Antimicrobial Properties and Mode of Action of Cryptdin-4, a Mouse α-Defensin Regulated by Peptide Redox Structures and Bacterial Cultivation Conditions
Abstract
1. Introduction
2. Results
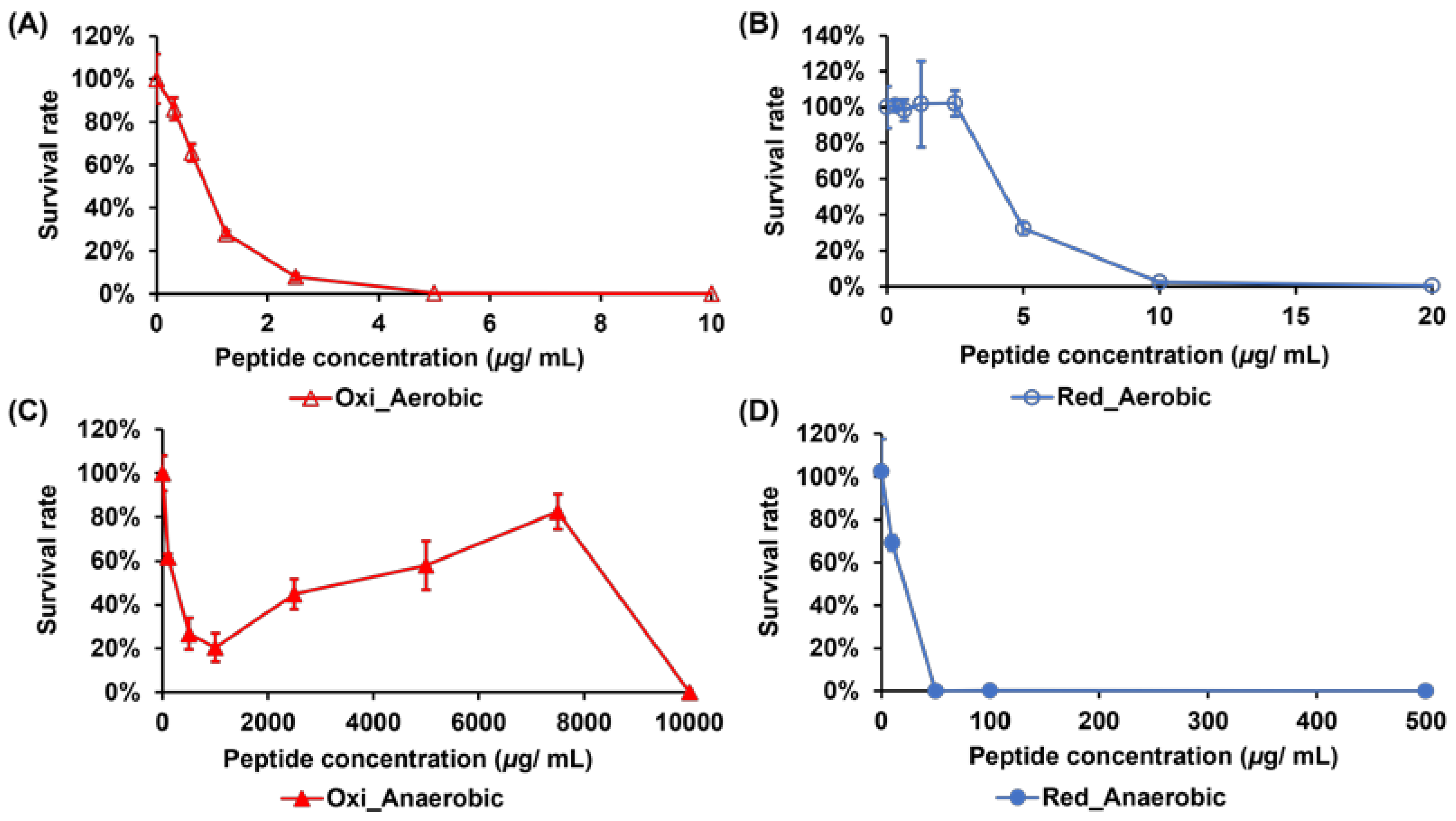
2.1. Comparing the Activities of Crp4oxi and Crp4red against E. coli Cultured under Aerobic and Anaerobic Conditions
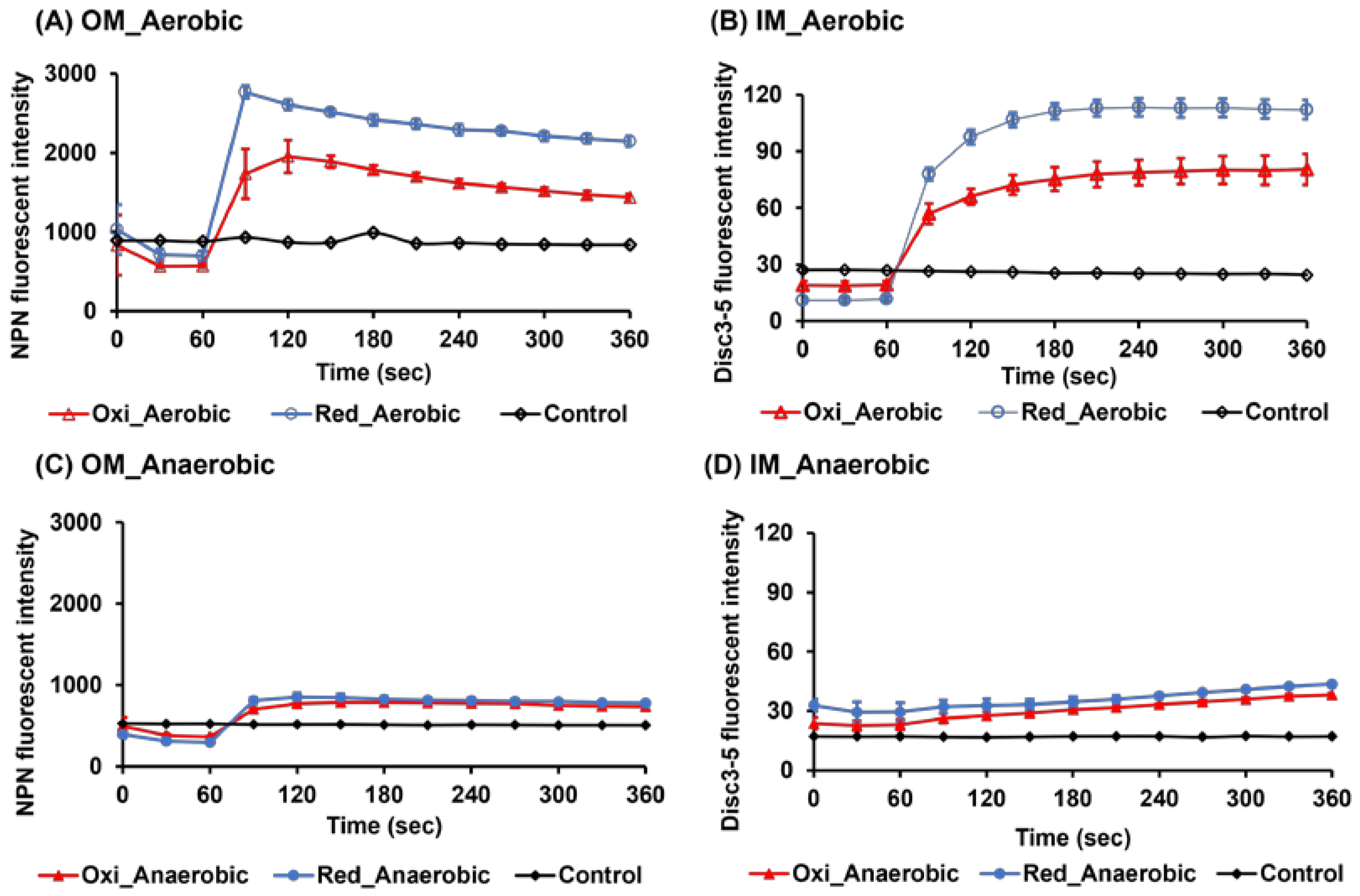
2.2. Evaluation of Outer and Inner Membrane Damages by Fluorescence Measurements
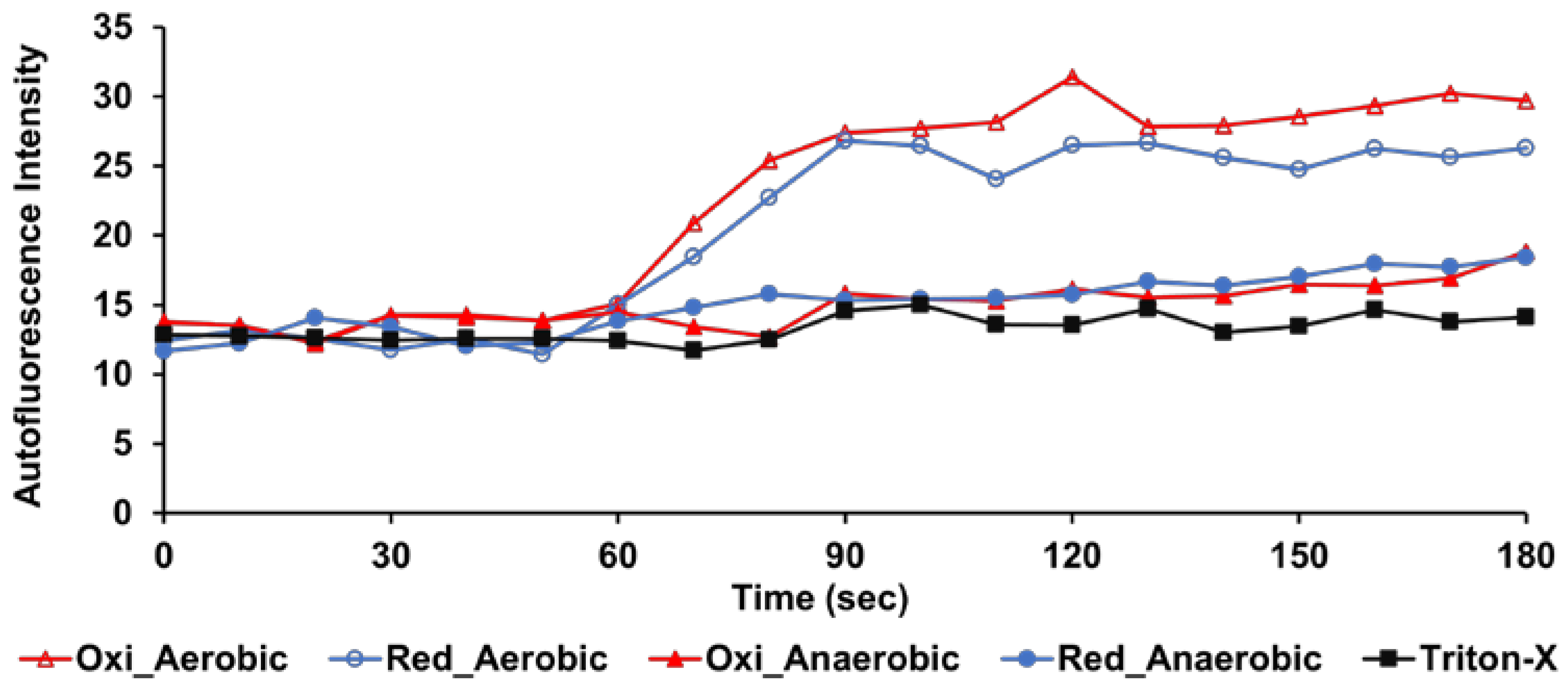
2.3. Detection of Crp4s-Induced Oxidative Stress by Intracellular Autofluorescence Assay
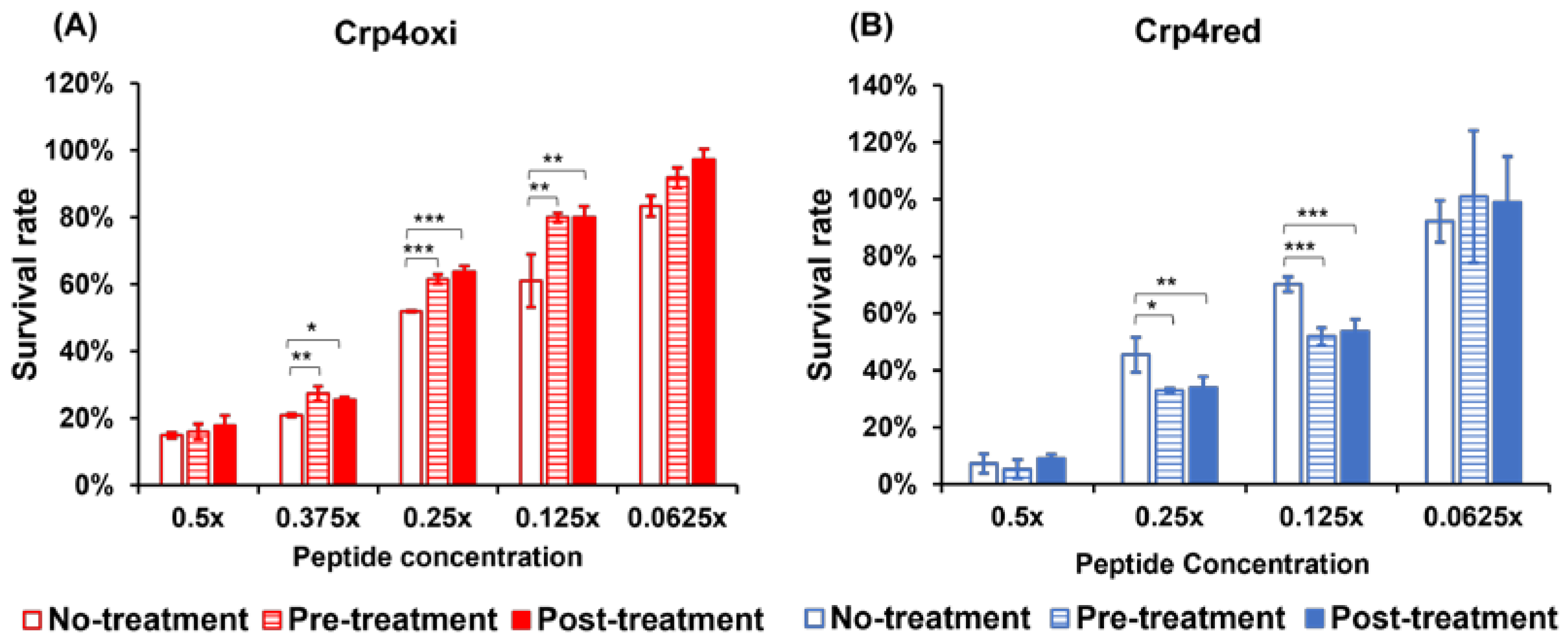
2.4. Evaluation of the Effects of Oxidative Stress and Its Removal on Antimicrobial Activity
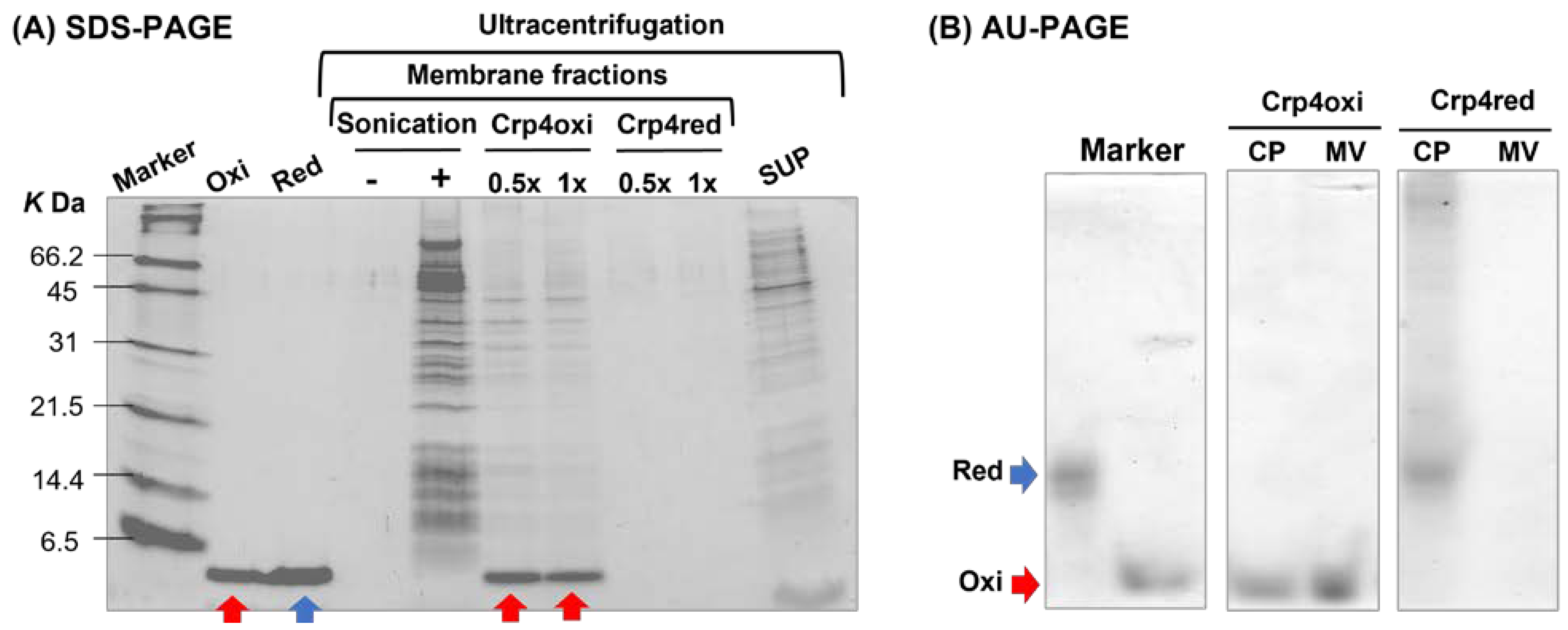
2.5. Crp4 Treatment-Induced Membrane Vesicles Production under Aerobic Conditions
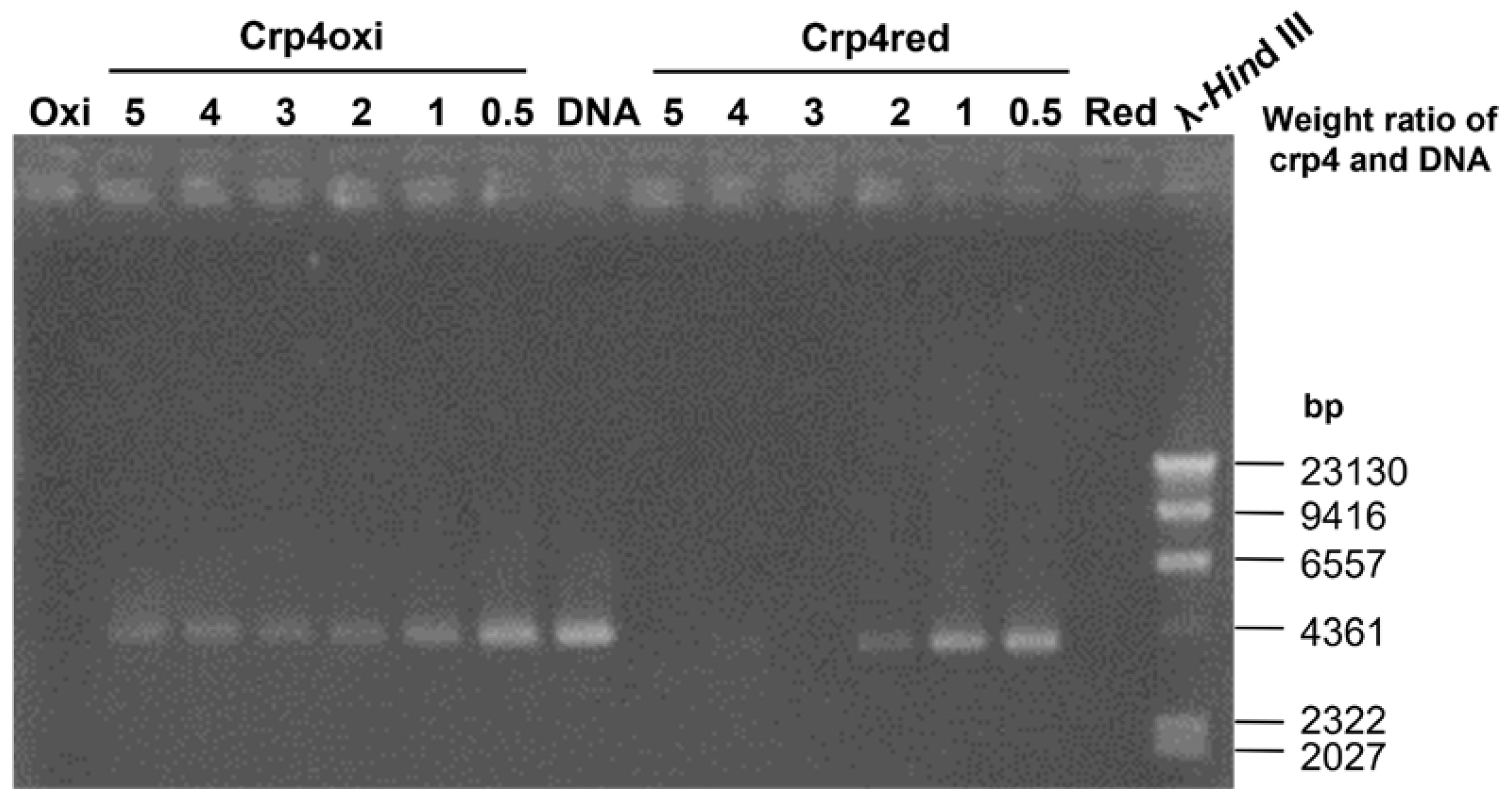
2.6. Evaluation of DNA-Binding Ability of Crp4s
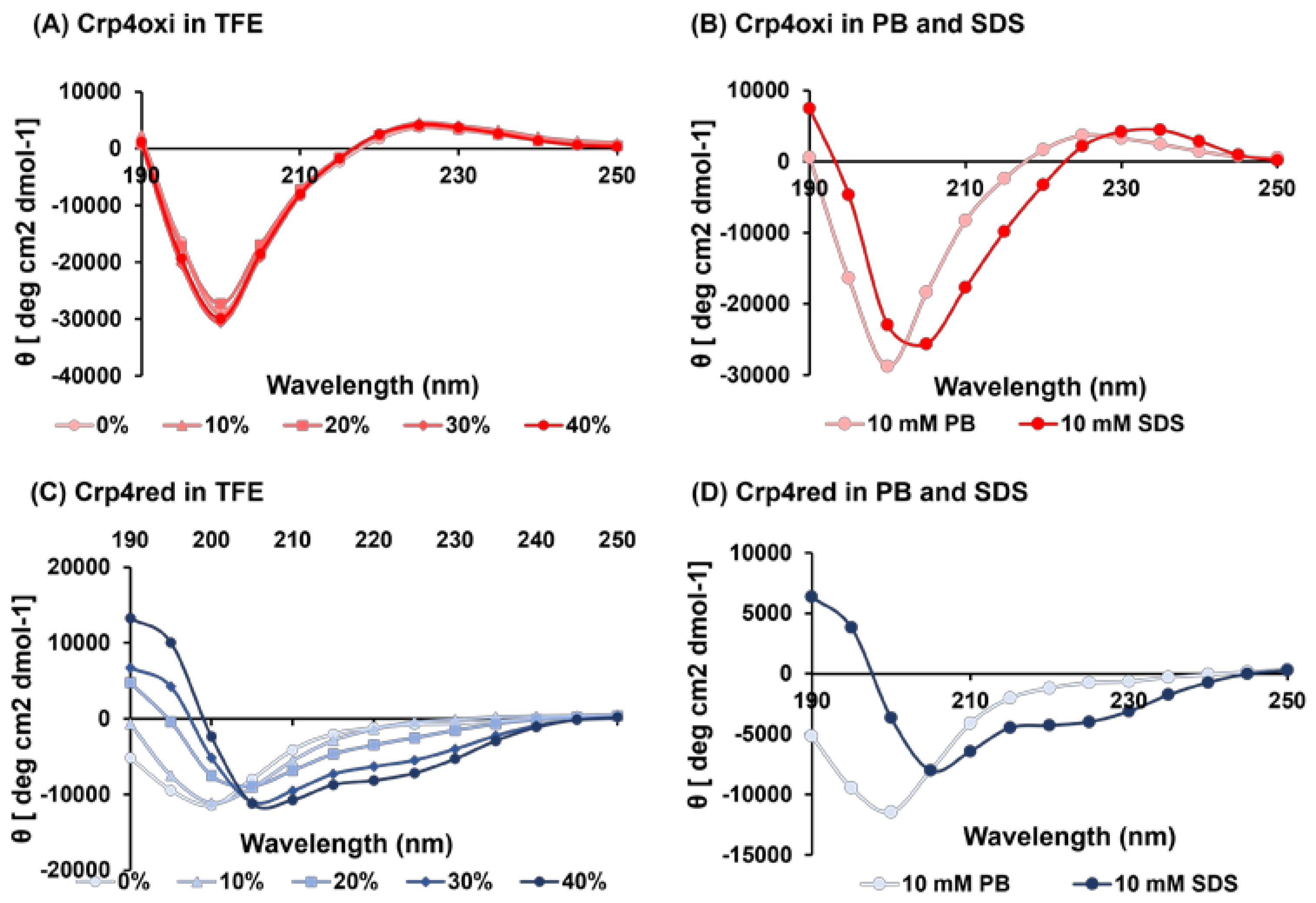
2.7. Structural Characterization of Crp4s by Circular Dichroism (CD) Spectroscopy
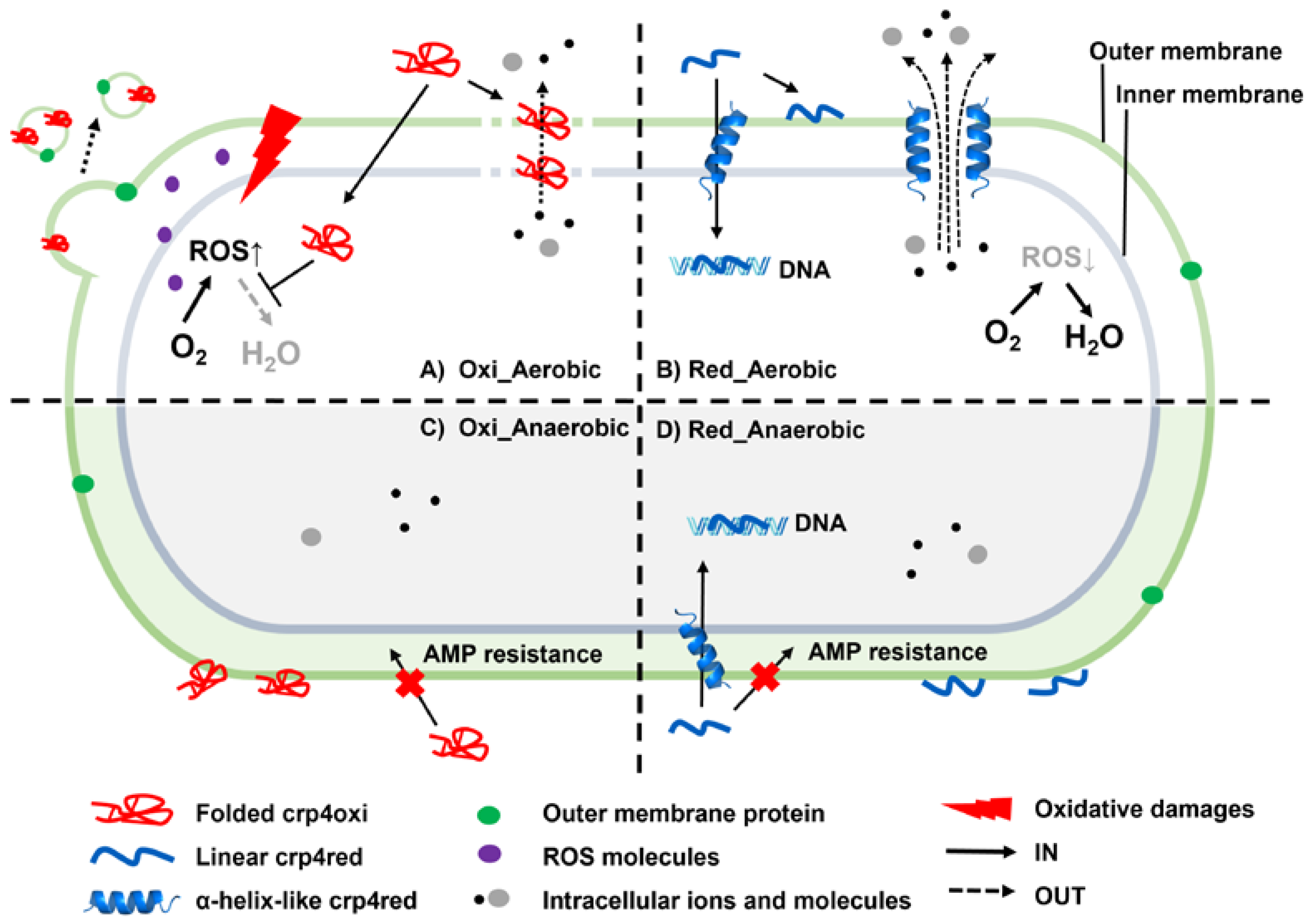
3. Discussion
4. Materials and Methods
4.1. Peptides
4.2. Bacteria Cultivation
4.3. Bactericidal Activity Assays
4.4. Assay for Peptide-Induced Membrane Damages
4.5. Isolation of Membrane Vesicles
4.6. Electrophoresis Assay
4.7. In Vitro DNA-Binding Studies
4.8. Cellular Autofluorescence Assay
4.9. Antioxidant Pre-Treatment
4.10. Antioxidant Post-Treatment
4.11. Circular Dichroism (CD) Spectrometry
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sabatier, J.M. Antibacterial peptides. Antibiotics 2020, 9, 142. [Google Scholar] [CrossRef]
- Ganz, T.; Lehrer, R.I. Defensins. Curr. Opin. Immunol. 1994, 6, 584–589. [Google Scholar] [CrossRef]
- Ouellette, A.J.; Selsted, M.E. Paneth cell defensins: Endogenous peptide components of intestinal host defense. FASEB J. 1996, 10, 1280–1289. [Google Scholar] [CrossRef]
