Antifungal Activity of Guiera senegalensis: From the Chemical Composition to the Mitochondrial Toxic Effects and Tyrosinase Inhibition
Abstract
1. Introduction
2. Results and Discussion
2.1. Chemical Composition of G. senegalensis CME, MF and EAF
2.2. Characterization of the Phenolic Profile of the G. senegalensis CME
2.3. Characterization of the Phenolic and Chlorophyll Profiles of the G. senegalensis EAF
2.4. Fungal Susceptibility to G. senegalensis CME, MF and EAF
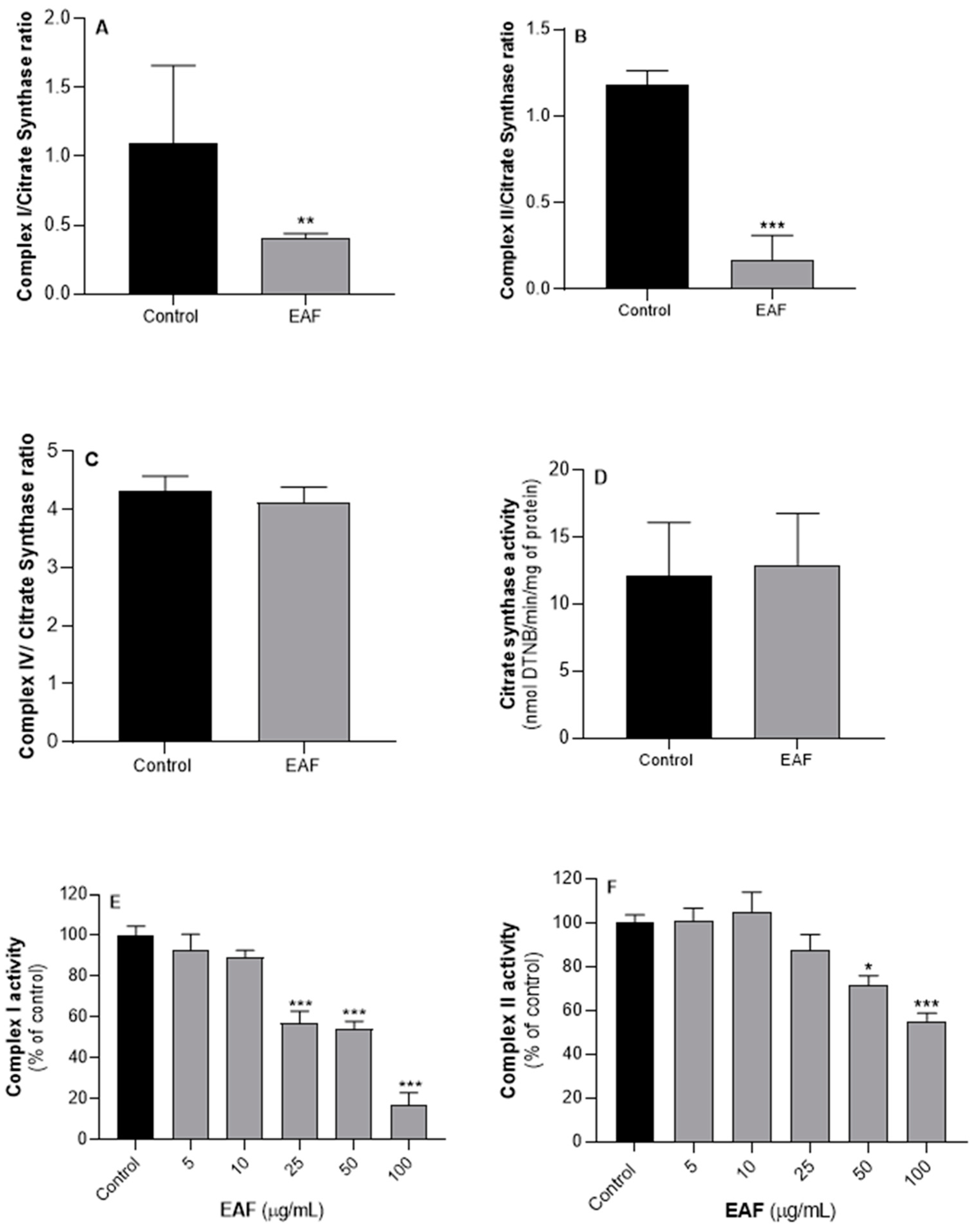
2.5. G. senegalensis EAF Exhibits Antifungal Activity Related to Mitochondrial Dysfunction
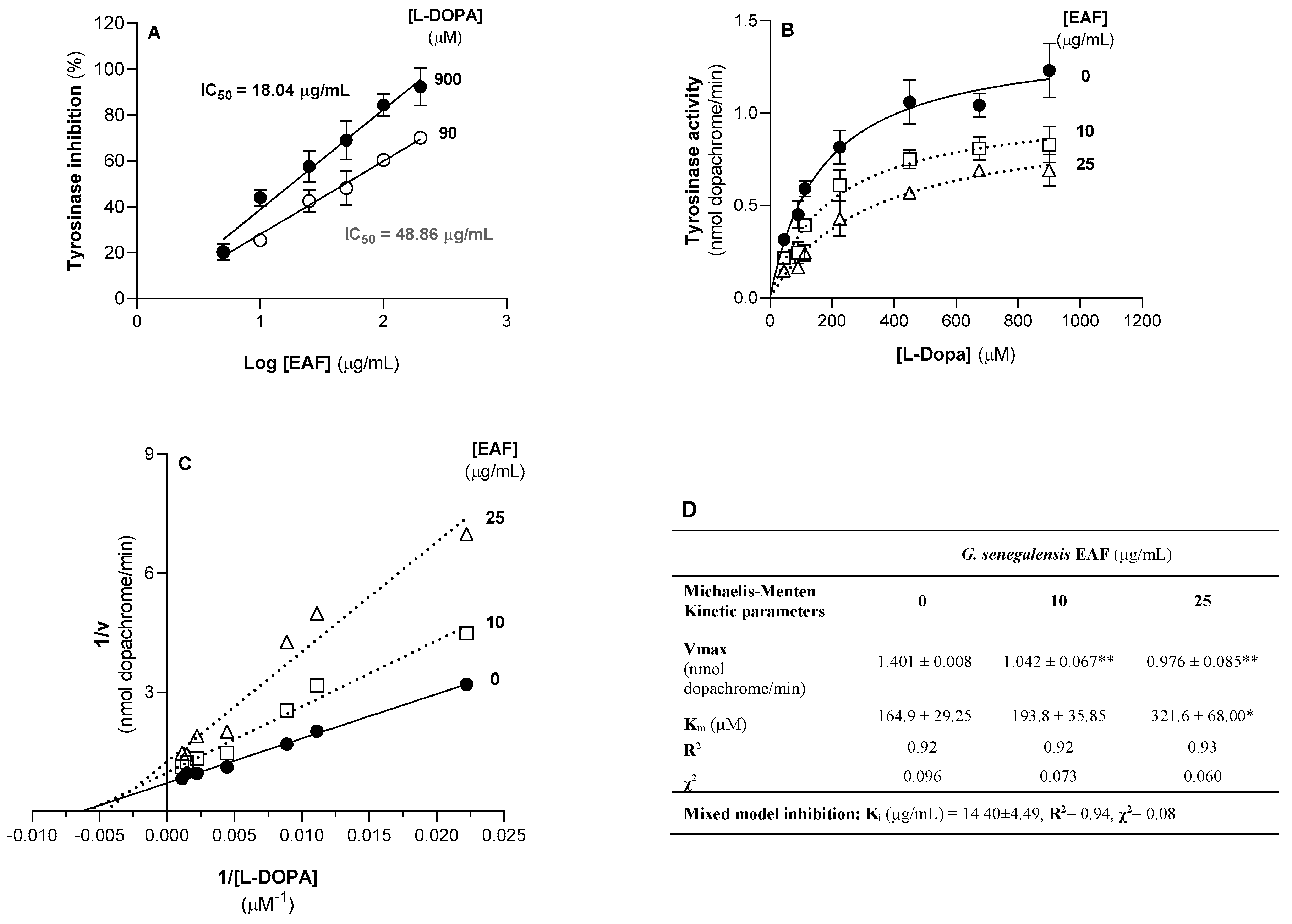
2.6. G. senegalensis EAF Inhibits the Activity of Tyrosinase
3. Materials and Methods
3.1. Standards and Reagents
3.2. Plant Material
3.3. Preparation and Fractionation of G. senegalensis Leaves Crude Methanol Extract
3.4. General Chemical Characterization
3.5. Characterization of the Phenolic Profile
3.5.1. HPLC-DAD-ESI(Ion Trap)-MSn and UPLC-ESI-QTOF-MS2 Analyses
3.5.2. HPLC-DAD Analysis
3.6. Characterization of the Chlorophyll Profile
3.6.1. HPLC-DAD Analysis
3.6.2. UPLC-ESI-QTOF-MS2 Analyses
3.7. Antifungal Activity Assessment
3.7.1. Fungal Organisms
3.7.2. Assessment of Antifungal Activity by Susceptibility Tests and Time-Dependent Growth Effects
3.8. Assessment of the Effects on Mitochondria by Measuring the Mitochondrial Respiratory Chain Complex Activities
3.9. Tyrosinase Activity
3.10. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Eurostat Sales of Pesticides in the EU. Available online: https://ec.europa.eu/eurostat/web/products-eurostat-news/-/ddn-20200603-1 (accessed on 10 January 2023).
