The COVID-19 Pandemic Enhanced the Decade-Long Trend of the Decreasing Utilization of Antibiotics
Abstract
1. Introduction
2. Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Huttner, A.; Harbarth, S.; Carlet, J.; Cosgrove, S.; Goossens, H.; Holmes, A.; Jarlier, V.; Voss, A.; Pittet, D. Antimicrobial resistance: A global view from the 2013 World Healthcare-Associated Infections Forum. Antimicrob. Resist. Infect. Control 2013, 2, 31. [Google Scholar] [CrossRef]
- Bell, B.G.; Schellevis, F.; Stobberingh, E.; Goossens, H.; Pringle, M. A systematic review and meta-analysis of the effects of antibiotic consumption on antibiotic resistance. BMC Infect. Dis. 2014, 14, 13. [Google Scholar] [CrossRef]
- Frost, I.; Van Boeckel, T.P.; Pires, J.; Craig, J.; Laxminarayan, R. Global geographic trends in antimicrobial resistance: The role of international travel. J. Travel Med. 2019, 26, taz036. [Google Scholar] [CrossRef]
- Nellums, L.B.; Thompson, H.; Holmes, A.; Castro-Sánchez, E.; Otter, J.A.; Norredam, M.; Friedland, J.S.; Hargreaves, S. Antimicrobial resistance among migrants in Europe: A systematic review and meta-analysis. Lancet Infect. Dis. 2018, 18, 796–811. [Google Scholar] [CrossRef]
- Costelloe, C.; Metcalfe, C.; Lovering, A.; Mant, D.; Hay, A. Effect of antibiotic prescribing in primary care on antimicrobial resistance in individual patients: Systematic review and meta-analysis. BMJ 2010, 340, c2096. [Google Scholar] [CrossRef]
