Abstract
Consumption of antimicrobials is an important driver of antimicrobial resistance. There is limited knowledge of the key determinants of antimicrobial prescribing behavior in hospitals. An understanding of these determinants is required for the successful design, adoption, and implementation of quality improvement interventions in Antimicrobial Stewardship Programs (ASP). This study aimed to describe the main factors that influence the doctor’s decision on antimicrobials prescribing and to identify the behaviors that drive physicians’ decision making. A structured web-based questionnaire focused on behavioral components of antimicrobial prescription was applied to the medical staff of three different departments—Internal Medicine, General Surgery, and Intensive Care Medicine—of a university hospital. All doctors agreed that inadequate use of antimicrobials increases AMR. A total of 77% of the surgeons and 100% of the internists and intensivists perceived antimicrobial prescription as a priority in the department. Full autonomy in antimicrobial prescription was preferred by internists (64%) but not by surgeons (18%) and intensivists (24%). Most physicians were keen to have ASP advice, but most did not want advice from colleagues of the same service. Almost all surgeons ask for advice when prescribing, but only 68% of the internists do it. Less than half of all physicians and only 25% of the surgeons felt free to prescribe contrary to guidelines. Most physicians, particularly in Intensive Care Medicine (94%), adopt the “wait and see” strategy when no microbiologic confirmation is available, but 27% of the surgeons start empirical therapy. In conclusion, the context of antimicrobial prescription, autonomy, and confidence in antimicrobial prescription demonstrated heterogeneity between the three departments and this should be considered when planning ASP.
1. Background
Antimicrobial resistance (AMR) is a serious and growing public health problem in both hospital and community-acquired infections worldwide, with a negative impact on health and economic outcomes. Several countries face high levels of antimicrobial resistance and increasing trends are expected in the coming years [1,2].
Inappropriate use of antimicrobials (including over-, under-, and misuse) is recognized as a key driver of AMR. The design of antimicrobial management programs should be based on the best current understanding of the relationship between antimicrobial use and resistance [3].
Worldwide, between 2000 and 2015, antibiotic consumption, expressed in DDD, increased by 65%, and the antibiotic consumption rate increased by 39%. Projections of global antibiotic consumption in 2030, assuming no policy changes, were up to 200% higher than in 2015. The global increase in antimicrobial consumption from 2000 to 2015 was driven by low- and middle-income countries, where rising consumption correlated with gross domestic product per capita growth and, although currently lower than in high-income countries, is rapidly converging to the rates of high-income countries [4]. There were an estimated 4.95 million deaths associated with bacterial AMR in 2019, including 1.27 million deaths attributable to bacterial AMR [5].
In Portugal, the rates of most of the bacteria/resistance combinations, such as methicillin-resistant Staphylococcus aureus, vancomycin-resistant Enterococcus faecium, carbapenem-resistant Acinetobacter spp., and fluoroquinolone resistant Escherichia coli have been decreasing in the last decade [6]. However, carbapenem-resistant Klebsiella pneumoniae rates have increased from 2 to 12% in the last 8 years [6], and infections due to antibiotic-resistant bacteria are estimated to be associated with 256 disability-adjusted life years (DALYs) and 10 attributable deaths per 100,000 population [7]. Despite the implementation of different policies, hospital antibiotic consumption has not significantly improved [6], and adequate use of antibiotics is a priority target of the national priority program to combat AMR [8].
Antimicrobial stewardship programs have been implemented worldwide, particularly in the hospital setting, aiming at the promotion of more prudent use of antibiotics. They are mandatory in all health units in Portugal. Several programs have been based on audit and feedback, educational strategies, and/or formulary restriction [9]. However, contextual factors that may influence antimicrobial prescription are often disregarded [10]. In their daily practice, physicians need to balance the possible benefits and the possible individual and collective adverse events associated with antibiotic prescription and such decision making is often difficult [11]. In this context, factors such as lack of knowledge, fear of an adverse outcome, especially in more complex patients, hierarchical relations, team organization, and normative beliefs seem to be relevant drivers of antibiotic prescription [11,12]. Thus, understanding what influences antimicrobial prescription using a context-specific approach is essential to guide and optimize stewardship interventions, effectively improving prescription behavior. The theory of planned behavior is one the most used models to explain and support antibiotic prescription behavior [13]. It considers that behavior intentions are the best predictors of actual behavior and that they result from attitudes towards behaviors, subjective norms (social norms), and perceived behavioral control. These intention drivers are, in turn, influenced by individual beliefs, namely, behavioral (behaviors), normative (subjective norms), and control (behavior control), and are shaped by individual and contextual conditions [13,14].