- Campoy, S.; Adrio, J.L. Antifungals. Biochem. Pharmacol. 2017, 133, 86–96. [Google Scholar] [CrossRef] [PubMed]
- Kainz, K.; Bauer, M.A.; Madeo, F.; Carmona-Gutierrez, D. Fungal infections in humans: The silent crisis. Microb. Cell 2020, 7, 143–145. [Google Scholar] [CrossRef] [PubMed]
- Silva, O.; Gomes, E.T. Guieranone A, a naphthyl butenone from the leaves of Guiera senegalensis with antifungal activity. J. Nat. Prod. 2003, 66, 447–449. [Google Scholar] [CrossRef] [PubMed]
- Bosisio, E.; Mascetti, D.; Verotta, L.; Zani, F.; Mazza, P.; Talbot, M. Guiera senegalensis J. F. Gmelin (Combretaceae): Biological activities and chemical investigation. Phytomedicine 1997, 3, 339–348. [Google Scholar] [CrossRef] [PubMed]
- Sombié, P.A.; Konate, K.; Youl, E.; Coulibaly, A.Y.; Kiendrébéogo, M.; Choudhary, M.I.; Nacoulma, O.G. GC-MS analysis and antifungal activity from galls of Guiera senegalensis J.F Gmel (Combretaceae). J. Appl. Pharm. Sci. 2013, 3, 006–012. [Google Scholar] [CrossRef]
- Adebiyi, A.; Ayo, R.; Bello, I.; Habila, J. Phytochemical screening and anti-TB activity of root extracts of Guiera senegalensis (J. F. Gmel). Am. J. Biosci. Bioeng. 2015, 3, 208–213. [Google Scholar] [CrossRef]
- Fiot, J.; Sanon, S.; Azas, N.; Mahiou, V.; Jansen, O.; Angenot, L.; Balansard, G.; Ollivier, E. Phytochemical and pharmacological study of roots and leaves of Guiera senegalensis J.F. Gmel (Combretaceae). J. Ethnopharmacol. 2006, 106, 173–178. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. Methods Enzymol. 1999, 299, 152–178. [Google Scholar] [CrossRef]
- Dorta, E.; Lobo, M.G.; Gonzalez, M. Reutilization of mango byproducts: Study of the effect of extraction solvent and temperature on their antioxidant properties. J. Food Sci. 2012, 77, C80–C88. [Google Scholar] [CrossRef]
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Monteiro-Cardoso, V.F.; Oliveira, M.M.; Melo, T.; Domingues, M.R.M.; Moreira, P.I.; Ferreiro, E.; Peixoto, F.; Videira, R.A. Cardiolipin profile changes are associated to the early synaptic mitochondrial dysfunction in Alzheimer’s Disease. J. Alzheimer’s Dis. 2014, 43, 1375–1392. [Google Scholar] [CrossRef] [PubMed]
- Kenny, A.P. The determination of cholesterol by the Liebermann-Burchard reaction. Biochem. J. 1952, 52, 611–619. [Google Scholar] [CrossRef] [PubMed]
- Lichtenthaler, H.K.; Buschmann, C. Chlorophylls and carotenoids: Measurement and characterization by UV-Vis spectroscopy. Curr. Protoc. Food Anal. Chem. 2001, 1, F4.3.1–F4.3.8. [Google Scholar] [CrossRef]
- Ancolio, C.; Azas, N.; Mahiou, V.; Ollivier, E.; Di Giorgio, C.; Keita, A.; Timon-David, P.; Balansard, G. Antimalarial activity of extracts and alkaloids isolated from six plants used in traditional medicine in Mali and Sao Tome. Phyther. Res. 2002, 16, 646–649. [Google Scholar] [CrossRef] [PubMed]
- Azas, N.; Laurencin, N.; Delmas, F.; Di Giorgio, C.; Gasquet, M.; Laget, M.; Timon-David, P. Synergistic in vitro antimalarial activity of plant extracts used as traditional herbal remedies in Mali. Parasitol. Res. 2002, 88, 165–171. [Google Scholar] [CrossRef] [PubMed]
- Bouchet, N.; Lévesque, J.; Pousset, J.-L. HPLC isolation, identification and quantification of tannins from Guiera senegalensis. Phytochem. Anal. 2000, 11, 52–56. [Google Scholar] [CrossRef]