- Gyssens, I.C. Role of Education in Antimicrobial Stewardship. Med. Clin. North Am. 2018, 102, 855–871. [Google Scholar] [CrossRef]
- Jang, W.; Hwang, H.; Jo, H.-U.; Cha, Y.-H.; Kim, B. Effect of discontinuation of an antimicrobial stewardship programme on the antibiotic usage pattern. Clin. Microbiol. Infect. 2021, 27, 1860.e1–1860.e5. [Google Scholar] [CrossRef]
- Llor, C.; Sierra, N.; Hernández, S.; Bayona, C.; Hernández, M.; Moragas, A.; Calviño, O. El cumplimiento del tratamiento antibiótico en la faringitis aguda es muy bajo, principalmente con antibióticos que se toman tres veces al día [Compliance rate of antibiotic therapy in patients with acute pharyngitis is very low, mainly when thrice-daily antibiotics are given]. Rev. Esp. Quim. 2009, 22, 20–24. (In Spanish) [Google Scholar]
- Llor, C.; Sierra, N.; Hernández, S.; Moragas, A.; Hernández, M.; Bayona, C.; Miravitlles, M. The higher the number of daily doses of antibiotic treatment in lower respiratory tract infection the worse the compliance. J. Antimicrob. Chemother. 2008, 63, 396–399. [Google Scholar] [CrossRef]
- Kuhnke, A.; Lode, H. Probleme der Pharmakotherapie bei Infektionen im Alter. Der Internist 2003, 44, 986–994. [Google Scholar] [CrossRef]
- Campion, M.; Scully, G. Antibiotic Use in the Intensive Care Unit: Optimization and De-Escalation. J. Intensiv. Care Med. 2018, 33, 647–655. [Google Scholar] [CrossRef]