- Ayabe, T.; Satchell, D.P.; Wilson, C.L.; Parks, W.C.; Selsted, M.E.; Ouellette, A.J. Secretion of microbicidal α-defensins by intestinal paneth cells in response to bacteria. Nat. Immunol. 2000, 1, 113–118. [Google Scholar] [CrossRef]
- Masuda, K.; Sakai, N.; Nakamura, K.; Yoshioka, S.; Ayabe, T. Bactericidal activity of mouse α-defensin cryptdin-4 predominantly affects noncommensal bacteria. J. Innate Immun. 2011, 3, 315–326. [Google Scholar] [CrossRef] [PubMed]
- Jing, W.; Hunter, H.N.; Tanabe, H.; Ouellette, A.J.; Vogel, H.J. Solution structure of cryptdin-4, a mouse paneth cell alpha-defensin. Biochemistry 2004, 43, 15759–15766. [Google Scholar] [CrossRef]
- Shimizu, Y.; Nakamura, K.; Yoshii, A.; Yokoi, Y.; Kikuchi, M.; Shinozaki, R.; Nakamura, S.; Ohira, S.; Sugimoto, R.; Ayabe, T. Paneth cell α-defensin misfolding correlates with dysbiosis and ileitis in Crohn’s disease model mice. Life Sci. Alliance 2020, 3, e201900592. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, K.; Nakamura, K.; Shimizu, Y.; Yokoi, Y.; Ohira, S.; Hagiwara, M.; Wang, Y.; Song, Y.; Aizawa, T.; Ayabe, T. Decrease of α-defensin impairs intestinal metabolite homeostasis via dysbiosis in mouse chronic social defeat stress model. Sci. Rep. 2021, 11, 9915. [Google Scholar] [CrossRef] [PubMed]
- Schroeder, B.O.; Ehmann, D.; Precht, J.C.; Castillo, P.A.; Küchler, R.; Berger, J.; Schaller, M.; Stange, E.F.; Wehkamp, J. Paneth cell α-defensin 6 (HD-6) is an antimicrobial peptide. Mucosal Immunol. 2015, 8, 661–671. [Google Scholar] [CrossRef]
- Espey, M.G. Role of oxygen gradients in shaping redox relationships between the human intestine and its microbiota. Free Radic. Biol. Med. 2013, 55, 130–140. [Google Scholar] [CrossRef]
- Liu, W.; Dong, S.L.; Xu, F.; Wang, X.Q.; Withers, T.R.; Yu, H.D.; Wang, X. Effect of intracellular expression of antimicrobial peptide LL-37 on growth of Escherichia coli strain TOP10 under aerobic and anaerobic conditions. Antimicrob. Agents Chemother. 2013, 57, 4707–4716. [Google Scholar] [CrossRef]