- Alam, P.; Parvez, M.K.; Arbab, A.H.; Al-Dosari, M.S. Quantitative analysis of rutin, quercetin, naringenin, and gallic acid by validated RP- and NP-HPTLC methods for quality control of anti-HBV active extract of Guiera senegalensis. Pharm. Biol. 2017, 55, 1317–1323. [Google Scholar] [CrossRef] [PubMed]
- Dirar, A.I.; Adhikari-Devkota, A.; Hassan, M.M.; Wada, M.; Watanabe, T.; Devkota, H.P. Phenolic compounds as potent free radical scavenging and enzyme inhibitory components from the leaves of Guiera senegalensis. Nat. Prod. Commun. 2019, 14, 1–4. [Google Scholar] [CrossRef]
- Makkar, H.P.S.; Becker, K. Isolation of tannins from leaves of some trees and shrubs and their properties. J. Agric. Food Chem. 1994, 42, 731–734. [Google Scholar] [CrossRef]
- Maleš, Ž.; Medić-Šarić, M.; Bucar, F. Flavonoids of Guiera senegalensis J. F. GMEL.—Thin-layer chromatography and numerical methods. Croat. Chem. Acta 1998, 71, 69–79. [Google Scholar]
- Ficarra, R.; Ficarra, P.; Tommasini, S.; Carulli, M.; Melardi, S.; Di Bella, M.R.; Calabrò, M.L.; De Pasquale, R.; Germanò, M.P.; Sanogo, R.; et al. Isolation and characterization of Guiera senegalensis J.F.Gmel. active principles. Boll. Chim. Farm. 1997, 136, 454–459. [Google Scholar] [PubMed]
- Lamien, C.E.; Meda, A.; Mans, J.; Romito, M.; Nacoulma, O.G.; Viljoen, G.J. Inhibition of fowlpox virus by an aqueous acetone extract from galls of Guiera senegalensis J. F. Gmel (Combretaceae). J. Ethnopharmacol. 2005, 96, 249–253. [Google Scholar] [CrossRef] [PubMed]
- Viera, I.; Roca, M.; Perez-Galvez, A. Mass Spectrometry of non-allomerized chlorophylls a and b derivatives from plants. Curr. Org. Chem. 2018, 22, 842–876. [Google Scholar] [CrossRef]
- Schelbert, S.; Aubry, S.; Burla, B.; Agne, B.; Kessler, F.; Krupinska, K.; Hörtensteiner, S. Pheophytin pheophorbide hydrolase (pheophytinase) is involved in chlorophyll breakdown during leaf senescence in Arabidopsis. Plant Cell 2009, 21, 767–785. [Google Scholar] [CrossRef] [PubMed]
- Uaciquete, A.; Korsten, L.; Van der Waals, J.E. Epidemiology of cashew anthracnose (Colletotrichum gloeosporioides Penz.) in Mozambique. Crop Prot. 2013, 49, 66–72. [Google Scholar] [CrossRef]
- Sanders, G.M.; Korsten, L. Comparison of cross inoculation potential of South African avocado and mango isolates of Colletotrichum gloeosporioides. Microbiol. Res. 2003, 158, 143–150. [Google Scholar] [CrossRef]
- Ishii, H.; Watanabe, H.; Yamaoka, Y.; Schnabel, G. Sensitivity to fungicides in isolates of Colletotrichum gloeosporioides and C. acutatum species complexes and efficacy against anthracnose diseases. Pestic. Biochem. Physiol. 2022, 182, 105049. [Google Scholar] [CrossRef]
- Maekawa, L.E.; Lamping, R.; Marcacci, S.; Maekawa, M.Y.; Nassri, M.R.G.; Koga-Ito, C.Y. Antimicrobial activity of chlorophyll-based solution on Candida albicans and Enterococcus faecalis. Rev. Sul-Bras. Odontol. 2007, 4, 36–40. [Google Scholar]
- Kustov, A.V.; Belykh, D.V.; Smirnova, N.L.; Venediktov, E.A.; Kudayarova, T.V.; Kruchin, S.O.; Khudyaeva, I.S.; Berezin, D.B. Synthesis and investigation of water-soluble chlorophyll pigments for antimicrobial photodynamic therapy. Dye. Pigment. 2018, 149, 553–559. [Google Scholar] [CrossRef]
