Abstract
The human gut is inhabited by a multitude of bacteria, yeasts, and viruses. A dynamic balance among these microorganisms is associated with the well-being of the human being, and a large body of evidence supports a role of dysbiosis in the pathogenesis of several diseases. Given the importance of the gut microbiota in the preservation of human health, probiotics, prebiotics, synbiotics, and postbiotics have been classically used as strategies to modulate the gut microbiota and achieve beneficial effects for the host. Nonetheless, several molecules not typically included in these categories have demonstrated a role in restoring the equilibrium among the components of the gut microbiota. Among these, rifaximin, as well as other antimicrobial drugs, such as triclosan, or natural compounds (including evodiamine and polyphenols) have common pleiotropic characteristics. On one hand, they suppress the growth of dangerous bacteria while promoting beneficial bacteria in the gut microbiota. On the other hand, they contribute to the regulation of the immune response in the case of dysbiosis by directly influencing the immune system and epithelial cells or by inducing the gut bacteria to produce immune-modulatory compounds, such as short-chain fatty acids. Fecal microbiota transplantation (FMT) has also been investigated as a procedure to restore the equilibrium of the gut microbiota and has shown benefits in many diseases, including inflammatory bowel disease, chronic liver disorders, and extraintestinal autoimmune conditions. One of the most significant limits of the current techniques used to modulate the gut microbiota is the lack of tools that can precisely modulate specific members of complex microbial communities. Novel approaches, including the use of engineered probiotic bacteria or bacteriophage-based therapy, have recently appeared as promising strategies to provide targeted and tailored therapeutic modulation of the gut microbiota, but their role in clinical practice has yet to be clarified. The aim of this review is to discuss the most recently introduced innovations in the field of therapeutic microbiome modulation.
1. Introduction
Interest in the human gut microbiota and its potential impact on human health has significantly increased since the development of metagenomic technologies that allow for the deep sequencing of microbial genomes. Trillions of bacteria live in the gut microbiota of the human digestive system and are established as a very complex environment. Beginning at birth, a wide range of genetic, nutritional, and environmental variables influence the composition of the gut microbiome [1]. After the beginning of the 20th century, Metchnikoff proposed that the human gut microbiome plays a part in both health and sickness [2]. Subsequently, the introduction of new molecular technologies that emerged in metagenomics spread a new perspective on microbiota research. The so-called “microbiota revolution” enabled the discovery of crucial links between pathogenic diseases and gut microbes [3].
The gut microbiota is composed of bacteria (generally including Firmicutes, Bacteroidetes, Actinobacteria, Proteobacteria, Fusobacteria, and Verrucomicrobia phila), yeasts, and viruses [4,5,6]. The gut bacteria perform a variety of functions, including vitamin production, pathogen defense, immune response stimulation, metabolism regulation and drug absorption [6]. High taxonomic diversity, microbial gene richness, and a stable core microbiota are frequently observed in healthy microbiota communities [6]. However, the relative distribution of microorganisms varies between individuals, even within the same person. Nearly every element of the host can be impacted by the microbiota, and dysbiosis is linked to a wide range of illnesses. In particular, dysbiosis has been implicated in cardiovascular and respiratory diseases, inflammatory bowel diseases, liver disorders, a variety of neoplasms, and metabolic illnesses via the intensification of a chronic inflammatory state [7,8,9]. It has also been suggested that gut microbes play a part in preserving the homeostasis of the gut–brain axis [9]. It has been supposed that different molecular patterns enhance microbiota-associated pathogenic states. Recently, it was discovered that the microbiota produces small molecules called genotoxins. In particular, the family of indolimines generated by the M. morganii strains associated with IBD–colorectal cancer can enhance colon carcinogenesis in mice and increase intestinal permeability [10]. Other small-molecule metabolites of Gram-positive and Gram-negative bacteria, such as those from Clostridium perfringens and Clostridium ramosum strains, directly damage DNA and cause the expression of double-strand break markers—as well as cell-cycle arrest—in epithelial cells [10]. Alterations in intestinal microbiota can therefore be correlated to pathological states, both through a non-specific mechanism of chronic inflammation and through specific molecular patterns (Figure 1); therefore, it is essential to achieve the ability to both modulate complex microbial communities and target individual members within these communities.
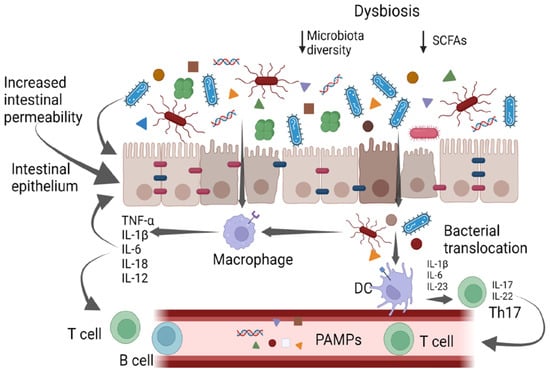
Figure 1.
Gut bacteria and systemic inflammation. PAMPs—pathogen-associated molecular patterns; DCs—dendritic cells. Created with BioRender.com.
The intestinal mucosa provides a selective, permeable barrier for nutrient absorption and protection from external factors. Pathogens, xenobiotics, and food can disrupt the intestinal barrier, while genetic and immune factors predispose individuals to gut barrier dysfunction. The gut microbiota participates in regulating the integrity and function of the intestinal barrier in a homeostatic balance in various ways: opposing colonization by pathogens, promoting the differentiation of regulatory T (Treg) cells (which induce tolerance to lumen antigens), and stimulating B cells to secrete immunoglobulin (Ig)A to avoid bacterial translocation. Commensals also convert dietary fiber into short-chain fatty acids (SCFAs), which protect the gut barrier in various ways, including by providing energy for colonocytes and stimulating the production of mucus, antimicrobial proteins, and Treg cells. Dysbiosis and chronic disruption of the gut barrier can lead to the translocation of microbial components, the activation of pro-inflammatory patterns, and the production of systemic, low-grade inflammation [11,12,13].
To date, a multitude of molecules with a modulatory effect on gut microbiota have been developed [14,15,16,17,18]. Most of these agents are prebiotics, probiotics, synbiotics, or postbiotics [14,15,16,17,18]. However, some molecules known for their antimicrobial effect have been shown to improve the balance of the gut microbiota [19]. Due to their properties, these agents could not be included in the previously mentioned therapeutic groups [19]. Nonetheless, rather than being antimicrobial, their activity may be classified as eubiotic [19]. The improved understanding of this host–microbiota relationship has allowed for the development of microbiota-based therapies such as bacteria modulation, fecal microbiota transplantation (FMT), and molecular techniques (phage therapy and engineered bacteria). However, despite great interest in their efficacy, most of these interventions are not available in clinical practice [20]. The purpose of this review is to examine new therapeutic perspectives for the modulation of microbiota, including molecules with a eubiotic effect, FMT, and molecular techniques. Given the enormous intra- and interpersonal variability of the human gut microbiota and its alterations in the context of related pathologies, it would be desirable to achieve a “personalization” in therapeutic modulation. The aim of this review is to analyze some of the most recent and innovative perspectives in the field of gut microbiota modulation in order to obtain such personalization.
2. Eubiotics: Drugs to Modulate the Gut Microbiota
Prebiotics and probiotics have historically played a crucial role in the regulation of the gut microbiota. Prebiotics are typically non-digestible food components that selectively encourage the growth and activity of a small number of bacteria in the digestive tract [14]. Probiotics are a group of advantageous microorganisms. Probiotics are live microorganisms which, when administered in adequate amounts, confer a health benefit to the host [15]. Synbiotics (a combination of prebiotics and probiotics) and postbiotics (inanimate microorganisms and/or their components that confer a health benefit to the host) have been recently introduced, showing a beneficial effect upon the dysbiotic state [16,17,18].
An overview of the conditions for which there is evidence that the oral administration of a specific prebiotic, probiotic, or synbiotic is effective is reported in Table 1. For a more comprehensive and detailed analysis, we recommend referring to The World Gastroenterology Organisation Global Guidelines 2023 [21].

Table 1.
Overview of clinical studies on probiotics, prebiotics, synbiotics in particular diseases.
Nevertheless, pharmacological agents not included in the above-mentioned groups demonstrate an interesting ability to alter microbiota, leading to dysbiosis, with a significant effect on associated diseases. Antimicrobials, natural compounds, and even metabolites of the same gut microbiota have been considered. A new group, eubiotics, has been proposed [54,55]. All these molecules have a somewhat antimicrobial activity; however, rather than determining a global depletion in bacteria abundance, the antimicrobial activity leads to a modulation of the composition of microbiota, which can be beneficial for the host [54,55].
2.1. Rifaximin: New Perspectives for an Old Antibiotic
During the last half of the 20th century, a number of new antibacterial agents came into clinical use, providing clinicians with a variety of options when treating many types of infectious diseases; among these options, antibiotics played a major role [56]. In addition to their beneficial effect against pathogenic bacteria, antibiotics are associated with significant short- and long-term alterations in the composition of the human microbiota [57]. The way an antibiotic affects the gut microbiota depends largely on its class, as well as the composition of the microbiota in the gut before the antibiotic is administered [58].