- Choi, H.; Yang, Z.; Weisshaar, J.C. Oxidative stress induced in E. coli by the human antimicrobial peptide LL-37. PLoS Pathog. 2017, 13, e1006481. [Google Scholar] [CrossRef]
- Rowe-Magnus, D.A.; Kao, A.Y.; Prieto, A.C.; Pu, M.; Kao, C. Cathelicidin peptides restrict bacterial growth via membrane perturbation and induction of reactive oxygen species. mBio 2019, 10, e02021-19. [Google Scholar] [CrossRef] [PubMed]
- Wendler, J.; Ehmann, D.; Courth, L.; Schroeder, B.O.; Malek, N.P.; Wehkamp, J. Bacterial periplasmic oxidoreductases control the activity of oxidized human antimicrobial β-defensin 1. Infect. Immun. 2018, 86, e00875-17. [Google Scholar] [CrossRef] [PubMed]
- Raschig, J.; Mailänder-Sánchez, D.; Berscheid, A.; Berger, J.; Strömstedt, A.A.; Courth, L.F.; Malek, N.P.; Brötz-Oesterhelt, H.; Wehkamp, J. Ubiquitously expressed Human Beta Defensin 1 (hBD1) forms bacteria-entrapping nets in a redox dependent mode of action. PLoS Pathog. 2017, 13, e1006261. [Google Scholar] [CrossRef]
- Mastroianni, J.R.; Lu, W.; Selsted, M.E.; Ouellette, A.J. Differential susceptibility of bacteria to mouse paneth cell α-defensins under anaerobic conditions. Antibiotics 2014, 3, 493–508. [Google Scholar] [CrossRef]
- Nakamura, K.; Yokoi, Y.; Fukaya, R.; Ohira, S.; Shinozaki, R.; Nishida, T.; Kikuchi, M.; Ayabe, T. Expression and localization of Paneth cells and their α-defensins in the small intestine of adult mouse. Front. Immunol. 2020, 11, 570296. [Google Scholar] [CrossRef]
- Konjar, Š.; Pavšič, M.; Veldhoen, M. Regulation of oxygen homeostasis at the intestinal epithelial barrier site. Int. J. Mol. Sci. 2021, 22, 9170. [Google Scholar] [CrossRef]
- Doherty, T.; Waring, A.J.; Hong, M. Dynamic structure of disulfide-removed linear analogs of tachyplesin-i in the lipid bilayer from solid-state NMR. Biochemistry 2008, 47, 1105–1116. [Google Scholar] [CrossRef] [PubMed]