- Septimus, E.J. Antimicrobial Resistance: An Antimicrobial/Diagnostic Stewardship and Infection Prevention Approach. Med. Clin. North Am. 2018, 102, 819–829. [Google Scholar] [CrossRef]
- Browne, A.J.; Chipeta, M.G.; Haines-Woodhouse, G.; A Kumaran, E.P.; Hamadani, B.H.K.; Zaraa, S.; Henry, N.J.; Deshpande, A.; Reiner, R.C.; Day, N.P.J.; et al. Global antibiotic consumption and usage in humans, 2000–18: A spatial modelling study. Lancet Planet. Health 2021, 5, e893–e904. [Google Scholar] [CrossRef]
- Rizvi, R.F.; Craig, K.J.T.; Hekmat, R.; Reyes, F.; South, B.; Rosario, B.; Kassler, W.J.; Jackson, G.P. Effectiveness of non-pharmaceutical interventions related to social distancing on respiratory viral infectious disease outcomes: A rapid evidence-based review and meta-analysis. SAGE Open Med. 2021, 9, 20503121211022973. [Google Scholar] [CrossRef]
- Kucharski, A.J.; Klepac, P.; Conlan, A.J.K.; Kissler, S.M.; Tang, M.L.; Fry, H.; Gog, J.R.; Edmunds, W.J.; Emery, J.C.; Medley, G.; et al. Effectiveness of isolation, testing, contact tracing, and physical distancing on reducing transmission of SARS-CoV-2 in different settings: A mathematical modelling study. Lancet Infect. Dis. 2020, 20, 1151–1160. [Google Scholar] [CrossRef]
- To, K.K.; Chan, J.F.; Yuen, K.Y. Viral lung infections: Epidemiology, virology, clinical features, and management of avian influenza A(H7N9). Curr. Opin. Pulm. Med. 2014, 20, 225–232. [Google Scholar] [CrossRef]