- Caesar, L.K.; Cech, N.B. Synergy and antagonism in natural product extracts: When 1 + 1 does not equal 2. Nat. Prod. Rep. 2019, 36, 869–888. [Google Scholar] [CrossRef]
- Silva, M.G.; Mendes, D.; Manuel Oliveira, M.; Andrade, P.B.; Videira, R.A.; Peixoto, F. Mitochondria as a toxicological target for fungicides. In Mitochondrial Intoxication; Elsevier: Amsterdam, The Netherlands, 2023; pp. 493–526. [Google Scholar]
- Zhao, R.Z.; Jiang, S.; Zhang, L.; Yu, Z. Bin Mitochondrial electron transport chain, ROS generation and uncoupling. Int. J. Mol. Med. 2019, 44, 3–15. [Google Scholar] [CrossRef] [PubMed]
- Du, S.; Hu, X.; Shao, X.; Qian, X. Novel trifluoromethyl sydnone derivatives: Design, synthesis and fungicidal activity. Bioorg. Med. Chem. Lett. 2021, 44, 128114. [Google Scholar] [CrossRef] [PubMed]
- Buhler, W. Raised Resistance Risks. Available online: https://pesticidestewardship.org/resistance/fungicide-resistance/raised-resistance-risks/ (accessed on 15 February 2023).
- Gisi, U.; Sierotzki, H.; Cook, A.; McCaffery, A. Mechanisms influencing the evolution of resistance to Qo inhibitor fungicides. Pest Manag. Sci. 2002, 58, 859–867. [Google Scholar] [CrossRef] [PubMed]
- Mounkoro, P.; Michel, T.; Benhachemi, R.; Surpateanu, G.; Iorga, B.I.; Fisher, N.; Meunier, B. Mitochondrial complex III Qi-site inhibitor resistance mutations found in laboratory selected mutants and field isolates. Pest Manag. Sci. 2019, 75, 2107–2114. [Google Scholar] [CrossRef] [PubMed]
- Muddathir, A.M.; Yamauchi, K.; Batubara, I.; Mohieldin, E.A.M.; Mitsunaga, T. Anti-tyrosinase, total phenolic content and antioxidant activity of selected Sudanese medicinal plants. S. Afr. J. Bot. 2017, 109, 9–15. [Google Scholar] [CrossRef]
- Dirar, A.I.; Alsaadi, D.H.M.; Wada, M.; Mohamed, M.A.; Watanabe, T.; Devkota, H.P. Effects of extraction solvents on total phenolic and flavonoid contents and biological activities of extracts from Sudanese medicinal plants. S. Afr. J. Bot. 2019, 120, 261–267. [Google Scholar] [CrossRef]
- Nairn, R.; Cresswell, W.; Nairn, J. Mushroom tyrosinase: A model system to combine experimental investigation of enzyme-catalyzed reactions, data handling using R, and enzyme-inhibitor structural studies. Biochem. Mol. Biol. Educ. 2015, 43, 370–376. [Google Scholar] [CrossRef]
- Rebelo, M.J.; Sousa, C.; Valentão, P.; Rego, R.; Andrade, P.B. Phenolic profile of Douro wines and evaluation of their NO scavenging capacity in LPS-stimulated RAW 264.7 macrophages. Food Chem. 2014, 163, 16–22. [Google Scholar] [CrossRef]
- Nielsen, S.S. Phenol-sulfuric acid method for total carbohydrates. In Food Analysis Laboratory Manual; Springer: Boston, MA, USA, 2010; pp. 47–53. ISBN 978-1-4419-1462-0. [Google Scholar]
- Bartlett, E.M.; Lewis, D.H. Spectrophotometric determination of phosphate esters in the presence and absence of orthophosphate. Anal. Biochem. 1970, 36, 159–167. [Google Scholar] [CrossRef]
- Araújo, L.B.D.C.; Silva, S.L.; Galvão, M.A.M.; Ferreira, M.R.A.; Araújo, E.L.; Randau, K.P.; Soares, L.A.L. Total phytosterol content in drug materials and extracts from roots of Acanthospermum hispidum by UV-VIS spectrophotometry. Rev. Bras. Farmacogn. 2013, 23, 736–742. [Google Scholar] [CrossRef]