- Bechinger, B.; Juhl, D.W.; Glattard, E.; Aisenbrey, C. Revealing the mechanisms of synergistic action of two magainin antimicrobial peptides. Front. Med. Technol. 2020, 2, 615494. [Google Scholar] [CrossRef]
- Loh, B.; Grant, C.; Hancock, R.E.W. Use of the fluorescent probe 1-N-phenylnaphthylamine to study the interactions of aminoglycoside antibiotics with the outer membrane of Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 1984, 26, 546–551. [Google Scholar] [CrossRef]
- Morin, N.; Lanneluc, I.; Connil, N.; Cottenceau, M.; Pons, A.M.; Sablé, S. Mechanism of bactericidal activity of microcin L in Escherichia coli and Salmonella enterica. Antimicrob. Agents Chemother. 2011, 55, 997–1007. [Google Scholar] [CrossRef]
- Kashyap, D.R.; Rompca, A.; Gaballa, A.; Helmann, J.D.; Chan, J.; Chang, C.J.; Hozo, I.; Gupta, D.; Dziarski, R. Peptidoglycan recognition proteins kill bacteria by inducing oxidative, thiol, and metal stress. PLoS Pathog. 2014, 10, e1004280. [Google Scholar] [CrossRef] [PubMed]
- Van Der Heijden, J.; Reynolds, L.A.; Deng, W.; Mills, A.; Scholz, R.; Imami, K.; Foster, L.J.; Duong, F.; Finlay, B.B.I. Salmonella rapidly regulates membrane permeability to survive oxidative stress. mBio 2016, 7, 1238–1254. [Google Scholar] [CrossRef]
- Hong, Y.; Zeng, J.; Wang, X.; Drlica, K.; Zhao, X. Post-stress bacterial cell death mediated by reactive oxygen species. Proc. Natl. Acad. Sci. USA 2019, 116, 10064–10071. [Google Scholar] [CrossRef]
- Lu, H.P.; Xun, L.; Xie, X.S. Single-molecule enzymatic dynamics. Science 1998, 282, 1877–1882. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.; Yang, Z.; Weisshaar, J.C. Single-cell, real-time detection of oxidative stress induced in Escherichia coli by the antimicrobial peptide CM15. Proc. Natl. Acad. Sci. USA 2015, 112, E303–E310. [Google Scholar] [CrossRef] [PubMed]
- Dwyer, D.J.; Belenky, P.A.; Yang, J.H.; MacDonald, I.C.; Martell, J.D.; Takahashi, N.; Chan, C.T.Y.; Lobritz, M.A.; Braff, D.; Schwarz, E.G.; et al. Antibiotics induce redox-related physiological alterations as part of their lethality. Proc. Natl. Acad. Sci. USA 2014, 111, E2100–E2109. [Google Scholar] [CrossRef] [PubMed]
