Epidemiological Impact on Use of Antibiotics in Patients Hospitalized for COVID-19: A Retrospective Cohort Study in Italy
Abstract
1. Introduction
Increased Incidence of Antibiotic Resistance in the COVID-19 Era
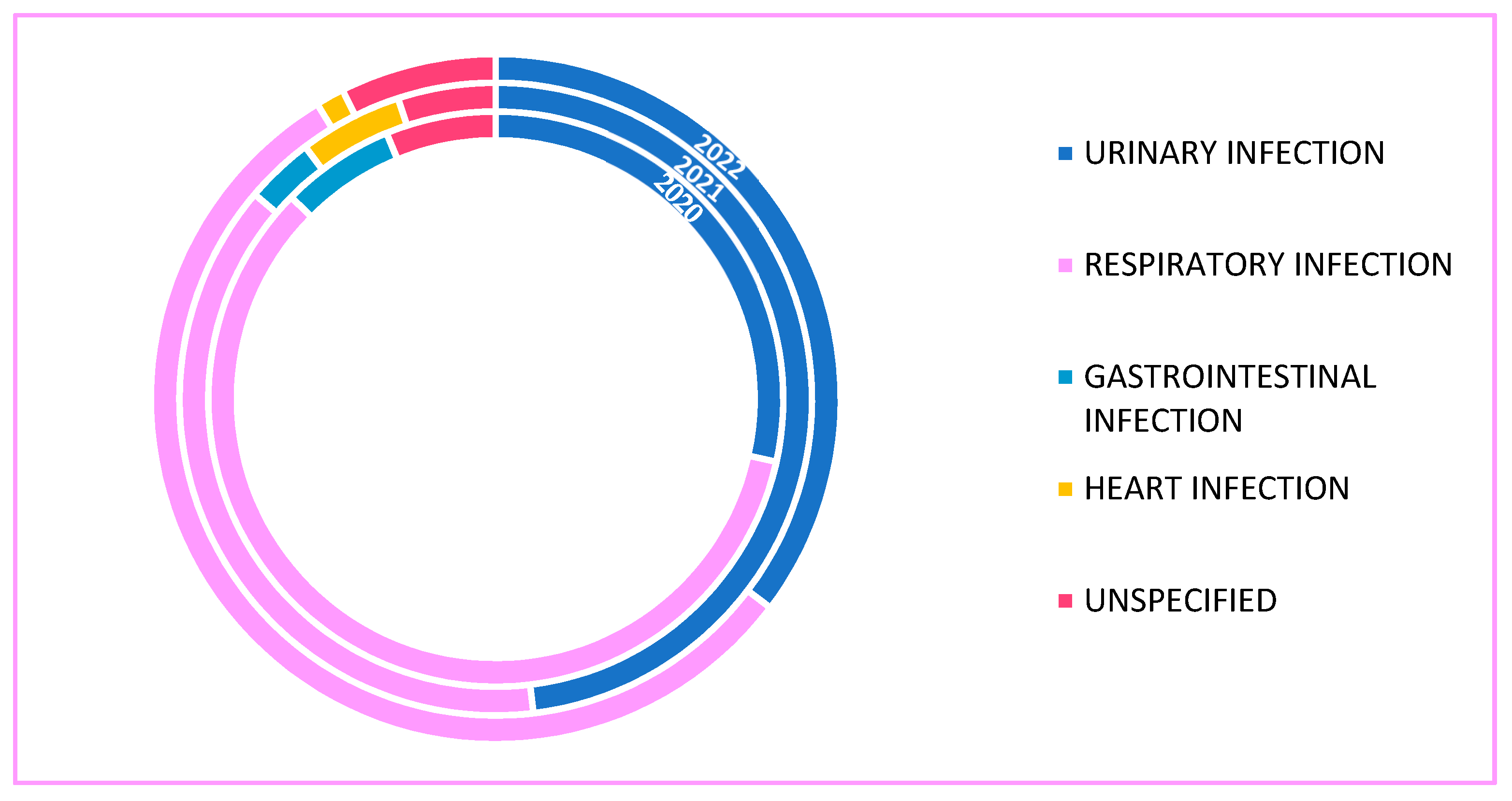
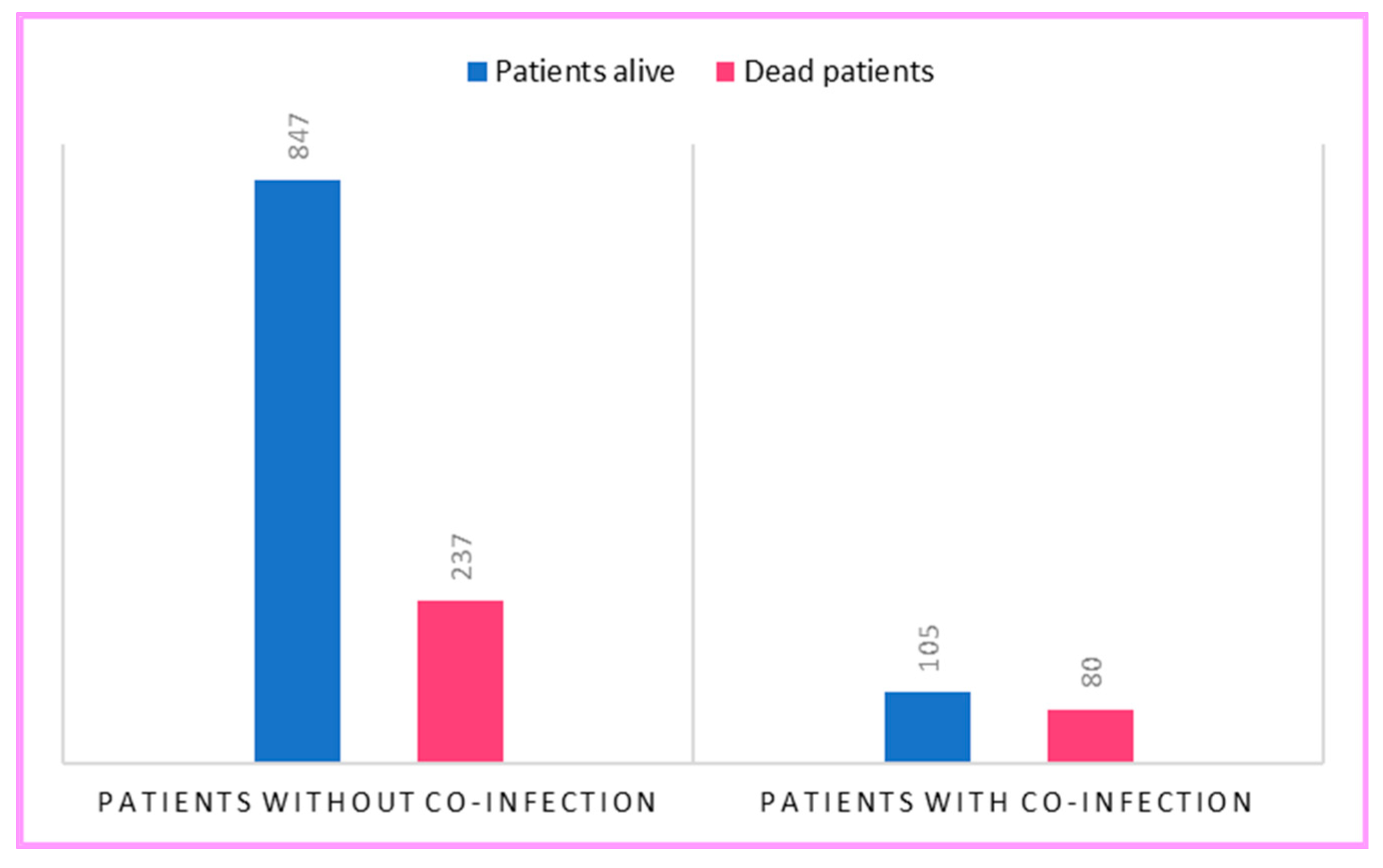
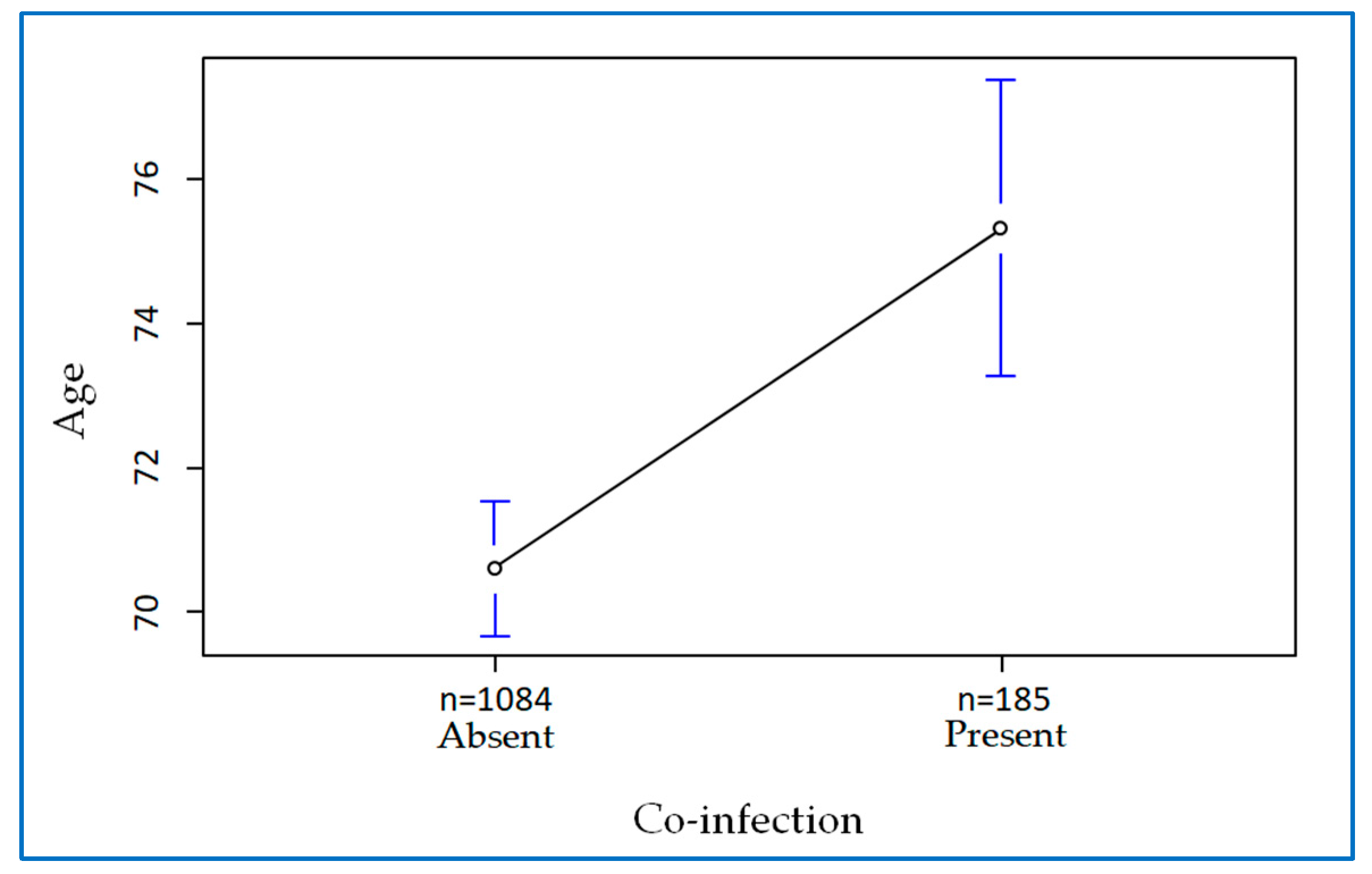
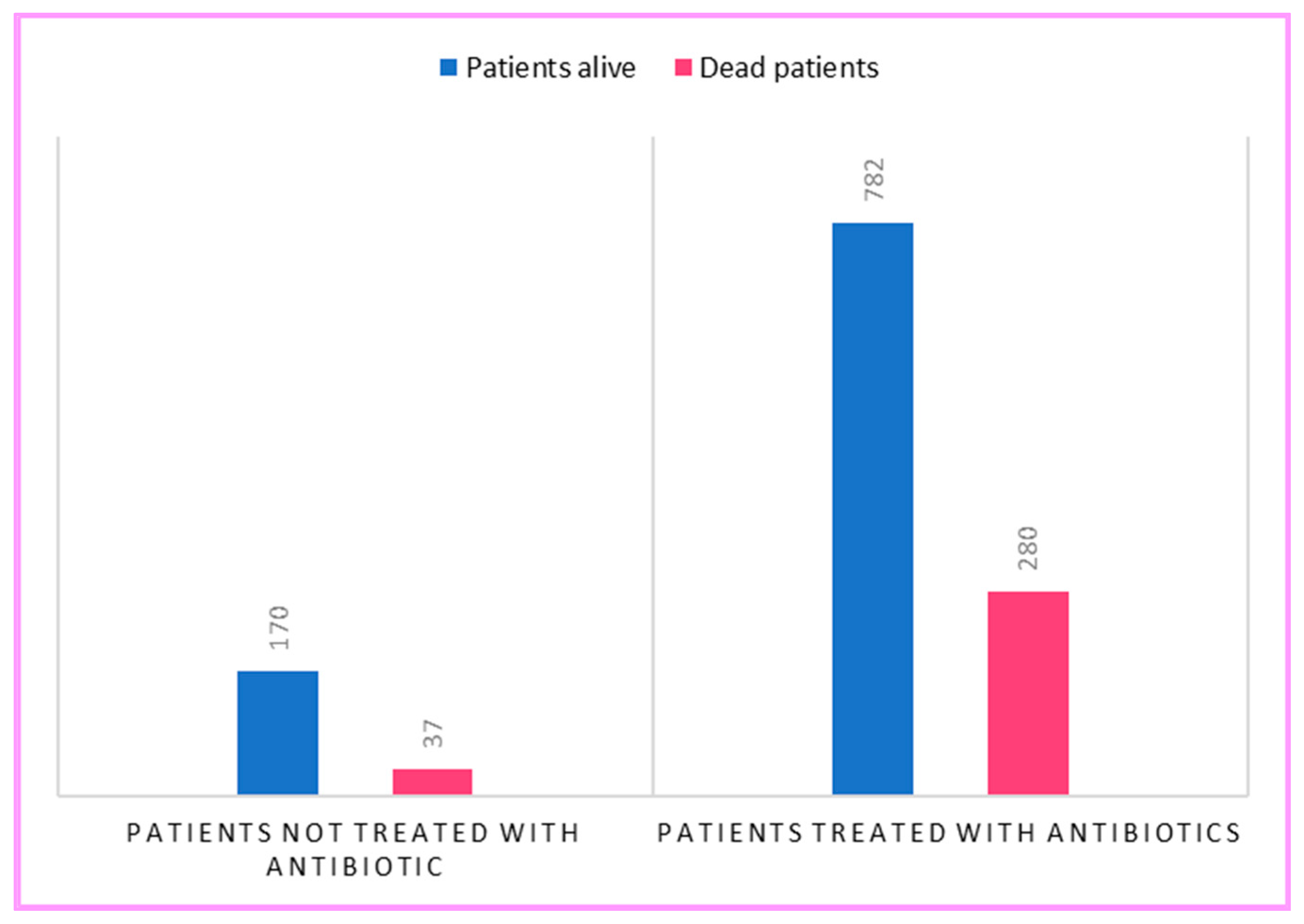
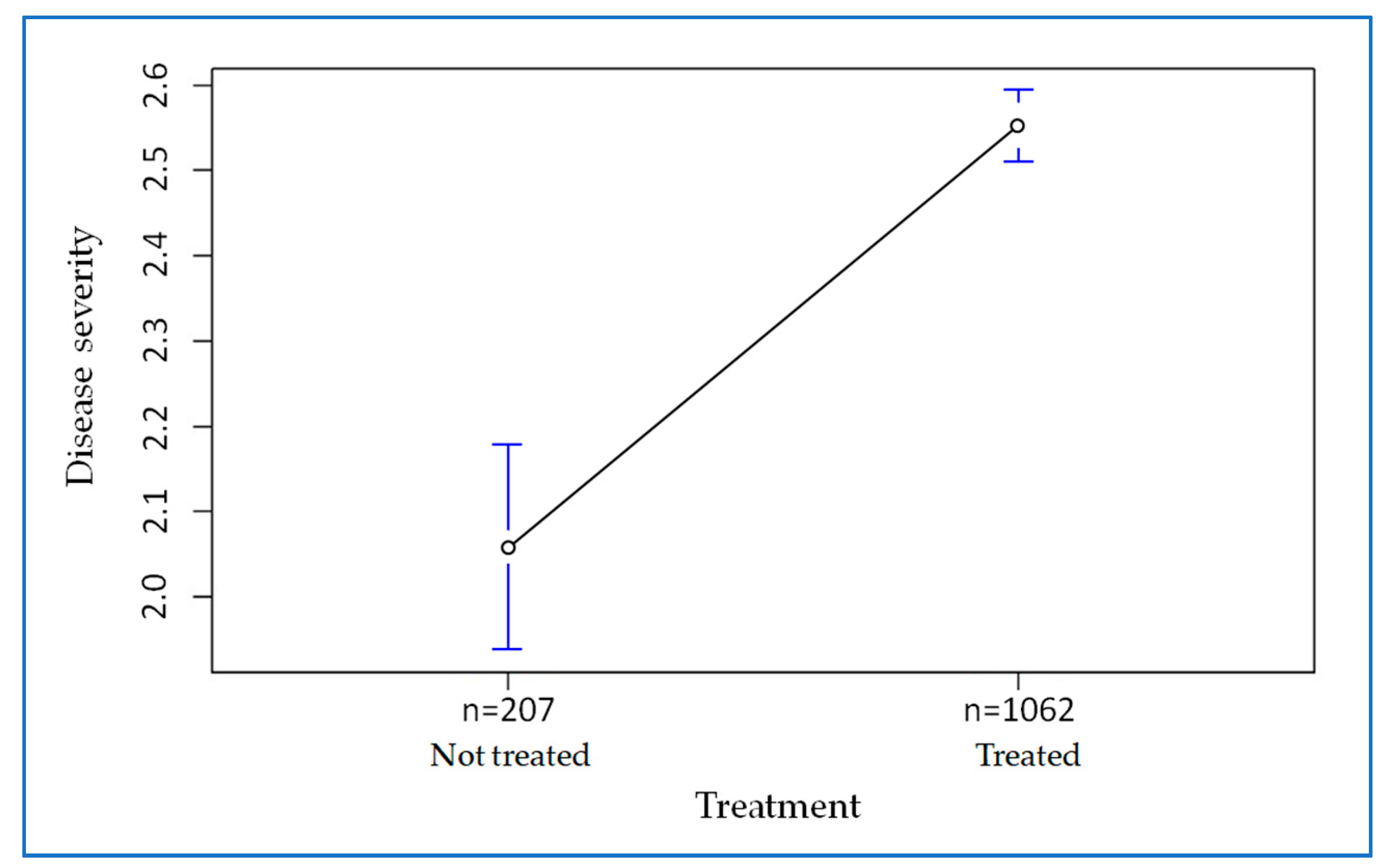
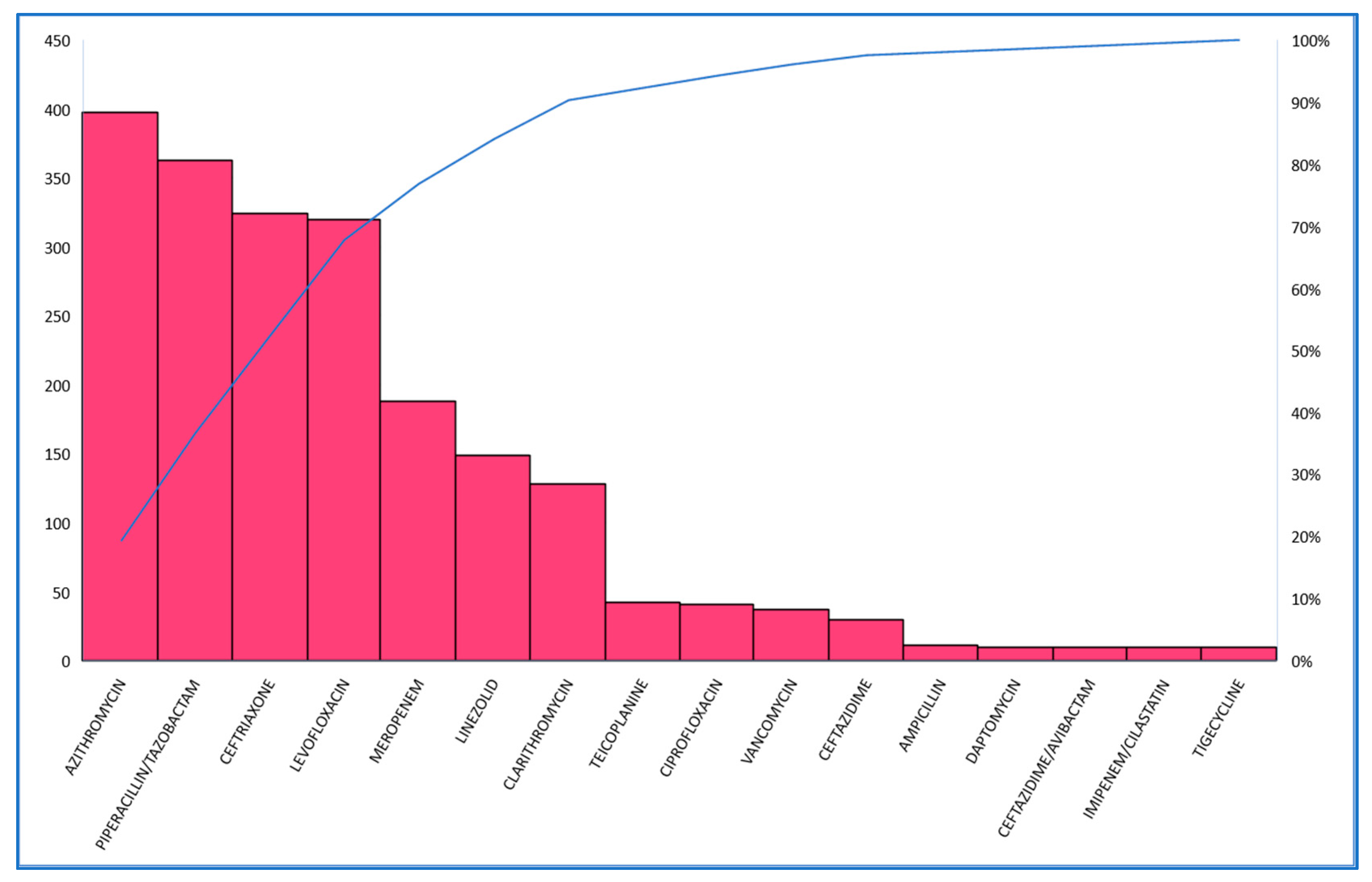
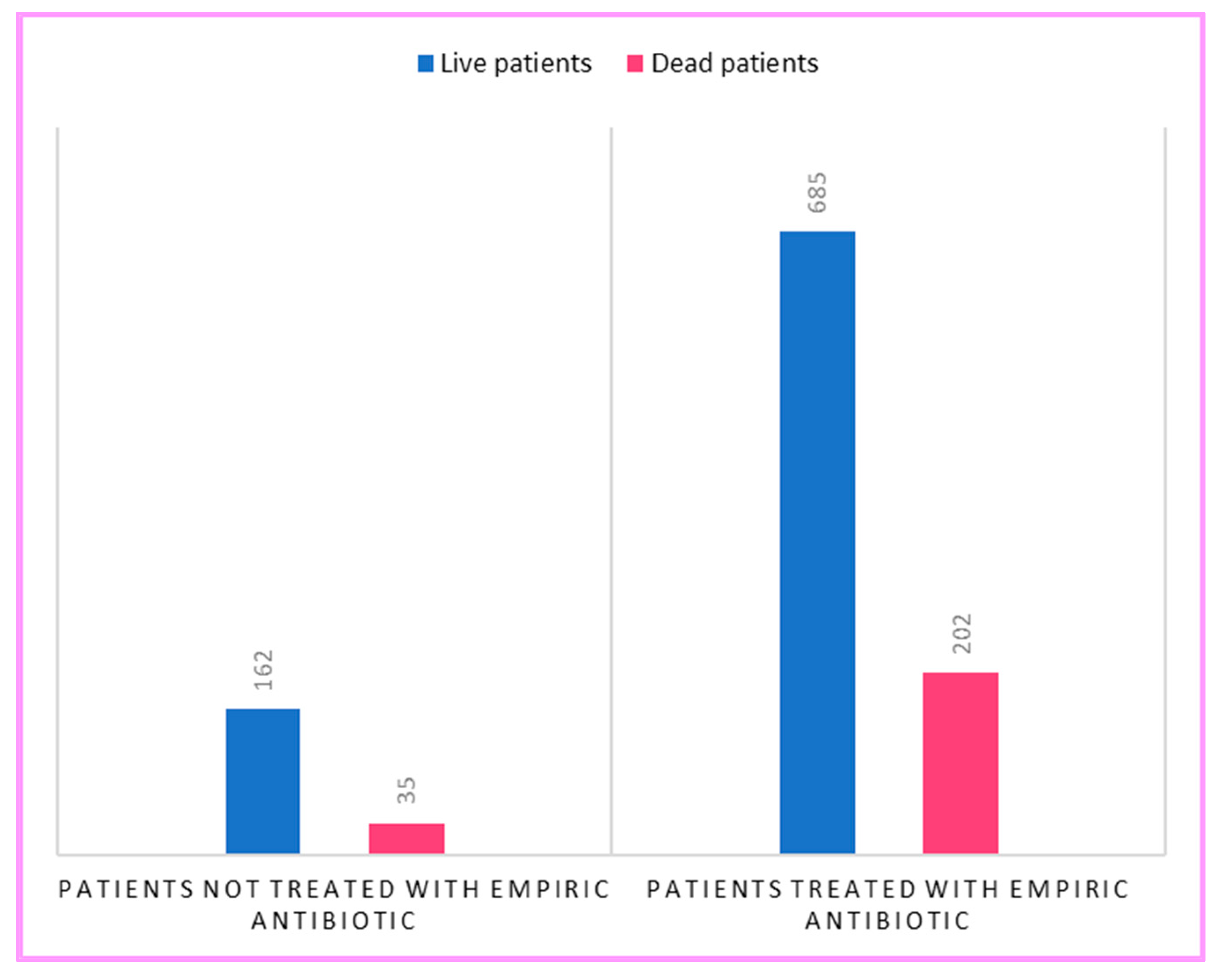
2. Results
3. Discussion
4. Materials and Methods
