Meropenem MICs at Standard and High Inocula and Mutant Prevention Concentration Inter-Relations: Comparative Study with Non-Carbapenemase-Producing and OXA-48-, KPC- and NDM-Producing Klebsiella pneumoniae
Abstract
1. Introduction
2. Results
2.1. Susceptibility Testing with Meropenem and K. pneumoniae at SI and HI
2.2. MPC Determination of Meropenem against K. pneumoniae
3. Discussion
4. Materials and Methods
4.1. Antimicrobial Agent and Bacterial Strains
4.2. Susceptibility Testing
4.3. Mutant Prevention Concentration (MPC) Determinations
4.4. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Magréault, S.; Jauréguy, F.; Carbonnelle, E.; Zahar, J.R. When and how to use MIC in clinical practice? Antibiotics 2022, 11, 1748. [Google Scholar] [CrossRef] [PubMed]
- Drlica, K.; Zhao, X. Mutant selection window hypothesis updated. Clin. Infect. Dis. 2007, 44, 681–688. [Google Scholar] [CrossRef] [PubMed]
- Cantón, R.; Morosini, M.-I. Emergence and spread of antibiotic resistance following exposure to antibiotics. FEMS Microbiol. Rev. 2011, 35, 977–991. [Google Scholar] [CrossRef] [PubMed]
- Croisier, D.; Etienne, M.; Bergoin, E.; Charles, P.E.; Lequeu, C.; Piroth, L.; Portier, H.; Chavanet, P. Mutant selection window in levofloxacin and moxifloxacin treatments of experimental pneumococcal pneumonia in a rabbit model of human therapy. Antimicrob. Agents Chemother. 2004, 48, 1699–1707. [Google Scholar] [CrossRef]
- Firsov, A.A.; Vostrov, S.N.; Lubenko, I.Y.; Drlica, K.; Portnoy, Y.A.; Zinner, S.H. In vitro pharmacodynamic evaluation of the mutant selection window hypothesis using four fluoroquinolones against Staphylococcus Aureus. Antimicrob. Agents Chemother. 2003, 47, 1604–1613. [Google Scholar] [CrossRef]
- Fujimura, S.; Nakano, Y.; Watanabe, A. A correlation between reduced susceptibilities to vancomycin and daptomycin among the MRSA isolates selected in mutant selection window of both vancomycin and daptomycin. J. Infect. Chemother. 2014, 20, 752–756. [Google Scholar] [CrossRef]
- Firsov, A.A.; Smirnova, M.V.; Lubenko, I.Y.; Vostrov, S.N.; Portnoy, Y.A.; Zinner, S.H. Testing the mutant selection window hypothesis with Staphylococcus aureus exposed to daptomycin and vancomycin in an in vitro dynamic model. J. Antimicrob. Chemother. 2006, 58, 1185–1192. [Google Scholar] [CrossRef]
- Firsov, A.A.; Alieva, K.N.; Strukova, E.N.; Golikova, M.V.; Portnoy, Y.A.; Dovzhenko, S.A.; Kobrin, M.B.; Romanov, A.V.; Edelstein, M.V.; Zinner, S.H. Testing the mutant selection window hypothesis with Staphylococcus aureus exposed to linezolid in an in vitro dynamic model. J. Antimicrob. Chemother. 2017, 72, 3100–3107. [Google Scholar] [CrossRef]
- Allen, G.P.; Deshpande, L.M. Determination of the mutant selection window for clindamycin, doxycycline, linezolid, moxifloxacin and trimethoprim/sulfamethoxazole against community-associated meticillin-resistant Staphylococcus aureus (MRSA). Int. J. Antimicrob. Agents 2010, 35, 45–49. [Google Scholar] [CrossRef]
- Li, Y.; Feng, B.; Gu, X.; Yang, D.; Zeng, Z.; Zhang, B.; Ding, H. Correlation of PK/PD Indices with resistance selection for cefquinome against Staphylococcus aureus in an in vitro model. Front. Microbiol. 2016, 7, 466. [Google Scholar] [CrossRef]
- Zinner, S.H.; Gilbert, D.; Greer, K.; Portnoy, Y.A.; Firsov, A.A. Concentration-resistance relationships with Pseudomonas aeruginosa exposed to doripenem and ciprofloxacin in an in vitro model. J. Antimicrob. Chemother. 2013, 68, 881–887. [Google Scholar] [CrossRef] [PubMed]
