Modulation of the Acute Inflammatory Response Induced by the Escherichia coli Lipopolysaccharide through the Interaction of Pentoxifylline and Florfenicol in a Rabbit Model
Abstract
1. Introduction
2. Results
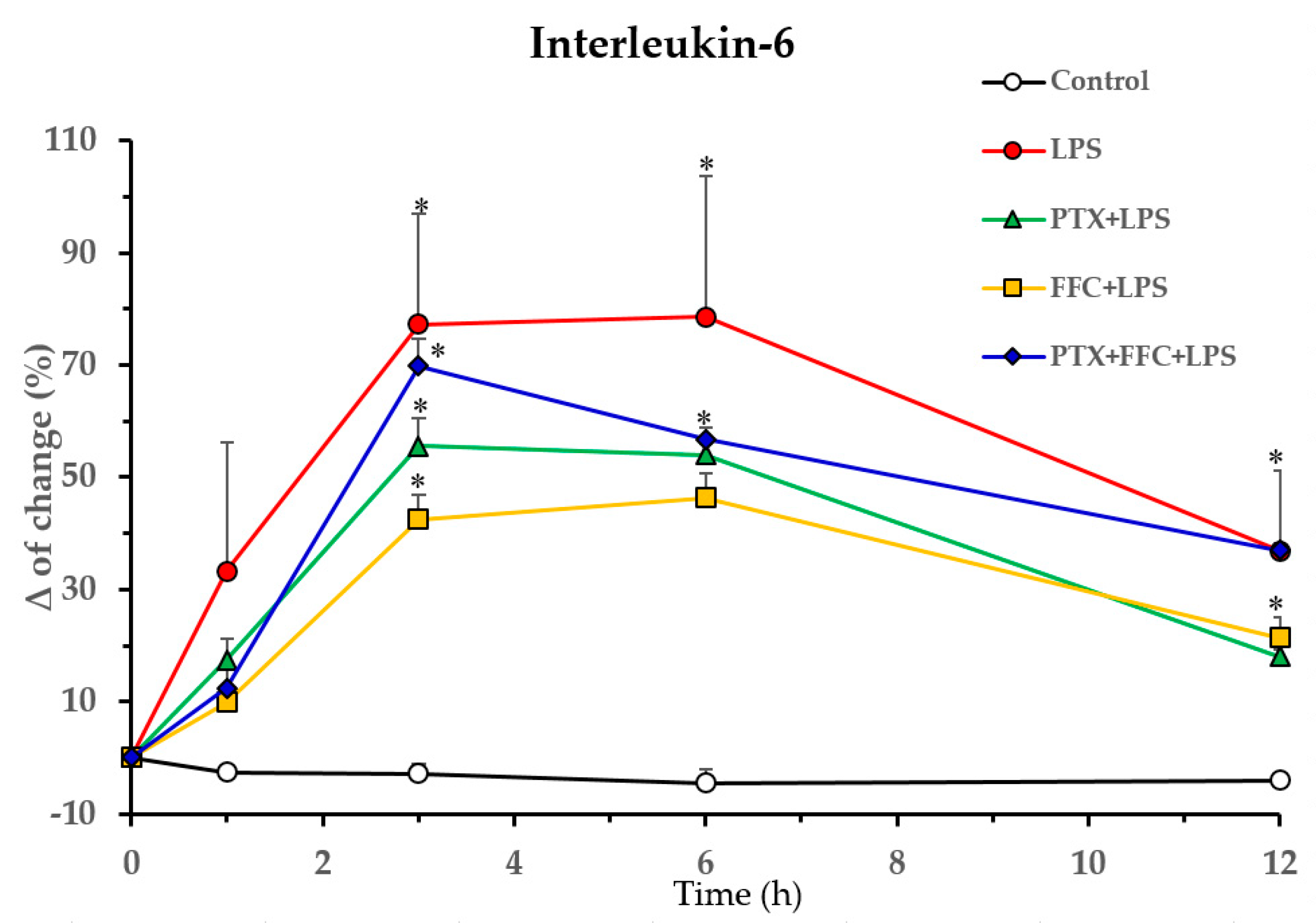
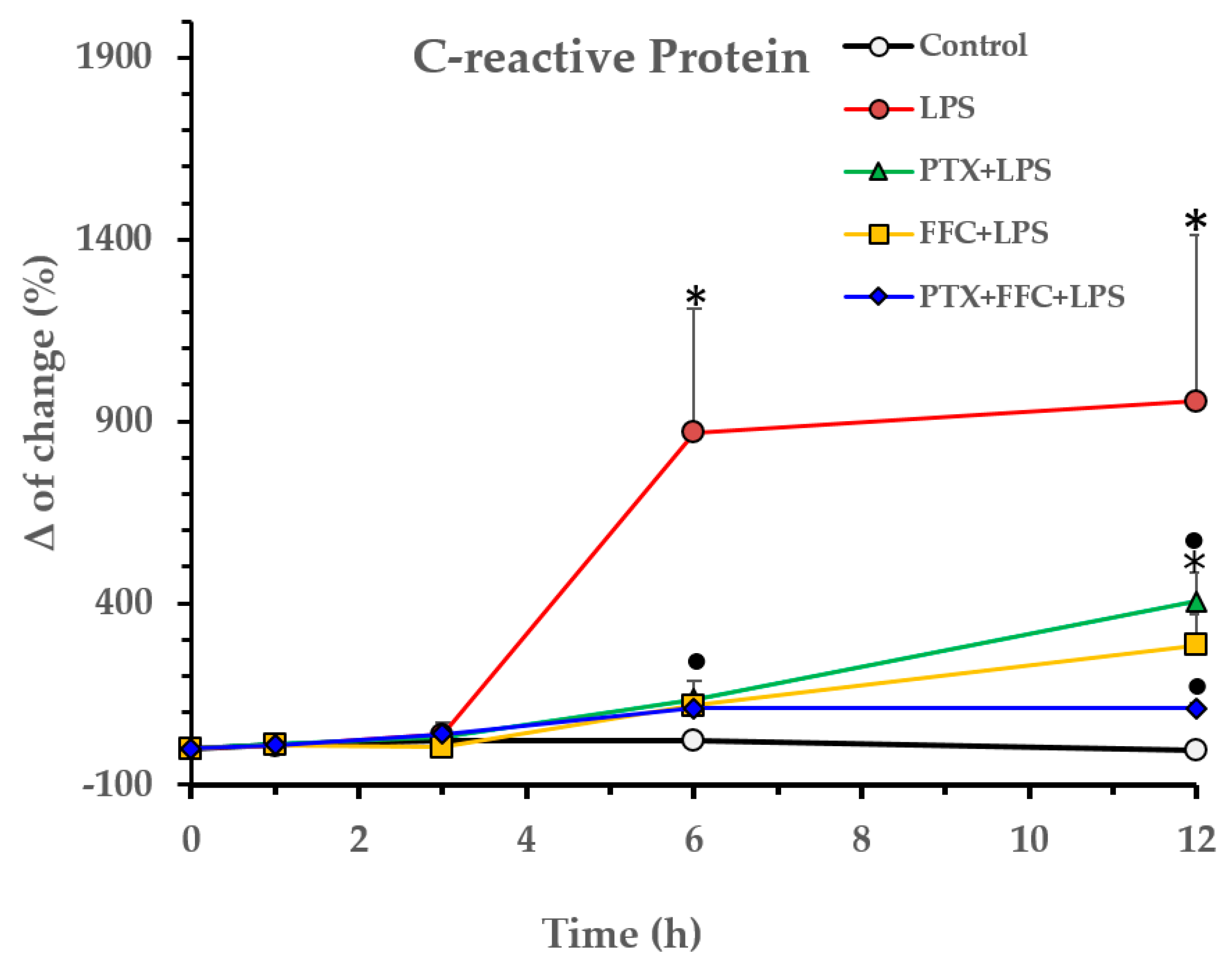
2.1. Effects of Florfenicol and Pentoxifylline Alone or Co-Administered on Cytokine Responses Induced by LPS In Vivo
2.2. Effects of Florfenicol and Pentoxifylline Alone or Co-Administered on Hematological and Clinical Biochemistry Responses Induced by LPS
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Drugs and Reagents
4.3. Experimental Design
4.4. Body Temperature Recording and Blood Samples Processing
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Guimaraes, E.T.; Barbosa dos Santos, T.; Carvalho Silva, D.K.; Meira, C.S.; Magalhães Moreira, D.R.; Da Silva, T.F.; Salmon, D.; Barreiro, E.J.; Botelho Pereira Soares, M. Potential immunosupresive of a phosphodiesterase-4 inhibitor N-acylhydrazone in models of lipopolysaccharide-induced shock and delayed-type hypersensitivity reaction. Int. Immunopharmacol. 2018, 65, 108–118. [Google Scholar] [CrossRef]
- Anuforom, O.; Wallace, G.R.; Piddock, L.V. The immune response and antibacterial therapy. Med. Microbiol. Immunol. 2015, 204, 151–159. [Google Scholar] [CrossRef] [PubMed]
- Dowling, P. Chloramphenicol, thiamphenicol and florfenicol. In Antmicrobial Therapy in Veterinary Medicine, 5th ed.; Giguère, S., Prescott, J.F., Dowling, P., Eds.; Wiley Blackwell: Ames, IA, USA, 2013; pp. 269–277. [Google Scholar]
- Papich, M.G. Chloramphenicol and derivatives, macrolides, lincosamides and miscellaneous antimicrobials. In Veterinary Pharmacology and Therapeutics, 10th ed.; Riviere, J.E., Papich, M.G., Eds.; John Wiley & Sons Inc.: Hoboken, NJ, USA, 2018; pp. 903–912. [Google Scholar]
- Huh, D.; Zhang, T.; Zhang, Z.; Wang, G.; Wang, F.; Qu, Y.; Niu, Y.; Liu, S. Toxicity to the hemathopoietic and lymphoid organs of piglets treated with a therapeutic dose of florfenicol. Vet. Immunol. Immunopathol. 2014, 162, 122–131. [Google Scholar] [CrossRef]
- Park, B.K.; Lim, J.-H.; Kim, M.-S.; Hwang, Y.H.; Yun, H.-I. Pharmacokinetics of florfenicol and its major metabolite, florfenicol-amine, in rabbits. J. Vet. Pharmacol. Ther. 2007, 30, 32–36. [Google Scholar] [CrossRef] [PubMed]
- Farag, V.M.; El-Shafei, R.A.; Elkenany, R.M.; Ali, H.S.; Eladl, A.H. Antimicrobial, immunological and biochemical efects of forfenicol and garlic (Allium sativum) on rabbits infected with Escherichia coli serotype O55: H7. Vet. Res. Commun. 2021, 46, 363–376. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Song, Y.; Xiong, H.; Xinxin, C.; An, N.; Junwen, F.; Junqing, C.; Deng, X. Effect of florfenicol on early cytokine responses and survival in murine endotoxemia. Int. Immunopharmacol. 2008, 8, 982–988. [Google Scholar] [CrossRef]
- Xinxin, C.; Chi, C.; Xiao, C.; Xue, X.; Yongjun, Y.; Jungqing, C.; Xuming, D. Florfenicol inhibits allergic airway inflammation in mice by p38 MAPK-mediated phosphorylation of GATA 3. Clin. Immunol. 2011, 138, 231–238. [Google Scholar] [CrossRef]