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wiersinga, W.J.; Rhodes, A.; Cheng, A.C.; Peacock, S.J.; Prescott, H.C. Pathophysiology, Transmission, Diagnosis, and Treatment of Coronavirus Disease 2019 (COVID-19): A Review. J. Am. Med. Assoc. 2020, 324, 782–793. [Google Scholar] [CrossRef]
- Chams, N.; Chams, S.; Badran, R.; Shams, A.; Araji, A.; Raad, M.; Mukhopadhyay, S.; Stroberg, E.; Duval, E.J.; Barton, L.M.; et al. COVID-19: A Multidisciplinary Review. Front. Public Health 2020, 8, 383. [Google Scholar] [CrossRef] [PubMed]
- Morris, D.E.; Cleary, D.W.; Clarke, S.C. Secondary bacterial infections associated with influenza pandemics. Front. Microbiol. 2017, 8, 1041. [Google Scholar] [CrossRef] [PubMed]
- Hanada, S.; Pirzadeh, M.; Carver, K.Y.; Deng, J.C. Respiratory viral infection-induced microbiome alterations and secondary bacterial pneumonia. Front. Immunol. 2018, 9, 2640. [Google Scholar] [CrossRef] [PubMed]
- Langford, B.J.; So, M.; Raybardhan, S.; Leung, V.; Westwood, D.; MacFadden, D.R.; Soucy, J.P.R.; Daneman, N. Bacterial co-infection and secondary infection in patients with COVID-19: A living rapid review and meta-analysis. Clin. Microbiol. Infect. 2020, 26, 1622–1629. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Vidal, C.; Sanjuan, G.; Moreno-García, E.; Puerta-Alcalde, P.; Garcia-Pouton, N.; Chumbita, M.; Fernandez-Pittol, M.; Pitart, C.; Inciarte, A.; Bodro, M.; et