The use of an antibiotic results in a decrease in alpha (α) diversity, which measures the variety of bacterial taxa present in a given individual’s microbiota, and in beta (β)-diversity, which is a marker of the homogeneity of bacterial relative abundance [58,59]. Since suppressing susceptible microbes can create an ecological niche for opportunistic pathogenic bacteria that are resistant to the antibiotic, increasing the host’s susceptibility to post-antibiotic infection, the majority of studies focus on the dysbiotic effect of antibiotics [57,60,61]. Additionally, it has been demonstrated that antibiotics enrich phage-encoded genes, which transfer resistance to the administered drug and unrelated antibiotics and encourage interactions between phages and bacteria, thereby enhancing the exchange of resistance genes [62].
Nonetheless, some antibiotics have shown an intriguing function in the clinical modulation of the gut microbiota. Taking into account changes in bacterial abundance, the non-systemic antibiotic rifaximin, which has bactericidal and bacteriostatic activity against both aerobic and anaerobic bacterial species, has demonstrated promising results [63]. Rifaximin also has bile-acid-dependent solubility, which increases its effectiveness in the small intestine while inhibiting colonic bacteria only moderately [64]. Rifaximin irreversibly binds the bacterial DNA-dependent RNA polymerase, inhibiting bacterial protein synthesis [65]. To date, rifaximin is usually administered with beneficial effects in the management of diseases associated with an alteration in the gut microbiota, such as irritable bowel syndrome (IBS), diverticular disease, and hepatic encephalopathy (HE) [66,67,68]. Orally administered rifaximin determines minimal changes in the composition of gut microbiota, promoting the growth of bacterial species with a beneficial impact [63]. In addition, rifaximin modulates the inflammatory response by upregulating the expression of NF-kB via the pregnane X receptor [69,70] and downregulating the pro-inflammatory cytokines interleukin-1B and tumor necrosis factor alpha (TNFα) [71,72]. As observed in a clinical trial based on a multi-tagged pyrosequencing analysis, rifaximin modulates the networks among several bacteria (Enterobacteriaceae, Bacteroidaceae, Veillonellaceae, Porphyromonadaceae, and Rikenellaceae) and bacterial metabolites when administered to patients with mild HE [73]. An analysis of the serum of treated patients revealed an increase in saturated and unsaturated fatty acids [73]. Furthermore, rifaximin increased bacterial diversity, the Bacteroidetes/Firmicutes ratio, and the abundance of Faecalibacterium prausnitzii, a butyrate producer with potent anti-inflammatory properties, in a second smaller study involving 15 patients with IBS [74]. An analysis of gut microbiota diversity showed that the clinical response was associated with a slight increase in α-diversity [74]. Treatment with 1200 mg of rifaximin daily for 10 days increased the abundance of Lactobacilli in another report on 19 patients with various gastrointestinal and liver disorders (inflammatory bowel disorder, IBS, diverticular disease, and HE), with no effects upon the α-diversity of the gut microbiota [54]. A recent clinical trial on patients with HE demonstrated that daily treatment with 1200 mg of rifaximin increased the abundance of Proteobacteria while decreasing the abundance of Firmicutes [75]. A metagenomic analysis highlighted that a decrease in the prevalence of Veillonella, Haemophilus, Streptococcus, Parabacteroides, Megamonas, Roseburia, Alistipes, Ruminococcus, and Lactobacillus was also associated with rifaximin administration. Despite this trend of bacterial abundance modification, the stability of the gut microbiota was not affected by rifaximin administration, and its minimal antibacterial effect in the intestine was confirmed in this report [75]. While the overall composition of the gut microbiota is unaffected by the treatment, rifaximin causes a relative rather than an absolute change in the abundance of these helpful bacteria. This might be because some microbes that are sensitive to rifaximin experienced a minimal reduction in abundance that is not statistically significant while others, such as Lactobacilli, developed resistance [54]. Interestingly, Brigidi et al. showed a significant initial decrease in the fecal abundance of Lactobacilli in individuals with mild or severe ulcerative colitis who were receiving a higher dosage of rifaximin (i.e., 1800 mg daily). However, the abundance of Lactobacilli returned after three cycles of therapy that each lasted 10 days. Moreover, Lactobacilli, which were particularly susceptible to rifaximin at the beginning of the study, developed resistance to the drug (to a mean value of 12 μg/mL) [76].
In order to improve the pharmacological approach and incorporate it into therapeutic options, it is necessary to investigate the mechanisms that underlie the clinical impact exerted by this beneficial gut microbiota perturbation.
2.2. The Multi-Layered Mechanisms of Rifaximin
Rifaximin, as previously mentioned, differs from other antibiotics as it does not affect the gut microbiota’s overall composition. Indeed, rifaximin increases the abundance of beneficial gut bacteria and promotes metabolic modifications, balancing the relationship between the host and the bacteria with a well-recognized positive clinical effect.
Although the underlying biological mechanisms are not fully understood, some metabolic networks may be suggested. Rifaximin treatment protected mice exposed to malathion from stress-induced oxidative damage in a pre-clinical study [77]. In the gut microbiota of rifaximin-treated mice, acetate- and propionate-producing bacteria, such as Lactobacillus spp., Bacteroides spp., Prevotella spp., Streptococcus spp., Phascolarctobacterium succinatutens, and Negativicutes spp., were found in high concentrations [77]. Therefore, treatment with rifaximin effectively increased the production of short-chain fatty acids (SCFAs) by modulating the gut microbiota [78]. Dupraz et al. showed that intestinal gamma-delta (γδ) T cells in mice and humans produce less IL-17 and IL-22 when microbiota-produced SCFAs, particularly propionate, are present. This results in a decrease in the inflammatory state [79]. Furthermore, the administration of rifaximin was linked to an increase in the expression of peroxisome proliferator-activated receptor gamma coactivator-1 alpha, a key metabolic regulator whose expression is elicited by SCFAs [77,80]. As a result, SCFAs might be the means through which rifaximin reduces systemic inflammation and exerts its beneficial effect. Another preclinical model highlighted rifaximin’s function in controlling systemic inflammatory responses. By inhibiting the activation of the TLR-4/NF-B signaling pathway and downregulating inflammatory factors such as TNF-α, IL-6, IL-17A, and IL-23, rifaximin significantly diminished the severity of clinical disease in mice with proteoglycan-induced ankylosing spondylitis. In this instance, a microbiological analysis showed an increase in Lactobacillus and Bacteroides, with a higher Bacteroidetes/Firmicutes ratio [81]. Additionally, in vitro studies performed in human gut epithelial cells indicated that rifaximin decreases apoptosis and increases tight junction protein expression by activating the TLR4, MyD88, and NF-kB pathways [82]. Meanwhile, increased populations of bacteria such as Lactobacilli have been shown to reduce the production of pro-inflammatory cytokines and TNF-α to prevent the spread of pathogenic bacteria and to modulate intestinal permeability [83,84,85].
As a result, rifaximin not only creates an anti-inflammatory environment by regulating intestinal metabolic pathways but also helps to reestablish the function of the intestinal barrier by reducing intestinal permeability, preventing the translocation and overgrowth of bacteria that could be harmful to the individual [55].
Patel et al. proposed that these preclinical implications could be transferred to a human model in a recent randomized controlled clinical trial [86]. In comparison to the placebo group, 90 days of rifaximin treatment decreased the plasma levels of tumor necrosis factor alpha (TNF-α) and circulating neutrophil TLR-4 expression in patients with cirrhosis and hepatic encephalopathy [86]. Additionally, metagenomic quantification techniques showed that rifaximin reduced the levels of sialidase-rich species that degrade mucin (i.e., Streptococcus spp., Veillonella atypica and parvula, Akkermansia, and Hungatella), preventing the so-called “oralization” of the gut microbiota [86]. These bacteria are present in the oral microbiota, but they are also more prevalent in the gut microbiota in conditions such as cirrhosis [87]. Sialidase alters the intestinal permeability by degrading O-glycans in the gut mucin barrier. Rifaximin also promoted an intestinal microenvironment rich in TNF-α and interleukin-17E (IL-17) [86]. It has been demonstrated that IL-17E (also known as IL-25), unlike other isoforms of IL-17, is implicated in the integrity of the gut barrier [88,89]. Intestinal tuft cells produce IL-17E in response to indole-acetic propionic acid, a tryptophan metabolite involved in gut homeostasis and anti-inflammatory pathways mediated by IL-10 [88,89]. It is also intriguing to note that plasma TNF-α levels decreased while intestinal TNF-α increased, demonstrating how the maintenance of gut homeostasis and the control of systemic inflammation can be achieved by increasing an inflammatory cytokine in a specific microenvironment. Clinically, rifaximin treatment resulted in a recovery of neurocognitive function, in addition to the complete resolution of hepatic encephalopathy. Additionally, rifaximin allowed for the preservation of beta (β) diversity in the gut microbiota, whereas the placebo group saw a decrease in this metric [86].