- Zhang, X.; Song, K.; Xiong, H.; Li, H.; Chu, X.; Deng, X. Protective effect of florfenicol on acute lung injury induced by lipopolysaccharide in mice. Int. Immunopharmacol. 2009, 9, 1525–1529. [Google Scholar] [CrossRef] [PubMed]
- Chin, A.C.; Morck, D.W.; Merril, J.K.; Ceri, H.; Olson, M.E.; Read, R.R.; Dick, P.; Buret, A.G. Anti-inflamatory benefits of tilmicosin in calves with Pasteurella haemolytica-infected lungs. Am. J. Vet. Res. 1998, 59, 165–171. [Google Scholar]
- Subbian, S.; Tsenova, L.; Holloway, J.; Peixoto, B.; O’Brien, P.; Dartois, V.; Khetani, V.; Zeldis, J.B.; Kaplan, G. Adjunctive phosphodiesterase-4 inhibitor therapy improves antibiotic response to pulmonary tuberculosis in a rabbit model. EBioMed 2016, 4, 104–114. [Google Scholar] [CrossRef] [PubMed]
- Adcock, K.G.; Kyle, P.B.; Deaton, J.S.; Olivier, J.H.; Hogan, S.M. Pharmacokinetics of intranasal and intratracheal pentoxifylline in rabbits. Pharmacotherapy 2007, 27, 200–206. [Google Scholar] [CrossRef] [PubMed]
- Mostafa-Hedeab, G.; Al-Kuraishy, H.; Al-Gareb, A.I.; Jeandet, P.; Saad, H.M.; Batiha, G.E.-S. A raising dawn of pentoxifylline in management of inflammatory disorders in Covid-19. Inflammopharmacology 2022, 30, 799–809. [Google Scholar] [CrossRef] [PubMed]
- Mokra, D.; Mokri, J. Phosphodierase inhibitors in acute lung injury: What are the perspectives? Int. J. Mol. Sci. 2021, 22, 1929. [Google Scholar] [CrossRef]
- Halloy, D.J.; Cambier, C.; Gustin, P.G. Efficacy of ceftiofur and flunixin in the early treatment of bronchopneumonia in weaners. Vet. Rec. 2006, 158, 291–296. [Google Scholar] [CrossRef]
- De Koster, J.D.; Tena, J.-K.; Stegmann, M.R. Treatment of bovine respiratory disease with single administration of tulathromycin and ketoprofen. Vet. Rec. 2022, 190, e834. [Google Scholar] [CrossRef] [PubMed]
- Thiry, J.; González-Martín, J.V.; Elvira, L.; Pagot, E.; Voisin, F.; Lequeux, G.; Weingarten, A.; de Haas, V. Treatment of naturally occurring bovine respiratory disease in juvenile calves with a single administration of a florfenicol plus flunixin meglumine formulation. Vet. Rec. 2014, 174, 430–436. [Google Scholar] [CrossRef]
- Achard, D.; Caruso-Vares, A.; Collin, J.-F.; McKelvie, J.; Reddick, D.; Ramage, C. Treatment of experimentally induced bovine respiratory disease in young calves with a single administration of a combination of florfenicol and meloxicam. Vet. Rec. 2018, 183, 535. [Google Scholar] [CrossRef]
- Turner, P.V.; Cheng Chen, H.; Taylor, W.M. Pharmacokinetics of meloxicam in rabbits after single and repeat oral dosing. Comp. Med. 2006, 56, 63–67. [Google Scholar]
- Curry, S.L.; Cogar, S.M.; Cook, J.L. Nonsteroidal antiinflammatory drugs: A review. J. Am. Anim. Hosp. Assoc. 2005, 41, 298–309. [Google Scholar] [CrossRef]
- Buras, J.A.; Holzmann, B.; Sitkovsky, M. Animal models of sepsis: Setting the stage. Nat. Rev. Drug Discov. 2005, 4, 854–865. [Google Scholar] [CrossRef]
- El-Sabrout, K.; Aggag, S.; Freire de Souza, B. Some recent applications of rabbit biotechnology—A review. Anim. Biotechnol. 2020, 31, 76–80. [Google Scholar] [CrossRef] [PubMed]