al. Incidence of co-infections and superinfections in hospitalized patients with COVID-19: A retrospective cohort study. Clin. Microbiol. Infect. 2021, 27, 83–88. [Google Scholar] [CrossRef] [PubMed]
- Karami, Z.; Knoop, B.T.; Dofferhoff, A.S.M.; Blaauw, M.J.T.; Janssen, N.A.; van Apeldoorn, M.; Kerckhoffs, A.P.M.; van de Maat, J.S.; Hoogerwerf, J.J.; Ten Oever, J. Few bacterial co-infections but frequent empiric antibiotic use in the early phase of hospitalized patients with COVID-19: Results from a multicentre retrospective cohort study in The Netherlands. Infect. Dis. 2021, 53, 102–110. [Google Scholar] [CrossRef]
- Westblade, L.F.; Simon, M.S.; Satlin, M.J. Bacterial Coinfections in Coronavirus Disease 2019. Trends Microbiol. 2021, 29, 930–941. [Google Scholar] [CrossRef]
- Lansbury, L.; Lim, B.; Baskaran, V.; Lim, W.S. Co-Infections in People with COVID-19: A Systematic Review and Meta-Analysis. SSRN Electron. J. 2020. [Google Scholar] [CrossRef]
- WHO. Clinical Management of Severe Acute Respiratory Infection (SARI) When COVID-19 Disease Is Suspected. Interim Guidance 13 March 2020. Available online: https://apps.who.int/iris/handle/10665/331446 (accessed on 10 March 2023).