- Goswami, M.; Narayana Rao, A.V.S.S. Transcriptome profiling reveals interplay of multifaceted stress response in Escherichia coli on exposure to glutathione and ciprofloxacin. mSystems 2018, 3, e00001-18. [Google Scholar] [CrossRef] [PubMed]
- Fahey, R.C.; Brown, W.C.; Adams, W.B.; Worsham, M.B. Occurrence of glutathione in bacteria. J. Bacteriol. 1978, 133, 1126–1129. [Google Scholar] [CrossRef]
- Marcén, M.; Cebrián, G.; Ruiz-Artiga, V.; Condón, S.; Mañas, P. Protective effect of glutathione on Escherichia coli cells upon lethal heat stress. Food Res. Int. 2019, 121, 806–811. [Google Scholar] [CrossRef]
- Schwechheimer, C.; Kuehn, M.J. Outer-membrane vesicles from Gram-negative bacteria: Biogenesis and functions. Nat. Rev. Microbiol. 2015, 13, 605–619. [Google Scholar] [CrossRef]
- Orench-Rivera, N.; Kuehn, M.J. Differential packaging into outer membrane vesicles upon oxidative stress reveals a general mechanism for cargo selectivity. Front. Microbiol. 2021, 12, 561863. [Google Scholar] [CrossRef]
- Balhuizen, M.D.; Veldhuizen, E.J.A.; Haagsman, H.P. Outer membrane vesicle induction and isolation for vaccine development. Front. Microbiol. 2021, 12, 629090. [Google Scholar] [CrossRef] [PubMed]
- Collins, S.M.; Brown, A.C. Bacterial outer membrane vesicles as antibiotic delivery vehicles. Front. Immunol. 2021, 12, 3773. [Google Scholar] [CrossRef] [PubMed]
- Mathew, B.; Nagaraj, R. Antimicrobial activity of human α-defensin 5 and its linear analogs: N-terminal fatty acylation results in enhanced antimicrobial activity of the linear analogs. Peptides 2015, 71, 128–140. [Google Scholar] [CrossRef]
- Anunthawan, T.; De La Fuente-Núñez, C.; Hancock, R.E.W.; Klaynongsruang, S. Cationic amphipathic peptides KT2 and RT2 are taken up into bacterial cells and kill planktonic and biofilm bacteria. Biochim. Biophys. Acta 2015, 1848, 1352–1358. [Google Scholar] [CrossRef]