- Vassilara, F.; Galani, I.; Souli, M.; Papanikolaou, K.; Giamarellou, H.; Papadopoulos, A. Mechanisms responsible for imipenem resistance among Pseudomonas aeruginosa clinical isolates exposed to imipenem concentrations within the mutant selection window. Diagn. Microbiol. Infect. Dis. 2017, 88, 276–281. [Google Scholar] [CrossRef] [PubMed]
- Brook, I. Inoculum effect. Rev. Infect. Dis. 1989, 11, 361–368. [Google Scholar] [CrossRef]
- Kebriaei, R.; Rice, S.A.; Singh, K.V.; Stamper, K.C.; Dinh, A.Q.; Rios, R.; Diaz, L.; Murray, B.E.; Munita, J.M.; Tran, T.T.; et al. Influence of inoculum effect on the efficacy of daptomycin monotherapy and in combination with β-Lactams against daptomycin-susceptible Enterococcus faecium harboring LiaSR substitutions. Antimicrob. Agents Chemother. 2018, 62, e00315-18. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.G.; Murakami, Y.; Andes, D.R.; Craig, W.A. Inoculum effects of ceftobiprole, daptomycin, linezolid, and vancomycin with Staphylococcus aureus and Streptococcus pneumoniae at inocula of 10(5) and 10(7) CFU injected into opposite thighs of neutropenic mice. Antimicrob. Agents Chemother. 2013, 57, 1434–1441. [Google Scholar] [CrossRef]
- Kim, T.; Lee, S.C.; Bae, M.; Sung, H.; Kim, M.-N.; Jung, J.; Kim, M.J.; Kim, S.-H.; Lee, S.-O.; Choi, S.-H.; et al. In vitro activities and inoculum effects of ceftazidime-avibactam and aztreonam-avibactam against carbapenem-resistant Enterobacterales isolates from South Korea. Antibiotics 2020, 9, 912. [Google Scholar] [CrossRef] [PubMed]
- Lenhard, J.R.; Bulman, Z.P. Inoculum effect of β-lactam antibiotics. J. Antimicrob. Chemother. 2019, 74, 2825–2843. [Google Scholar] [CrossRef]
- Lenhard, J.R.; Gall, J.S.; Bulitta, J.B.; Thamlikitkul, V.; Landersdorfer, C.B.; Forrest, A.; Nation, R.L.; Li, J.; Tsuji, B.T. Comparative pharmacodynamics of four different carbapenems in combination with polymyxin B against carbapenem-resistant Acinetobacter Baumannii. Int. J. Antimicrob. Agents 2016, 48, 719–724. [Google Scholar] [CrossRef]
- Harada, Y.; Morinaga, Y.; Kaku, N.; Nakamura, S.; Uno, N.; Hasegawa, H.; Izumikawa, K.; Kohno, S.; Yanagihara, K. In vitro and in vivo activities of piperacillin-tazobactam and meropenem at different inoculum sizes of ESBL-producing Klebsiella pneumoniae. Clin. Microbiol. Infect. 2014, 20, O831-9. [Google Scholar] [CrossRef]
- Miller, W.R.; Singh, K.V.; Arias, C.A.; Murray, B.E. Adjunctive clavulanic acid abolishes the cefazolin inoculum effect in an experimental rat model of methicillin-sensitive Staphylococcus aureus endocarditis. Antimicrob. Agents Chemother. 2018, 62, e01158-18. [Google Scholar] [CrossRef]
- Nannini, E.C.; Singh, K.V.; Arias, C.A.; Murray, B.E. In Vivo effects of cefazolin, daptomycin, and nafcillin in experimental endocarditis with a methicillin-susceptible Staphylococcus aureus strain showing an inoculum effect against cefazolin. Antimicrob. Agents Chemother. 2013, 57, 4276–4281. [Google Scholar] [CrossRef] [PubMed]
- Singh, K.V.; Tran, T.T.; Nannini, E.C.; Tam, V.H.; Arias, C.A.; Murray, B.E. Efficacy of ceftaroline against methicillin-susceptible Staphylococcus aureus exhibiting the cefazolin high-inoculum effect in a rat model of endocarditis. Antimicrob. Agents Chemother. 2017, 61, e00324-17. [Google Scholar] [CrossRef] [PubMed]