Rifaximin demonstrates great potential for maintaining the balance between the host and the gut microbiota. Molecular and microbiological data are strongly consistent with clinical evidence. Rifaximin’s effectiveness, however, may be impacted by various disruptions to the gut homeostasis that are related to various pathological conditions. On the other hand, rifaximin is a versatile drug because it has the ability to restore balance instead of acting on a single target. Regarding one of the main issues with antibiotic therapy, rifaximin showed no long-term effects, and its impact on bacterial resistance appears to be absolutely negligible [75]. The eubiotic effects of rifaximin are summarized in Figure 2.
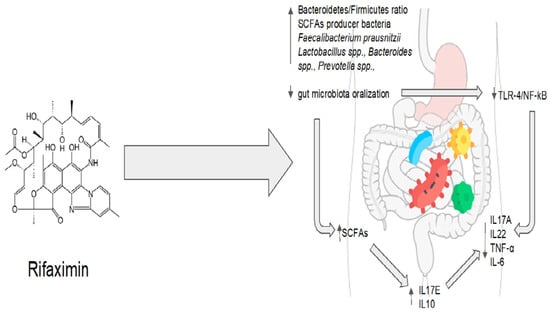
Figure 2.
Rifaximin’s eubiotic effects. IL17A: interleukin 17A; IL22: interleukin 22; TNF-α: tumor necrosis factor alpha; IL-6: interleukin 6; IL10: interleukin 10; IL17E: interleukin 17E.
Rifaximin therapy is associated with the modulation of the gut microbiota, which increases the production of short-chain fatty acids (SCFAs) and reduces the stimulation of the Toll-like receptor 4 (TLR4) pathway via pathogen-associated molecular patterns (PAMPs). This leads to a decrease in the production of pro-inflammatory cytokines such as IL17A, IL22, TNF-α, and IL-6 and an increase in the production of anti-inflammatory cytokines such asIL10, and IL17E.
2.3. Other Antimicrobial Agents: Triclosan
Triclosan is a nonionic broad-spectrum antimicrobial agent. Its chemical name is 2,4,4′-trichloro-2′-hydroxydiphenyl ether. It is widely used in toothpaste, food storage containers, medical products, personal care products, and plastic cutting boards [90]. Triclosan acts as a detergent, directly disrupting the integrity of the bacterial membrane [91]. Moreover, it interferes with the synthesis of bacterial fatty acids by inhibiting the enoyl-acyl carrier protein (enoyl-ACP) reductase [92]. At low concentrations, it limits bacterial growth, while at high concentrations, it is bactericidal [93].
Since its introduction, numerous studies have examined the effects of triclosan on living organisms to determine its safety. A randomized study of triclosan-containing household and personal care products carried out to evaluate the safety of triclosan showed that triclosan exposure did not induce global reconstruction or the loss of microbial diversity in the gut microbiota [94]. Nevertheless, because triclosan-containing products included toothpaste, the oral microbiota was also examined. So far, even in sites where triclosan was applied directly, no significant differences in α-diversity were detected [94]. Triclosan seems to not have an antibacterial effect at the dosage used in household and personal care products, despite the fact that this effect may be present at higher concentrations and for longer periods of exposure [94,95].
Another preclinical model sought to define perturbations to the gut microbiota caused by triclosan. Microbiota composition was evaluated at three, twenty-one, and fifty-two weeks after the administration of a low dose of triclosan. Following exposure to triclosan (50 mg/kg/day), the abundance of Bacteroidetes increased at week 52. At the same dose after 52 weeks, triclosan slightly (but not significantly) reduced the abundance of Firmicutes. At 21 and 52 weeks of age, triclosan decreased the levels of Akkermansia muciniphila at the species level. Low doses of triclosan increased α-diversity after three weeks when compared to the control group [96]. Triclosan resistance mechanisms include changes in the enoyl-ACP reductase and efflux pumps. However, despite the widespread use of products containing triclosan, the community’s overall resistance and cross-resistance rates are low [97].
Triclosan consequently gained attention as a modulator of the gut microbiota, and it was considered as a drug for the treatment of dysbiosis in a recent preclinical study [98]. Mice received a continuous high-fat diet for 20 weeks and were then treated with triclosan at a dose of 400 mg/kg/d for the final 8 weeks [98]. In mice fed a high-fat diet, triclosan increased the ratio of Bacteroidetes/Firmicutes and decreased the number of pathogenic Gram-negative bacteria, such as Helicobacter, Erysipelatoclostridium, and Citrobacter, as shown by a metagenomic analysis [98]. Triclosan also increased the relative abundance of Lactobacillus, Bifidobacterium, and Lachnospiraceae, which protect against abnormal metabolic processes [98]. The increase in α- and β-diversity also demonstrated triclosan’s improvement of bacterial diversity and richness [98].
On the other hand, a cohort study showed that triclosan exposure causes a decrease in the diversity of the gut microbiota in breast-fed infants [99]. This study, however, is not representative of the general population due to the small sample size and the high vulnerability of the infant gut microbiota to antimicrobial agents. Additionally, rather than thinking of triclosan as a perturbator of a balanced microenvironment, it would be interesting to consider it as a modulator of the gut microbiota in patients with a state of gut dysbiosis [99]. Indeed, triclosan is employed in periodontal disease, which could be considered a model of oral dysbiosis [100]. Nonetheless, oral dysbiosis, which is characterized by an increased abundance of Porphyromonas gingivalis and Aggregatibacter actinomycetemcomitans, has been associated with the impairment of the gut microbiota equilibrium and the loss of gut microbial diversity [101,102]. Moreover, these oral pathobionts have been associated with NAFLD and NASH progression in preclinical models [101,103]. As a consequence, periodontal therapy can improve oral and gut dysbiosis in patients with chronic liver diseases [104]. In particular, periodontal therapy in cirrhotic individuals reduces blood levels of inflammatory cytokines and lipopolysaccharide (LPS), with beneficial effects upon both quality of life and in mitigating the development of hepatic encephalopathy [104]. Triclosan, as a treatment for periodontal disease [105], has been shown to reduce the production of pro-inflammatory cytokines (such as IL-8, IL-1 α, and TNFα) in a human epithelial cells, monocytes, and fibroblast cultures exposed to LPS [106]. More specifically, triclosan treatment inhibits the TLR-4 pathway by inducing the microRNA miR146a to downregulate IRAK1 and TRAF6 proteins. Conversely, triclosan exposure increases the epithelial cells’ production of other bioactive anti-microbial molecules, such as β-dsefensins [106]. Interestingly, β-defensins have been proposed as a key factor in the physiological homeostasis between the host and the microbiome [107].
In conclusion, triclosan, an antimicrobial agent with a long history, has been suggested to have a positive impact on the microbiota in the human gut. In addition to its well-known antibacterial action, triclosan could have a pleiotropic effect on different cell types, directly orchestrating physiological homeostasis between microbiota and their host. However, more investigations are necessary.
Triclosan’s eubiotic effects are summarized in Figure 3.
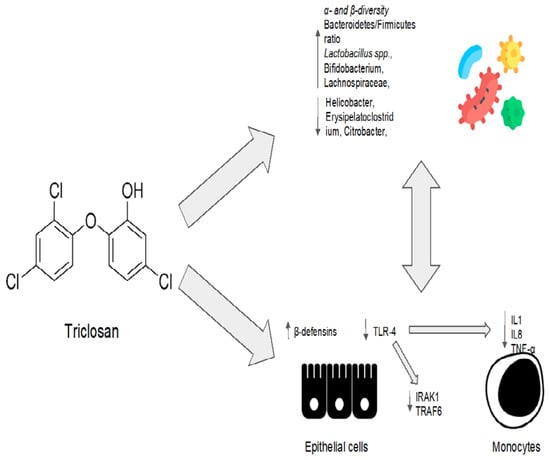
Figure 3.
Triclosan’s eubiotic effects. IL1: interleukin 1; IL8: interleukin 8; TNF-α: tumor necrosis factor alpha; IRAK1: interleukin 1 receptor-associated kinase 1; TRAF6: TNF-receptor-associated factor 6.
Some studies suggest that triclosan may have a beneficial modulatory effect on the gut microbiota, promoting bacterial diversity and the abundance of “good” bacteria. In vitro studies have shown that triclosan can promote the epithelial–microbiota balance by inducing the production of β-defensins and inhibiting the activation of the Toll-like receptor 4 (TLR4) pathway. Furthermore, triclosan treatment is associated with a decrease in the production of inflammatory cytokines by immune cells.
2.4. Natural Products: Promising Agents for the Modulation of Microbiota
Evodiamine is an alkaloid, mainly present in the Evodia Fructus, which is officially listed in the Chinese Pharmacopoeia as a remedy for patients suffering from viral hepatitis, cholangitis, and gastric ulcers, among other disorders [108]. Evodiamine has been demonstrated to have an antimicrobial activity in vitro, inhibiting bacterial topoisomerase I [108].
