In Vivo Evaluation of an Ivermectin and Allicin Combination Treatment for Eradicating Poultry Red Mite
Abstract
1. Introduction
2. Results and Discussion
3. Materials and Methods
3.1. Samples
3.2. In Vitro PRM-Eradication Effect
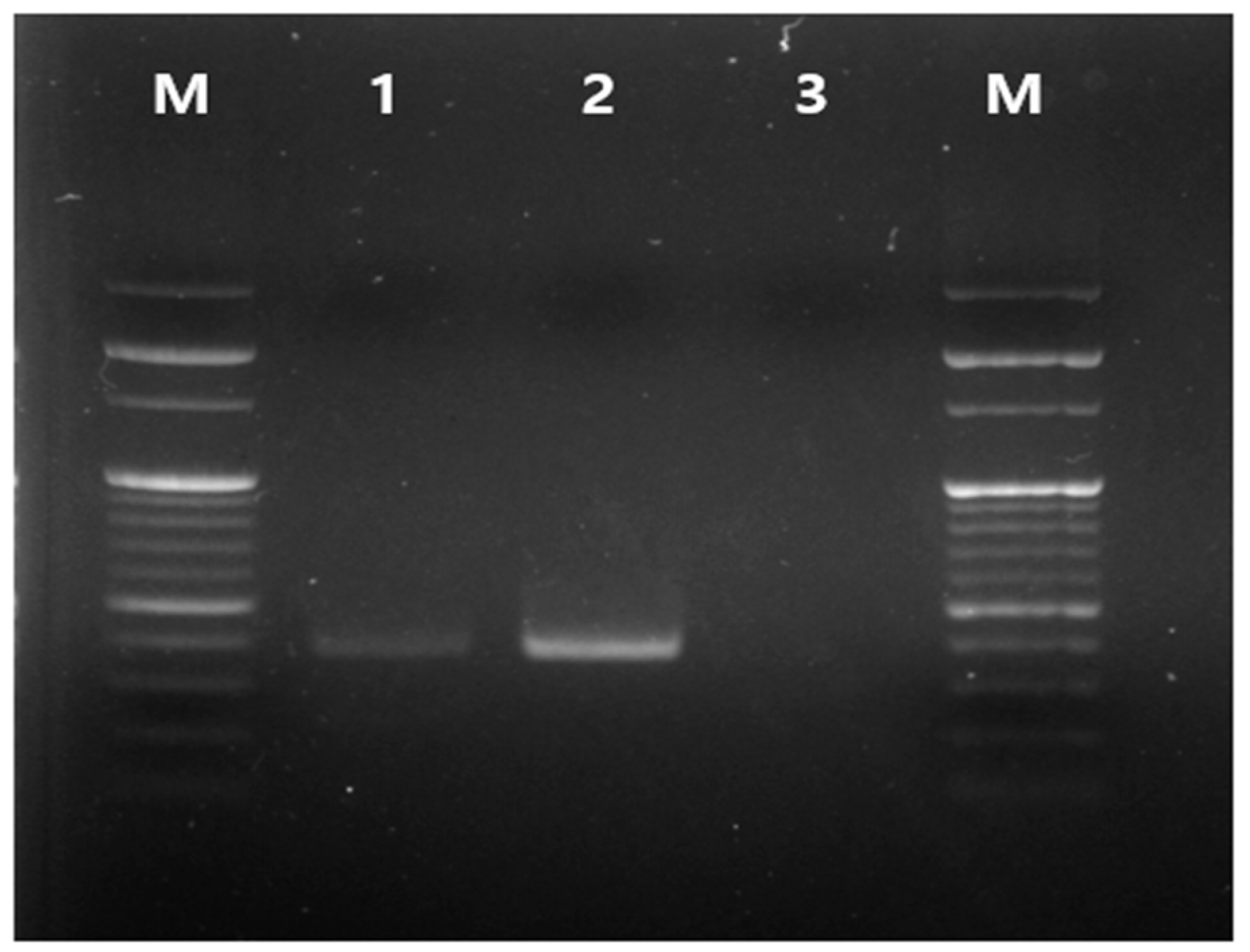
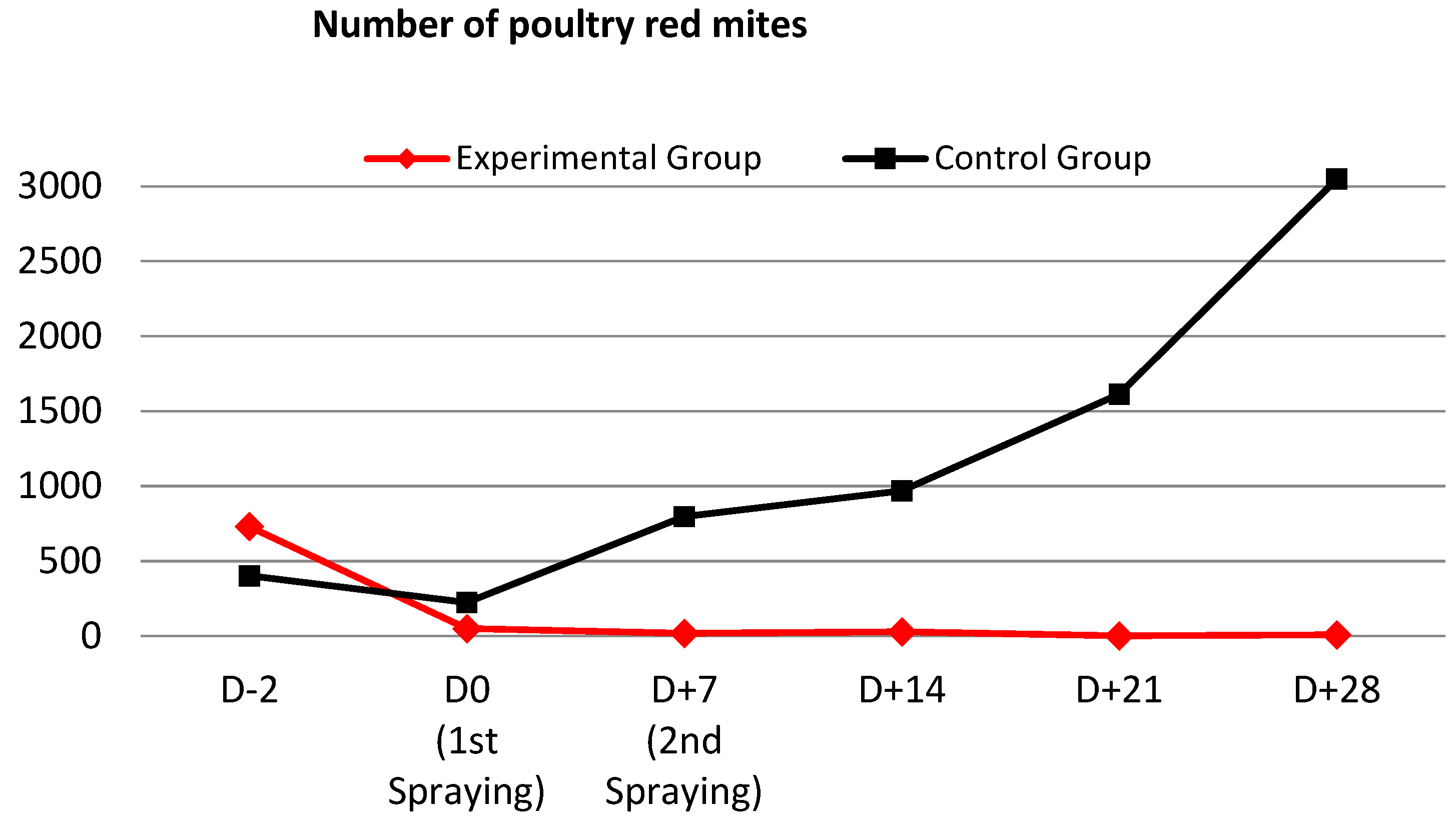
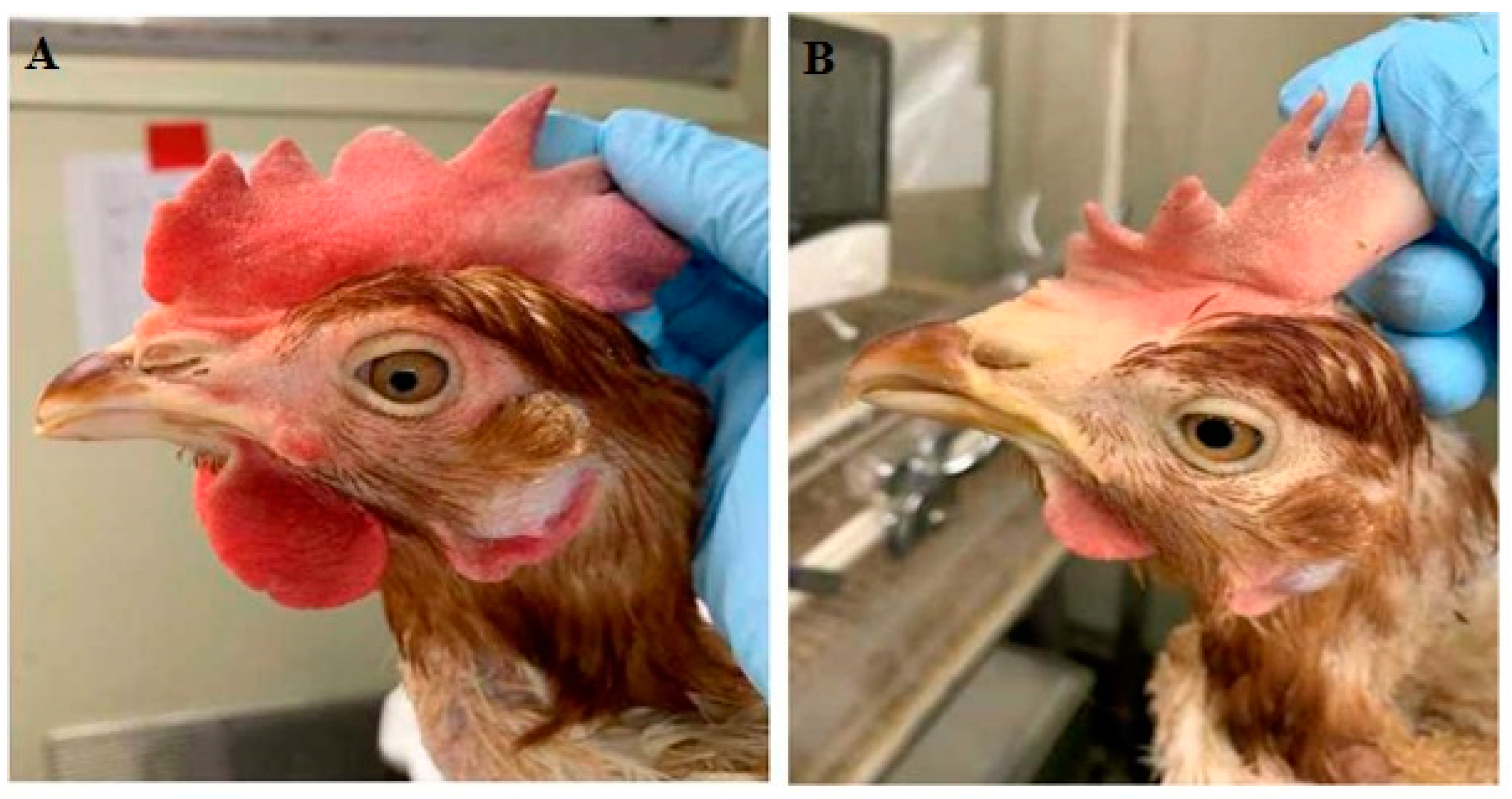
3.3. In Vivo PRM-Eradication Effect
3.4. Evaluation of Ivermectin Residue in Chickens
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Abbas, R.Z.; Colwell, D.D.; Iqbal, Z.; Khan, A. Acaricidal drug resistance in poultry red mite (Dermanyssus gallinae) and approaches to its management. Worlds Poult. Sci. J. 2014, 70, 113–124. [Google Scholar] [CrossRef]
- Kirkwood, A.C. Anemia in poultry infested with red mite Dermanyssus gallinae. Vet. Rec. 1968, 80, 514–516. [Google Scholar] [CrossRef] [PubMed]
- Lancaster, J.L.; Meisch, M.V. Mites. In Arthropods in Livestock and Poultry Production, (Ellis Horwood Series in Acarology), 1st ed.; Halsted Press: Chichester, UK, 1986; pp. 289–320. [Google Scholar]
- Hearle, E. Insects and Allied Parasites Injurious to Livestock and Poultry in Canada; Farmers’ Bulletin: Ottawa, ON, Canada, 1938; p. 88. [Google Scholar]