- Docobo-Pérez, F.; López-Cerero, L.; López-Rojas, R.; Egea, P.; Domínguez-Herrera, J.; Rodríguez-Baño, J.; Pascual, A.; Pachón, J. Inoculum effect on the efficacies of amoxicillin-clavulanate, piperacillin-tazobactam, and imipenem against extended-spectrum β-lactamase (ESBL)-producing and non-ESBL-producing Escherichia coli in an experimental murine sepsis model. Antimicrob. Agents Chemother. 2013, 57, 2109–2113. [Google Scholar] [CrossRef] [PubMed]
- Soriano, F.; Santamaría, M.; Ponte, C.; Castilla, C.; Fernández-Roblas, R. In vivo significance of the inoculum effect of antibiotics on Escherichia coli. Eur. J. Clin. Microbiol. Infect. Dis. 1988, 7, 410–412. [Google Scholar] [CrossRef] [PubMed]
- Smelter, D.; McCrone, S.; Rose, W. Cefazolin inoculum effect predicts reduced susceptibility to other antibiotics and patient outcomes in MSSA endovascular infections. Open Forum Infect. Dis. 2020, 7 (Suppl. 1), S617. [Google Scholar] [CrossRef]
- Miller, W.R.; Seas, C.; Carvajal, L.P.; Diaz, L.; Echeverri, A.M.; Ferro, C.; Rios, R.; Porras, P.; Luna, C.; Gotuzzo, E.; et al. The cefazolin inoculum effect is associated with increased mortality in methicillin-susceptible Staphylococcus aureus bacteremia. Open Forum Infect. Dis. 2018, 5, ofy123. [Google Scholar] [CrossRef]
- Poirel, L.; Bonnin, R.A.; Nordmann, P. Genetic features of the widespread plasmid coding for the carbapenemase OXA-48. Antimicrob. Agents Chemother. 2012, 56, 559–562. [Google Scholar] [CrossRef]
- Rodrigues, Y.C.; Lobato, A.R.F.; Quaresma, A.J.P.G.; Guerra, L.M.G.D.; Brasiliense, D.M. The spread of NDM-1 and NDM-7-producing Klebsiella pneumoniae is driven by multiclonal expansion of high-risk clones in healthcare institutions in the state of Pará, Brazilian Amazon Region. Antibiotics 2021, 10, 1527. [Google Scholar] [CrossRef]
- Mulvey, M.R.; Grant, J.M.; Plewes, K.; Roscoe, D.; Boyd, D.A. New Delhi metallo-β-lactamase in Klebsiella pneumoniae and Escherichia coli, Canada. Emerg. Infect. Dis. 2011, 17, 103–106. [Google Scholar] [CrossRef]
- Cuzon, G.; Naas, T.; Truong, H.; Villegas, M.V.; Wisell, K.T.; Carmeli, Y.; Gales, A.C.; Venezia, S.N.; Quinn, J.P.; Nordmann, P. Worldwide diversity of Klebsiella pneumoniae that produce beta-lactamase blaKPC-2 gene. Emerg. Infect. Dis. 2010, 16, 1349–1356. [Google Scholar] [CrossRef]
- Reale, M.; Strazzulla, A.; Quirino, A.; Rizzo, C.; Marano, V.; Postorino, M.C.; Mazzitelli, M.; Greco, G.; Pisani, V.; Costa, C.; et al. Patterns of multi-drug resistant bacteria at first culture from patients admitted to a third level University hospital in Calabria from 2011 to 2014: Implications for empirical therapy and infection control. Infez. Med. 2017, 25, 98–107. [Google Scholar] [PubMed]
- Scaglione, V.; Reale, M.; Davoli, C.; Mazzitelli, M.; Serapide, F.; Lionello, R.; La Gamba, V.; Fusco, P.; Bruni, A.; Procopio, D.; et al. Prevalence of antibiotic resistance over time in a third-level University Hospital. Microb. Drug Resist. 2022, 28, 425–435. [Google Scholar] [CrossRef] [PubMed]
- Golikova, M.V.; Alieva, K.N.; Filimonova, A.V.; Ageevets, V.A.; Sulian, O.S.; Avdeeva, A.A.; Sidorenko, S.V.; Zinner, S.H. Klebsiella pneumoniae susceptibility to carbapenem/relebactam combinations: Influence of inoculum density and carbapenem-to-inhibitor concentration ratio. Biomedicines 2022, 10, 1454. [Google Scholar] [CrossRef] [PubMed]
- The European Committee on Antimicrobial Susceptibility Testing. Breakpoint Tables for Interpretation of MICs and Zone Diameters. Version 13.0, 2023. Available online: http://www.eucast.org (accessed on 12 April 2023).