Evodiamine’s impact on intestinal inflammation and the gut microbiota was examined in a preclinical study [109]. In order to create a intestinal inflammatory tumor mouse model, azomethane/sodium dextran sulfate was used [109]. The tumor model was then treated with evodiamine and 5-aminosalicylic acid. Evodiamine and 5-aminosalicylic acid both prevented the growth of tumors and induced the death of tumor cells [109]. A quantitative polymerase chain reaction analysis showed that evodiamine and 5-aminosalicylic acid reduced the number of Enterococcus faecalis and Escherichia coli while increasing the abundance of Bifidobacterium, Campylobacter, and Lactobacillus when compared to the control group [109]. The IL6/STAT3/P65 signaling pathway was inhibited, and levels of inflammatory factor, d-lactic acid, and serum endotoxin were all significantly decreased in the evodiamine group [109]. Evodiamine also showed efficacy in preventing colorectal tumors in a mouse model of chemically induced colitis [110]. In this model, evodiamine increased the abundance of SCFA-producing bacteria, inhibiting the harmful bacteria [110]. After evodiamine treatment, histological analysis showed a remarkable reversion of intestinal epithelial structure destruction, inflammatory cells infiltration, and crypt loss. The intestinal barrier was also restored, with an increased expression of occludin, zonula occludens-1, and E-cadherin [110,111]. Additionally, evodiamine decreased the expression of pro-inflammatory genes involved in the Wnt signaling pathway, the Hippo signaling pathway, and the IL-17 signaling pathway [110]. This anti-inflammatory effect could be mediated by the downregulation of the nuclear factor-kappa B (NF-κB) signal and the inhibition of NLRP3 inflammasome activation, as shown in another sodium-dextran-sulfate-induced colitis murine model [111].
The effect of evodiamine was also evaluated on H. pylori in an in vitro gastric adenocarcinoma model [112]. Evodiamine treatment decreased the bacterial production of the type IV secretion system components and the system subunit protein A protein and the expression of cytotoxin-associated antigen A (CagA) and vacuolating cytotoxin A (VacA). This resulted in a reduction in CagA end VacA proteins in tumor cells. In addition, evodiamine specifically prevented the H. pylori-infection-induced stimulation of signaling proteins such as NF-κB and the mitogen-activated protein kinase (MAPK) pathway. As a result, IL-8 secretion in tumoral cells was reduced [112].
Evodiamine and berberine were combined in order to determine their effect on the gut microbiota of rats fed a high-fat diet [113]. The treatment with evodiamine and berberine increased the diversity of the gut microbiota globally [113]. The gut microbiota profiles were determined via the high-throughput sequencing of the bacterial 16S ribosomal RNA gene [113]. In comparison to the control group, there were higher proportions of SCFA-producing bacteria (Lactobacillus, Prevotella, Ruminococcaceae, and Bacteroides). In contrast, the key bacteria responsible for the imbalance in the gut microbiota in the group with a high-fat diet were Fusobacteria and Lachnospiraceae [113]. The high-fat-diet group’s Firmicutes/Bacteroidetes ratio was significantly higher than that of the control group [113]. The administration of evodiamine and berberine reverted the increasing trend of the ratio. Evodiamine–berberine also lowered the intestinal submucosal edema typical of a high-fat diet and the mucosal inflammatory cell infiltration [113]. As a result, the rats treated with evodiamine and berberine showed a significant reduction in body weight, as well as plasma triglyceride and total cholesterol levels [113]. Liver injury (measured via plasma levels of aspartate aminotransferase, alanine aminotransferase, and gamma-glutamyl transpeptidase) was significantly reduced [113]. Evodiamine’s eubiotic effects are summarized in Figure 4.
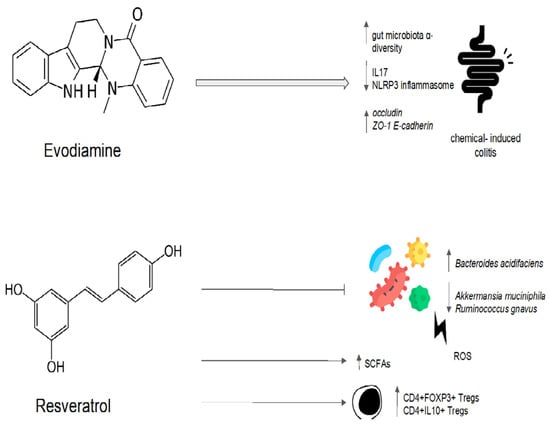
Figure 4.
Possible mechanisms of the eubiotic effect of evodiamine and resveratrol. IL17: interleuchina-17; NLRP3: NOD-like receptor family, pyrin domain-containing protein 3; ROS: reactive oxygen species; ZO-1: zonula occludens-1; E-cadherin: epithelial cadherin.
The capacity of other substances to alter the microbiota has been researched in relation to their potential role in the treatment of colorectal cancer. Natural products, and in particular natural extracts, are frequently the source of anticancer agents. In mice with heterotopic xenograft colorectal cancer, an ethanol extract of Euphorbia lathyris was found to reduce tumor size [114]. When compared to mice without colorectal cancer, a gut microbiota analysis showed a significant decrease in the abundance of Lactobacilli, with predominance of colitogenic bacteria such as Akkermansia and Turicibacter. Turicibacter disappeared after treatment with an ethanol extract of Euphorbia lathyris, and Lactobacillus abundance recovered [114]. Nevertheless, it is still unclear whether the Euphorbia lathyris extract impacted the microbiota directly or whether it modulated gut microorganisms as a result of its anti-neoplastic effects.
Propolis is a resinous material collected by bees from the buds and resins of plants and mixed with bee enzymes, pollen, and wax [115]. It has been used as a traditional remedy worldwide. In some countries, it is considered a complementary medicine, while it is regulated as a food supplement in others [116]. It contains a variety of molecular compounds, such as flavonoids, terpenes, phenolic acids, β-steroids, and the derivatives of sesquiterpenes, naphthalene, and stilbenes [117]. Its antioxidant, anti-inflammatory, and antimicrobial activities are probably due to its polyphenol contents. Concerning the microbiota, propolis has been demonstrated to modulate intestinal bacteria, resulting in an improvement in intestinal barrier function in diabetic rats [117]. However, due to the content variability of propolis, studies require the standardization of its content. A recent preclinical trial showed that a standardized polyphenol propolis mixture modulated the gut microbiota in an in vitro human model, determining an increase in the production of SCFAs [116]. A propolis mix with 7.21 g of total polyphenols/g was specifically used. The propolis extract’s polyphenols included quercetin, apigenin, pinobanskin, chrysin, pinocembrin, and galangin at defined dosages. The standardized polyphenol combination underwent an in vitro digestion process which mimicked human digestion before being fermented by fecal microbiota isolated from healthy adults and children, obese children, celiac children, and children with a food allergy. The production of SCFAs significantly increased following these processes, as shown by a combined chromatographic and UV detection technique. Particularly, samples from the allergic, obese, and celiac patients had significantly higher levels of acetic acid production than the samples from healthy people. On the other hand, compared to other samples, the samples from individuals with allergies had higher levels of propionic acid [116].
Polyphenols are primarily produced by plants as defense against pathogenic microorganisms [118]. As different microbes have different metabolisms, polyphenols appear to selectively inhibit bacterial growth. Tannic acid, for instance, did not affect the growth of Bifidobacterium infantis and Lactobacillus acidophilus, but it inhibited the growth of Clostridium clostridiiforme, E. coli ATCC 25,922, and Enterobacter cloacae ATCC 13,047 in anaerobic conditions. Tannic acid is a potent iron chelator, and its ability to inhibit bacterial growth may be due to the fact that it deprives bacteria of the iron that they require for metabolism (Bifidobacterium infantis and Lactobacillus acidophilus do not) [119]. Other polyphenols have a more specific mechanism of action. Epigallocatechin directly binds the peptidoglycan in the S. aureus cellular wall, causing osmotic damage and bacterial death [120]. Furthermore, tea polyphenols exert direct damage on the outer and inner membranes of S. marcescens, dramatically increasing cellular permeability and thus inhibiting bacterial growth [121].
One of the most investigated polyphenols is resveratrol, a phytoalexin present in many plants such as grapes, peanuts, and berries [122]. Resveratrol has demonstrated antimicrobial and antifungal activity by preventing the formation of biofilms [123,124,125]. Resveratrol, previously administered to mice receiving intrarectal treatment with the oxidizing agent 2,4,6-trinitrobenzenesulfonic acid, demonstrates a beneficial effect on the gut microbiota. Genomic DNA analysis and 16S rRNA gene sequencing revealed that oxidative stress increased species such as Bacteroides acidifaciens and decreased species such as Ruminococcus gnavus and Akkermansia muciniphila, according to an analysis of stool microbiota. The administration of resveratrol returned the gut bacteria to their homeostatic levels and increased the production of butyric acid. A mesenteric lymph node analysis via cytometry showed a significant increase in the percentages of both anti-inflammatory CD4+FOXP3+ Tregs and CD4+IL10+ in the resveratrol-treated group. Meanwhile, inflammatory Th1/Th17 cells were suppressed, and mucosal inflammation was also significantly reduced [126]. A microarray analysis of microRNA profiles in mesenteric lymph node cells highlighted that resveratrol treatment was associated with miR-31, Let-7a, and miR-132 downregulation [127]. All these microRNAs target anti-inflammatory T cells. In particular, miR-31 inhibits the production of FoxP3 [128]. It is noteworthy to observe that miR-31 expression in tissues connected to the disease is considerably greater among individuals with ulcerative colitis [127].