- Sopena, N.; Sabrià, M.; Pedro-Botet, M.L.; Manterola, J.M.; Matas, L.; Domínguez, J.; Modol, P.T.J.M.; Ausina, V.; Foz, M. Prospective Study of Community-Acquired Pneumonia of Bacterial Etiology in Adults. Eur. J. Clin. Microbiol. Infect. Dis. 1999, 18, 852–858. [Google Scholar] [CrossRef]
- Tanislav, C.; Kostev, K. Fewer non-COVID-19 respiratory tract infections and gastrointestinal infections during the COVID-19 pandemic. J. Med. Virol. 2021, 94, 298–302. [Google Scholar] [CrossRef]
- Ronald, A. The etiology of urinary tract infection: Traditional and emerging pathogens. Disease-A-Month 2003, 49, 71–82. [Google Scholar] [CrossRef]
- Tanislav, C.; Jacob, L.; Kostev, K. Consultations Decline for Stroke, Transient Ischemic Attack, and Myocardial Infarction during the COVID-19 Pandemic in Germany. Neuroepidemiology 2021, 55, 70–78. [Google Scholar] [CrossRef]
- Wang, T.; Shen, L.; Yin, J.; Zhou, L.; Sun, Q. Antibiotic use in township hospitals during the COVID-19 pandemic in Shandong, China. Antimicrob. Resist. Infect. Control 2022, 11, 164. [Google Scholar] [CrossRef]
- Akmatov, M.K.; Kohring, C.; Dammertz, L.; Heuer, J.; Below, M.; Bätzing, J.; Holstiege, J. The Effect of the COVID-19 Pandemic on Outpatient Antibiotic Prescription Rates in Children and Adolescents—A Claims-Based Study in Germany. Antibiotics 2022, 11, 1433. [Google Scholar] [CrossRef]