- Langford, B.J.; So, M.; Raybardhan, S.; Leung, V.; Soucy, J.P.R.; Westwood, D.; Daneman, N.; MacFadden, D.R. Antibiotic prescribing in patients with COVID-19: Rapid review and meta-analysis. Clin. Microbiol. Infect. 2021, 27, 520–531. [Google Scholar] [CrossRef]
- Lai, C.C.; Chen, S.Y.; Ko, W.C.; Hsueh, P.R. Increased antimicrobial resistance during the COVID-19 pandemic. Int. J. Antimicrob. Agents 2021, 57, 106324. [Google Scholar] [CrossRef]
- Pinte, L.; Ceasovschih, A.; Niculae, C.M.; Stoichitoiu, L.E.; Ionescu, R.A.; Balea, M.I.; Cernat, R.C.; Vlad, N.; Padureanu, V.; Purcarea, A.; et al. Antibiotic Prescription and InHospital Mortality in COVID-19: A Prospective Multicentre Cohort Study. J. Pers. Med. 2022, 12, 877. [Google Scholar] [CrossRef] [PubMed]
- Lingas, E.C. Empiric Antibiotics in COVID 19: A Narrative Review. Cureus 2022, 14, 2–7. [Google Scholar] [CrossRef] [PubMed]
- Rezasoltani, S.; Yadegar, A.; Hatami, B.; Asadzadeh Aghdaei, H.; Zali, M.R. Antimicrobial Resistance as a Hidden Menace Lurking behind the COVID-19 Outbreak: The Global Impacts of Too Much Hygiene on AMR. Front. Microbiol. 2020, 11, 590683. [Google Scholar] [CrossRef] [PubMed]
- Zaman, S.B.; Hussain, M.A.; Nye, R.; Mehta, V.; Mamun, K.T.; Hossain, N. A Review on Antibiotic Resistance: Alarm Bells are Ringing. Cureus 2017, 9, e1403. [Google Scholar] [CrossRef]
- Teich, V.D.; Klajner, S.; Almeida, F.A.S.D.; Dantas, A.C.B.; Laselva, C.R.; Torritesi, M.G.; Canero, T.R.; Berwanger, O.; Rizzo, L.V.; Reis, E.P.; et al. Epidemiologic and clinical features of patients with COVID-19 in Brazil. Einstein 2020, 18, eAO6022. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Hu, B.; Hu, C.; Zhu, F.; Liu, X.; Zhang, J.; Wang, B.; Xiang, H.; Cheng, Z.; Xiong, Y.; et al. Clinical Characteristics of 138 Hospitalized Patients with 2019 Novel Coronavirus-Infected Pneumonia in Wuhan, China. JAMA 2020, 323, 1061–1069. [Google Scholar] [CrossRef]
- Rawson, T.M.; Moore, L.S.; Zhu, N.; Ranganathan, N.; Skolimowska, K.; Gilchrist, M.; Satta, G.; Cooke, G.; Holmes, A. Bacterial and fungal co-infection in individuals with coronavirus: A rapid review to support COVID-19 antimicrobial prescribing Timothy. Clin. Infect. Dis. 2020, 71, 2459–2468. [Google Scholar]
- Tiri, B.; Sensi, E.; Marsiliani, V.; Cantarini, M.; Priante, G.; Vernelli, C.; Martella, L.A.; Costantini, M.; Mariottini, A.; Andreani, P.; et al. Antimicrobial stewardship program, COVID-19, and infection control: Spread of carbapenem-resistant klebsiella pneumoniae colonization in ICU COVID-19 patients. What did not work? J. Clin. Med. 2020, 9, 2744. [Google Scholar] [CrossRef]
- Karnoukh, K.I.; Drozdov, V.N.; Shikh, E.V.; Zhilina, S.V.; Lazareva, N.B. Etiology and Antimicrobial Resistance of Secondary Bacterial Infections in Patients Hospitalized with COVID-19: A Retrospective Analysis. Vestn. Ross. Akad. Meditsinskikh Nauk. 2022, 77, 25–32. [Google Scholar] [CrossRef]
- Contou, D.; Claudinon, A.; Pajot, O.; Micaëlo, M.; Longuet Flandre, P.; Dubert, M.; Cally, R.; Logre, E.; Fraissé, M.; Mentec, H.; et al. Bacterial and viral co-infections in patients with severe SARS-CoV-2 pneumonia admitted to a French ICU. Ann. Intensive Care 2020, 10, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Global Antimicrobial Resistance and Use Surveillance System (GLASS) Report 2022. GENEVA: World Health Organization. 2022. Available online: https://www.who.int/publications/i/item/9789240062702 (accessed on 15 March 2023).