- Abdel-Ghaffar, F.; Semmler, M.; Al-Rasheid, K.; Mehlhorn, H. In vitro efficacy of ByeMite® and Mite-Stop® on developmental stages of the red chicken mite Dermanyssus gallinae. Parasitol. Res. 2009, 105, 1469–1471. [Google Scholar] [CrossRef] [PubMed]
- Zeman, P.; Stika, V.; Skalka, B.; Bártík, M.; Dusbábck, F.; Lávicková, M. Potential role of Dermanyssus gallinae de Geer, 1778 in the circulation of the agent of pullurosis-typhus in hens. Folia Parasitol. 1982, 29, 371–374. [Google Scholar]
- Hungerford, T.G.; Hart, L. Fowl tick fever. Agric. Gazette 1937, 48, 591–592. [Google Scholar]
- Chauve, C. The poultry red mite Dermanyssus gallinae (De Geer, 1778): Current situation and future prospective for control. Vet. Parasitol. 1998, 79, 239–245. [Google Scholar] [CrossRef]
- Beugnet, F.; Chauve, C.; Gauthey, M.; Beert, L. Resistance of the red poultry mite to pyrethroids in France. Vet Rec. 1997, 140, 577–579. [Google Scholar] [CrossRef]
- Marangi, M.; Cafiero, M.A.; Capelli, G.; Camarda, A.; Sparagano, O.A.E.; Giangaspero, A. Evaluation of the poultry red mite Dermanyssus gallinae (Acari: Dermanyssidae) susceptibility to some acaricides in field populations from Italy. Exp. Appl. Acarol. 2009, 48, 11–18. [Google Scholar] [CrossRef]