- Mouton, J.W.; van den Anker, J.N. Meropenem clinical pharmacokinetics. Clin. Pharmacokinet. 1995, 28, 275–286. [Google Scholar] [CrossRef] [PubMed]
- Dandekar, P.K.; Maglio, D.; Sutherland, C.A.; Nightingale, C.H.; Nicolau, D.P. Pharmacokinetics of meropenem 0.5 and 2 g every 8 hours as a 3-hour infusion. Pharmacotherapy 2003, 23, 988–991. [Google Scholar] [CrossRef]
- Adler, A.; Ben-Dalak, M.; Chmelnitsky, I.; Carmeli, Y. Effect of resistance mechanisms on the inoculum effect of carbapenem in Klebsiella pneumoniae isolates with borderline carbapenem resistance. Antimicrob. Agents Chemother. 2015, 59, 5014–5017. [Google Scholar] [CrossRef]
- Lozano-Huntelman, N.A.; Singh, N.; Valencia, A.; Mira, P.; Sakayan, M.; Boucher, I.; Tang, S.; Brennan, K.; Gianvecchio, C.; Fitz-Gibbon, S.; et al. Evolution of antibiotic cross-resistance and collateral sensitivity in Staphylococcus epidermidis using the mutant prevention concentration and the mutant selection window. Evol. Appl. 2020, 13, 808–823. [Google Scholar] [CrossRef]
- Gugel, J.; Dos Santos Pereira, A.; Pignatari, A.C.; Gales, A.C. beta-Lactam MICs correlate poorly with mutant prevention concentrations for clinical isolates of Acinetobacter spp. and Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 2006, 50, 2276–2277. [Google Scholar] [CrossRef]