- Costa, A.F. Farmacognosia—Volume III, 3rd ed.; Fundação Calouste Gulbenkian: Lisbon, Portugal, 2001; ISBN 9789723109139. [Google Scholar]
- Ferreres, F.; Grosso, C.; Gil-Izquierdo, A.; Fernandes, A.; Valentão, P.; Andrade, P.B. Comparing the phenolic profile of Pilocarpus pennatifolius Lem. by HPLC–DAD–ESI/MS n with respect to authentication and enzyme inhibition potential. Ind. Crop. Prod. 2015, 77, 391–401. [Google Scholar] [CrossRef]
- Garcia, C.J.; García-Villalba, R.; Garrido, Y.; Gil, M.I.; Tomás-Barberán, F.A. Untargeted metabolomics approach using UPLC-ESI-QTOF-MS to explore the metabolome of fresh-cut iceberg lettuce. Metabolomics 2016, 12, 138. [Google Scholar] [CrossRef]

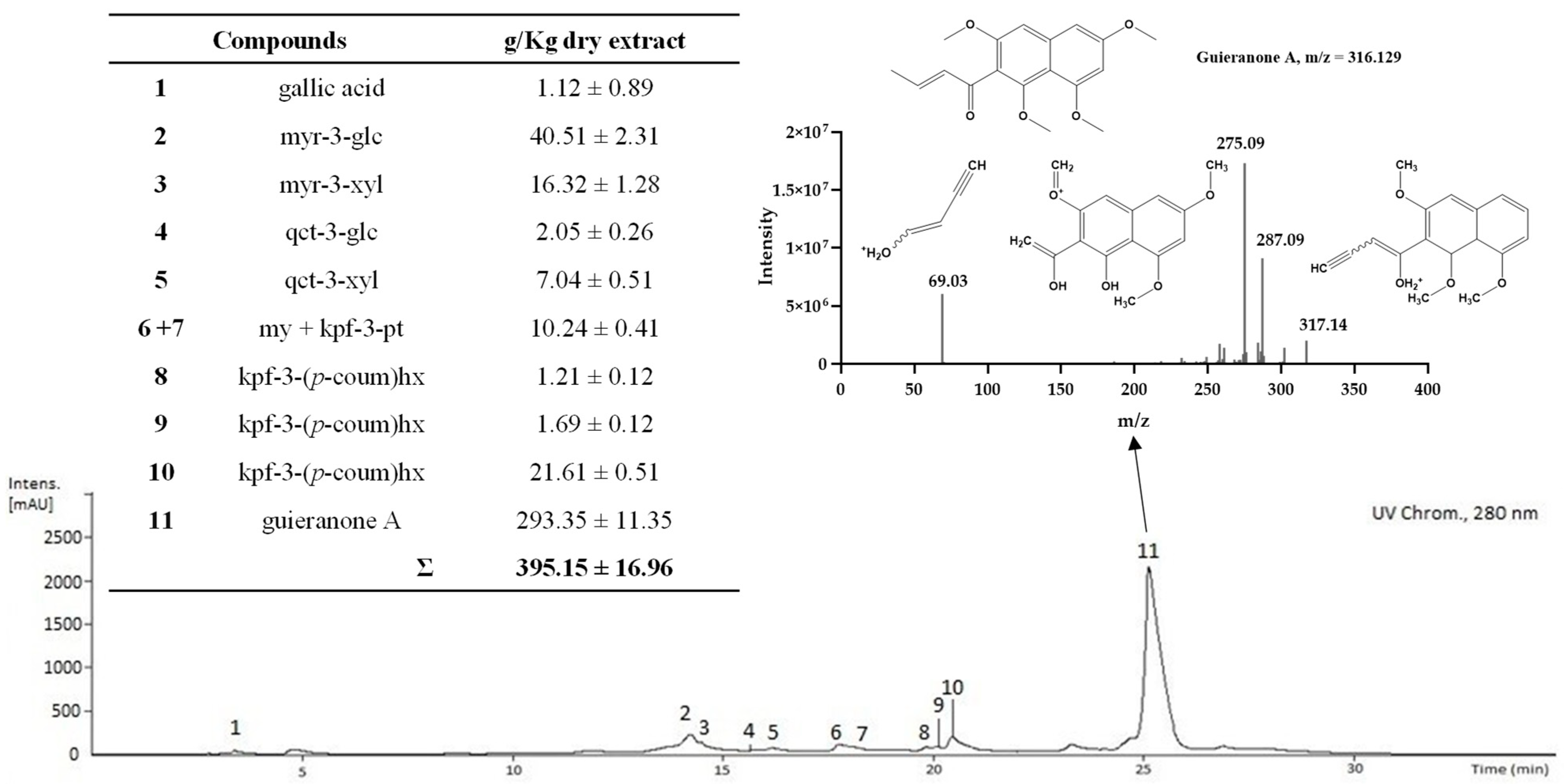
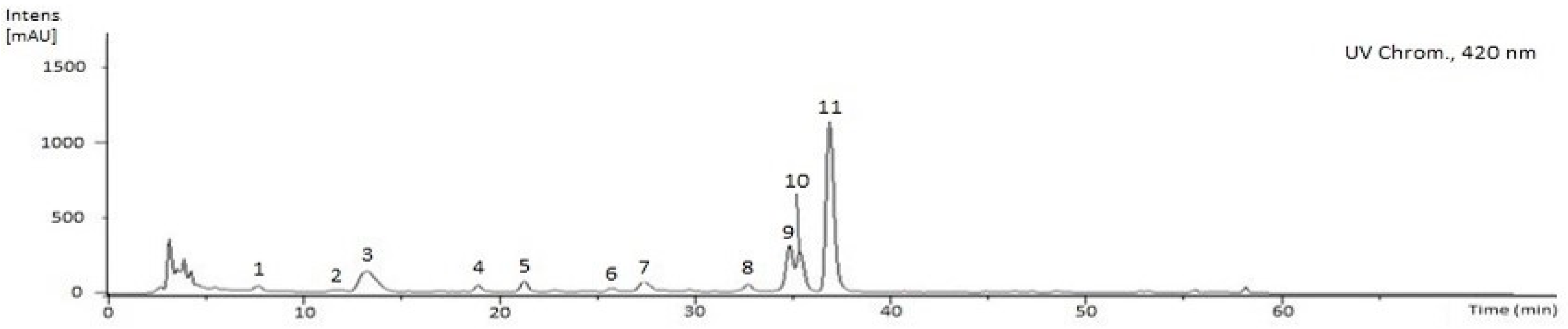

| (µg Compound Equivalents per mg Extract) | CME | EAF | E.F. | MF | E.F. |
|---|---|---|---|---|---|
| Extraction/fraction yield (%) | 17.58 ± 5.43 | 24.15 ± 16.43 | n. a. | 11.44 ± 6.89 | n. a. |
| Folin–Ciocalteu reactive phenolic compounds | 37.63 ± 3.48 | 46.12 ± 5.98 | 1.23 | 17.7 ± 1.13 | 0.47 |
| Total Sugars | 58.91 ± 4.56 | 76.45 ± 20.90 | 1.30 | 33.70 ± 3.44 | 0.57 |
| Phospholipids | 37.70 ± 6.02 | 22.38 ± 6.20 | 0.59 | 8.45 ± 6.38 | 0.22 |
| Phytosterols | 67.39 ± 6.30 | 193.65 ± 20.38 | 2.87 | 17.17 ± 8.16 | 0.25 |
| Chlorophylls | 5.83 ± 1.00 | 28.63 ± 1.36 | 4.91 | Not detected | n. a. |
| Alkaloids | Not detected | Not detected | n. a. | Not detected | n. a. |
| Compounds 1 | Rt (min) | UV (nm) | Molecular Formula | [M−H]−, m/z | MS2 Fragments−, m/z (%) | g/Kg Dry Extract 3 | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| galloyl derivatives | ||||||||||
| 1 | gall-quinc ac (GQA) | 2.2 | 272 | C14H15O10 | 343.0673 | 191.0565(Quinc-H), 169.0145(GA-H) | 1.33 ± 0.01 | |||
| 2 | digall-Quinc ac (dGQA) | 2.4 | 272 | C21H19O14 | 495.0780 | 343.0669(GQA-H), 191.0568(QA-H), 169.0149(GA-H) | 2.06 ± 0.02 | |||
| 3 | gallic ac (GA) | 2.9 | 273 | C7H6O5 | 169.0147 | 125.0244(GA-H-44) | 11.64 ± 0.25 | |||
| 4 | gall-quinc ac (GQA) | 3.1 | 274 | C14H15O10 | 343.0675 | 191.0563(Quinc-H), 169.0146(GA-H) | 0.37 ± 0.004 | |||
| 5 | digall-quinc ac (dGQA) | 5.0 | 273 | C21H19O14 | 495.0782 | 343.0670(GQA-H), 191.0569(QA-H), 169.0152(GA-H) | 0.46 ± 0.02 | |||
| 6 | digall-quinc ac (dGQA) | 6.6 | 275 | C21H19O14 | 495.0779 | 343.0668(GQA-H), 191.0569(QA-H), 169.0150(GA-H) | 0.59 ± 0.03 | |||
| 7 | trigall-quinc ac (tGQA) | 6.8 | 272 | C28H23O18 | 647.0892 | 495.0774(dGQA-H), 343.0670(GQA-H), 169.0147(GA-H) | 2.25 ± 0.05 | |||
| 8 | digall-quinc ac (dGQA) | 6.9 | 275 | C21H19O14 | 495.0780 | 343.0671(GQA-H), 191.0570(QA-H), 169.0146(GA-H) | 1.60 ± 0.04 | |||
| 9 | trigall-quinc ac (tGQA) | 9.1 | 275 | C28H23O18 | 647.0895 | 495.0770(dGQA-H), 343.0672(GQA-H), 307.0270, 169.0147(GA-H) | 1.98 ± 0.006 | |||
| 10 | tetragall-quinc ac | 10.1 | 276 | C35H27O22 | 799.1000 | 647.0894(tGQA-H), 629.0768(tGQA-H-W), 601.0818(tGQA-H-W-28), 495.0780(dGQA-H), 343.0665(GQA-H), 169.0145(GA-H) | 11.48 ± 0.07 | |||
| Σ | 33.76 ± 0.50 | |||||||||
| flavonoid glycosides and aglycones | ||||||||||