- Dong, W.; Luo, X.; Sun, Y.; Li, Y.; Wang, C.; Guan, Y.; Shang, D. Binding properties of DNA and antimicrobial peptide Chensinin-1b containing lipophilic alkyl tails. J. Fluoresc. 2020, 30, 131–142. [Google Scholar] [CrossRef] [PubMed]
- Seebach, D.; Beck, A.K.; Bierbaum, D.J. The world of beta- and gamma-peptides comprised of homologated proteinogenic amino acids and other components. Chem. Biodivers. 2004, 1, 1111–1239. [Google Scholar] [CrossRef]
- Sato, Y.; Wang, Y.; Song, Y.; Geng, W.; Yan, S.; Nakamura, K.; Kikukawa, T.; Demura, M.; Ayabe, T.; Aizawa, T. Potent bactericidal activity of reduced cryptdin-4 derived from its hydrophobicity and mediated by bacterial membrane disruption. Amino Acids 2022, 54, 289–297. [Google Scholar] [CrossRef]
- Song, Y.; Wang, Y.; Yan, S.; Nakamura, K.; Kikukawa, T.; Ayabe, T.; Aizawa, T. Efficient recombinant production of mouse-derived cryptdin family peptides by a novel facilitation strategy for inclusion body formation. Microb. Cell Fact. 2023, 22, 9. [Google Scholar] [CrossRef] [PubMed]
- Bonucci, A.; Balducci, E.; Pistolesi, S.; Pogni, R. The defensin-lipid interaction: Insights on the binding states of the human antimicrobial peptide HNP-1 to model bacterial membranes. Biochim. Biophys. Acta 2013, 1828, 758–764. [Google Scholar] [CrossRef] [PubMed]
- Scudiero, O.; Galdiero, S.; Cantisani, M.; Di Noto, R.; Vitiello, M.; Galdiero, M.; Naclerio, G.; Cassiman, J.J.; Pedone, C.; Castaldo, G.; et al. Novel synthetic, salt-resistant analogs of human beta-defensins 1 and 3 endowed with enhanced antimicrobial activity. Antimicrob. Agents Chemother. 2010, 54, 2312–2322. [Google Scholar] [CrossRef]
- Cummings, J.E.; Vanderlick, T.K. Kinetics of cryptdin-4 translocation coupled with peptide-induced vesicle leakage. Biochemistry 2007, 46, 11882–11891. [Google Scholar] [CrossRef] [PubMed]
