than before it. However, decreasing trends in both Eco-ESBL and Kp-ESBL in the community setting were observed during the pandemic. |
| Topic | Search Terms |
|---|---|
| Context | COVID-19 COVID-19 pandemic SARS-CoV2 Coronavirus pandemic |
| Bacteria | Enterobacterales AND (ESBL OR ESBL-positive OR ESBL-producing OR Enterobacteriaceae OR extended-spectrum beta-lactamase OR extended spectrum beta lactamase OR extended spectrum beta lactamases) |
| Outcomes | ‘COVID-19 ESBL’ OR ‘COVID-19 extended-spectrum beta-lactamase’ OR ‘pandemic associated esbl’ OR ‘pandemic associated ESBL’ OR ‘COVID-19 Enterobacterales’ OR ‘COVID-19 enterobacterales’ OR ‘COVID-19 enterobacteriaceae’ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mai, H.T.T.; Espinoza, J.L. The Impact of COVID-19 Pandemic on ESBL-Producing Enterobacterales Infections: A Scoping Review. Antibiotics 2023, 12, 1064. https://doi.org/10.3390/antibiotics12061064
Mai HTT, Espinoza JL. The Impact of COVID-19 Pandemic on ESBL-Producing Enterobacterales Infections: A Scoping Review. Antibiotics. 2023; 12(6):1064. https://doi.org/10.3390/antibiotics12061064
Chicago/Turabian StyleMai, Ha Thi Thao, and J. Luis Espinoza. 2023. "The Impact of COVID-19 Pandemic on ESBL-Producing Enterobacterales Infections: A Scoping Review" Antibiotics 12, no. 6: 1064. https://doi.org/10.3390/antibiotics12061064
APA StyleMai, H. T. T., & Espinoza, J. L. (2023). The Impact of COVID-19 Pandemic on ESBL-Producing Enterobacterales Infections: A Scoping Review. Antibiotics, 12(6), 1064. https://doi.org/10.3390/antibiotics12061064