Moreover, by lowering inflammation and regulating hepatic lipid metabolism, resveratrol has been demonstrated to lower the risk of non-alcoholic fatty liver disease (NAFLD). Resveratrol treatment reduced hepatic steatosis in male mice fed with a high-fat diet and modified the composition of the gut bacteria by enhancing the growth of SCFA-producing bacteria such as Allobaculum, Bacteroides, and Blautia [129]. A clinical trial investigated the impact of resveratrol on the fecal microbiota of overweight men and women [130]. In comparison to women, men have a higher fecal abundance of Bacteroidetes. Resveratrol (282 and 80 mg/day, respectively) and another phenolic compound called epigallocatechin-3-gallate were combined for 12 weeks [130]. Men experienced a decline in the relative abundance of Bacteroidetes, but women did not. Administration had no effect on firmicutes, actinobacteria, gammaproteobacteria, Akkermansia muciniphila, sulfate-reducing bacteria, or acetogenic bacteria in either men or women. By using indirect calorimetry, it was demonstrated that fat oxidation was increased in men compared to women [130]. These findings highlight the importance of a gut microbiota modulator’s ability to restore an altered equilibrium. Instead of acting against a specific target, a good modulator suppresses a deregulated element until it resumes its homeostatic role [130]. Resveratrol’s eubiotic effects are summarized in Figure 4.
The Mediterranean diet, with a plant-based profile, is distinguished by a notable richness in polyphenols [131]. Indeed, a diet similar to the Mediterranean diet was strongly linked to a microbial composition in which SCFA-producing bacteria, such as Ruminococcus, predominated. When compared to other dietary patterns, it was also linked to lower levels of fecal calprotectin [132]. The majority of research on polyphenols use purified and more concentrated polyphenol extracts [133]. However, it has been shown that foods rich in polyphenols, such as almonds and cranberries, can modulate the gut microbiota as well [134,135].
Other relevant substances have been discovered to be elevated in the serum of people eating a Mediterranean diet. Indole-3-propionic acid is one specific example. As previously mentioned, this tryptophan metabolite, which is produced by gut bacteria, induces tuft cells to produce IL-17E, which has a modulatory effect on the gut microbiota, preventing lipoperoxidation and oxidative stress injury and reducing the synthesis of proinflammatory cytokines [136]. The administration of indole-3-propionic acid resulted in a significantly different composition of the intestinal microbiota in a sepsis mouse model, with an enrichment of the Bifidobacteriaceae family and a depletion of the Enterobacteriaceae family. In comparison to the control group, it resulted in lower serum inflammatory mediator levels and a higher survival rate [137]. This emphasizes once more how a compound that affects the gut microbiota can modify a systemic condition. In a different preclinical study, indole-3-propionic acid prevented the development of nonalcoholic steatohepatitis in mice fed a high-fat diet [138]. An analysis of the fecal microbiota via DNA sequencing techniques revealed a decrease in the Firmicutes/Bacteroidetes ratio as well as a decrease in the overall population of the pathobiont Streptococcus [138]. Rats fed a high-fat diet showed a loss of the normal villus structure of the ileum epithelium at the histological level. Treatment with indole-3-propionic acid improved the expression of tight junction proteins and restored the height of the ileum villus [138]. In addition, endotoxin plasma levels were lower in mice treated with indole-3-propionic acid than in the control group. As a result, when indole-3-propionic acid was administered, a significant histological reduction in steatohepatitis was seen [138]. Nonetheless, indole-3-propionic acid has been found to reverse dysbiosis induced by total abdominal irradiation in a mouse model, which was characterized by a decrease in the relative abundance of Lactobacillus and an increase in Bacteroides acidifaciens and Ruminococcus gauvreauii [139].
Evodiamine administration in murine models of chemically induced colitis has confirmed its potential role as a eubiotic. In fact, the modulatory effect of evodiamine results in an increase in α-diversity and a consequent inhibition of inflammation, as demonstrated by the decrease in IL17 production and NLRP3 inflammasome inhibition. Moreover, evodiamine can improve the epithelial barrier by restoring the expression of occludin, ZO-1, and E-cadherin after chemical damage. Resveratrol has also been shown to improve disruption to the gut microbiota after oxidative stress damage. The eubiotic effects of resveratrol are associated with an increase in the production of short-chain fatty acids (SCFAs) and the activation of the anti-inflammatory phenotype regulatory T cells (Tregs).
The dynamic actions of pharmaceutical agents are a fascinating topic. Monodirectional approaches are insufficient when dealing with the complex microenvironment that is the microbiota. It is necessary to think about an intervention that can restore the disrupted balance, enhancing the resilience of the gut microenvironment. Far from being considered merely antimicrobial, these drugs are contributing to the definition of a new therapeutic paradigm. Single-target therapies have the risk of worsening the already fragile equilibrium. To restore the organism’s balance, rather than eliminating the bacteria thought to be responsible for the disruption, it is reasonable to promote the harmonious growth of all bacterial species, including the “harmful” ones. In this regard, a strategy that promotes a healthy metabolic and immunologic cooperation with the host, providing a quantitative and qualitative harmonic balance of the gut microbial components, is required. This is known as eubiosis because it is an ideal condition for a living system. Eubiotics refer to the strategies that can be used to achieve this goal. Antibiotics, but also chemically produced antimicrobials, plant extracts, or compounds and even metabolites of the same gut microbiota, could play a role. Thus, the pharmacological approach, along with dietary changes and lifestyle intervention, should be integrated into a complex model, leaving the door open to other techniques, among which the possibility of directly changing the composition of the microbiota is playing an increasingly intriguing role. A summary of the main eubiotics studied and their mechanisms of action is reported in Table 2.

Table 2.
Eubiotics, mechanisms of action, and a shift in microbial composition.
3. Fecal Microbiota Transplantation
Fecal microbiota transplantation (FMT) is defined as the infusion of feces from a healthy, screened donor to a recipient to restore a disorder associated with a perturbation of the gut microbiota (dysbiosis). Over time, FMT has been proven to be an effective and safe procedure and has been established as a reliable treatment option for recurrent Clostridioides difficile infection (CDI) [140,141,142,143,144].
The FMT process is characterized by several key steps that may differ among different centers. The first cornerstone of the FMT framework is represented by the selection of stool donors [145]. Current guidelines for FMT in clinical practice [146] recommend a specific four-step selection process, including a clinical interview, blood and stool testing, a further questionnaire, and a direct molecular stool testing on the day of each donation [147]. In the last year, donor screening has been updated to prevent the risk of COVID-19 dissemination [148,149]. Recently, the need to make FMT an equitable, accessible, widespread, and secure procedure have led to the development of stool banks [150], with the principle aim of providing FMT to health centers in a safe and traceable manner. FMT protocols may also differ according to the route of delivery. More specifically, FMT could be administered by upper GI routes (including upper GI endoscopy and nasogastric/nasoduodenal tube) [151,152], capsules [153,154], and via lower GI routes (enema [155] and colonoscopy [142,156]). Colonoscopy and capsule administration are known to be the most effective routes in the treatment of recurrent CDI, with a cure rate of nearly 90% for colonoscopies [157] and 85% for capsules [158,159].
At present, and over the course of the years, FMT has been established as a reliable therapeutic alternative to vancomycin and fidaxomicin for the treatment of recurrent Clostridioides difficile infection (CDI) [140,141,142], as recommended by international guidelines and consensus reports [146,150,160]. In this setting, a large body of evidence supports the efficacy of FMT also in the treatment of severe and complicated CDI. Particularly, FMT has been proven to be effective in reducing the risk of CDI- associated bloodstream infections [161] and in decreasing the need for surgical treatment [162], with an increase of overall survival in patients with recurrent CDI [161]. It was also shown to be a cost-effective strategy for this disorder. FMT has also proven to be highly effective and superior to the standard of care alone, vancomycin, in achieving a sustained resolution of the first episode of CDI [163]. After the satisfactory results obtained in CDI, in recent years, a growing number of studies have been carried out to investigate the role of FMT in the treatment of noncommunicable chronic disorders, including inflammatory bowel disease (IBD) [164,165], irritable bowel syndrome (IBS) [152], psychiatric disorders [166], metabolic disorders [167], liver disease [168], autoimmune disorders, and cancer [169,170,171]. In these areas, FMT has achieved promising but heterogenous results, making it impossible to draw any firm clinical conclusions as to its efficacy in these settings.