The objective of this study is to characterize behaviors in prescribing antimicrobial drugs to hospitalized patients at a public university tertiary hospital in northern Portugal, closely examining attitudes, subjective norms, perceived behavioral control, and physicians’ intention to prescribe.
2. Results
The overall response rate was 61%, namely, 44% in General Surgery (n = 22), 60% in Internal Medicine (n = 39), and 82% in Intensive Care Medicine (n = 33).
The participants were stratified into specialists (less than 5 years as specialist); graduated specialists (between 5 to 8 years as specialist); and senior specialists (more than 8 years as specialist).
Almost 60% of the sample was under 45 years old, ranging from 50% in General Surgery to 66% in Intensive Care Medicine. Around half of participants were females, and most were specialists or graduated specialists (64%). Senior specialists represented 11% of respondents. Almost all doctors worked full-time at CHUSJ, and most had more than 10 years of experience (65%) (Table 1).

Table 1.
Sociodemographic data.
Figure 1 and Figure 2 show the proportion of physicians that agreed or totally agreed with different concepts of antimicrobial use and of AMR. Results are presented as proportions for each department. Detailed results are available in Supplementary Table S1.
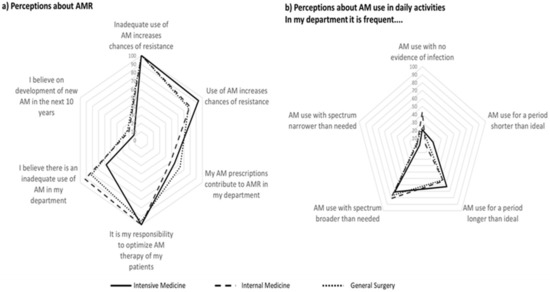
Figure 1.
Proportion of participants that agree or totally agree with statements regarding perceptions on antimicrobial resistance (a) and use of antimicrobials in daily activities (b).
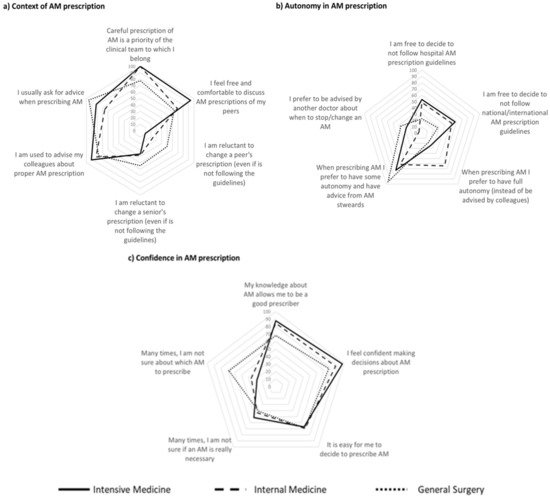
Figure 2.
Contextual and individual characteristics influencing antimicrobial (AM) prescription decision in the three different Services assessed, in terms of (a) context of AM prescription; (b) autonomy in AM prescription and (c) confidence in AM prescription.
All doctors agreed that inadequate use of antimicrobials increases AMR, while for 84%, overall use of antimicrobials increases AMR (almost 95% in Intensive Medicine and 77% in General Surgery). A smaller proportion reported that his/her own prescriptions contribute to AMR—from 49% in Internal Medicine to 64% in General Surgery.
Less than 60% of the intensivists, 82% of the surgeons, and 92% of the internists agreed that inadequate antimicrobial prescription occurs in their department (Figure 1a). Around half of physicians reported departments’ use of antimicrobials for periods longer than ideal. Three out of four physicians from General Surgery and from Intensive Care Medicine reported frequent use of antimicrobials with spectra broader than needed (50% in Internal Medicine). The perception of antimicrobial use with no evidence of infection varied from 41% in Internal Medicine to 23% in General Surgery and 17% in Intensive Care Medicine (Figure 1b). Most physicians reported that it was easy to access local or national guidelines (Table 2).

Table 2.
Detailed results of the questionnaire.
The context of antimicrobial prescription (Figure 2a) and autonomy (Figure 2b) and confidence (Figure 2c) in its prescription showed some heterogeneity between departments.