- Mouton, J.M.; Punt, N. Use of the t > MIC to choose between different dosing regimens of β-lactam antibiotics. J. Antimicrob. Chemother. 2001, 47, 500–501. [Google Scholar] [CrossRef]
- Van der Zwaluw, K.; de Haan, A.; Pluister, G.N.; Bootsma, H.J.; de Neeling, A.J.; Schouls, L.M. The carbapenem inactivation method (CIM), a simple and low-cost alternative for the Carba NP test to assess phenotypic carbapenemase activity in gram-negative rods. PLoS ONE 2015, 10, e0123690. [Google Scholar] [CrossRef]
- Clinical Laboratory Standards Institute (CLSI). Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically, 11th ed.; M07; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2019. [Google Scholar]
- Blondeau, J.M. New concepts in antimicrobial susceptibility testing: The mutant prevention concentration and mutant selection window approach. Vet. Dermatol. 2009, 20, 383–396. [Google Scholar] [CrossRef] [PubMed]
- Firsov, A.A.; Portnoy, Y.A.; Strukova, E.N.; Shlykova, D.S.; Zinner, S.H. Predicting bacterial resistance using the time inside the mutant selection window: Possibilities and limitations. Int. J. Antimicrob. Agents 2014, 44, 301–305. [Google Scholar] [CrossRef] [PubMed]
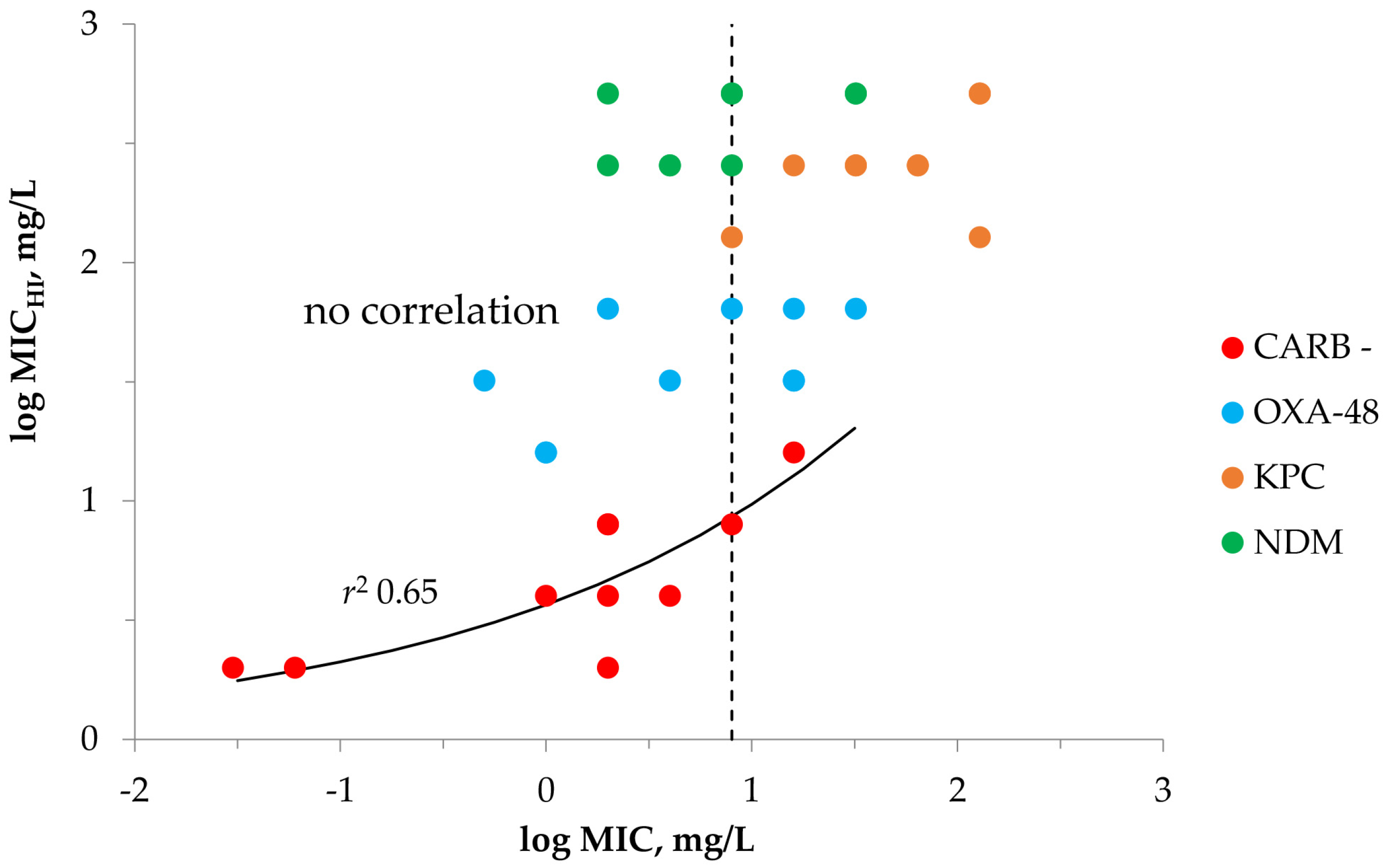
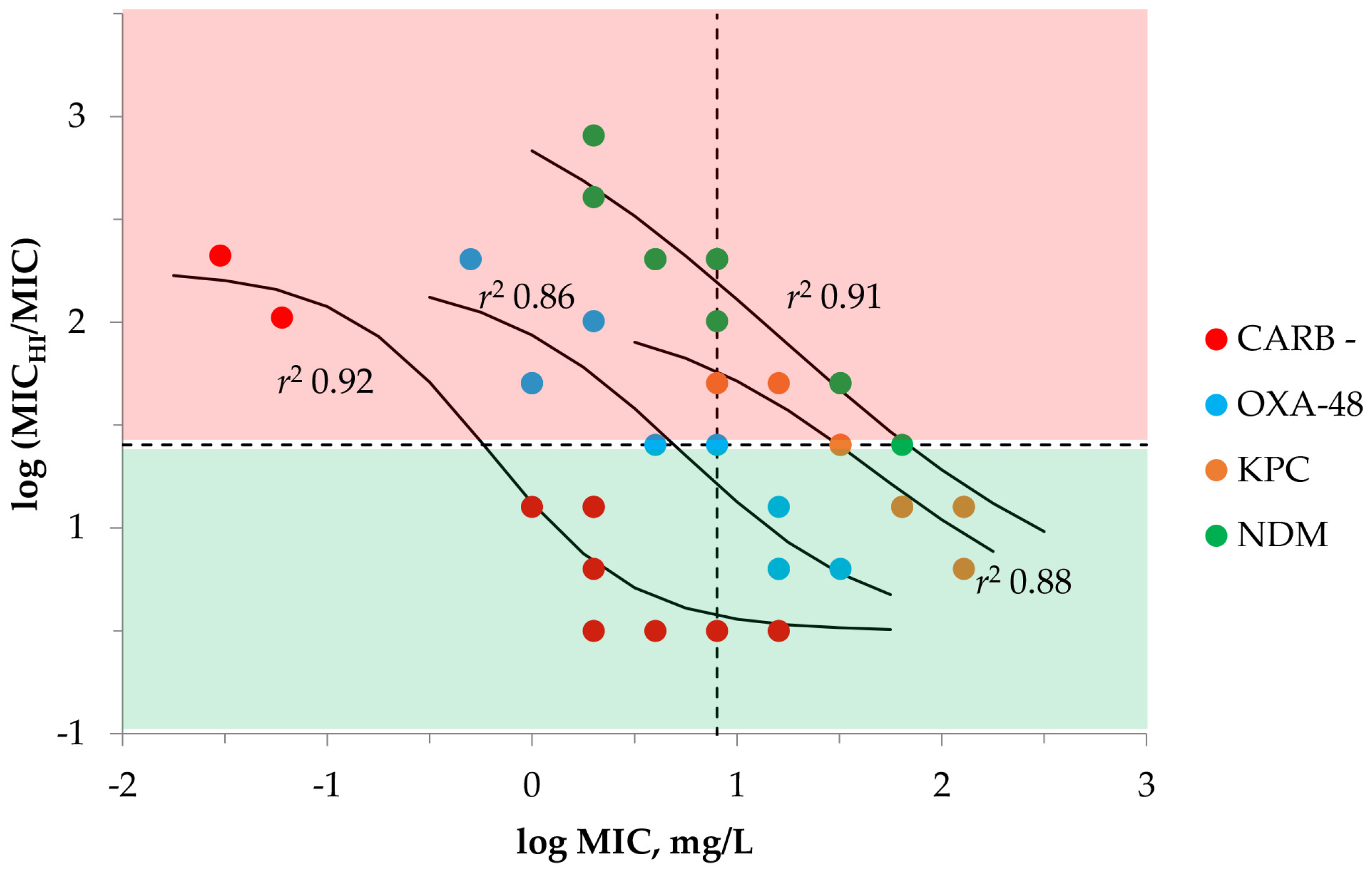
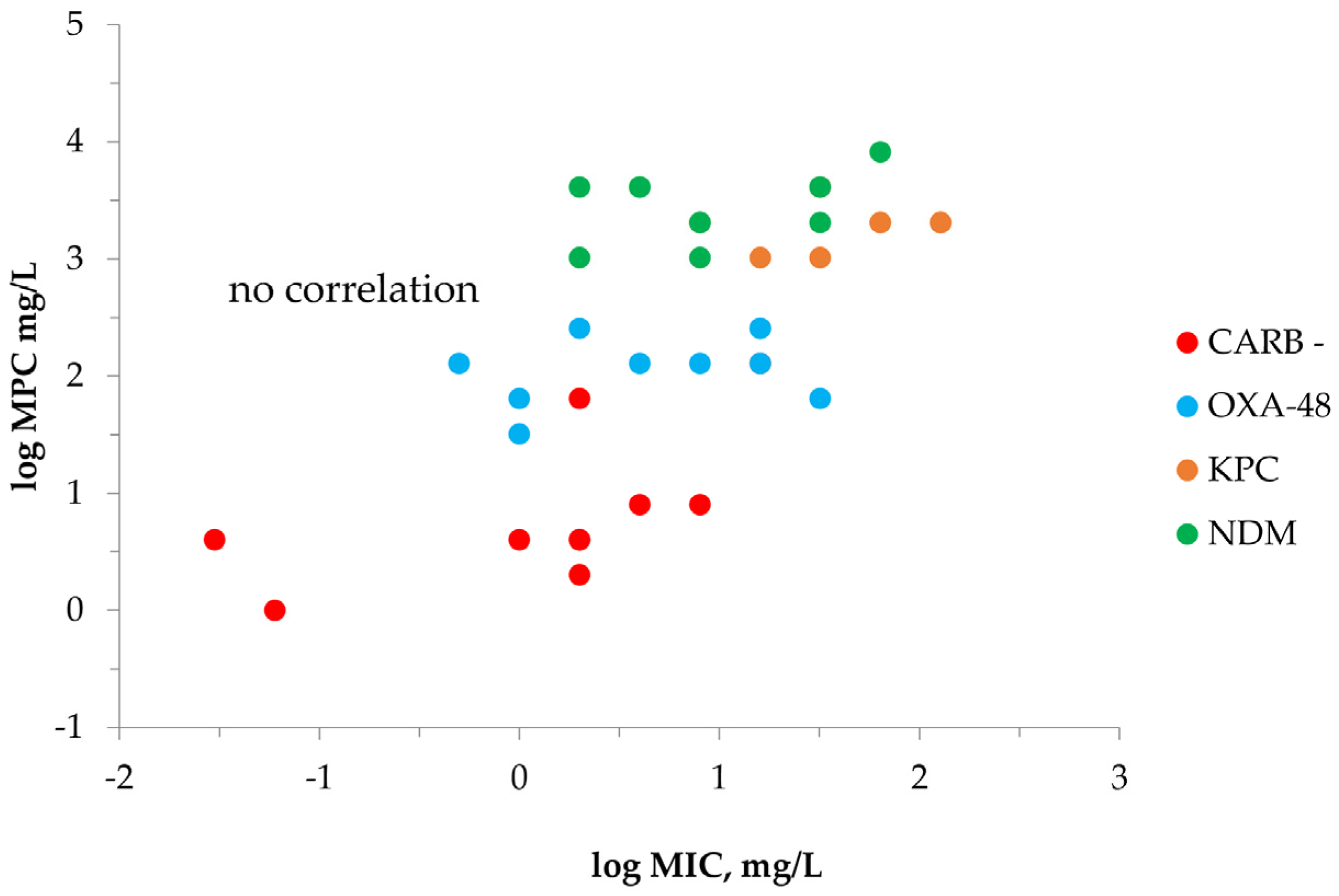

| Carbapenemase | K. pneumoniae | MIC | MICHI | MICHI/MIC | IE 1 | MPC |
|---|---|---|---|---|---|---|
| Carbapenemase-negative (n = 10) | 700,603 | 0.03 | 2 | 64 | + | 4 |
| 188 | 0.06 | 2 | 32 | + | 1 | |
| 782 | 1 | 4 | 4 | − | 4 | |
| 2286 | 2 | 2 | 1 | − | 2 | |
| 2684 | 2 | 4 | 2 | − | 4 | |
| 3093 | 2 | 8 | 4 | − | 64 | |
| 3101 | 2 | 8 | 4 | − | 4 | |
| 1676 | 4 | 4 | 1 | − | 8 | |
| 844 | 8 | 8 | 1 | − | 8 | |
| 2895 | 16 | 16 | 1 | − | 128 | |
| OXA-48 carbapenemase producers (n = 10) | 1278 | 0.5 | 32 | 64 | + | 128 |
| 1128 | 1 | 16 | 16 | + | 32 | |
| 215 | 1 | 16 | 16 | + | 64 | |
| 1456 | 2 | 64 | 32 | + | 256 | |
| 1170 | 4 | 32 | 8 | + | 128 | |
| 3111 | 8 | 64 | 8 | + | 128 | |
| 202 | 16 | 32 | 2 | − | 128 | |
| 38 | 16 | 32 | 2 | − | 256 | |
| 485 | 16 | 64 | 4 | − | 256 | |
| 75 | 32 | 64 | 2 | − | 64 | |
| KPC carbapenemase producers (n = 9) | BAA 1904 | 8 | 128 | 16 | + | 2048 |