- Nordenfors, H.; Hoglund, J.; Tauson, R.; Chirico, J. Effect of permethrin impregnated plastic strips on Dermanyssus gallinae in loose-housing systems for laying hens. Vet. Parasitol. 2001, 102, 121–131. [Google Scholar] [CrossRef]
- Zeman, P. Encounter the poultry red mite resistance to acaricides in Czechoslovak poultry farming. Folia Parasitol. 1987, 34, 369–373. [Google Scholar]
- European Food Safety Authority (EFSA). Occurrence of residues of fipronil and other acaricides in chicken eggs and poultry muscle/fat. EFSA J. 2018, 16, 5164. [Google Scholar] [CrossRef]
- Mozafar, F.; Tierzucht, L. Tackling red mite in laying hens remains a challenge. World Poult. Mag. 2014, 30, 22–24. [Google Scholar]
- Deeks, L.S.; Naunton, M.; Currie, M.J.; Bowden, F.J. Topical ivermection 0.5% lotion for treatment of head lice. Ann. Pharmacother. 2013, 47, 1161–1167. [Google Scholar] [CrossRef] [PubMed]
- Birrenkott, G.P.; Brockenfelt, G.E.; Greer, J.A.; Owens, M.D. Topical application of garlic reduces Northern fowl mite infestation in laying hens. Poult. Sci. 2000, 79, 1575–1577. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.J.; Rhee, D.K.; Pyo, S. Allicin, a major component of garlic, inhibits apoptosis of macrophage in a depleted nutritional state. Nutrition 2006, 22, 1177–1184. [Google Scholar] [CrossRef]