A total of 77% of the surgeons and 100% of the internists and intensivists perceived antimicrobial prescription as a priority in the department. Being comfortable discussing prescription with peers and debating colleagues’ (even if seniors) prescriptions was more frequent among intensivists, followed by internists and general surgeons. Conversely, almost all general surgeons ask for advice when prescribing but fewer doctors (68%) from Internal medicine exhibited the same behavior (Figure 2a).
Less than half of all physicians and 25% of the surgeons felt free to prescribe contrary to guidelines. Full autonomy in antimicrobial prescription was preferred by internists (64%) but not by surgeons and intensivists (18% and 24%, respectively). Most physicians declared themselves to be keen to have advice from antimicrobial stewards, but most preferred not to be advised by colleagues of the same service to change or stop antibiotics (Figure 2b). Most physicians agreed that their knowledge about antimicrobials allows them to be good prescribers, although almost 1/3 of general surgeons did not agree with such a statement. Almost all intensivists feel confident making decisions about antimicrobial prescription, even though 50% are frequently not sure if the antimicrobial is really necessary. In General Surgery, there was a lower proportion of uncertainty regarding the need of an antimicrobial but indecision about which one to prescribe was more frequent than in the other services (68% vs. 27% in intensive care medicine). The highest confidence occurred in internal medicine, where around 43% of physicians were uncertain about the need to prescribe an antimicrobial and 36% were uncertain about which one to prescribe (Figure 2c).
Antimicrobial prescription attitudes are presented in Figure 3. Most physicians, particularly in Intensive Care Medicine (94%), adopt the “wait and see” strategy when no microbiologic confirmation is available, while 27% of general surgeons start empirical therapy in that case. When deciding which antimicrobial to prescribe, almost all consider the patients’ probability of developing resistance. When different antimicrobials are possible and appropriate, most opt to choose the one with the highest likelihood of curing the infection, almost 40% of intensivists decide for the one with lower likelihood of inducing resistance (26% in internal medicine and 18% in general surgery), and about 1/5 of general surgeons opt for the one with lowest individual adverse effects. The uncertainty of an infection and the proximity of the weekend lead 27% of surgeons to empirically prescribe an antimicrobial. On the contrary, only a residual proportion of internists and intensivists do it. When facing laboratory delays, 64% of surgeons feel they should prescribe an antimicrobial, while only 36% of intensivists and 21% of internists feel similarly.
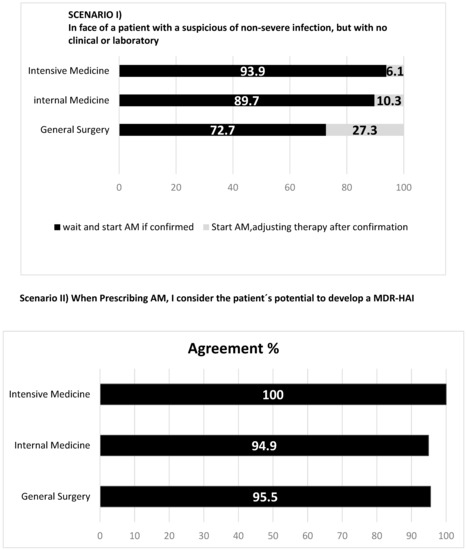
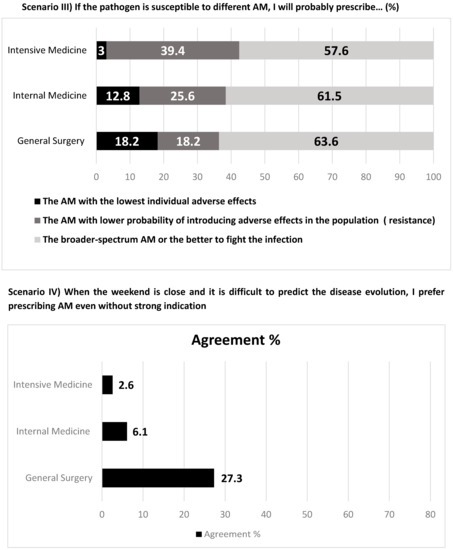
Figure 3.
Antimicrobial (AM) prescription attitudes towards different scenarios.