- Rathmann, W.; Bongaerts, B.; Carius, H.J.; Kruppert, S.; Kostev, K. Basic characteristics and representativeness of the German Disease Analyzer database. Int. J. Clin. Pharmacol. Ther. 2018, 56, 459–466. [Google Scholar] [CrossRef]
- Kern, W.V.; Kostev, K. Prevalence of and Factors Associated with Antibiotic Prescriptions in Patients with Acute Lower and Upper Respiratory Tract Infections—A Case-Control Study. Antibiotics 2021, 10, 455. [Google Scholar] [CrossRef]
- Bittner, C.B.; Plach, M.; Steindl, H.; Abramov-Sommariva, D.; Abels, C.; Kostev, K. Prevalence of Antibiotic Prescription in Patients with Acute Rhinosinusitis Treated by General Practitioners and Otolaryngologists in Germany—A Retrospective Cohort Study. Antibiotics 2022, 11, 1576. [Google Scholar] [CrossRef]
- Holstiege, J.; Schulz, M.; Akmatov, M.K.; Kern, W.V.; Steffen, A.; Bätzing, J. The Decline in Outpatient Antibiotic Use. Dtsch Arztebl Int. 2020, 117, 679–686. [Google Scholar] [CrossRef]
- Holstiege, J.; Schulz, M.; Akmatov, M.K.; Steffen, A.; Bätzing, J. Marked reductions in outpatient antibiotic prescriptions for children and adolescents–a population-based study covering 83% of the paediatric population, Germany, 2010 to 2018. Eurosurveillance 2020, 25, 1900599. [Google Scholar] [CrossRef]
- Masters, G.A.; Asipenko, E.; Bergman, A.L.; Person, S.D.; Brenckle, L.; Simas, T.A.M.; Ko, J.Y.; Robbins, C.L.; Byatt, N. Impact of the COVID-19 pandemic on mental health, access to care, and health disparities in the perinatal period. J. Psychiatr. Res. 2021, 137, 126–130. [Google Scholar] [CrossRef]