- Cummings, J.E.; Vanderlick, T.K. Aggregation and hemi-fusion of anionic vesicles induced by the antimicrobial peptide cryptdin-4. Biochim. Biophys. Acta—Biomembr. 2007, 1768, 1796–1804. [Google Scholar] [CrossRef]
- Guerrero Montero, I.; Dolata, K.M.; Schlüter, R.; Malherbe, G.; Sievers, S.; Zühlke, D.; Sura, T.; Dave, E.; Riedel, K.; Robinson, C. Comparative proteome analysis in an Escherichia coli CyDisCo strain identifies stress responses related to protein production, oxidative stress and accumulation of misfolded protein. Microb. Cell Fact. 2019, 18, 19. [Google Scholar] [CrossRef]
- Duperthuy, M.; Sjöström, A.E.; Sabharwal, D.; Damghani, F.; Uhlin, B.E.; Wai, S.N. Role of the Vibrio cholerae matrix protein Bap1 in cross-resistance to antimicrobial peptides. PLoS Pathog. 2013, 9, e1003620. [Google Scholar] [CrossRef]
- Beloin, C.; Roux, A.; Ghigo, J.M. Escherichia coli biofilms. Curr. Top. Microbiol. Immunol. 2008, 322, 249. [Google Scholar] [CrossRef]
- Kanjee, U.; Houry, W.A. Mechanisms of acid resistance in Escherichia coli. Annu. Rev. Microbiol. 2013, 67, 65–81. [Google Scholar] [CrossRef]
- Dombek, K.M.; Ingram, L.O. Effects of ethanol on the Escherichia coli plasma membrane. J. Bacteriol. 1984, 157, 233–239. [Google Scholar] [CrossRef]
- Xu, H.; Yuan, Z.; Sun, L. A non-canonical teleost nk-lysin: Antimicrobial activity via multiple mechanisms. Int. J. Mol. Sci. 2022, 23, 12722. [Google Scholar] [CrossRef] [PubMed]
- Hassett, D.J.; Imlay, J.A. Bactericidal antibiotics and oxidative stress: A radical proposal. ACS Chem. Biol. 2007, 2, 708–710. [Google Scholar] [CrossRef] [PubMed]
- Ehmann, D.; Wendler, J.; Koeninger, L.; Larsen, I.S.; Klag, T.; Berger, J.; Marette, A.; Schaller, M.; Stange, E.F.; Malek, N.P.; et al. Paneth cell α-defensins HD-5 and HD-6 display differential degradation into active antimicrobial fragments. Proc. Natl. Acad. Sci. USA 2019, 116, 3746–3751. [Google Scholar] [CrossRef]