- Giannella, M.; Rinaldi, M.; Tesini, G.; Gallo, M.; Cipriani, V.; Vatamanu, O.; Campoli, C.; Toschi, A.; Ferraro, G.; Horna, C.S.; et al. Predictive model for bacterial co-infection in patients hospitalized for COVID-19: A multicenter observational cohort study. Infection 2022, 50, 1243–1253. [Google Scholar] [CrossRef] [PubMed]
- Casqueiro, J.; Casqueiro, J.; Alves, C. Infections in patients with diabetes mellitus: A review of pathogenesis. Indian J. Endocrinol. Metab. 2012, 16 (Suppl. S1), S27–S36. [Google Scholar] [CrossRef] [PubMed]
- Nasir, N.; Rehman, F.; Omair, S.F. Risk factors for bacterial infections in patients with moderate to severe COVID-19: A case-control study. J. Med. Virol. 2021, 93, 4564–4569. [Google Scholar] [CrossRef] [PubMed]
- Olry de Labry-Lima, A.; Bermúdez-Tamayo, C.; Martinez-Olmos, J.; Martin-Ruiz, E. The use of masks to protect against respiratory infections: An umbrella review. Enferm. Infecc. Microbiol. Clin. 2021, 39, 436–444. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.Y.; Chang, P.H.; Chen, K.Y.; Lin, I.F.; Hsih, W.H.; Tsai, W.L.; Chen, J.A.; Lee, S.S.; GREAT working group. Coronavirus disease 2019 (COVID-19) associated bacterial coinfection: Incidence, diagnosis and treatment. J. Microbiol. Immunol. Infect. 2022, 55, 985–992. [Google Scholar] [CrossRef] [PubMed]
- Giacomelli, A.; Ridolfo, A.L.; Oreni, L.; Vimercati, S.; Albrecht, M.; Cattaneo, D.; Rimoldi, S.G.; Rizzardini, G.; Galli, M.; Antinori, S. Consumption of antibiotics at an Italian university hospital during the early months of the COVID-19 pandemic: Were all antibiotic prescriptions appropriate? Pharmacol. Res. 2021, 164, 105403. [Google Scholar] [CrossRef]
- RECOVERY Collaborative Group. Azithromycin in patients admitted to hospital with COVID-19 (RECOVERY): A randomised, controlled, open-label, platform trial. Lancet 2021, 397, 605–612. [Google Scholar] [CrossRef] [PubMed]
- Cong, W.; Poudel, A.N.; Alhusein, N.; Wang, H.; Yao, G.; Lambert, H. Antimicrobial Use in COVID-19 Patients in the First Phase of the SARS-CoV-2 Pandemic: A Scoping Review. Antibiotics 2021, 10, 745. [Google Scholar] [CrossRef]
- Perrella, A.; Fortinguerra, F.; Pierantozzi, A.; Capoluongo, N.; Carannante, N.; Lo Vecchio, A.; Bernardi, F.F.; Trotta, F.; Cangini, A. Hospital Antibiotic Use during COVID-19 Pandemic in Italy. Antibiotics 2023, 12, 168. [Google Scholar] [CrossRef]
- Sakeena, M.H.F.; Bennett, A.A.; McLachlan, A.J. Enhancing pharmacists’ role in developing countries to overcome the challenge of antimicrobial resistance: A narrative review. Antimicrob. Resist. Infect. Control 2018, 7, 63. [Google Scholar] [CrossRef] [PubMed]
- Fortinguerra, F.; Ambrosino, F.; Pierantozzi, A.; Da Cas, R.; Trotta, F.; Cangini, A. The use of antibiotics in Italy. The national OsMed report 2019. Recenti. Prog. Med. 2021, 112, 186–190. [Google Scholar] [CrossRef] [PubMed]
- Gunjan Vidic, J.; Manzano, M.; Raj, V.S.; Pandey, R.P.; Chang, C.M. Comparative meta-analysis of antimicrobial resistance from different food sources along with one health approach in Italy and Thailand. One Health 2022, 16, 100477. [Google Scholar] [CrossRef] [PubMed]
- Napolitano, F.; Izzo, M.T.; Di Giuseppe, G.; Angelillo, I.F. Public knowledge, attitudes, and experience regarding the use of antibiotics in Italy. PLoS ONE 2013, 8, e84177. [Google Scholar] [CrossRef] [PubMed]
- Buetti, N.; Mazzuchelli, T.; Priore, E.L.; Balmelli, C.; Llamas, M.; Pallanza, M.; Elzi, L.; Consonni, V.; Trimboli, P.; Forni-Ogna, V.; et al. Early administered antibiotics do not impact mortality in critically ill patients with COVID-19. J. Infect. 2020, 81, e148–e149. [Google Scholar] [CrossRef] [PubMed]
- Petteys, M.M.; Medaris, L.A.; Williamson, J.E.; Soman, R.S.; Denmeade, T.A.; Anderson, W.E.; Leonard, M.K.; Polk, C.M. Outcomes and antibiotic use in patients with coronavirus disease 2019 (COVID-19) admitted to an intensive care unit. Antimicrob. Steward. Healthc. Epidemiol. 2022, 2, e12. [Google Scholar] [CrossRef] [PubMed]