- Assi, L.; Deal, J.A.; Samuel, L.; Reed, N.S.; Ehrlich, J.R.; Swenor, B.K. Access to food and health care during the COVID-19 pandemic by disability status in the United States. Disabil. Health J. 2022, 15, 101271. [Google Scholar] [CrossRef]
- Balhara, Y.P.S.; Singh, S.; Narang, P. Effect of lockdown following COVID-19 pandemic on alcohol use and help-seeking behavior: Observations and insights from a sample of alcohol use disorder patients under treatment from a tertiary care center. Psychiatry Clin Neurosci. 2020, 74, 440–441. [Google Scholar] [CrossRef]
- Watson, G.; Pickard, L.; Williams, B.; Hargreaves, D.; Blair, M. ‘Do I, don’t I?’ A qualitative study addressing parental perceptions about seeking healthcare during the COVID-19 pandemic. Arch. Dis. Child. 2021, 106, 1118–1124. [Google Scholar] [CrossRef]
- Malcolm, W.; Seaton, R.A.; Haddock, G.; Baxter, L.; Thirlwell, S.; Russell, P.; Cooper, L.; Thomson, A.; Sneddon, J. Impact of the COVID-19 pandemic on community antibiotic prescribing in Scotland. JAC-Antimicrob. Resist. 2020, 2, dlaa105. [Google Scholar] [CrossRef]
- Gillies, M.B.; Burgner, D.P.; Ivancic, L.; Nassar, N.; Miller, J.E.; Sullivan, S.G.; Todd, I.M.F.; Pearson, S.; Schaffer, A.L.; Zoega, H. Changes in antibiotic prescribing following COVID-19 restrictions: Lessons for post-pandemic antibiotic stewardship. Br. J. Clin. Pharmacol. 2022, 88, 1143–1151. [Google Scholar] [CrossRef]