- Kang, J.W.; Chae, H.Y.; Hossain, M.A. Poultry red mite eradication potential of ivermectin and allicin combination treatment. Vet. Med. Sci. 2023, 1–5. [Google Scholar] [CrossRef]
- Hamscher, G.; Priess, B.; Harting, J.; Nogossek, M.I.; Glünder, G.; Nau, H. Determination of propoxur residues in eggs by liquid chromatography-diode array detection after treatment of stocked housing facilities for the poultry red mite (Dermanyssus gallinae). Anal. Chim. Acta 2003, 483, 19–26. [Google Scholar] [CrossRef]
- Zeman, P. Systemic efficacy of ivermectin against Dermanyssus gallinae (De Geer, 1778) in fowls. Vet. Parasitol. 1987, 23, 141–146. [Google Scholar] [CrossRef]
- Desloire, S.; Moro, C.V.; Chauve, C.; Zenner, L. Comparison of four methods of extracting DNA from D. gallinae (Acari: Dermanyssidae). Vet. Res. 2006, 37, 725–732. [Google Scholar] [CrossRef]
- Animal and Plant Agency (APQA). Guidelines for Safety and Efficacy of Veterinary Drugs. 2021. Available online: https://ebook.qia.go.kr/20211116_094500 (accessed on 12 February 2023).