- Galang-De Leon, W.A.M.; Buensalido, J.A.L. Prevalence of Empiric Antibacterial Therapy, Community-Acquired Bacterial Superinfection, and Antibiotic-Associated Adverse Reactions among Patients with COVID-19 Pneumonia Admitted in Makati Medical Center from March 2020 to March 2021. Infect. Chemother. 2022, 54, 266–274. [Google Scholar] [CrossRef]
- Park, D.H.; Lee, C.M.; Chang, E.; Kang, C.K.; Park, W.B.; Kim, N.J.; Choe, P.G.; Oh, M.D. Clinical Impact of Empirical Antibiotic Therapy in Patients with Coronavirus Disease 2019 Requiring Oxygen Therapy. J. Korean Med. Sci. 2022, 37, e238. [Google Scholar] [CrossRef]
- Micoli, F.; Bagnoli, F.; Rappuoli, R.; Serruto, D. The role of vaccines in combatting antimicrobial resistance. Nat. Rev. Microbiol. 2021, 19, 287–302. [Google Scholar] [CrossRef]

| February 2020–December 2020 | January 2021–December 2021 | January 2022–December 2022 | |
|---|---|---|---|
| Total (%) | 421 (100) | 475 (100) | 373 (100) |
| Average age (SD) | 70.7 (14.4) | 68 (14.7) | 76 (16) |
| Males (%) | 244 (58) | 297 (62.5) | 171 (45.8) |
| Females (%) | 117 (42) | 178 (37.4) | 202 (54.2) |
| Asthma (%) | 3 (0.7) | 2 (0.4) | 6 (1.6) |
| COPD (%) | 20 (4.7) | 11 (2.3) | 9 (2.4) |
| Obesity (%) | 22 (5.2) | 34 (7.1) | 22 (5.9) |
| Diabetes (%) | 60 (14.3) | 91 (19.1) | 56 (15) |
| Renal failure (%) | 6 (1.4) | 8 (1.7) | 44 (11.8) |
| Cancer pathology (%) | 10 (2.4) | 7 (1.5) | 34 (9.1) |
| Hypertension (%) | 63 (15) | 81 (17) | 29 (7.7) |
| Atrial fibrillation (%) | 25 (6) | 37 (7.8) | 53 (14.2) |
| Ischemic heart disease (%) | 19 (4.5) | 25 (5.3) | 37 (9.9) |
| Heart failure (%) | 31 (7.4) | 24 (5) | 15 (4) |
| Hospitalization for COVID-19 symptomatic (%) | 28 (6.7) | 34 (7.2) | 132 (35.4) |
| Hospitalization for COVID-19 pneumonia (%) | 113 (26.8) | 123 (25.8) | 47 (12.6) |
| Hospitalization for ARDS by COVID-19 (%) | 280 (66.5) | 318 (67) | 194 (52) |
| Average days of hospitalization (SD) | 13.2 (11.4) | 13.1 (12.5) | 15.2 (14) |
| Deaths (%) | 132 (31.3) | 106 (22.3) | 79 (21.2) |
| β | Standard Error | z | p | |
|---|---|---|---|---|
| Age | 0.083 | 0.007 | 11.582 | 0.000 |
| Comorbidity | 0.606 | 0.158 | 3.828 | 0.000 |
| Co-infection | 1.221 | 0.194 | 6.281 | 0.000 |
| Severity of COVID-19 | 0.783 | 0.112 | 6.569 | 0.000 |
| Admission department | 0.930 | 0.170 | 5.466 | 0.000 |
| β | Standard Error | z | p | |
|---|---|---|---|---|
| Age | 0.062 | 0.006 | 10.144 | 0.001 |
| Comorbidity | 0.645 | 0.152 | 4.221 | 0.001 |
| Co-infection | 0.850 | 0.181 | 4.698 | 0.001 |
| Antibiotic use | 0.493 | 0.210 | 2.343 | 0.019 |
| β | Standard Error | z | p | |
|---|---|---|---|---|
| Age | 0.071 | 0.006 | 10.664 | 0.001 |
| Comorbidity | 0.597 | 0.157 | 3.804 | 0.001 |
| Co-infection | 0.620 | 0.188 | 3.286 | 0.001 |
| Azithromycin | 0.121 | 0.162 | 0.746 | 0.455 |
| Piperacillin/Tazobactam | 0.556 | 0.158 | 3.503 | 0.001 |
| Ceftriaxone | −0.046 | 0.137 | −0.339 | 0.734 |
| Levofloxacin | −0.201 | 0.173 | −1.160 | 0.246 |
| Meropenem | 0.766 | 0.211 | 3.627 | 0.001 |
| Linezolid | 0.903 | 0.237 | 3.814 | 0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maraia, Z.; Mazzoni, T.; Turtora, M.P.; Tempera, A.; Spinosi, M.; Vagnoni, A.; Mazzoni, I. Epidemiological Impact on Use of Antibiotics in Patients Hospitalized for COVID-19: A Retrospective Cohort Study in Italy. Antibiotics 2023, 12, 912. https://doi.org/10.3390/antibiotics12050912
Maraia Z, Mazzoni T, Turtora MP, Tempera A, Spinosi M, Vagnoni A, Mazzoni I. Epidemiological Impact on Use of Antibiotics in Patients Hospitalized for COVID-19: A Retrospective Cohort Study in Italy. Antibiotics. 2023; 12(5):912. https://doi.org/10.3390/antibiotics12050912
Chicago/Turabian StyleMaraia, Zaira, Tony Mazzoni, Miriana Pia Turtora, Alessandra Tempera, Marco Spinosi, Anita Vagnoni, and Isidoro Mazzoni. 2023. "Epidemiological Impact on Use of Antibiotics in Patients Hospitalized for COVID-19: A Retrospective Cohort Study in Italy" Antibiotics 12, no. 5: 912. https://doi.org/10.3390/antibiotics12050912
APA StyleMaraia, Z., Mazzoni, T., Turtora, M. P., Tempera, A., Spinosi, M., Vagnoni, A., & Mazzoni, I. (2023). Epidemiological Impact on Use of Antibiotics in Patients Hospitalized for COVID-19: A Retrospective Cohort Study in Italy. Antibiotics, 12(5), 912. https://doi.org/10.3390/antibiotics12050912