- Abad-Díez, J.M.; Calderón-Larrañaga, A.; Poncel-Falcó, A.; Poblador-Plou, B.; Calderón-Meza, J.M.; Sicras-Mainar, A.; Clerencia-Sierra, M.; Prados-Torres, A. Age and gender differences in the prevalence and patterns of multimorbidity in the older population. BMC Geriatr. 2014, 14, 75. [Google Scholar] [CrossRef]
- Kirchberger, I.; Meisinger, C.; Heier, M.; Zimmermann, A.-K.; Thorand, B.; Autenrieth, C.S.; Peters, A.; Ladwig, K.-H.; Döring, A. Patterns of Multimorbidity in the Aged Population. Results from the KORA-Age Study. PLoS ONE 2012, 7, e30556. [Google Scholar] [CrossRef]
- Yeoh, Y.K.; Zuo, T.; Lui, G.C.-Y.; Zhang, F.; Liu, Q.; Li, A.Y.; Chung, A.C.; Cheung, C.P.; Tso, E.Y.; Fung, K.S.; et al. Gut microbiota composition reflects disease severity and dysfunctional immune responses in patients with COVID-19. Gut 2021, 70, 698–706. [Google Scholar] [CrossRef]
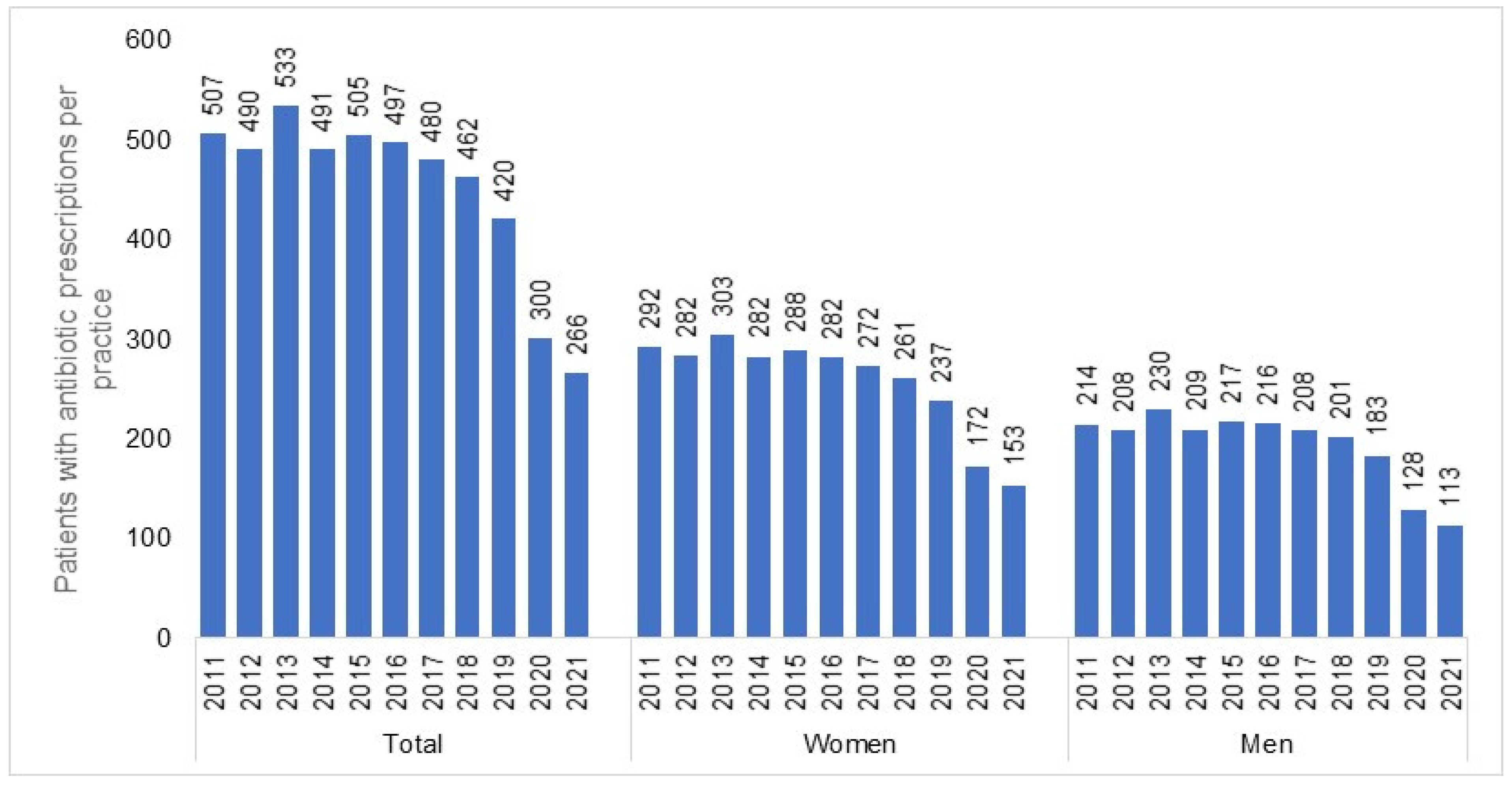
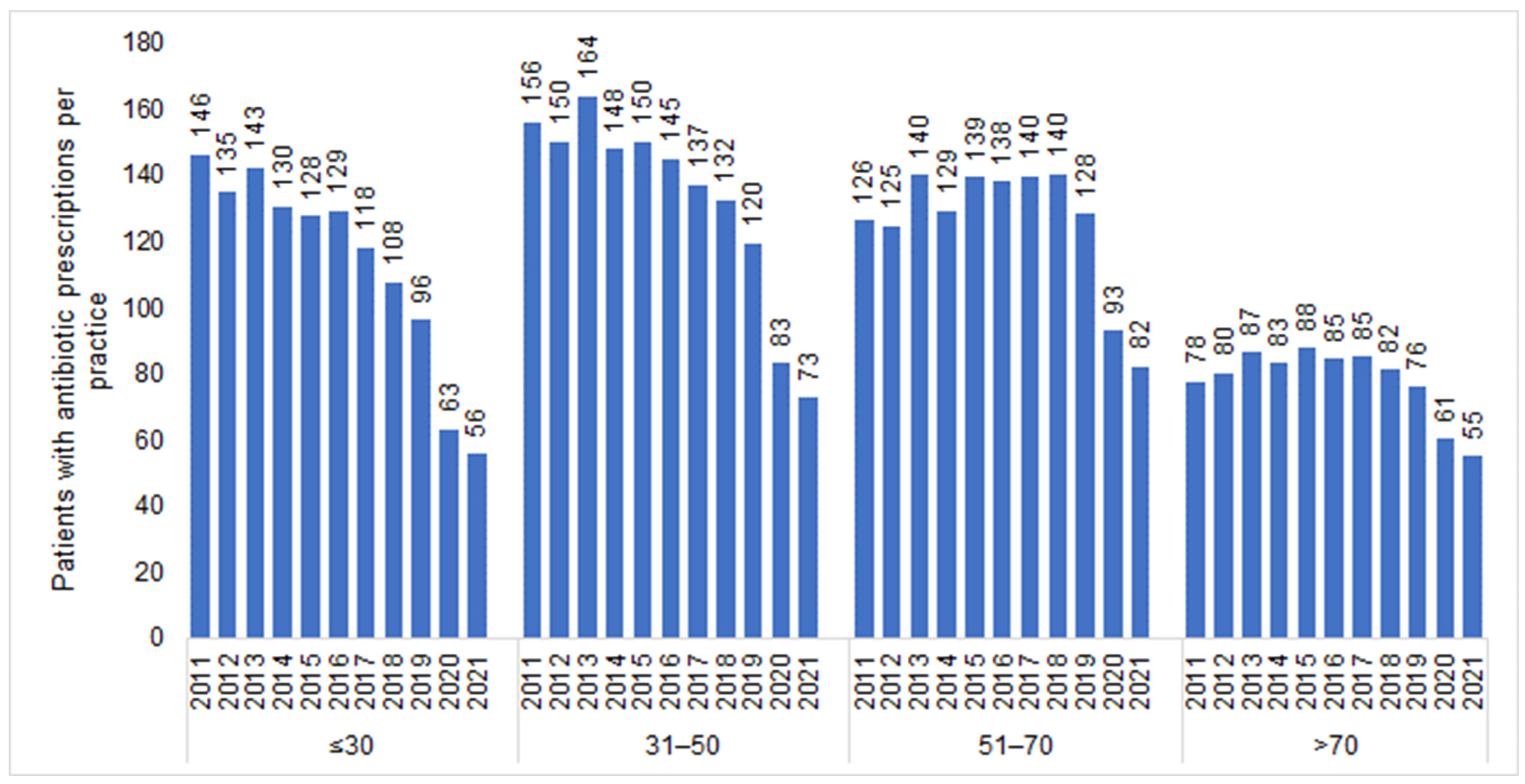
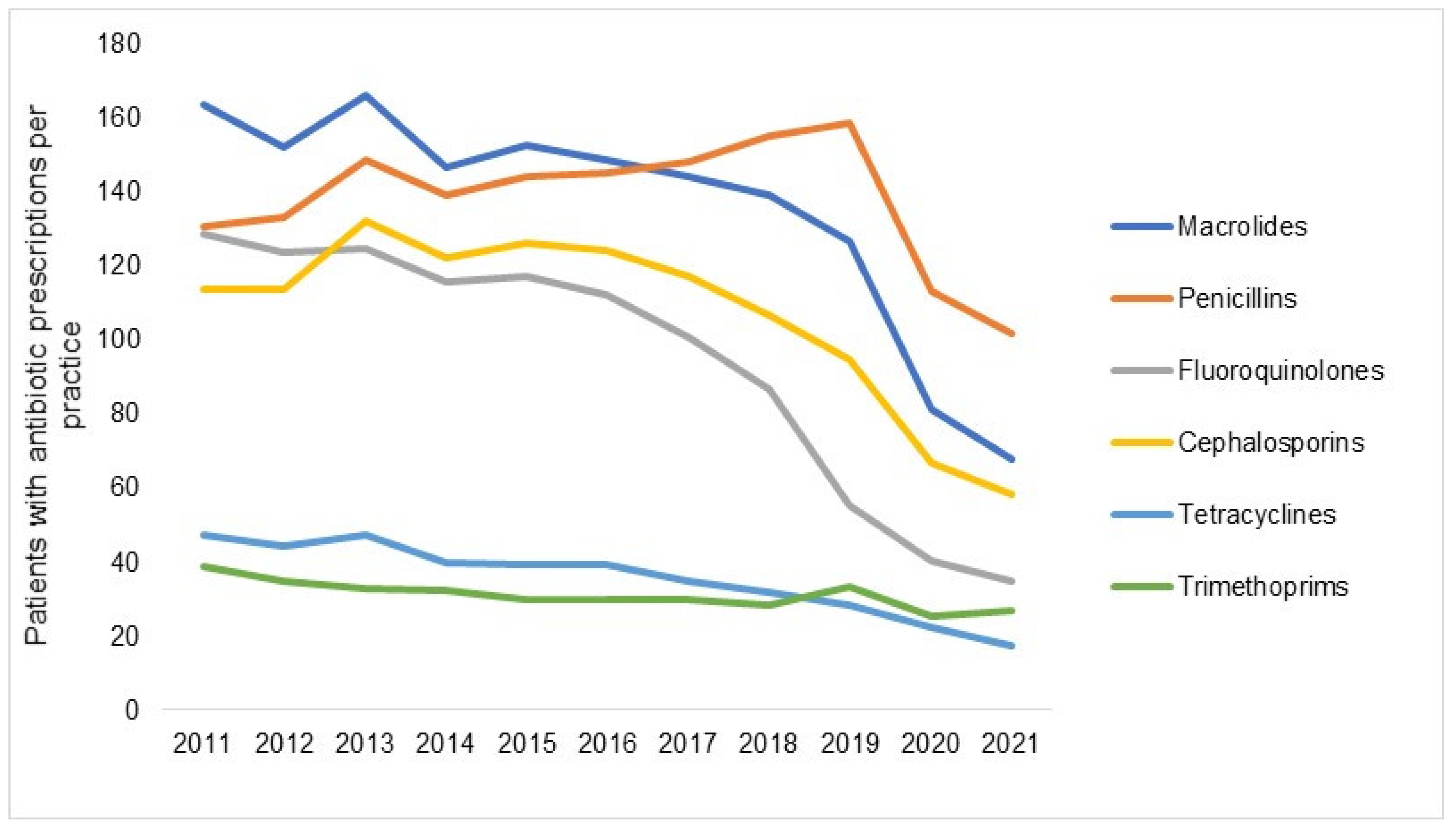
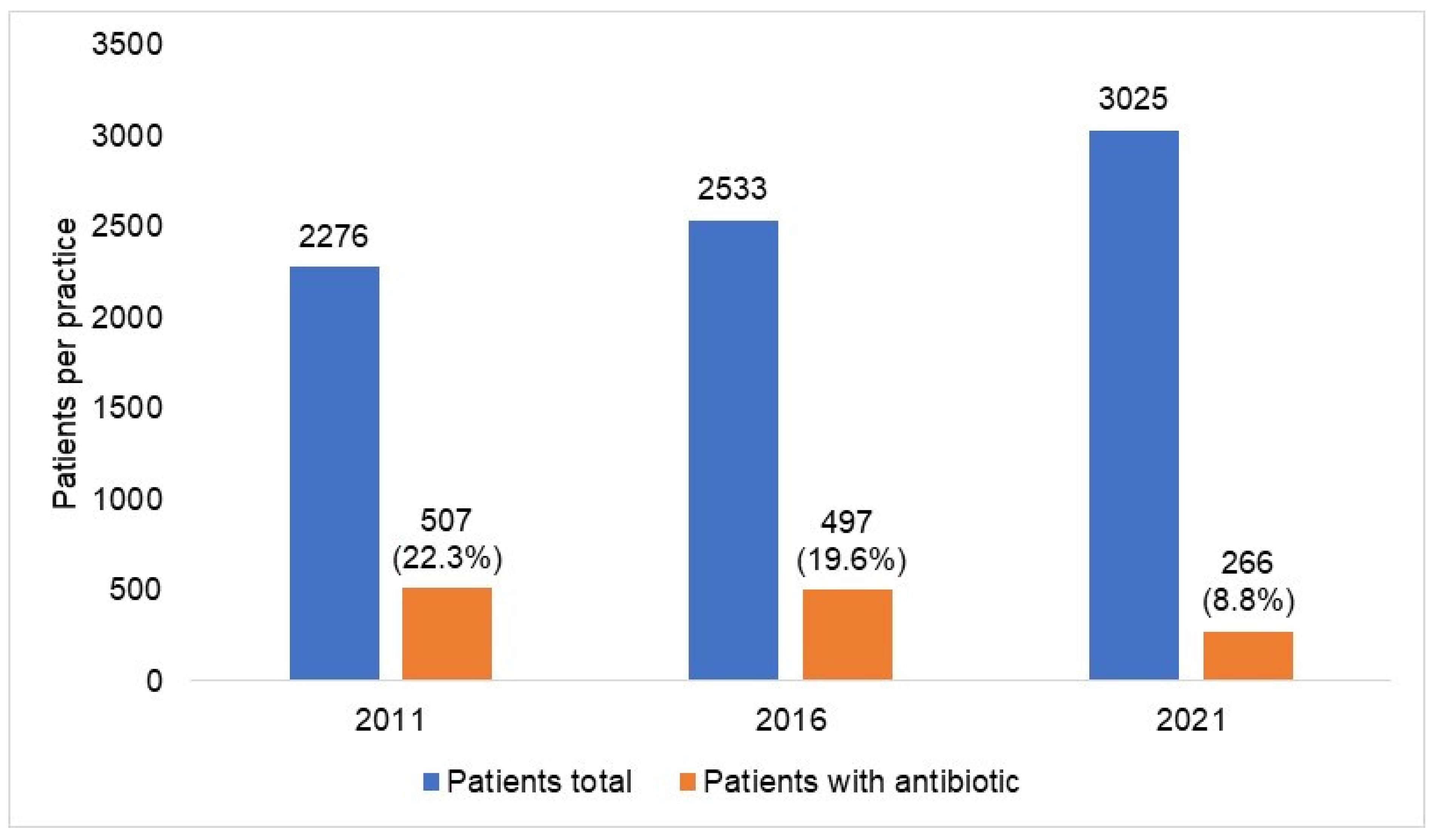
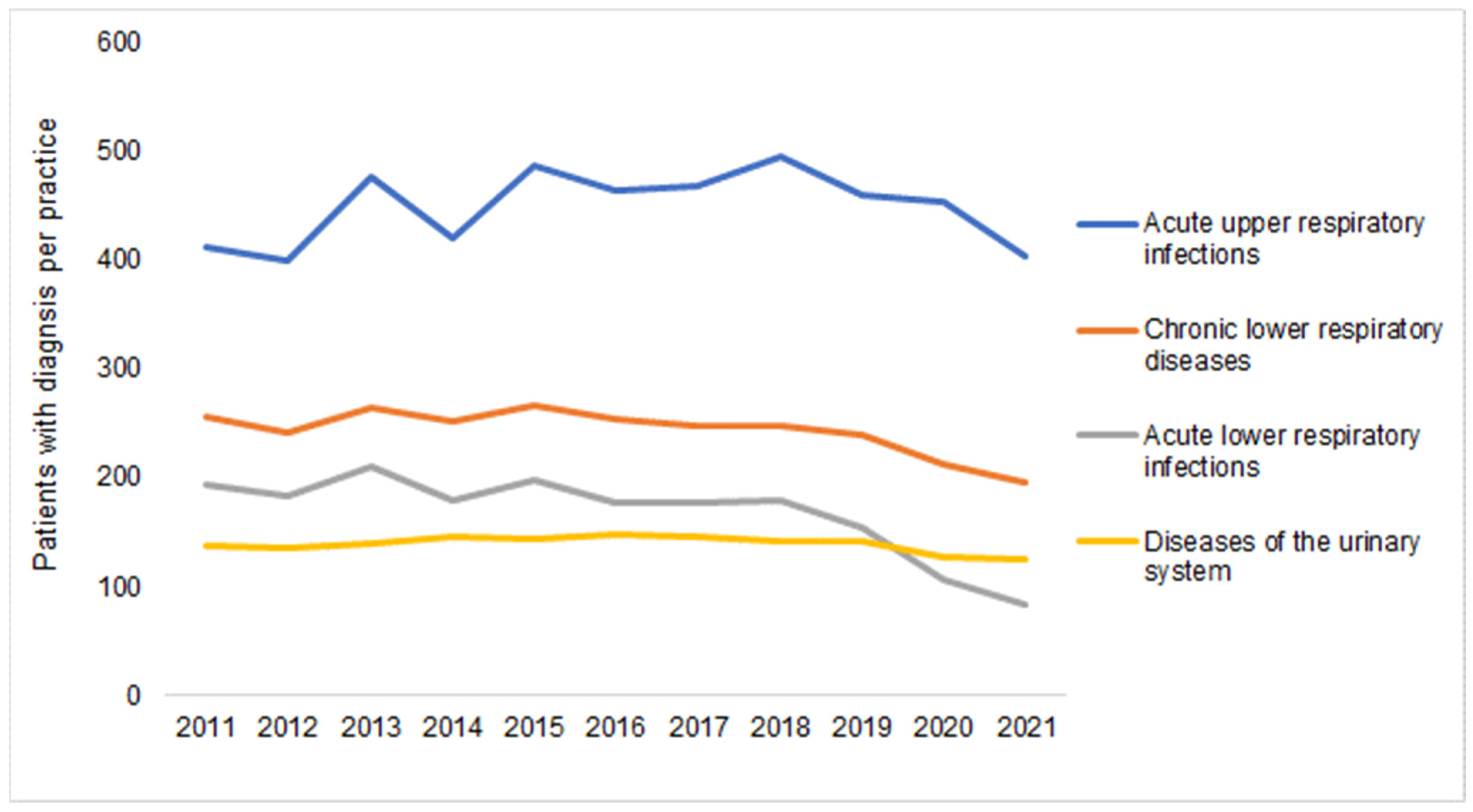
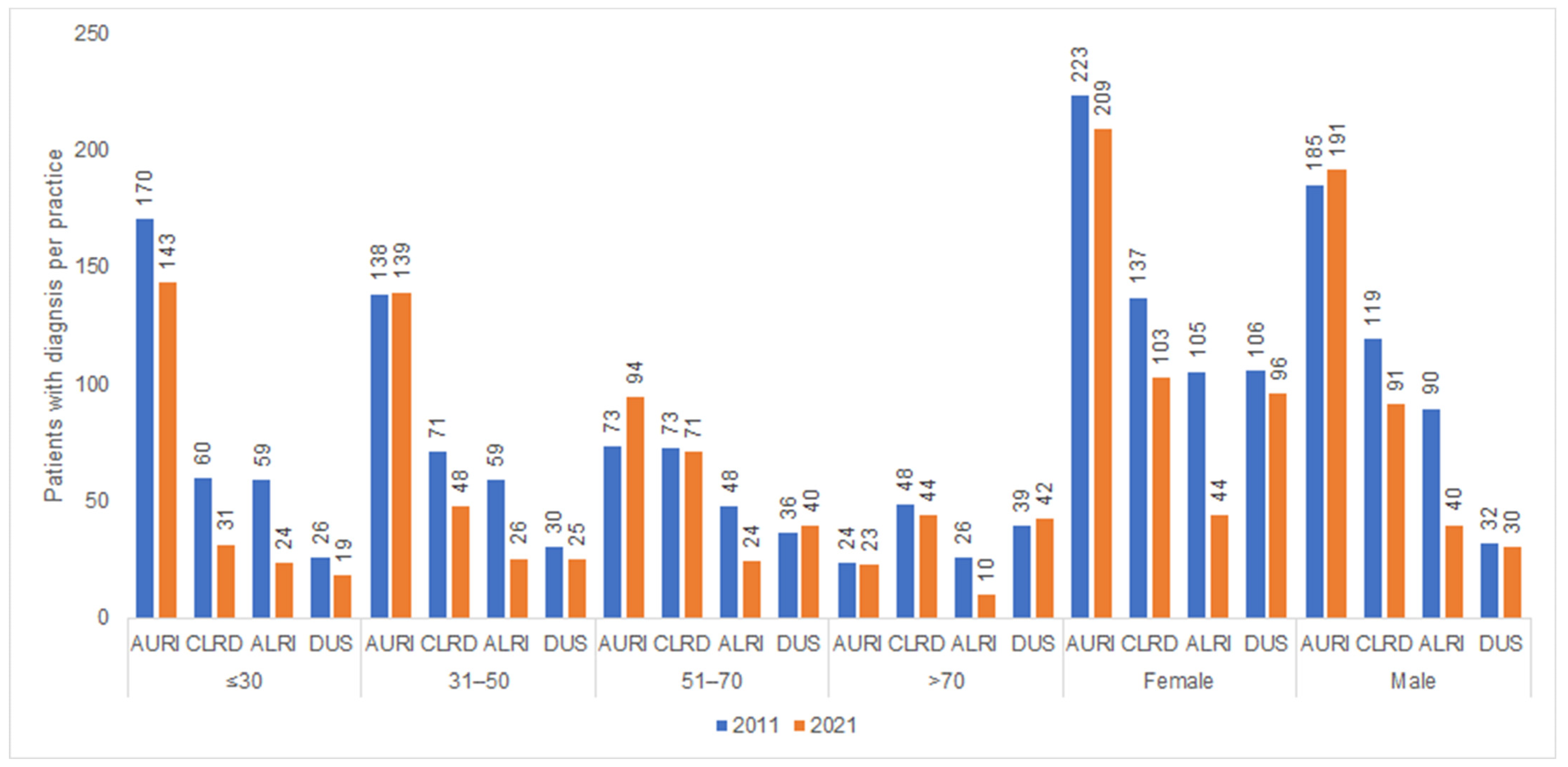
| Yearly Difference | ||||||
|---|---|---|---|---|---|---|
| Patients with Antibiotic Prescriptions | 2016–2015 | 2017–2016 | 2018–2017 | 2019–2018 | 2020–2019 | 2021–2020 |