- Poli-de-Figueiredo, L.F.; Garrido, A.; Nakagawa, N.; Sannomiya, P. Experimental modelss of sepsis and their clinical relevance. Shock 2008, 30, 53–59. [Google Scholar] [CrossRef] [PubMed]
- Mota, C.M.D.; Madden, C.J. Neural circuits mediating circulating interleukin-1-β-evoked fever in the absence of prostaglandin E2 productin. Brain Behav. Immun. 2022, 103, 109–121. [Google Scholar] [CrossRef]
- Li, P.; Ye, J.; Zeng, S.; Yang, C. Florfenicol alleviated lipopolysaccharide-induced inflammatory responses in Ctenofaryngodon idella through inhibiting toll/NF-kB signalling pathways. Fish Shellfish Immunol. 2019, 94, 479–484. [Google Scholar] [CrossRef]
- Haas, C.E.; Nelsen, J.T. Drug-cytokine interactions. In Drug Interactions in Infectious Diseases, 2nd ed.; Piscitelli, S.C., Rodvold, K.A., Eds.; Humana Press Inc.: Tolowa, NJ, USA, 2005; pp. 431–462. [Google Scholar]
- Marques, L.J.; Zheng, L.; Poulakis, N.; Guzman, J.; Costabel, U. Pentoxifylline inhibits TNF-alpha production from human alveolar macrophages. J. Respir. Crit. Care Med. 1999, 159, 508–511. [Google Scholar] [CrossRef]
- Webster, J.D.; Vucic, D. The balance of tnf mediated pathways regulates inflammatory cell death signaling in healthy and diseased tissues. Front Cell Dev. Biol. 2020, 8, 365. [Google Scholar] [CrossRef]
- Ebersole, J.L.; Cappelli, D. Acute-phase reactants in infections and inflammatory diseases. Periodontology 2000, 40, 19–49. [Google Scholar] [CrossRef]
- Remick, D.G.; Ward, P.A. Evaluation of endotoxin models for the study of sepsis. Shock 2005, 24 (Suppl. 1), 7–11. [Google Scholar] [CrossRef]
- Aliyu, M.; Zohora, F.T.; Anka, A.U.; Ali, K.; Maleknia, S.; Saffarioun, M.; Azizi, G. Interleukin-6 cytokine: An overview of the immune regulation, immune dysregulation, and therapeutic approach. Int. Immunopharmacol. 2022, 111, 109130. [Google Scholar] [CrossRef]
- Pritts, T.; Hungness, E.; Wang, Q.; Robb, B.; Hershko, D.; Hasselgren, P.-O. Mucosal and enterocyte IL-6 production during sepsis and endotoxemia-role of transcription factors and regulation by the stress response. Am. J. Surg. 2002, 183, 372–383. [Google Scholar] [CrossRef]
- Bode, C.; Diedrich, B.; Muenster, S.; Hentschel, V.; Weisheit, C.; Rommelsheim, K.; Hoeft, A.; Meyer, R.; Boehm, O.; Knuefermann, P.; et al. Antibiotics Regulate the Immune Response in Both Presence and Absence of Lipopolysaccharide through Modulation of Toll-like Receptors, Cytokine Production and Phagocytosis in Vitro. Int. Immunopharmacol. 2014, 18, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Sauer, A.; Peukert, K.; Putensen, C.; Bode, C. Antibiotics as Immunomodulators: A Potential Pharmacologic Approach for ARDS Treatment. Eur. Respir. Rev. 2021, 30, 210093. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Xiong, H.; Li, H.; Yu, L.; Deng, X. Effects of florfenicol on LPS-induced nitric oxide and prostaglandin E2 production in RAW 364.7 macrophages. Fundam. Clin. Pharmacol. 2011, 25, 591–598. [Google Scholar] [CrossRef] [PubMed]
- Marnell, L.; Mold, C.; Du Clos, T.W. C-reactive protein: Ligands, receptors and role in inflammation. Clin. Immunol. 2005, 117, 104–111. [Google Scholar] [CrossRef] [PubMed]