| −152 | [M−H/2H]− | |||||||||
| 11 | my-3-(gall)galc | 10.4 | 264, 300sh, 356 | C28H24O17 | 631.0917 | 479.0843(100) | 316.0228(15) | 5.36 ± 0.16 | ||
| 12 | my-3-(gall)glc | 10.5 | 264, 300sh, 355 | C28H24O17 | 631.0911 | 479.0845(100) | 316.0226(15) | 1.45 ± 0.60 | ||
| 13 | my-3-galc | 11.2 | 254sh, 265, 300sh, 356 | C21H20O13 | 479.8361 | 316.0229(100) | 28.63 ± 0.53 | |||
| 14 | my-3-glc | 11.4 | 253, 264, 302, 356 | C21H20O13 | 479.0834 | 316.0230(100) | 29.64 ± 1.39 | |||
| 15 | my-3-xyl | 11.8 | 255sh, 265, 302sh, 354 | C20H18O12 | 449.0716 | 316.0225(100) | 5.09 ± 0.21 | |||
| 16 | qct-3-(gall)galc | 12.0 | 254sh, 266, 300sh, 356 | C28H24O16 | 615.1025 | 463.0877(100) | 301.0350(20) | 2.63 ± 0.06 | ||
| 17 | qct-3-(gall)glc | 12.2 | 254sh, 265, 300sh, 355 | C28H24O16 | 615.1018 | 463.0881(100) | 301.0352(30) | 1.86 ± 0.02 | ||
| 18 | my-3-arab | 12.4 | 254sh, 264, 302sh, 356 | C20H18O12 | 449.0720 | 316.0227(100) | 12.35 ± 0.07 | |||
| 19 | my-3-rh | 12.5 | 254, 264sh, 300sh, 352 | C21H20O12 | 463.0882 | 316.0228(100) | 36.94 ± 1.21 | |||
| 20 | qct-3-galc | 12.9 | 256, 266sh, 300sh, 354 | C21H20O12 | 463.0876 | 301.0349(100) | 7.00 ± 0.11 | |||
| 21 | qct-3-glc | 13.1 | 255, 266sh, 302sh, 354 | C21H20O12 | 463.0880 | 301.0353(100) | 21.06 ± 0.16 | |||
| 22 | qct-3-xyl | 13.7 | 255, 266sh, 302sh, 352 | C20H18O11 | 433.0782 | 301.0350(100) | 1.24 ± 0.04 | |||
| 23 | qct-3-arab | 14.0 | 255, 266sh, 300sh, 352 | C20H18O11 | 433.0780 | 301.0351(100) | 10.70 ± 0.25 | |||
| 24 | qct-3-rh | 14.5 | 255, 265sh, 302sh, 352 | C21H20O11 | 447.0938 | 301.0348(100) | 8.66 ± 0.07 | |||
| 25 | my | 15.2 | ---- 2 | C15H10O8 | 317.0307 | 11.40 ± 0.04 | ||||
| 26 | kpf-3-pt | 15.2 | ---- 2 | C20H18O10 | 417.0831 | 284.0338(100) | ||||
| 27 | kpf-3-rh | 16.1 | 266, 288sh, 348 | C21H20O10 | 431.0975 | 285.0397(100) | 1.87 ± 0.09 | |||
| 28 | qct | 17.7 | 256, 265sh, 300sh, 368 | C15H10O7 | 301.0357 | 0.37 ± 0.02 | ||||
| 32 | hespt | 21.5 | 285, 232sh | C16H14O6 | 301.0728 | 0.07 ± 0.002 | ||||
| 33 | isorhmn | 22.4 | 256, 266sh, 298sh, 370 | C16H12O7 | 315.0536 | 1.05 ± 0.03 | ||||
| Σ | 187.37 ± 5.06 | |||||||||
| flavonols glycosides-cinnamoyl derivatives | ||||||||||
| −146 | ||||||||||
| 29 | kpf-3-(p-coum)hx | 18.3 | 266, 296sh, 314, 350sh | C30H25O13 | 593.1301 | 447.0935(15) | 284.0338(100) | 2.16 ± 0.10 | ||
| 30 | kpf-3-(p-coum)hx | 18.5 | 265, 295sh, 314, 352sh | C30H25O13 | 593.1302 | 447.0935(10) | 284.0340(100) | 5.35 ± 0.23 | ||
| 31 | kpf-3-(p-coum)hx | 18.7 | 266, 294sh, 316, 350sh | C30H25O13 | 593.1299 | 447.0935(25) | 284.0339(100) | 1.11 ± 0.04 | ||
| Σ | 8.62 ± 0.37 | |||||||||
| other compounds—positive ion mode | [M+H]+, m/z | |||||||||
| 34 | guieranone A | 23.1 | 266sh, 274, 312, 326, 380 | C18H20O5 | 317.1290 | 287.0913(60) | 275.0913(100) | 69.0335(40) | 36.46 ± 2.87 | |
| Σ | 266.21 ± 8.79 | |||||||||
| Compounds | Rt (min) | UV (nm) | Molecular Formula | [M+H]+ (m/z) | MS2[M+H]+, m/z (%) | g/Kg Dry Extract 1 | |
|---|---|---|---|---|---|---|---|
| 1 | HO-lactone-chlorophyll a | 7.71 | 407, 664 | C55H72MgN4O7 | 925.00 | 647.2269(60) 588.2128(20) 553.1991(35) | 1.78 ± 0.34 |
| 2 | chlorophyll b | 11.91 | 435 | C55H70MgN4O6 | 907.55 | 553.1992(30) 227.1603(100) | 0.67 ± 0.03 |