Regarding subjective norms, different sources of pressure towards AM prescription were observed (Figure 4). Around 40% of general surgery and internal medicine physicians felt pressure from patients to prescribe AM (12% among intensivists), and pressure not to prescribe was not felt. Peer pressure to prescribe antimicrobials was similar among internal medicine and intensive care physicians (82% and 85%), respectively. However, that number drops to 68% among surgeons.
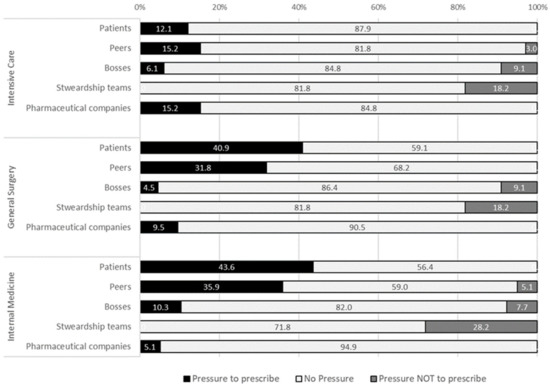
Figure 4.
Sources of pressure towards antimicrobial prescription felt by physicians.
A total of 15% of intensivists, 10% of surgeons, and 5% of internists felt pressure form pharmaceutical companies towards prescription. Finally, around 1/5 felt pressure against prescription from the local stewardship teams, mostly in the internal medicine department (28%).
For the future, most physicians intend to stop other doctors from prescribing antimicrobials without proper indication (68% in general surgery and 97% in intensive care medicine). More than 85% of general surgeons, 58% of internists, and 45% of intensivists plan to ask for local stewardship teams’ advice (Table 1).
3. Discussion
Our study brings to light key aspects related with the context of antimicrobial prescription. First, it reveals that departments’ leadership, prioritization of adequate antibiotic use, and team support may be related to the recognition of the problem of AMR and the ability to prescribe adequately; second, it reinforces that quality improvement, including stewardship activities, needs to be adapted to each department according to its needs, expectations, resources, and processes; third, it identifies some targets that can help the design of future effective quality improvement interventions.
Countless individual and contextual aspects can influence prescription. As observed in other settings, our study showed that professionals’ hierarchical relations, knowledge, autonomy, subjective norms, and control beliefs influence their clinical decisions [12,15,16,17,18].
This was also expressed in the pressure felt from peers towards antibiotic prescription that was lower in intensive care medicine, a department where physicians suggested less hierarchical structure. Moreover, the craving and intention to request advice differed between departments, which may limit the buy-in and the effectiveness of external stewardship interventions. While general surgeons seem to be willing to have external support, internal medicine and intensive care medicine physicians are less likely to request advice. This may be related with cultural boundaries across specialties [19], the perception of a deeper knowledge on antibiotics prescription (slightly higher in both departments when compared with general surgery), the preference for full autonomy (as particularly expressed in internal medicine), or a culture of internal discussion without the need of external peers (as observed in intensive care medicine).
Such organizational idiosyncrasies must be specifically addressed when designing quality improvement interventions, in line with what was observed in other hospitals; prescribing etiquette and clinical leadership should be understood and taken into account before planning and implementing the intervention [12,16,20]. Understanding is a prerequisite for change; therefore, it is a necessary step before any antimicrobial stewardship interventions [21,22].
This study also demonstrates that the pressure felt by doctors can be an important factor, which brings vulnerability to actions. Pressure comes from different factors and from different directions. The pressure felt from patients (although not so strongly felt in intensive medicine, as expected because of the patients’ clinical condition) towards prescription is an interesting result that suggests the need for interventions focusing on physicians’ communication skills and patients’ engagement and literacy. It is recognized that shared clinical decision making improves the adequacy of antibiotic prescription [23]. Although this study did not evaluate the relation between doctors and pharmaceuticals’ representatives, the drug industry was still be considered a source of pressure—albeit in a lesser extent—as it has been observed in other settings [24,25].
We observed full awareness of the impact of inadequate use in antimicrobial resistance but the proportion of physicians that agreed on the influence of overall antibiotic use was lower, suggesting that the drivers of antimicrobial resistance are not fully recognized. Additionally, the perception of their own contribution to the departments’ resistance patterns was even lower in all departments. This may reflect a gap between theoretical and real risks and ascription of responsibility to others [12,15,26]. More than formal training, periodic reports on setting-specific resistance maps and feedback on physician’s prescription may be useful to decrease such a gap [12,15].