| Absolute number of patients per practice | 497−505 = −8 | 480−497 = −17 | 462−480 = −18 | 420−462 = −42 | 300−420 = −120 | 266−300 = −34 |
| Percentage reduction | −1.6% | −3.4% | −3.8% | −9.1% | −28.6% | −11.3% |
| Infectious diseases * | 2016−2015 | 2017−2016 | 2018−2017 | 2019−2018 | 2020−2019 | 2021−2020 |
| Absolute number of patients per practice | 1044−1094 = −51 | 1039−1044 = −4 | 1065−1039 = +26 | 997−1065 = −98 | 901−997 = −96 | 810−901 = −91 |
| Percentage reduction | −4.6% | −0.4% | 2.5% | −6.4% | −9.6% | −10.1% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tanislav, C.; Rosenbauer, J.; Kostev, K. The COVID-19 Pandemic Enhanced the Decade-Long Trend of the Decreasing Utilization of Antibiotics. Antibiotics 2023, 12, 927. https://doi.org/10.3390/antibiotics12050927
Tanislav C, Rosenbauer J, Kostev K. The COVID-19 Pandemic Enhanced the Decade-Long Trend of the Decreasing Utilization of Antibiotics. Antibiotics. 2023; 12(5):927. https://doi.org/10.3390/antibiotics12050927
Chicago/Turabian StyleTanislav, Christian, Josef Rosenbauer, and Karel Kostev. 2023. "The COVID-19 Pandemic Enhanced the Decade-Long Trend of the Decreasing Utilization of Antibiotics" Antibiotics 12, no. 5: 927. https://doi.org/10.3390/antibiotics12050927
APA StyleTanislav, C., Rosenbauer, J., & Kostev, K. (2023). The COVID-19 Pandemic Enhanced the Decade-Long Trend of the Decreasing Utilization of Antibiotics. Antibiotics, 12(5), 927. https://doi.org/10.3390/antibiotics12050927