- Hong, E.; Park, K.T.; Kang, B.S.; Kang, H.K.; Jeon, J.J.; Kim, H.S.; Son, J.; Kim, C.H. Acaricidal effect of mixtures of hydrated lime and ethanol on poultry red mite (Dermanyssus gallinae). Korean J. Poult. Sci. 2020, 47, 21–27. [Google Scholar] [CrossRef]
- Henderson, C.F.; Tilton, E.W. Tests with acaricides against the brown wheat mite. J. Econ. Entomol. 1955, 48, 157–161. [Google Scholar] [CrossRef]
- Committee for Medicinal Products for Veterinary Use (CVMP). Guideline on Statistical Principles for Clinical Trials for Veterinary Medicinal Products (Pharmaceuticals); EMA/CVMP/EWP/81976/2010; European Medicine Agency: London, UK, 2012. [Google Scholar]
- Animal and Plant Agency (APQA). Guidance for Residues of Veterinary Medicinal Products. 2016. Available online: https://ebook.qia.go.kr/20211116_094500 (accessed on 12 February 2023).
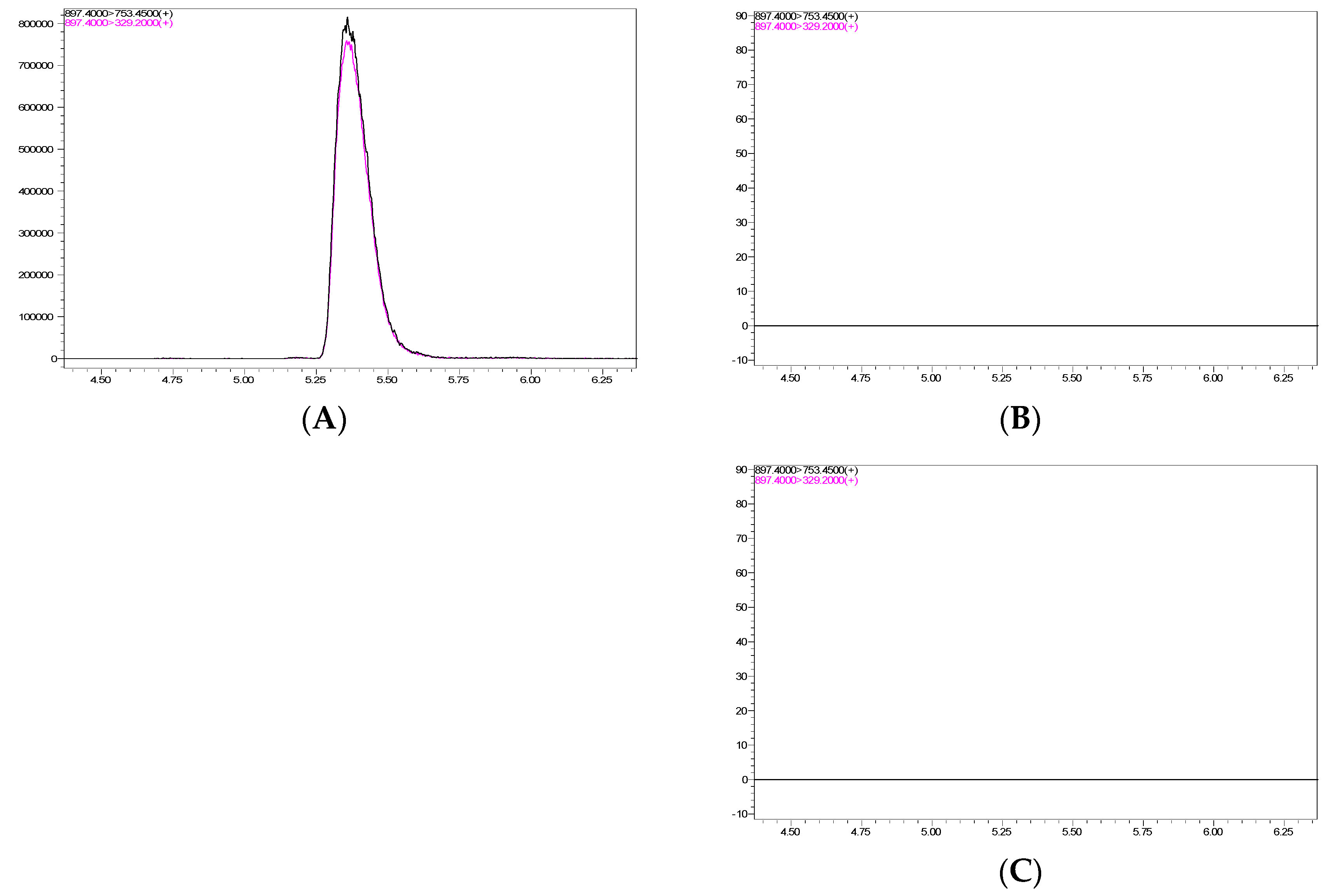


| Group | Plate No. | Number of Dead Red Mites | (a) Mortality Rate (%) | Average (n = 3) | Standard Deviation | (b) Corrected Eradication Rate (%) | |
|---|---|---|---|---|---|---|---|
| 24 h | 48 h | ||||||
| Untreated control (PEG400) | 1 | 0 | 0 | 0 | 0 | - | 0 |
| 2 | 0 | 0 | 0 | ||||
| 3 | 0 | 0 | 0 | ||||
| Clove extract | 1 | 3 | 3 | 15 | 20 | 8.7 | 20 |
| 2 | 1 | 3 | 15 | ||||
| 3 | 3 | 6 | 30 | ||||
| Cypress oil | 1 | 6 | 11 | 55 | 68.3 | 15.3 | 68.3 |
| 2 | 13 | 13 | 65 | ||||
| 3 | 16 | 17 | 85 | ||||
| Shrurry sophora extract | 1 | 7 | 13 | 65 | 40 | 27.8 | 40 |
| 2 | 9 | 9 | 45 | ||||
| 3 | 0 | 2 | 10 | ||||
| Ivermectin (1.0 mg/mL) + allicin (0.5 mg/mL) | 1 | 20 | 20 | 100 | 100.0 | - | 100.0 |
| 2 | 20 | 20 | 100 | ||||
| 3 | 20 | 20 | 100 | ||||