Stewardship should focus on excessively long and excessively broad-spectrum antimicrobial therapy. Although not so frequent, the use of antibiotics with no evidence of infection was also perceived as a reality, particularly in Internal Medicine. In fact, almost half of physicians are often not sure if an antibiotic is needed. These aspects may be addressed with interventions to improve the accuracy of the diagnosis, not only with the development and good use of tests but also with training on, and behavioral interventions in, dealing and tolerating the risk of a watchful waiting strategy [12]. Antimicrobial stewardship teams may provide the support and comfort for this strategy. In some departments, namely, in General Surgery, training and advice may be useful to promote the correct antibiotic to be used. In this department, physicians seem to crave support to improve their knowledge and confidence in antibiotic prescription.
Our study has some limitations. The questionnaire was developed after an extensive review of the literature and included questions designed to reflect constructs of the theory of planned behavior. However, although we tested the questionnaire in a small sample of physicians, we did not perform a formal statistical evaluation to ensure its validity and reliability. We opted to focus on only three different services of the hospital; therefore, the sample may not represent the global medical population of the hospital. However, these were the largest medical, surgical, and critical care services of the hospital, and the strategy involving heads of department resulted in a high response rate, which would not be feasible if we opted to deliver the questionnaire to all hospital physicians. Additionally, our results suggest that hospital-based approaches may lack departments’ specificities and may lead to non-effective interventions. It is also possible that physicians with some interest or knowledge in the topic tended to respond more than less interested or less informed physicians. We tried to minimize this by ensuring response anonymity with an online self-reported questionnaire. Finally, the questionnaire was opened between May and June 2020, after the first wave of COVID-19 pandemic. In that first wave, there was an increase in antimicrobial consumption at the hospital, mainly to deal with bacterial super-infections [27]. This timing may have impacted on physicians’ answers.
4. Participants and Methods
A cross-sectional web-survey was conducted at Centro Hospitalar Universitário São João (CHUSJ). CHUSJ is a public university and tertiary hospital with 1083 beds, and around 40,000 hospitalizations per year. The hospital has antibiotic therapy guidelines elaborated by the hospital unit for the prevention of infection and antimicrobial resistance (UPCIRA) which promotes antimicrobial stewardship activities in several clinical services.
To represent medical, surgical, and critical care areas, the three largest services of each type were selected: General Surgery (n = 50 medical doctors), Internal Medicine (n = 65 medical doctors), and Intensive Care Medicine (n = 40 medical doctors).
The project was presented to the heads of services together with a link to the online questionnaire (developed in Google Forms). The questionnaire was sent to the physicians by the head of service, so researchers did not have direct contact with potential participants and had no access to their contacts. The questionnaire was written in Portuguese and the form was opened for 5 weeks (between 24 May and 30 June 2020). One reminder was sent by the head of each department at the end of the fourth week (except in General Surgery).
Participation was voluntary and anonymous. Participants were informed about the purpose, methods, and intended uses of the research and what their participation in the research entailed. Before starting the questionnaire, participants registered their acceptance in the online form. The CHUSJ ethical committee approved the study (number 55/2020).
The survey consisted of a structured questionnaire with statements that could reflect physician’s opinions. It was based in the available literature on similar surveys [15,16,17,18,19,28,29,30,31,32,33] that focused on behavioral components of antimicrobial prescription and were adapted to the Portuguese context by the research team. The theory underlying the formulation of most of the questions was based on the “Theory of Planned Behavior” [13,14], one of the theories in behavioral sciences thar describe individual behavioral patterns. Most questions were designed using a 4-point Likert Scale (strongly disagree, disagree, agree, strongly agree), and the remaining were dichotomous or multiple-choice (Supplementary Materials Table S1—English version). It was piloted in a small sample of physicians to correct for ambiguous or misunderstood questions and assess if all the relevant issues were included.
Globally, six main sections were included in the survey:
- (1)
- Demographic and general information: age, sex, level of training, time of experience (years), and doctor’s career position;
- (2)
- Perceptions about local and global AMR and if physicians’ practices influence AMR;
- (3)
- Perceived control behaviors that address how easy a prescriber feels in making a decision on antimicrobial prescription; these include self-efficiency and self-confidence and the capacity to prescribe in good practice and with a sense of control over the situation [12];
- (4)
- Subjective norms, aimed to identify normative influences that drive doctors’ behaviors: position under different types of pressure (from peers, patients or industry, patient clinical condition, etc.) to prescribe antimicrobials;
- (5)
- Habits and perceived knowledge regarding prescribing behavior, autonomy to change other prescribers’ decisions, attitudes towards antimicrobial prescribing;
- (6)
- Intentions: the degree to which a prescriber is willing to change antimicrobial prescriptions and to ask for support of the hospital antimicrobial stewardship team.