Increasing evidence suggests that FMT’s clinical success in chronic disorders may be influenced by several factors related to the microbial characteristics of both donors and recipients or closely related to FMT working protocols. Recently, the role of ecological parameters and the taxonomic composition of the donor microbiome, including bacteria, bacteriophages, and fungi, has been investigated in several studies. In FMT trials [172,173] enrolling patients with IBD, the clinical response to FMT was very closely associated with higher donor evenness and richness. This finding correlates with the known evidence that alpha diversity can be considered a marker of human health and is indirectly related to the engraftment success [174,175,176]. Beyond ecological parameters, the compositional characteristics of the donor microbiome also appear to influence FMT efficacy. In a recent randomized trial [152] of patients with IBS, the use of a “super donor” was associated with a significantly higher success rate than a placebo. Moreover, the route of delivery and the amount of infused feces also appear to be other parameters able to influence clinical response after FMT. The clinical success of FMT is higher when the administration is performed via colonoscopy [157] or capsules [153,154], with the use of a suitable amount of feces (>50 g) [152] or with a protocol employing multiple infusions [142,177]. Furthermore, pre-conditioning with antibiotics may improve FMT’s clinical efficacy, probably modifying alpha diversity and improving engraftment. Unfortunately, nowadays, the interpretation of the reproducibility and effectiveness of FMT are fragmented by the adoption of different protocols.
The recent understanding of FMT’s mechanisms has allowed us to associate the role of donor microbiome engraftment (defined as the number of engrafting strains by the total detected strains in the donor and recipient [178]) with the clinical response [151,178,179]. The close association of engraftment with the clinical response to FMT has been identified in a recent metagenomic metanalysis of 24 FMT trials, including communicable and non-communicable disorders, in which engraftment was found to be higher in communicable disorders and after the antibiotic pre-conditioning of the recipient [178]. The increasingly crucial role of engraftment in FMT has created the need to assess it using precise tools, mainly whole-genome sequencing (WGS), which provides a higher taxonomic resolution than 16S rRNA gene sequencing. Despite its enormous value, a widespread diffusion of WGS is still limited by its high costs and analytical complexity which require staff with bioinformatics and computational skills [180].
Beyond the knowledge of FMT’s mechanisms for improving clinical response, another relevant issue in this field is represented by the precision and reproducibility of FMT, as the more we need targeted donor microbiomes, the more it will be hard to find and, in particular, keep them (as the donor microbiome can be perturbed over time). In recent years, novel, advanced FMT preparations [153,154]—including lyophilized fecal material, bacterial consortia, and live biotherapeutic products (LBPs) [181,182]—have emerged as alternatives to “classical” FMT and are also appearing in clinical practice for the prevention of CDI recurrence; however, their efficacy for noncommunicable disorders has to be proved yet.
4. Engineered Bacteria and Phage Therapy
One of the most significant limits of the current techniques used to modulate the gut microbiota is due to a lack of tools that can precisely modulate specific members of complex microbial communities. To the date, two different approaches have been hypothesized to overcome these limits: the first approach is based on delivering ex vivo-engineered bacteria into the gut, and the alternative approach involves taking advantage of the rich microbial ecosystem in the gut by genetically modifying the microbiome in situ through use of engineered bacteriophages [183].
4.1. Engineered Bacteria
The “ex vivo” approach provides bacteria that are engineered ex vivo to secrete therapeutic molecules or to sense one or more biomarkers and can be introduced to the microbiome. Engineered gut bacteria can be divided into three main classes: drug factory probiotics, diagnostic gut bacteria, and smart probiotics [184] (Table 3).

Table 3.
Classification and main studies of engineered bacteria.
Drug factory probiotics are bacteria engineered to constitutively produce a therapeutic molecule within the body [184]. In 2000, a natural probiotic, Lactococcus lactis, was genetically modified to constitutively secrete the human anti-inflammatory cytokine protein interleukin-10 (IL-10); when orally administrated in rat models of colitis, a significant reduction of inflammation was assessed [185]. This evidence inspired countless subsequent studies: the same natural probiotic, L. lactis, was modified to secrete a variety of anti-inflammatory molecules, including anti-tumor necrosis factor (TNF) nanobodies (certolizumab) [186], IL-27 [187] and recombinant mouse heme oxygenase-1 (rmHO-1) [188]. All these studies used drug factory probiotics, which were administered orally, and they all showed that this method reduced inflammation in the mouse gut more than systemically administered drugs. These probiotics have been studied in a wide range of disorders: for example, Lactobacillus gasseri, which secreted full-length protein GLP1, induced the differentiation of rat epithelial cells into functional glucose-responsive insulin-producing cells, and this improved glucose control in a rat model of diabetes mellitus [189];
On the other hand, diagnostic gut bacteria are defined as bacteria that sense one or more biomarkers, compute that those biomarkers are present in a combination indicative of disease, and produce a reporter that can be externally measured by a clinician [184]. Using engineered bacteria to sense transient molecules that are degraded, modified, or absorbed before exiting the gut and thus cannot be easily captured and quantified by traditional non-invasive tests, is an exciting prospect for measuring novel biomarkers. In 2015, an engineered E. coli Nissle 1917 was used as diagnostic tool to detect the presence of liver metastasis in mouse models: the probiotic was engineered to express an enzyme that could cleave a systemically administered substrate, leading to a color change that was detected in urine. After oral delivery, this modified E. coli Nissle 1917 generated a high-contrast urine signal through the selective expansion of the probiotic in liver metastases, demonstrating that probiotics can be programmed to safely and selectively deliver synthetic gene circuits to diseased tissue microenvironments in vivo [190]. In 2017 [191], Daffler and his collaborators hypothesized that thiosulfate could serve as a novel biomarker of colitis and engineered E. coli Nissle 1917 to carry sensors that detected increased thiosulfate levels during chemically induced colitis in mice. Furthermore, the greater the extent of inflammation, the greater the thiosulfate receptor activity was, suggesting that thiosulfate may be a novel colitis biomarker.
Smart probiotics are bacteria that sense one or more biomarkers, compute that those biomarkers are present in a combination indicative of disease, and respond by delivering a precise dose of one or more appropriate therapeutics at the diseased tissue [184]. To date, such smart probiotics still are not available: further progress in synthetic biology is needed to create such a complex model of engineered bacteria. Selecting the chassis for smart probiotics requires careful evaluation: the capacity to survive transit through the gastrointestinal tract, colonize macroscopic or microscopic geographical locations within the gut, and reach desired densities there must be considered. Most engineered probiotics use a small set of bacterial species; Lactococcus lactis and Escherichia coli are commonly used for oral delivery to the gut; attenuated versions of Salmonella enterica subsp. enterica serovar Typhimurium, a pathogen capable of protein expression and immune stimulation in the human body, target the hypoxic tumor environment when administered systemically and the gut mucosa when provided orally [192,193]; attenuated Listeria monocytogenes, another pathogen, activates the immune system through growth within circulating immune cells to elicit antitumor responses [194].
There are several drawbacks that have limited this bacterial “cell therapy” approach. First, achieving stable engraftment of engineered microbes into the endogenous microbiota can be difficult. For example, the bacteria used in the clinic to date either have short or no colonization capacity in humans (L. lactis and E. coli) or are capable of being cleared by routine antibiotic administration (S. Typhimurium and L. monocytogenes). Moreover, there will likely be substantial variations in engraftment efficiency between different individuals due to inherent variations in their existing microbiomes. Thus, it is difficult to control or predict the long-term engraftment of exogenously delivered bacteria within any given patient population. Finally, even if the introduced strains can achieve stable engraftment, they may outcompete endogenous commensals and adversely alter the balance of the microbial ecosystem [183]. Furthermore, another concern is the uncontrolled growth of engineered bacteria within the gut. In order to reach the safe use of smart probiotics in clinical practice, biocontainment strategies are needed.
4.2. Phage Therapy
In addition to this “ex vivo” strategy, an “in vivo” approach has been developed and is one of the most cutting-edge frontiers in the manipulation of gut microbiota: phage therapy. Phages have been widely studied as a possible therapy against bacterial infection [195,196]; however, the aim of this review is to focus exclusively on their potential use as modulators of the gut microbiota. In the past decade, phages have been used for the precision editing of the gut microbiota. In 2017, phages against adherent-invasive E. coli (a bacteria implicated in the pathogenesis of inflammatory bowel disease) were administered in mice models of dextran-sodium-sulfate-induced colitis: mice receiving phage treatment were found to be protected from DSS-induced colitis, and E. coli colonization was reduced [197]. A phase I/IIa randomized, double-blind, placebo-controlled clinical trial is underway to assess the safety and efficacy of the oral administration of phages that target intestinal adherent–invasive E. coli in patients with Crohn’s disease in remission (NCT03808103) [198]. Enterococcus faecalis is significantly increased in patients with alcoholic hepatitis compared with patients without alcohol use disorder and in particular, the presence of E. faecalis strains that produce cytolysin (a bacterial exotoxin) correlates with worse outcomes: in 2019, a study showed that mouse models treated via oral gavage with phages specifically targeting cytolysin-positive E. faecalis showed reductions in ethanol-induced liver disease [199]. These are great examples that highlight the potential of phage therapy in the modulation of gut microbiota, but both these studies make use of natural phages: the next step in the field of phage therapy is represented by the possibility of engineering phages to deliver recombinant DNA to target bacteria or deliver drugs to a specific location with specificity down to the strain level.