- Destexhe, E.; Prinsen, M.K.; van Schöll, I.; Frieke Kuper, C.; Garcon, N.; Veenstra, S.; Segal, L. Evaluation of C-reactive protein as an inflammatory biomarker in rabbits for vaccine nonclinical safety. J. Pharmacol. Toxicol. Methods 2013, 68, 367–373. [Google Scholar] [CrossRef] [PubMed]
- Sproston, N.R.; Ashworth, J.J. Role of C-reactive protein at sites of inflammation and infection. Front. Immunol. 2018, 9, 754. [Google Scholar] [CrossRef] [PubMed]
- Roth, J. Endogenous antipyretics. Clin. Chim. Acta 2006, 371, 13–24. [Google Scholar] [CrossRef]
- Roth, J.; Blatteis, C.M. Mechanism of fever production and lysis: Lessons fron experimental LPS fever. Compr. Physiol. 2014, 4, 1563–1604. [Google Scholar]
- Romanovsky, A.A.; Blatteis, C.M. Biphasic fever: Whats triggers the second temperature rise? Am. J. Physiol. 1995, 269, R280–R286. [Google Scholar] [CrossRef]
- Roth, J.; de Souza, G.E.P. Fever induction pathways: Evidence from responses to systemic or local cytokine formation. Braz. J. Med. Biol. Res. 2001, 34, 301–314. [Google Scholar] [CrossRef]
- Blatteis, C.M. The cytokine-prostaglandin cascade in fever production: Fact or fancy? J. Therm. Biol. 2004, 29, 359–368. [Google Scholar] [CrossRef]
- Klinger, M.H.F.; Jelkmann, W. Role of the blood platelet in infection and inflammation. J. Interferon Cytok. Res. 2002, 22, 913–922. [Google Scholar] [CrossRef]
- Van Miert, A.S.; van Duin, C.T.; Verheijden, J.H.; Schotman, A.J. Staphylococcal enterotoxin B and Escherichia coli endotoxin: Comparative observations in goats on fever and associated clinical hematologic and blood biochemical changes after intravenous and intramammary administration. Am. J. Vet. Res. 1983, 44, 955–963. [Google Scholar] [PubMed]
- Deldar, A.; Naylor, J.M.; Bloom, J.C. Effects of Escherichia coli endotoxin on leukocyte and platelet counts, fibrinogen concentrations, and blood clotting in colostrum-fed and colostrun-deficient neonatal calves. Am. J. Vet. Res. 1984, 45, 670–677. [Google Scholar] [PubMed]
- Ward, D.S.; Fessler, J.F.; Bottoms, G.D.; Turek, J. Equine endotoxemia: Cardiovascular, eicosanoid, hematologic, blood chemical, and plasma enzyme alterations. Am. J. Vet. Res. 1987, 48, 1150–1156. [Google Scholar]
- Chandler, T.L.; Westhoff, T.A.; Sipka, A.S.; Overton, T.R.; Mann, S. Lipopolysaccharide challenge following intravenous amino acid infusion in postpartum dairy cows: II—Clinical and inflammatory responses. J. Dairy Sci. 2022, 105, 4611–4623. [Google Scholar] [CrossRef] [PubMed]
- Summers, C.; Rankin, S.M.; Condliffe, A.M.; Singh, N.; Peters, A.M.; Chilvers, E.R. Neutrophil kinetics in health and disease. Trends Immunol. 2010, 31, 318–324. [Google Scholar] [CrossRef] [PubMed]
- Nourshargh, S.; Alon, R. Leukocyte migration into inflamed tissues. Immunity 2014, 41, 694–707. [Google Scholar] [CrossRef] [PubMed]
- Boone, L.; Meyer, D.; Cusick, P.; Ennulat, D.; Provencher Bollinger, A.; Everds, N.; Meador, V.; Elliot, G.; Honor, D.; Bounous, D.; et al. Selection and interpretation of clinical pathology indicators of hepatic injury in preclinical studies. Vet. Clin. Pathol. 2005, 34, 182–188. [Google Scholar] [CrossRef]