| Ivermectin (0.25 mg/mL) + allicin (1.0 mg/mL) | 1 | 20 | 20 | 100 | 100.0 | - | 100.0 |
| 2 | 20 | 20 | 100 | ||||
| 3 | 20 | 20 | 100 | ||||
| Positive control (pyrethrin) | 1 | 20 | 20 | 100 | 100.0 | - | 100.0 |
| 2 | 20 | 20 | 100 | ||||
| 3 | 20 | 20 | 100 | ||||
| Class | D0 (After Initial Spraying) | D + 7 (Second Spraying) | D + 14 | D + 21 | D + 28 |
|---|---|---|---|---|---|
| Experimental group | 93.1% | 97.4% | 96.1% | 99.7% | 98.9% |
| Control group | 44.0% | −97.8% | −140.6% | −300.6% | −656.6% |
| IA efficacy | 87.6% | 98.7% | 98.4% | 99.4% | 99.9% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kang, J.; Chae, M.; Chae, H.; Kwon, Y.; Lee, J.; Hossain, M.A. In Vivo Evaluation of an Ivermectin and Allicin Combination Treatment for Eradicating Poultry Red Mite. Antibiotics 2023, 12, 876. https://doi.org/10.3390/antibiotics12050876
Kang J, Chae M, Chae H, Kwon Y, Lee J, Hossain MA. In Vivo Evaluation of an Ivermectin and Allicin Combination Treatment for Eradicating Poultry Red Mite. Antibiotics. 2023; 12(5):876. https://doi.org/10.3390/antibiotics12050876
Chicago/Turabian StyleKang, JeongWoo, MyeongJu Chae, HyunYoung Chae, YongKuk Kwon, JiYoun Lee, and Md Akil Hossain. 2023. "In Vivo Evaluation of an Ivermectin and Allicin Combination Treatment for Eradicating Poultry Red Mite" Antibiotics 12, no. 5: 876. https://doi.org/10.3390/antibiotics12050876
APA StyleKang, J., Chae, M., Chae, H., Kwon, Y., Lee, J., & Hossain, M. A. (2023). In Vivo Evaluation of an Ivermectin and Allicin Combination Treatment for Eradicating Poultry Red Mite. Antibiotics, 12(5), 876. https://doi.org/10.3390/antibiotics12050876








