First Case of VIM-1-like-Producing Pseudomonas putida Bacteremia in an Oncohematological Pediatric Patient in Italy
Abstract
1. Introduction
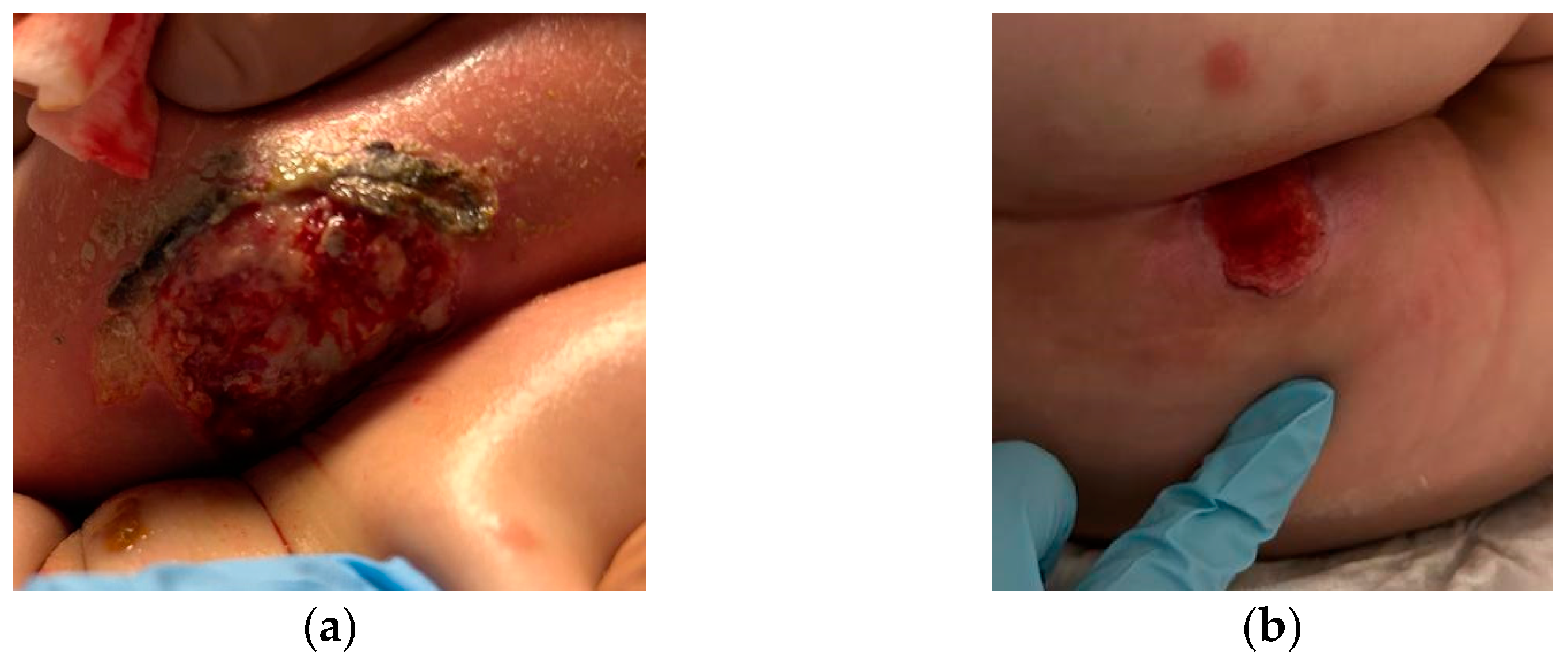
2. Case Description
3. Microbiological Investigation and Relevant Findings
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Queenan, A.M.; Bush, K. Carbapenemases: The versatile beta-lactamases. Clin. Microbiol. Rev. 2007, 20, 440–458. [Google Scholar] [CrossRef] [PubMed]
- Hammoudi Halat, D.; Ayoub Moubareck, C. The Current Burden of Carbapenemases: Review of Significant Properties and Dissemination among Gram-Negative Bacteria. Antibiotics 2020, 9, 186. [Google Scholar] [CrossRef] [PubMed]
- Yoshino, Y.; Kitazawa, T.; Kamimura, M.; Tatsuno, K.; Ota, Y.; Yotsuyanagi, H. Pseudomonas putida bacteremia in adult patients: Five case reports and a review of the literature. J. Infect. Chemother. 2011, 17, 278–282. [Google Scholar] [CrossRef] [PubMed]
- Fernández, M.; Porcel, M.; de la Torre, J.; Molina-Henares, M.A.; Daddaoua, A.; Llamas, M.A.; Roca, A.; Carriel, V.; Garzón, I.; Ramos, J.L.; et al. Analysis of the pathogenic potential of nosocomial Pseudomonas putida strains. Front. Microbiol. 2015, 6, 871. [Google Scholar] [CrossRef] [PubMed]
- Almuzara, M.; Radice, M.; de Gárate, N.; Kossman, A.; Cuirolo, A.; Santella, G.; Famiglietti, A.; Gutkind, G.; Vay, V. VIM-2-producing Pseudomonas putida, Buenos Aires. Emerg. Infect. Dis. 2007, 13, 668–669. [Google Scholar] [CrossRef] [PubMed]
- Bennett, J.W.; Herrera, M.L.; Lewis, J.S., 2nd; Wickes, B.W.; Jorgensen, J.H. KPC-2-producing Enterobacter cloacae and pseudomonas putida coinfection in a liver transplant recipient. Antimicrob. Agents Chemother. 2009, 53, 292–294. [Google Scholar] [CrossRef] [PubMed]
- Almeida, A.C.; Vilela, M.A.; Cavalcanti, F.L.; Martins, W.M.; Morais, M.A., Jr.; Morais, M.M. First description of KPC-2-producing Pseudomonas putida in Brazil. Antimicrob. Agents Chemother. 2012, 56, 2205–2206. [Google Scholar] [CrossRef] [PubMed]