Data were collected in Google Forms and stored in Microsoft Excel®. Data were exported to IBM SPSS Statistics 25 for analysis. The frequency of each point class of the Likert scale was calculated and then collapsed into two categories: strongly agree or agree, and strongly disagree or disagree. All variables were described using absolute (n) and relative (%) frequencies, presented for the overall sample and stratified by department.
Due to the sample characteristics, age groups were collapsed into three categories—from 25–34 years, 35–54 years, and above 55 years old—and CHUSJ working years into up to 2, 3–4, and 5–10 years; and ≥10 years of experience, namely, 10–30 years and ≥30 years of experience.
5. Conclusions
In summary, this study demonstrates the relevance of behavioral, normative, and control aspects, together with individual and contextual conditions, on doctors’ decision-making when prescribing antimicrobials. There is not a superior strategy or a magic bullet that fits all settings, but the sum of different efforts and approaches can lead to the best outcome concerning the quality of antimicrobial prescription.
On top of the organizational and contextual department characteristics that should be taken into account in tailoring stewardship interventions towards behavioral changes, specific areas for improvement were identified regarding knowledge, the need for support, type and duration of the antimicrobials, and risk management to deal with uncertainty.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/antibiotics12061032/s1, Table S1: Survey Instrument, version in English.
Author Contributions
A.P.M.A.-C. performed the operational part of the study and drafted the first version of the manuscript. S.C. and J.-A.P. conceptualized the study and supervised and reviewed the manuscript. A.J.S.A. and E.B. participated in the operational part of the study. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was authorized by the Centro Hospitalar Universitário Sao Joao Ethical Committee (authorization no. 55/2020) and by the Administration Council of the institution.
Informed Consent Statement
All medical doctors who anonymously answered the questionnaire gave their implicit consent by completing the formulary.
Data Availability Statement
Data will be available upon request to the research team.
Acknowledgments
The authors want to thank all medical doctors from the Intensive Care Medicine, Internal Medicine, and General Surgery departments who have answered the questionnaire for participating in the study.
Conflicts of Interest
The authors declare no conflict of interest.
References
- OECD. Stemming the Superbug Tide: Just A Few Dollars More. OECD Health Policy Studies. 2018. Available online: https://www.oecdilibrary.org/content/publication/9789264307599-en (accessed on 3 January 2023).
- Cassini, A.; Högberg, L.D.; Plachouras, D.; Quattrocchi, A.; Hoxha, A.; Simonsen, G.S.; Colomb-Cotinat, M.; Kretzschmar, M.E.; Devleesschauwer, B.; Cecchini, M.; et al. Attributable deaths and disability-adjusted life-years caused by infections with antibiotic-resistant bacteria in the EU and the European Economic Area in 2015: A population-level modelling analysis. Lancet Infect. Dis. 2019, 19, 56–66. [Google Scholar] [CrossRef]
- MacDougall, C.; Polk, R.E. Antimicrobial stewardship programs in health care systems. Clin. Microbiol. Rev. 2005, 18, 638–656. [Google Scholar] [CrossRef] [PubMed]
- Klein, E.Y.; Van Boeckel, T.P.; Martinez, E.M.; Pant, S.; Gandra, S.; Levin, S.A.; Goossens, H.; Laxminarayan, R. Global increase and geographic convergence in antibiotic consumption between 200 and 2015. Proc. Natl. Acad. Sci. USA 2018, 115, E3463–E3470. [Google Scholar] [CrossRef] [PubMed]