| BAA 1705 | 16 | 256 | 16 | + | 1024 | |
| BAA 1900 | 32 | 256 | 8 | + | 1024 | |
| 14 | 32 | 256 | 8 | + | 4096 | |
| BAA 1898 | 64 | 256 | 4 | − | 2048 | |
| BAA 1899 | 64 | 256 | 4 | − | 2048 | |
| BAA 1902 | 128 | 256 | 2 | − | 2048 | |
| BAA 1905 | 128 | 512 | 4 | − | 2048 | |
| 16 | 128 | 512 | 4 | − | 2048 | |
| NDM carbapenemase producers (n = 10) | 1326 | 2 | 256 | 128 | + | 1024 |
| 2228 | 2 | 512 | 256 | + | 4096 | |
| 2342 | 4 | 256 | 64 | + | 4096 | |
| 35 | 4 | 256 | 64 | + | 4096 | |
| 1167 | 8 | 256 | 32 | + | 1024 | |
| 2131 | 8 | 512 | 64 | + | 2048 | |
| 3204 | 8 | 512 | 64 | + | 2048 | |
| 1961 | 32 | 512 | 16 | + | 4096 | |
| 3166 | 32 | 512 | 16 | + | 2048 | |
| 2863 | 64 | 512 | 8 | + | 8192 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Golikova, M.V.; Strukova, E.N.; Alieva, K.N.; Ageevets, V.A.; Avdeeva, A.A.; Sulian, O.S.; Zinner, S.H. Meropenem MICs at Standard and High Inocula and Mutant Prevention Concentration Inter-Relations: Comparative Study with Non-Carbapenemase-Producing and OXA-48-, KPC- and NDM-Producing Klebsiella pneumoniae. Antibiotics 2023, 12, 872. https://doi.org/10.3390/antibiotics12050872