- Melillo, A. Rabbit clinical pathology. J. Exot. Pet Med. 2007, 16, 135–145. [Google Scholar] [CrossRef]
- Jenkins, J.R. Rabbits diagnostic testing. J. Exot. Pet Med. 2008, 17, 4–15. [Google Scholar] [CrossRef]
- Elmas, M.; Yazar, E.; Uney, K.; Er Karabacak, A.; Traş, B. Pharmacokinetics of enrofloxacin and flunixin meglumine and interactions between both drugs after intravenous co-administration in healthy and endotoxaemic rabbits. Vet. J. 2008, 177, 418–424. [Google Scholar] [CrossRef] [PubMed]
- La Mura, V.; Pasarín, M.; Rodriguez-Vilarrupla, A.; García-Pagán, J.C.; Bosch, J.; Abraldes, J.G. Liver sinusoidal endothelial dysfunction after LPS administration: A role for inducible-nitric oxide synthase. J. Hepatol. 2014, 61, 1321–1327. [Google Scholar] [CrossRef] [PubMed]
- Cannon, J.G.; Tompkins, R.G.; Gelfand, J.A.; Michie, H.R.; Stanford, G.G.; van der Meer, J.W.M.; Endre, S.; Lonnemann, G.; Corsetti, J.; Chernow, B.; et al. Circulating Interlukin-1| and Tumor Necrosis Factor in septic shock and experimental endotoxin fever. J. Infect. Dis. 1990, 161, 79–84. [Google Scholar] [CrossRef] [PubMed]
- Flemming, S.; Schlegel, N.; Wunder, C.; Meier, M.; Baar, W.; Wollborn, J.; Roever, N.; Germer, C.-R.; Schick, M.A. Phosphodiesterase 4 inhibition dose dependently stabilizes microvascular barrier functions and microcirculation in a rodent model of polymicrobial sepsis. Schock 2014, 41, 537–545. [Google Scholar] [CrossRef] [PubMed]
- Van Duin, C.T.M.; Wensing, T.; Van Miert, A.S.J.P.A.M. Pentoxifylline pretreatment fails to block the acute-phase response to Escherichia coli endotoxin in dwarf goats. Vet. Res. Commun. 1995, 19, 149–157. [Google Scholar] [CrossRef] [PubMed]
- Pal, R.; Chaudhary, M.J.; Tiwari, P.C.; Nath, R.; Babu, S.; Pant, K.K. Pharmacological studies on the anti-inflammatory and immunomodulatory role of Pentoxifylline and its interaction with nitric oxide (NO) in experimental arthritis in rats. Inflammopharmacology 2016, 24, 221–231. [Google Scholar] [CrossRef]
- Corum, O.; Corum, D.D.; Atik, A.; Er, A.; Uney, K. Pharmacokinetics of pentoxifylline and its 5-hydroxyhexyl metabolite after intravenous administration of increasing doses to sheep. Am. J. Vet. Res. 2019, 80, 702–770. [Google Scholar] [CrossRef]
- Myers, M.J.; Baarsch, M.J.; Murtaugh, P. Effects of pentoxifylline on inflammatory cytokine expression and acute pleuropneumonia in swine. Immunobiology 2002, 205, 17–34. [Google Scholar] [CrossRef]
- Shahraky, A.R.; Pourjafar, M.; Chalmeh, A.; Badei, K.; Heidari, S.M.M.; Zamiri, M.J.; Nazifi, S. Attenuating the endotoxin induced acute phase response by pentoxifylline in comparison with dexamethasone and ketoprofen in sheep. Small Rumin. Res. 2016, 136, 156–160. [Google Scholar] [CrossRef]
- Zabel, P.; Wolter, D.T.; Schönharting, M.M.; Schade, U.F. Oxpentifylline in endotoxemia. Lancet 1989, 30, 1474–1477. [Google Scholar] [CrossRef] [PubMed]
- Waage, A.; Sorensen, M.; Stordal, B. Differential effect of oxpentifylline on tumour necrosis factor and interleukin-6 production. Lancet 1990, 335, 543. [Google Scholar] [CrossRef]