- Christopher Murray for the Antimicrobial Resistance Collaborators. Global burden of bacterial antimicrobial resistance in 2019: A systematic analysis. Lancet 2022, 399, 629–655. [Google Scholar] [CrossRef] [PubMed]
- European Centre for Disease Prevention and Control. Antimicrobial Consumption in the EU/EEA—Annual Epidemiological Report 2019; ECDC: Stockholm, Sweden, 2020. [Google Scholar]
- European Centre for Disease Prevention and Control. Assessing the Health Burden of Infections with Antibiotic-Resistant Bacteria in the EU/EEA, 2016–2020; ECDC: Stockholm, Sweden, 2022. [Google Scholar]
- DGS. Plano Nacional de Combate à Resistência aos Antimicrobianos 2019–2023. In Âmbito do Conceito “Uma só Saúde”; Direção Geral da Saúde: Lisboa, Portugal, 2019. [Google Scholar]
- Davey, P.; Marwick, C.A.; Scott, C.L.; Charani, E.; McNeil, K.; Brown, E.; Gould, I.M.; Ramsay, C.R.; Michie, S. Interventions to improve antibiotic prescribing practices for hospital inpatients. Cochrane Database Syst. Rev. 2017, 2, CD003543. [Google Scholar] [CrossRef] [PubMed]
- Donisi, V.; Sibani, M.; Carrara, E.; Del Piccolo, L.; Rimondini, M.; Mazzaferri, F.; Bovo, C.; Tacconelli, E. Emotional, cognitive and social factors of antimicrobial prescribing: Can antimicrobial stewardship intervention be effective without addressing psycho-social factors? J. Antimicrob. Chemother. 2019, 74, 2844–2847. [Google Scholar] [CrossRef]
- Teixeira Rodrigues, A.; Roque, F.; Falcão, A.; Figueiras, A.; Herdeiro, M.T. Understanding physician antibiotic prescribing behaviour: A systematic review of qualitative studies. Int. J. Antimicrob. Agents 2013, 41, 203–212. [Google Scholar] [CrossRef]
- Warremana, E.; Lambregtsa, M.; Wouters, R.; Visser, L.; Staats, H.; van Dijk, E.; de Boer, M. Determinants of in-hospital antibiotic prescription behaviour: A systematic review and formation of a comprehensive framework. Clin. Microbiol. Infect. Off. Publ. Eur. Soc. Clin. Microbiol. Infect. Dis. 2019, 25, 538–545. [Google Scholar] [CrossRef]
- Ajzen, I. The theory of planned behaviour: Reactions and reflections. Psychol. Health 2011, 26, 1113–1127. [Google Scholar] [CrossRef]
- Kiriakidis, S.P. (Ed.) Theory of Planned Behaviour: The Intention-Behaviour Relationship and the Perceived Behavioural Control (PBC) Relationship with Intention and Behaviour. Int. J. Strateg. Innov. Mark. 2015, 3, 40–51. [Google Scholar] [CrossRef]
- Labricciosa, F.M.; Sartelli, M.; Correia, S.; Abbo, L.M.; Severo, M.; Ansaloni, L.; Coccolini, F.; Alves, C.; Melo, R.B.; Baiocchi, G.L.; et al. Emergency surgeons’ perceptions and attitudes towards antibiotic prescribing and resistance: A worldwide cross-sectional survey. World J. Emerg. Surg. 2018, 13, 27. [Google Scholar] [CrossRef]
- Parker, H.M.; Mattick, K. The determinants of antimicrobial prescribing among hospital doctors in England: A framework to inform tailored stewardship interventions. Br. J. Clin. Pharmacol. 2016, 82, 431–440. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Liu, C.; Wang, D.; Deng, Z.; Tang, Y.; Zhang, X. Determinants of antibiotic prescribing behaviors of primary care physicians in Hubei of China: A structural equation model based on the theory of planned behavior. Antimicrob. Resist. Infect. Control 2019, 8, 23. [Google Scholar] [CrossRef]
- Sheeran, P.; Orbell, S. Implementation intentions and repeated behaviour: Augumenting the predictive validity of theory of planned behaviour. Eur. J. Soc. Psychol. 1999, 29, 349–369. [Google Scholar] [CrossRef]
- Ponnet, K.; Wouters, E.; Van Hal, G.; Heirman, W.; Walrave, M. Determinants of physicians prescribing behaviour of methylphenidate for cognitive enhancement. Psychol. Health Med. 2014, 19, 286–295. [Google Scholar] [CrossRef] [PubMed]