Golikova MV, Strukova EN, Alieva KN, Ageevets VA, Avdeeva AA, Sulian OS, Zinner SH. Meropenem MICs at Standard and High Inocula and Mutant Prevention Concentration Inter-Relations: Comparative Study with Non-Carbapenemase-Producing and OXA-48-, KPC- and NDM-Producing Klebsiella pneumoniae. Antibiotics. 2023; 12(5):872. https://doi.org/10.3390/antibiotics12050872
Chicago/Turabian StyleGolikova, Maria V., Elena N. Strukova, Kamilla N. Alieva, Vladimir A. Ageevets, Alisa A. Avdeeva, Ofeliia S. Sulian, and Stephen H. Zinner. 2023. "Meropenem MICs at Standard and High Inocula and Mutant Prevention Concentration Inter-Relations: Comparative Study with Non-Carbapenemase-Producing and OXA-48-, KPC- and NDM-Producing Klebsiella pneumoniae" Antibiotics 12, no. 5: 872. https://doi.org/10.3390/antibiotics12050872
APA StyleGolikova, M. V., Strukova, E. N., Alieva, K. N., Ageevets, V. A., Avdeeva, A. A., Sulian, O. S., & Zinner, S. H. (2023). Meropenem MICs at Standard and High Inocula and Mutant Prevention Concentration Inter-Relations: Comparative Study with Non-Carbapenemase-Producing and OXA-48-, KPC- and NDM-Producing Klebsiella pneumoniae. Antibiotics, 12(5), 872. https://doi.org/10.3390/antibiotics12050872