- Schandené, L.; Vandenbussche, P.; Crusiaux, A.; Alègre, M.L.; Abramowickz, D.; Dupont, E.; Content, J.; Goldman, M. Differential effects of pentoxifylline on the production of tumos nerosis factor-alpha and interleukin-6 by monocytes and T cells. Immunology 1992, 76, 30–34. [Google Scholar] [PubMed]
- Brie, D.; Sahebkar, A.; Penson, P.E.; Dinca, M.; Ursoniu, S.; Serban, M.-C.; Zanchetti, A.; Howard, G.; Ahmed, A.; Aronow, W.S.; et al. Effect of pentoxifylline on inflammatory markers and blood pressure: A systematic review and meta-analysis of randomized controlled trials. J. Hypertens. 2016, 34, 2318–2329. [Google Scholar] [CrossRef]
- Shaw, S.M.; Shah, M.K.; Williams, S.G.; Fildes, J.F. Immunological mechanism of pentoxifylline in chronic heart failure. Eur. J. Heart Fail. 2009, 11, 113–118. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Yu, J.; Zhang, L.-T.; Li, S.-Y.; Yan, J. The combination of ciprofloxacin and indomethacin suppresses the level of inflammatory cytokines secreted by macrophages in vitro. Chin. J. Traumatol. 2022, 25, 379–388. [Google Scholar] [CrossRef] [PubMed]
- Shapira, L.; Soskolne, W.A.; Houri, Y.; Barak, V.; Hala-Bi, A.; Stabholz, A. Protection against endo-toxic shock and lipopolysaccharide-induced local inflammation by tetracycline: Correlation with cytokine secretion. Infect. Immunol. 1996, 64, 825–828. [Google Scholar] [CrossRef]
- Kwak, H.J.; Song, J.S.; Yang, S.D.; Nam, J.-Y.; Cheon, H.G. Roflumilast inhibits lipopolysaccharide-induced inflammatory mediators via suppression of nuclear Factor-kB, p38 Mitogen-Activated Protein Kinase, and c-Jun NH2-Terminal Kinase activation. J. Pharmacol. Exp. Ther. 2005, 315, 1188–1195. [Google Scholar] [CrossRef]
- Al Kharfy, K.M.; Kellum, J.A.; Matzke, G.R. Unintended immunomodulation: Part II—Effects of pharmacological agents on cytokine activity. Shock 2000, 5, 346–360. [Google Scholar] [CrossRef]
- Coimbra, R.; Melbostad, H.; Loomis, W.; Tobar, M.; Hoyt, D.B. Phosphodiesterase inhibition decrease nuclear factor-kB activation +and shift the cytokine response toward anti-inflammatory activity in acute endotoxemia. J. Trauma 2005, 59, 575–582. [Google Scholar]
- Hand, W.L.; Hand, D.L. Influence of pentoxifylline and its derivatives on antibiotic uptake and superoxide generation by human phagocytic cell. Antimicrob. Agents Chemother. 1995, 39, 1574–1579. [Google Scholar] [CrossRef] [PubMed]
- Eng, J. Sample size estimation: How many individuals should be studied? Radiology 2003, 227, 309–313. [Google Scholar] [CrossRef] [PubMed]
- Peñailillo, A.K.; Sepulveda, M.A.; Palma, C.; Espinoza, A.; Aguilera, M.; Burgos, R.; Carretta, D.; Islas, A.; Pérez, R. Hematological and blood biochemical changes induced by the administration of low doses of Escherichia coli lipopolysaccharide, in rabbits. Arch. Med. Vet. 2016, 48, 315–320. [Google Scholar] [CrossRef]
 p < 0.05 vs. PTX/FFC/LPS.
p < 0.05 vs. PTX/FFC/LPS.
 p < 0.05 vs. PTX/FFC/LPS.
p < 0.05 vs. PTX/FFC/LPS.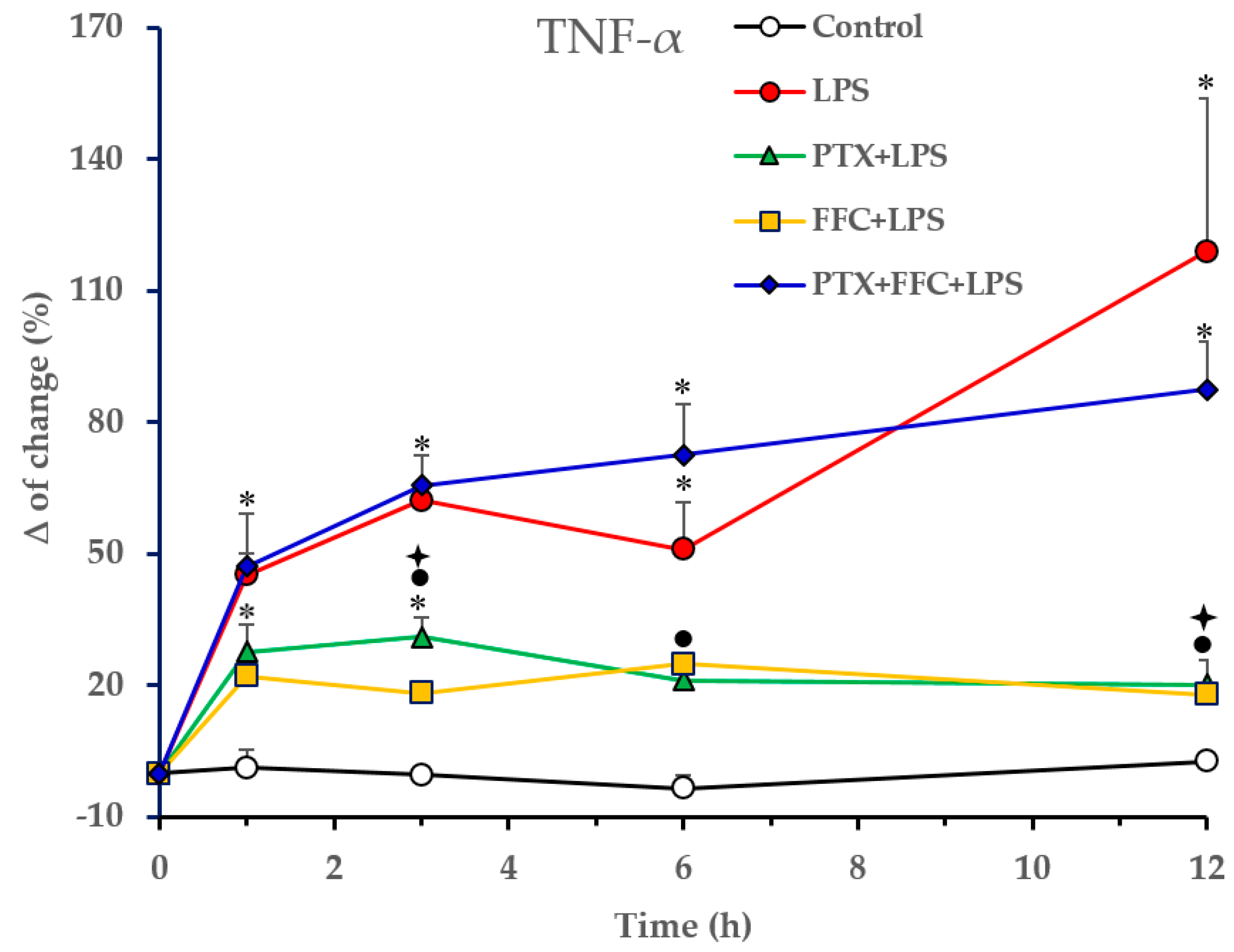
 p < 0.05 vs. PTX/FFC/LPS.