In situ microbiome engineering involves the delivery of transgenes into specific members of the endogenous microbiota. Since it directly modifies the endogenous microbiota, this type of therapy overcomes all the major drawbacks of the “ex vivo” strategy: by editing species that have long established long-term colonization, the genetically altered bacteria will likely be retained in the gut for much longer than the exogenously dosed bacteria, possibly achieving stable engraftment. Additionally, since the microbiome’s composition is not directly altered, there is probably less of an effect on other microbiome members that could endanger the delicate balance of this microbial ecosystem [183]. Although engineered bacteriophages represent an interesting and promising perspective in the field of gut microbiota modulation, current clinical trials involving phages are limited to their use as antimicrobials or diagnostics [183]. This is due to several difficulties that have yet to be resolved as of the time of writing, including the need to modulate bacterial target specificity, overcome bacterial immunity, and limit the spread of any exogenous DNA and engineered organisms using appropriate biocontainment systems. Phages’ exceptional specificity (often only a few strains of a given bacterial species are targeted by these viruses, and even closely related strains of the same bacterial species cannot be infected by them) [200,201,202] is indeed an advantage when a specific pathogen must be eliminated without disturbing other members of the same genus or species, but it can be an obstacle when the objective is to broadly affect multiple members of the microbiome among multiple individuals: to achieve a therapeutic effect, phage specificity must be adjusted to a level that permits the transduction of a sufficient number of bacterial strains [201].
Engineering the phage’s host recognition domain (HDR), a virus structure that controls the interface between phages and bacterial surface molecules, can achieve this objective [203]: several investigators have attempted to rationally design phages with an altered host range through HRD engineering [204,205,206,207,208]. Moreover, to achieve a stable modification in the gut microbiota though the use of phages, overcoming bacterial innate immunity is indispensable. Of all the known bacterial phage defense systems, the one called restriction modification (R-M) is the most abundant and is found in 75% to 95% of all known bacterial genomes [209,210,211]. This system functions through two components: endonucleases, which recognize and cleave specific DNA sequences, and cognate methyltransferases, which protect host DNA of the same sequence via methylation. In 2015, Robert et al. created a database called REBASE for the components of R-M systems. Covering recognition and cleavage sites of both restriction enzymes and methyltransferases of all completely sequenced genomes, REBASE is a powerful tool for designing plasmids with the proper methylation patterns necessary for known bacterial targets [212].
Overall, phage-based therapies could become promising and powerful approaches for modulating the gut microbiota, but more basic and preclinical studies, as well as properly designed randomized, double-blind, placebo-controlled trials, are required to help the field move forward.
5. Conclusions
Collectively, a growing suite of strategies for manipulating the gut microbiome—both highly targeted, and whole-ecosystem-based—have recently appeared in mainstream medicine. Eubiotics, mainly rifaximin, are increasingly becoming a cornerstone of specific disorders, including IBS and HE. Novel eubiotics, including other antimicrobials (e.g., triclosan) and natural products (e.g., polyphenols and propolis), appear to be a promising approach, but further studies are needed to confirm their value as microbiome modulators. FMT, after becoming a well-established therapy against recurrent CDI, has been investigated in other non-communicable disorders with variable results. To improve the outcomes of FMT in disorders beyond CDI, an understanding of the therapeutic mechanisms (e.g., microbial engraftment), as well as the application of novel technologies (e.g., whole genome sequencing), is advocated. Finally, novel approaches, e.g., the use of engineered probiotic bacteria or a bacteriophage-based therapy, have recently appeared as promising opportunities for providing the targeted and tailored therapeutic modulation of gut microbiota, but their role in clinical practice has yet to be clarified.
Author Contributions
Conceptualization, G.I. and F.R.P.; writing—original draft preparation, C.A., A.S., S.P. and W.F.; writing—review and editing, C.A., A.S., B.H.M., S.P. and W.F.; supervision, A.G., G.C., G.I. and F.R.P. All authors have read and agreed to the published version of the manuscript.
Funding
BHM is the recipient of an NIHR Academic Clinical Lectureship; the Division of Digestive Diseases at Imperial College London receives financial and infrastructure support from the NIHR Imperial Biomedical Research Centre (BRC) based at Imperial College Healthcare NHS Trust and Imperial College London (funding number: CL-2019-21-002).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Gomaa, E.Z. Human gut microbiota/microbiome in health and diseases: A review. Antonie Van Leeuwenhoek Int. J. Gen. Mol. Microbiol. 2020, 113, 2019–2040. [Google Scholar] [CrossRef] [PubMed]
- Metchnikoff, E. The Prolongation of Life: Optimistic Studies; Springer Publishing Company: New York, NY, USA, 2004. [Google Scholar]
- Blaser, M.J. The microbiome revolution. J. Clin. Investig. 2014, 124, 4162–4165. [Google Scholar] [CrossRef] [PubMed]
- Ianiro, G.; Bruno, G.; Lopetuso, L.; Bartoli Beghella, F.; Laterza, L.; D’Aversa, F.; Gigante, G.; Cammarota, G.; Gasbarrini, A. Role of yeasts in healthy and impaired gut microbiota: The gut mycome. Curr. Pharm. Des. 2014, 20, 4565–4569. [Google Scholar] [CrossRef] [PubMed]
- Ianiro, G.; Iorio, A.; Porcari, S.; Masucci, L.; Sanguinetti, M.; Perno, C.F.; Gasbarrini, A.; Putignani, L.; Cammarota, G. How the gut parasitome affects human health. Ther. Adv. Gastroenterol. 2022, 15, 17562848221091524. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Pedersen, O. Gut microbiota in human metabolic health and disease. Nat. Rev. Microbiol. 2021, 19, 55–71. [Google Scholar] [CrossRef]
- D’Aversa, F.; Tortora, A.; Ianiro, G.; Ponziani, F.R.; Annicchiarico, B.E.; Gasbarrini, A. Gut microbiota and metabolic syndrome. Intern. Emerg. Med. 2013, 8 (Suppl. 1), S11–S15. [Google Scholar] [CrossRef]
- Bibbò, S.; Ianiro, G.; Dore, M.P.; Simonelli, C.; Newton, E.E.; Cammarota, G. Gut Microbiota as a Driver of Inflammation in Nonalcoholic Fatty Liver Disease. Mediat. Inflamm. 2018, 2018, 9321643. [Google Scholar] [CrossRef]
- Hou, K.; Wu, Z.-X.; Chen, X.-Y.; Wang, J.-Q.; Zhang, D.; Xiao, C.; Zhu, D.; Koya, J.B.; Wei, L.; Li, J.; et al. Microbiota in health and diseases. Signal Transduct. Target. Ther. 2022, 7, 135. [Google Scholar] [CrossRef]
- Cao, Y.; Oh, J.; Xue, M.; Huh, W.J.; Wang, J.; Gonzalez-Hernandez, J.A.; Rice, T.A.; Martin, A.L.; Song, D.; Crawford, J.M.; et al. Commensal microbiota from patients with inflammatory bowel disease produce genotoxic metabolites. Science 2022, 378, eabm3233. [Google Scholar] [CrossRef]
- Malesza, I.J.; Malesza, M.; Walkowiak, J.; Mussin, N.; Walkowiak, D.; Aringazina, R.; Bartkowiak-Wieczorek, J.; Mądry, E. High-Fat, Western-Style Diet, Systemic Inflammation, and Gut Microbiota: A Narrative Review. Cells 2021, 10, 3164. [Google Scholar] [CrossRef]
- Martel, J.; Chang, S.-H.; Ko, Y.-F.; Hwang, T.-L.; Young, J.D.; Ojcius, D.M. Gut barrier disruption and chronic disease. Trends Endocrinol. Metab. 2022, 33, 247–265. [Google Scholar] [CrossRef] [PubMed]
- Di Tommaso, N.; Gasbarrini, A.; Ponziani, F.R. Intestinal Barrier in Human Health and Disease. Int. J. Environ. Res. Public Health 2021, 18, 12836. [Google Scholar] [CrossRef] [PubMed]
- da Silva, T.F.; Casarotti, S.N.; de Oliveira, G.L.V.; Penna, A.L.B. The impact of probiotics, prebiotics, and synbiotics on the biochemical, clinical, and immunological markers, as well as on the gut microbiota of obese hosts. Crit. Rev. Food Sci. Nutr. 2020, 61, 337–355. [Google Scholar] [CrossRef] [PubMed]
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canani, R.B.; Flint, H.J.; Salminen, S.; et al. Expert consensus document: The international scientific association for probiotics and prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514. [Google Scholar] [CrossRef] [PubMed]
- Salminen, S.; Collado, M.C.; Endo, A.; Hill, C.; Lebeer, S.; Quigley, E.M.M.; Sanders, M.E.; Shamir, R.; Swann, J.R.; Szajewska, H.; et al. The International Scientific Association of Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of postbiotics. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 649–667. [Google Scholar] [CrossRef] [PubMed]
- Vallianou, N.; Stratigou, T.; Christodoulatos, G.S.; Tsigalou, C.; Dalamaga, M. Probiotics, Prebiotics, Synbiotics, Postbiotics, and Obesity: Current Evidence, Controversies, and Perspectives. Curr. Obes. Rep. 2020, 9, 179–192. [Google Scholar] [CrossRef]
- Mosca, A.; Abreu, A.T.A.Y.; Gwee, K.A.; Ianiro, G.; Tack, J.; Nguyen, T.V.H.; Hill, C. The clinical evidence for postbiotics as microbial therapeutics. Gut Microbes 2022, 14, 2117508. [Google Scholar] [CrossRef]
- Rizzatti, G.; Ianiro, G.; Gasbarrini, A. Antibiotic and Modulation of Microbiota. J. Clin. Gastroenterol. 2018, 52 (Suppl. 1), S74–S77. [Google Scholar] [CrossRef]
- Fong, W.; Li, Q.; Yu, J. Gut microbiota modulation: A novel strategy for prevention and treatment of colorectal cancer. Oncogene 2020, 39, 4925–4943. [Google Scholar] [CrossRef]
- Guarner, F.; Khan, A.G.; Garisch, J.; Eliakim, R.; Gangl, A.; Thomson, A.; Krabshuis, J.; Lemair, T.; Kaufmann, P.; De Paula, J.A.; et al. World Gastroenterology Organisation Global Guidelines: Probiotics and Prebiotics. World Gastroenterology Organisation Global Guidelines. 2023. Available online: https://www.worldgastroenterology.org/UserFiles/file/guidelines/probiotics-and-prebiotics-english-2023.pdf (accessed on 11 April 2023).