- Chamon, R.C.; Abel da Rocha, J.; Araujo Martins, I.; Lopes Pires, L.; Macêdo de Almeida, B.; Souza Leite, N.; Rezende Vieira de Mendonça Souza, C.; Zahner, V.; Leite Ribeiro, R.; Pavoni Gomes Chagas, T.; et al. KPC-2 producing Pseudomonas putida as an unexpected pathogen of catheter-associated bloodstream infection. J. Infect. Dev. Ctries. 2020, 14, 411–414. [Google Scholar] [CrossRef] [PubMed]
- Treviño, M.; Moldes, L.; Hernández, M.; Martínez-Lamas, L.; García-Riestra, C.; Regueiro, B.J. Nosocomial infection by VIM-2 metallo-beta-lactamase-producing Pseudomonas putida. J. Med. Microbiol. 2010, 59 Pt 7, 853–855. [Google Scholar] [CrossRef] [PubMed]
- Lucignano, B.; Cento, V.; Agosta, M.; Ambrogi, F.; Albitar-Nehme, S.; Mancinelli, L.; Mattana, G.; Onori, M.; Galaverna, F.; Di Chiara, L.; et al. Effective Rapid Diagnosis of Bacterial and Fungal Bloodstream Infections by T2 Magnetic Resonance Technology in the Pediatric Population. J. Clin. Microbiol. 2022, 60, e0029222. [Google Scholar] [CrossRef] [PubMed]
- O’Hagan, S.; Nelson, P.; Speirs, L.; Moriarty, P.; Mallett, P. How to interpret a paediatric blood culture. Arch. Dis. Child. Educ. Pract. Ed. 2021, 106, 244–250. [Google Scholar] [CrossRef] [PubMed]
- Kirn, T.J.; Weinstein, M.P. Update on blood cultures: How to obtain, process, report, and interpret. Clin. Microbiol. Infect. 2013, 19, 513–520. [Google Scholar] [CrossRef] [PubMed]
- Miller, J.M.; Binnicker, M.J.; Campbell, S.; Carroll, K.C.; Chapin, K.C.; Gilligan, P.H.; Gonzalez, M.D.; Jerris, R.C.; Kehl, S.C.; Patel, R.; et al. A Guide to Utilization of the Microbiology Laboratory for Diagnosis of Infectious Diseases: 2018 Update by the Infectious Diseases Society of America and the American Society for Microbiology. Clin. Infect. Dis. 2018, 67, 813–816. [Google Scholar] [CrossRef] [PubMed]
- EUCAST. Available online: https://www.eucast.org/clinical_breakpoints/ (accessed on 13 March 2023).