p < 0.05 vs. PTX/FFC/LPS.
 p < 0.05 vs. PTX/FFC/LPS.
p < 0.05 vs. PTX/FFC/LPS.


| Total Proteins (g/L) | |||||
|---|---|---|---|---|---|
| 0 h | 1 h | 3 h | 6 h | 12 h | |
| Control | 62.2 ± 1.4 a | 58.8 ± 2.7 a | 61.8 ± 2.5 a | 58.6 ± 0.9 a | 58.2 ± 2.2 a |
| LPS | 61.2 ± 2.9 a | 59.4 ± 3.0 a | 61.2 ± 2.0 a | 56.0 ± 1.7 a | 56.8 ± 2.1 a |
| PTX + LPS | 62.4 ± 3.5 a | 59.2 ± 4.6 a | 57.2 ± 4.4 a | 53.2 ± 3.7 a * | 55.8 ± 2.9 a |
| FFC + LPS | 58.2 ± 0.5 a | 55.6 ± 1.2 a | 51.4 ± 1.2 b | 48.4 ± 3.9 b * | 50.2 ± 3.0 a * |
| FFC + PTX + LPS | 57.0 ± 2.3 a | 54.0 ± 3.5 a | 50.6 ± 2.4 b | 49.8 ± 2.9 b * | 49.2 ± 2.2 b * |
| ALT (U/L) | |||||
|---|---|---|---|---|---|
| 0 h | 1 h | 3 h | 6 h | 12 h | |
| Control | 35.2 ± 5.9 a | 33.4 ± 6.4 a | 38.8 ± 7.0 a | 38.6 ± 5.9 a | 39.2 ± 6.7 a |
| LPS | 39.6 ± 5.9 a | 37.4 ± 4.8 a | 43.8 ± 3.7 a | 44.6 ± 6.6 a | 52.0 ± 5.2 a |
| PTX + LPS | 46.7 ± 3.7 a | 54.3 ± 7.5 a | 54.0 ± 9.4 a | 70.0 ± 14.0 a | 148.6 ± 25.9 b * |
| FFC + LPS | 40.6 ± 3.9 a | 36.2 ± 4.1 a | 36.4 ± 3.5 a | 37.6 ± 7.0 a | 41.0 ± 6.4 a,c |
| FFC + PTX + LPS | 38.8 ± 4.5 a | 41.8 ± 9.3 a | 40.8 ± 7.9 a | 56.2 ± 15.1 a | 59.2 ± 17.7 a,c |
| AST (U/L) | |||||
| 0 h | 1 h | 3 h | 6 h | 12 h | |
| Control | 13.8 ± 1.1 a | 13.8 ± 1.5 a | 19.6 ± 2.9 a | 19.2 ± 2.1 a | 21.8 ± 1.9 a |
| LPS | 18.6 ± 2.9 a | 19.8 ± 3.9 a | 41.0 ± 6.7 a | 67.6 ± 14.3 b * | 77.2 ± 10.7 b * |
| PTX + LPS | 22.0 ± 2.7 a | 27.6 ± 6.0 a | 48.6 ± 9.2 a | 121.0 ± 27.6 b * | 189.2 ± 41.6 c * |
| FFC + LPS | 19.2 ± 3.5 a | 29.2 ± 5.2 a | 51.0 ± 10.5 a | 69.2 ± 11.7 b * | 105.0 ± 20.0 b,c * |
| FFC + PTX + LPS | 15.4 ± 2.8 a | 32.2 ± 8.1 a | 49.8 ± 7.9 a | 68.4 ± 10.2 b * | 101.6 ± 16.3 b,c * |
| GGT (U/L) | |||||
| 0 h | 1 h | 3 h | 6 h | 12 h | |
| Control | 13.4 ± 2.9 a | 9.6 ± 1.0 a | 10.0 ± 1.3 a | 9.4 ± 1.1 a | 9.6 ± 1.1 a |
| LPS | 9.0 ± 1.5 a | 9.2 ± 1.2 a | 12.8 ± 1.6 a | 13.4 ± 2.2 a | 19.4 ± 4.0 b * |
| PTX + LPS | 8.4 ± 1.4 a | 8.8 ± 1.4 a | 10.6 ± 1.4 a | 11.2 ± 2.2 a | 13.4 ± 2.1 b |
| FFC + LPS | 6.8 ± 1.5 a | 6.8 ± 1.9 a | 9.2 ± 0.8 a | 10.0 ± 0.9 a | 11.4 ± 0.6 a |
| FFC + PTX + LPS | 10.2 ± 1.3 a | 10.8 ± 1.3 a | 11.2 ± 1.1 a | 11.6 ± 1.2 a | 10.2 ± 0.8 a |
| Total bilirubin (µmol/L) | |||||
| 0 h | 1 h | 3 h | 6 h | 12 h | |
| Control | 3.8 ± 0.3 a | 3.8 ± 0.5 a | 4.4 ± 0.6 a | 4.3 ± 0.7 a | 3.3 ± 0.7 a |
| LPS | 3.7 ± 0.8 a | 4.5 ± 0.8 a | 4.2 ± 0.7 a | 5.4 ± 1.5 a | 7.1 ± 2.7 a |
| PTX + LPS | 5.0 ± 0.7 a | 4.2 ± 0.9 a | 4.6 ± 0.8 a | 5.2 ± 0.6 a | 5.4 ± 0.8 a |
| FFC + LPS | 3.8 ± 0.3 a | 2.4 ± 0.2 a | 4.0 ± 1.1 a | 4.6 ± 0.7 a | 3.5 ± 0.7 a |
| FFC + PTX + LPS | 2.4 ± 0.3 a | 3.3 ± 0.4 a | 4.8 ± 0.4 a | 7.3 ± 2.3 a | 4.7 ± 0.8 a |
| Creatinine (µmol/L) | |||||