- Tomisawa, S.; Sato, Y.; Kamiya, M.; Kumaki, Y.; Kikukawa, T.; Kawano, K.; Demura, M.; Nakamura, K.; Ayabe, T.; Aizawa, T. Efficient production of a correctly folded mouse α-defensin, cryptdin-4, by refolding during inclusion body solubilization. Protein Expr. Purif. 2015, 112, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Balhuizen, M.D.; van Dijk, A.; Jansen, J.W.A.; van de Lest, C.H.A.; Veldhuizen, E.J.A.; haagsman, h.p. outer membrane vesicles protect gram-negative bacteria against host defense peptides. mSphere 2021, 6, e0052321. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Song, Y.; Yan, S.; Hiramine, R.; Ohnishi, Y.; Yokoi, Y.; Nakamura, K.; Kikukawa, T.; Ayabe, T.; Aizawa, T. Antimicrobial Properties and Mode of Action of Cryptdin-4, a Mouse α-Defensin Regulated by Peptide Redox Structures and Bacterial Cultivation Conditions. Antibiotics 2023, 12, 1047. https://doi.org/10.3390/antibiotics12061047
Wang Y, Song Y, Yan S, Hiramine R, Ohnishi Y, Yokoi Y, Nakamura K, Kikukawa T, Ayabe T, Aizawa T. Antimicrobial Properties and Mode of Action of Cryptdin-4, a Mouse α-Defensin Regulated by Peptide Redox Structures and Bacterial Cultivation Conditions. Antibiotics. 2023; 12(6):1047. https://doi.org/10.3390/antibiotics12061047
Chicago/Turabian StyleWang, Yi, Yuchi Song, Shaonan Yan, Rina Hiramine, Yuki Ohnishi, Yuki Yokoi, Kiminori Nakamura, Takashi Kikukawa, Tokiyoshi Ayabe, and Tomoyasu Aizawa. 2023. "Antimicrobial Properties and Mode of Action of Cryptdin-4, a Mouse α-Defensin Regulated by Peptide Redox Structures and Bacterial Cultivation Conditions" Antibiotics 12, no. 6: 1047. https://doi.org/10.3390/antibiotics12061047
APA StyleWang, Y., Song, Y., Yan, S., Hiramine, R., Ohnishi, Y., Yokoi, Y., Nakamura, K., Kikukawa, T., Ayabe, T., & Aizawa, T. (2023). Antimicrobial Properties and Mode of Action of Cryptdin-4, a Mouse α-Defensin Regulated by Peptide Redox Structures and Bacterial Cultivation Conditions. Antibiotics, 12(6), 1047. https://doi.org/10.3390/antibiotics12061047