- Poirel, L.; Walsh, T.R.; Cuvillier, V.; Nordmann, P. Multiplex PCR for detection of acquired carbapenemase genes. Diagn. Microbiol. Infect. Dis. 2011, 70, 119–123. [Google Scholar] [CrossRef] [PubMed]
- Bisiklis, A.; Papageorgiou, F.; Frantzidou, F.; Alexiou-Daniel, S. Specific detection of blaVIM and blaIMP metallo-beta-lactamase genes in a single real-time PCR. Clin. Microbiol. Infect. 2007, 13, 1201–1203. [Google Scholar] [CrossRef] [PubMed]
- Ellington, M.J.; Kistler, J.; Livermore, D.M.; Woodford, N. Multiplex PCR for rapid detection of genes encoding acquired metallo-beta-lactamases. J. Antimicrob. Chemother. 2007, 59, 321–322. [Google Scholar] [CrossRef] [PubMed]
- Harbarth, S.; Garbino, J.; Pugin, J.; Romand, J.A.; Lew, D.; Pittet, D. Inappropriate initial antimicrobial therapy and its effect on survival in a clinical trial of immunomodulating therapy for severe sepsis. Am. J. Med. 2003, 115, 529–535. [Google Scholar] [CrossRef] [PubMed]


| Antimicrobial Agent | Antimicrobial MIC 1 (µg/mL) and Interpretation for: | |||||||
|---|---|---|---|---|---|---|---|---|
| E. cloacae | P.aeruginosa | P. putida | E. brevis | |||||
| MIC 1 | INT 2 | MIC 1 | INT 2 | MIC 1 | INT 2 | MIC 1 | INT 2 | |
| Amikacin | 4 | S | (4) | - | (4) | - | ≤4 | - |
| Amoxicillin/clavulanic | ≥32 | R | - | - | - | - | 8 | - |
| Aztreonam | - | - | 4 | I | 8 | I | 1 | - |
| Cefepime | ≥32 | R | - | - | - | - | - | - |
| Cefotaxime | ≥64 | R | - | - | - | - | 8 | - |
| Ceftazidime | 32 | R | 8 | I | >16 | R | 4 | - |
| Ceftazidime/avibactam | 0.5 | S | 4/4 | S | >16/4 | R | 4 | - |
| Ceftolozane/tazobactam | 1 | S | 2/4 | S | >32/4 | R | 4 | - |
| Ciprofloxacin | ≥4 | R | 0.5 | I | 0.25 | I | ≥2 | - |
| Colistin | - | - | 2 | S | 0.5 | S | ≥8 | - |
| Gentamicin | ≥16 | R | 4 | IE | ≤0.5 | IE | ≤0.5 | - |
| Imipenem | ≤0.25 | S | ≤0.5 | I | >16 | R | 1 | - |
| Meropenem | ≤0.25 | S | 0.25 | S | >16 | R | 2 | - |
| Piperacillin/tazobactam | - | - | 0.5 | I | >32/4 | R | 2 | - |
| Tobramycin | ≥16 | R | (≤1) | - | (≤1) | - | ≥8 | - |
| Trimethopim/sulfametoxazole | ≥320 | R | - | - | - | - | ≤1 | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cortazzo, V.; Agosta, M.; Gaspari, S.; Vrenna, G.; Lucignano, B.; Onori, M.; Di Ruscio, V.; Mancinelli, L.; Domo, D.; Perno, C.F.; et al. First Case of VIM-1-like-Producing Pseudomonas putida Bacteremia in an Oncohematological Pediatric Patient in Italy. Antibiotics 2023, 12, 1033. https://doi.org/10.3390/antibiotics12061033
Cortazzo V, Agosta M, Gaspari S, Vrenna G, Lucignano B, Onori M, Di Ruscio V, Mancinelli L, Domo D, Perno CF, et al. First Case of VIM-1-like-Producing Pseudomonas putida Bacteremia in an Oncohematological Pediatric Patient in Italy. Antibiotics. 2023; 12(6):1033. https://doi.org/10.3390/antibiotics12061033
Chicago/Turabian StyleCortazzo, Venere, Marilena Agosta, Stefania Gaspari, Gianluca Vrenna, Barbara Lucignano, Manuela Onori, Valentina Di Ruscio, Livia Mancinelli, Danielle Domo, Carlo Federico Perno, and et al. 2023. "First Case of VIM-1-like-Producing Pseudomonas putida Bacteremia in an Oncohematological Pediatric Patient in Italy" Antibiotics 12, no. 6: 1033. https://doi.org/10.3390/antibiotics12061033
APA StyleCortazzo, V., Agosta, M., Gaspari, S., Vrenna, G., Lucignano, B., Onori, M., Di Ruscio, V., Mancinelli, L., Domo, D., Perno, C. F., & Bernaschi, P. (2023). First Case of VIM-1-like-Producing Pseudomonas putida Bacteremia in an Oncohematological Pediatric Patient in Italy. Antibiotics, 12(6), 1033. https://doi.org/10.3390/antibiotics12061033