|---|---|---|---|---|---|
| 0 h | 1 h | 3 h | 6 h | 12 h | |
| Control | 94.4 ± 9.7 a | 99.0 ± 10.3 a | 97.8 ± 11.7 a | 87.4 ± 10.6 a | 91.2 ± 8.4 a |
| LPS | 89.6 ± 5.6 a | 90.4 ± 8.2 a | 103.4 ± 6.8 a | 106.2 ± 9.9 a | 99.4 ± 6.5 a |
| PTX + LPS | 81.8 ± 4.7 a | 95.6 ± 5.3 a | 107.4 ± 6.7 a | 105.6 ± 6.1 a | 99.6 ± 6.1 a |
| FFC + LPS | 87.4 ± 5.9 a | 112.0 ± 4.8 a | 122.0 ± 7.9 a | 130.8 ± 17.3 a | 118.2 ± 11.4 a |
| FFC + PTX + LPS | 80.4 ± 6.7 a | 89.6 ± 7.0 a | 102.2 ± 1.4 a | 101.2 ± 5.0 a | 92.6 ± 5.0 a |
| Urea (mmol/L) | |||||
| 0 h | 1 h | 3 h | 6 h | 12 h | |
| Control | 6.6 ± 0.2 a | 7.1 ± 0.2 a | 7.5 ± 0.3 a | 8.3 ± 0.4 a | 7.4 ± 0.3 a |
| LPS | 7.0 ± 0.6 a | 5.7 ± 0.6 a | 7.2 ± 0.8 a | 8.7 ± 1.2 a | 8.8 ± 1.1 a |
| PTX + LPS | 7.7 ± 0.5 a | 6.7 ± 0.6 a | 7.7 ± 0.7 a | 8.9 ± 1.0 a | 9.5 ± 0.7 a |
| FFC + LPS | 6.7 ± 1.1 a | 5.4 ± 0.9 a | 7.2 ± 0.5 a | 8.8 ± 0.9 a | 10.2 ± 0.8 a |
| FFC + PTX + LPS | 6.5 ± 0.2 a | 5.5 ± 0.6 a | 7.1 ± 0.4 a | 7.8 ± 0.1 a | 8.1 ± 0.6 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cazanga, V.; Palma, C.; Casanova, T.; Rojas, D.; Barrera, K.; Valenzuela, C.; Acevedo, A.; Ascui-Gac, G.; Pérez-Jeldres, T.; Pérez-Fernández, R. Modulation of the Acute Inflammatory Response Induced by the Escherichia coli Lipopolysaccharide through the Interaction of Pentoxifylline and Florfenicol in a Rabbit Model. Antibiotics 2023, 12, 639. https://doi.org/10.3390/antibiotics12040639
Cazanga V, Palma C, Casanova T, Rojas D, Barrera K, Valenzuela C, Acevedo A, Ascui-Gac G, Pérez-Jeldres T, Pérez-Fernández R. Modulation of the Acute Inflammatory Response Induced by the Escherichia coli Lipopolysaccharide through the Interaction of Pentoxifylline and Florfenicol in a Rabbit Model. Antibiotics. 2023; 12(4):639. https://doi.org/10.3390/antibiotics12040639
Chicago/Turabian StyleCazanga, Victoria, Cristina Palma, Tomás Casanova, Daniela Rojas, Karin Barrera, Cristhian Valenzuela, Aracelly Acevedo, Gabriel Ascui-Gac, Tamara Pérez-Jeldres, and Rubén Pérez-Fernández. 2023. "Modulation of the Acute Inflammatory Response Induced by the Escherichia coli Lipopolysaccharide through the Interaction of Pentoxifylline and Florfenicol in a Rabbit Model" Antibiotics 12, no. 4: 639. https://doi.org/10.3390/antibiotics12040639
APA StyleCazanga, V., Palma, C., Casanova, T., Rojas, D., Barrera, K., Valenzuela, C., Acevedo, A., Ascui-Gac, G., Pérez-Jeldres, T., & Pérez-Fernández, R. (2023). Modulation of the Acute Inflammatory Response Induced by the Escherichia coli Lipopolysaccharide through the Interaction of Pentoxifylline and Florfenicol in a Rabbit Model. Antibiotics, 12(4), 639. https://doi.org/10.3390/antibiotics12040639