| 3 | HO-chlorophyll a | 13.44 | 407, 665 | C55H72MgN4O6 | 909.53 | 555.2154(100) 227.1608(20) | 13.08 ± 1.63 |
| 4 | chlorophyll a | 18.84 | 418, 442, 470 | C55H72MgN4O5 | 893.70 | 539.2418(100) 227.1598(30) | 1.07 ± 0.18 |
| 5 | pheophorbide a | 21.19 | 418, 442, 471 | C35H36N4O5 | 593.27 | 533.2544(80) | 1.52 ± 0.40 |
| 6 | HO-lactone-pheophytin b | 25.68 | 434, 410 | C55H72N4O8 | 917.70 | 830.5706(5) 639.2801(2) 579.2597(6) 551.2652(100) | 0.63 ± 0.03 |
| 7 | HO-lactone-pheophytin a | 27.27 | 408, 664 | C55H74N4O7 | 903.65 | 625.2648(20) 607.2546(20) 565.2444 (80) | 2.50 ± 1.77 |
| 8 | HO-pheophytin b | 32.60 | 435, 412, 654 | C55H72N4O7 | 901.65 | 623.2682(5) 563.2349 (5) 227.1575(100) | 5.28 ± 2.89 |
| 9 | pheophytin b | 34.88 | 434, 412, 655 | C55H72N4O6 | 885.55 | 826.5379(100) 547.2350(30) 535.2348(80) | 12.67 ± 1.82 |
| 10 | HO-pheophytin a | 35.38 | 407, 666 | C55H74N4O6 | 887.65 | 609.2703(40) 591.2598(60) 531.2389(100) | 11.23 ± 0.72 |
| 11 | pheophytin a | 36.95 | 407, 665 | C55H74N4O5 | 871.57 | 593.2754(100) 533.2546(80) 519.2385(5) | 44.76 ± 1.78 |
| Σ | 95.19 ± 11.59 | ||||||
| Filamentous Fungi | MIC 1 (µg/mL) | MFC (µg/mL) | ||
|---|---|---|---|---|
| CME | EAF | MF | EAF | |
| Aspergillus fumigatus | >2000 | 2000 | >2000 | >2000 |
| Fusarium oxysporum | >2000 | 2000 | >2000 | >2000 |
| Colletotrichum gloeosporioides | >2000 | 500 | >2000 | 1000 |
| Yeast | MIC (µg/mL) | MFC (µg/mL) | ||||
|---|---|---|---|---|---|---|
| EAF | Penconazole | Fluconazole | EAF | Penconazole | Fluconazole | |
| Sacharomyces cerevisiae | 8 | 1 | 8 | 500 | 1 | 16 |
| Candida albicans | 250 | 32 | 2 | >1000 | >32 | >128 |
| Candida krusei | 16 | 16 | 32 | 500 | ≥32 | >128 |
| Cryptococcus neoformans | 8 | 16 | 8 | ≥125 | >16 | >32 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moreira, R.; Ferreres, F.; Gil-Izquierdo, Á.; Gomes, N.G.M.; Araújo, L.; Pinto, E.; Andrade, P.B.; Videira, R.A. Antifungal Activity of Guiera senegalensis: From the Chemical Composition to the Mitochondrial Toxic Effects and Tyrosinase Inhibition. Antibiotics 2023, 12, 869. https://doi.org/10.3390/antibiotics12050869
Moreira R, Ferreres F, Gil-Izquierdo Á, Gomes NGM, Araújo L, Pinto E, Andrade PB, Videira RA. Antifungal Activity of Guiera senegalensis: From the Chemical Composition to the Mitochondrial Toxic Effects and Tyrosinase Inhibition. Antibiotics. 2023; 12(5):869. https://doi.org/10.3390/antibiotics12050869
Chicago/Turabian StyleMoreira, Rute, Federico Ferreres, Ángel Gil-Izquierdo, Nelson G. M. Gomes, Luísa Araújo, Eugénia Pinto, Paula B. Andrade, and Romeu A. Videira. 2023. "Antifungal Activity of Guiera senegalensis: From the Chemical Composition to the Mitochondrial Toxic Effects and Tyrosinase Inhibition" Antibiotics 12, no. 5: 869. https://doi.org/10.3390/antibiotics12050869
APA StyleMoreira, R., Ferreres, F., Gil-Izquierdo, Á., Gomes, N. G. M., Araújo, L., Pinto, E., Andrade, P. B., & Videira, R. A. (2023). Antifungal Activity of Guiera senegalensis: From the Chemical Composition to the Mitochondrial Toxic Effects and Tyrosinase Inhibition. Antibiotics, 12(5), 869. https://doi.org/10.3390/antibiotics12050869