- Labi, A.K.; Obeng-Nkrumah, N.; Bjerrum, S.; Aryee, N.A.A.; Ofori-Adjei, Y.A.; Yawson, A.E.; Newman, M.J. Understanding the determinants of antimicrobial prescribing within hospitals: The role of “prescribing etiquette”. Clin. Infect. Dis. 2013, 57, 188–196. [Google Scholar]
- Saleem, Z.; Hassali, M.A.; Godman, B.; Hashmi, F.K.; Saleem, F. Antimicrobial prescribing and determinants of antimicrobial resistance: A qualitative study among physicians in Pakistan. Int. J. Clin. Pharm. 2019, 41, 1348–1358. [Google Scholar] [CrossRef]
- Charani, E.; Holmes, A. Antibiotic Stewardship-Twenty Years in the Making. Antibiotics 2019, 8, 7. [Google Scholar] [CrossRef]
- Rawson, T.M.; Moore, L.S.P.; Castro-Sanchez, E.; Charani, E.; Hernandez, B.; Alividza, V.; Husson, F.; Toumazou, C.; Ahmad, R.; Georgiou, P.; et al. Development of a patient-centred intervention to improve knowledge and understanding of antibiotic therapy in secondary care. Antimicrob. Resist. Infect. Control 2018, 7, 43. [Google Scholar] [CrossRef]
- Fischer, M.A.; Keough, M.E.; Baril, J.L.; Saccoccio, L.; Mazor, K.M.; Ladd, E.; Worley, A.V.; Gurwitz, J.H. Prescribers and pharmaceutical representatives: Why are we still meeting? J. Gen. Intern. Med. 2009, 24, 795–801. [Google Scholar] [CrossRef]
- Jandhyala, R. Influence of Pharmaceutical Company Engagement Activities on the Decision to Prescribe: A Pilot Survey of UK Rare Disease Medicine Prescribers. Pharm. Med. 2020, 34, 127–134. [Google Scholar] [CrossRef]
- Navarro-San Francisco, C.; Del Toro, M.D.; Cobo, J.; De Gea-García, J.H.; Vanó-Galván, S.; Moreno-Ramos, F.; Rodríguez-Bano, J.; Pano-Pardo, J.R. Knowledge and perceptions of junior and senior Spanish resident doctors about antibiotic use and resistance: Results of a multicenter survey. Enferm. Infecc. Y Microbiol. Clin. 2013, 31, 199–204. [Google Scholar] [CrossRef] [PubMed]
- Castro-Lopes, A.; Correia, S.; Leal, C.; Resende, I.; Soares, P.; Azevedo, A.; Paiva, J.-A. Increase of Antimicrobial Consumption in a Tertiary Care Hospital during the First Phase of the COVID-19 Pandemic. Antibiotics 2021, 10, 778. [Google Scholar] [CrossRef]
- Doron, S.; Nadkarni, L.; Price, L.L.; Lawrence, K.; Davidson, L.E.; Evans, J.; Garber, C.; Snydman, D. A nationwide survey of antimicrobial stewardship practices. Clin. Ther. 2013, 35, 758–765.e20. [Google Scholar] [CrossRef] [PubMed]
- Allenback, G.L. A Survey of Antimicrobial Stewardship Practices in the Western United States: Successes and Challenges Repository Citation [Internet]. 2014. Available online: https://digitalscholarship.unlv.edu/thesesdissertations/2054 (accessed on 3 January 2023).
- Thakolkaran, N.; Shetty, A.V.; D’Souza, N.R.; Shetty, A. Antibiotic prescribing knowledge, attitudes, and practice among physicians in teaching hospitals in South India. J. Fam. Med. Prim. Care 2017, 6, 526–532. [Google Scholar]
- Dyar, O.J.; Howard, P.; Nathwani, D.; Pulcini, C. Knowledge, attitudes, and beliefs of French medical students about antibiotic prescribing and resistance. Med. Mal. Infect. 2013, 43, 423–430. [Google Scholar] [CrossRef]
- Baadani, A.M.; Baig, K.; Alfahad, W.A.; Aldalbahi, S.; Omrani, A.S. Physicians’ knowledge, perceptions, and attitudes toward antimicrobial prescribing in Riyadh, Saudi Arabia. Saudi. Med. J. 2015, 36, 613–619. [Google Scholar] [CrossRef] [PubMed]
- Labi, A.K.; Obeng-Nkrumah, N.; Bjerrum, S.; Aryee, N.A.A.; Ofori-Adjei, Y.A.; Yawson, A.E.; Newman, M.J. Physician’s knowledge, attitudes, and perceptions concerning antibiotic resistance; A survey in a Ghanaian tertiary care hospital. BMC Health Serv. Res. 2018, 18, 126. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).