- Grossi, E.; Buresta, R.; Abbiati, R.; Cerutti, R. Clinical Trial on the Efficacy of a New Symbiotic Formulation, Flortec, in Patients with Acute Diarrhea. J. Clin. Gastroenterol. 2010, 44 (Suppl. 1), S35–S41. [Google Scholar] [CrossRef]
- McFarland, L.V. Systematic review and meta-analysis of Saccharomyces boulardii in adult patients. World J. Gastroenterol. 2010, 16, 2202–2222. [Google Scholar] [CrossRef] [PubMed]
- Greuter, T.; Michel, M.C.; Thomann, D.; Weigmann, H.; Vavricka, S.R. Randomized, Placebo-Controlled, Double-Blind and Open-Label Studies in the Treatment and Prevention of Acute Diarrhea with Enterococcus faecium SF68. Front. Med. 2020, 7, 276. [Google Scholar] [CrossRef]
- Liao, W.; Chen, C.; Wen, T.; Zhao, Q. Probiotics for the Prevention of Antibiotic-associated Diarrhea in Adults. J. Clin. Gastroenterol. 2021, 55, 469–480. [Google Scholar] [CrossRef] [PubMed]
- Newberry, S.J.; Hempel, S.; Maher, A.R.; Wang, Z.; Miles, J.N.V.; Shanman, R.; Johnsen, B.; Shekelle, P.G. Probiotics for the Prevention and Treatment of Antibiotic-Associated Diarrhea. JAMA 2012, 307, 1959–1969. [Google Scholar] [CrossRef]
- Shen, N.T.; Maw, A.; Tmanova, L.L.; Pino, A.; Ancy, K.; Crawford, C.V.; Simon, M.S.; Evans, A.T. Timely Use of Probiotics in Hospitalized Adults Prevents Clostridium difficile Infection: A Systematic Review With Meta-Regression Analysis. Gastroenterology 2017, 152, 1889–1900.e9. [Google Scholar] [CrossRef]
- Johnson, S.; Maziade, P.-J.; McFarland, L.V.; Trick, W.; Donskey, C.; Currie, B.; Low, D.E.; Goldstein, E.J. Is primary prevention of Clostridium difficile infection possible with specific probiotics? Int. J. Infect. Dis. 2012, 16, e786–e792. [Google Scholar] [CrossRef] [PubMed]
- Goldenberg, J.Z.; Yap, C.; Lytvyn, L.; Lo, C.K.-F.; Beardsley, J.; Mertz, D.; Johnston, B.C. Probiotics for the prevention of Clostridium difficile-associated diarrhea in adults and children. Cochrane Database Syst. Rev. 2017, 12, CD006095. [Google Scholar] [CrossRef]
- Yu, M.; Zhang, R.; Ni, P.; Chen, S.; Duan, G. Efficacy of Lactobacillus-supplemented triple therapy for H. pylori eradication: A meta-analysis of randomized controlled trials. PLoS ONE 2019, 14, e0223309. [Google Scholar] [CrossRef]
- Hauser, G.; Salkic, N.; Vukelic, K.; JajacKnez, A.; Stimac, D. Probiotics for Standard Triple Helicobacter pylori Eradication. Medicine 2015, 94, e685. [Google Scholar] [CrossRef]
- Delia, P. Use of probiotics for prevention of radiation-induced diarrhea. World J. Gastroenterol. 2007, 13, 912. [Google Scholar] [CrossRef]
- Liu, M.-M.; Li, S.-T.; Shu, Y.; Zhan, H.-Q. Probiotics for prevention of radiation-induced diarrhea: A meta-analysis of randomized controlled trials. PLoS ONE 2017, 12, e0178870. [Google Scholar] [CrossRef] [PubMed]
- Wei, D.; Heus, P.; van de Wetering, F.T.; van Tienhoven, G.; Verleye, L.; Scholten, R.J. Probiotics for the prevention or treatment of chemotherapy- or radiotherapy-related diarrhoea in people with cancer. Cochrane Database Syst. Rev. 2018, 2018, CD008831. [Google Scholar] [CrossRef] [PubMed]
- Chitapanarux, I.; Chitapanarux, T.; Traisathit, P.; Kudumpee, S.; Tharavichitkul, E.; Lorvidhaya, V. Randomized controlled trial of live lactobacillus acidophilus plus bifidobacterium bifidum in prophylaxis of diarrhea during radiotherapy in cervical cancer patients. Radiat. Oncol. 2010, 5, 31. [Google Scholar] [CrossRef] [PubMed]
- Zhao, R.; Wang, Y.; Huang, Y.; Cui, Y.; Xia, L.; Rao, Z.; Zhou, Y.; Wu, X. Effects of fiber and probiotics on diarrhea associated with enteral nutrition in gastric cancer patients. Medicine 2017, 96, e8418. [Google Scholar] [CrossRef] [PubMed]
- Gluud, L.L.; Vilstrup, H.; Morgan, M.Y. Non-absorbable disaccharides versus placebo/no intervention and lactulose versus lactitol for the prevention and treatment of hepatic encephalopathy in people with cirrhosis. In Cochrane Database of Systematic Reviews; Gluud, L.L., Ed.; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2016. [Google Scholar] [CrossRef]
- Lunia, M.K.; Sharma, B.C.; Sharma, P.; Sachdeva, S.; Srivastava, S. Probiotics Prevent Hepatic Encephalopathy in Patients with Cirrhosis: A Randomized Controlled Trial. Clin. Gastroenterol. Hepatol. 2014, 12, 1003–1008.e1. [Google Scholar] [CrossRef]
- Dhiman, R.K.; Thumburu, K.K.; Verma, N.; Chopra, M.; Rathi, S.; Dutta, U.; Singal, A.K.; Taneja, S.; Duseja, A.; Singh, M. Comparative Efficacy of Treatment Options for Minimal Hepatic Encephalopathy: A Systematic Review and Network Meta-Analysis. Clin. Gastroenterol. Hepatol. 2019, 18, 800–812.e25. [Google Scholar] [CrossRef]
- Eslamparast, T.; Poustchi, H.; Zamani, F.; Sharafkhah, M.; Malekzadeh, R.; Hekmatdoost, A. Synbiotic supplementation in nonalcoholic fatty liver disease: A randomized, double-blind, placebo-controlled pilot study. Am. J. Clin. Nutr. 2014, 99, 535–542. [Google Scholar] [CrossRef]
- Guglielmetti, S.; Mora, D.; Gschwender, M.; Popp, K. Randomised clinical trial: Bifidobacterium bifidum MIMBb75 significantly alleviates irritable bowel syndrome and improves quality of life -- a double-blind, placebo-controlled study. Aliment. Pharmacol. Ther. 2011, 33, 1123–1132. [Google Scholar] [CrossRef]
- Andresen, V.; Gschossmann, J.; Layer, P. Heat-inactivated Bifidobacterium bifidum MIMBb75 (SYN-HI-001) in the treatment of irritable bowel syndrome: A multicentre, randomised, double-blind, placebo-controlled clinical trial. Lancet Gastroenterol. Hepatol. 2020, 5, 658–666. [Google Scholar] [CrossRef]
- Ducrotté, P. Clinical trial: Lactobacillus plantarum 299v (DSM 9843) improves symptoms of irritable bowel syndrome. World J. Gastroenterol. 2012, 18, 4012–4018. [Google Scholar] [CrossRef]
- Ford, A.C.; Harris, L.A.; Lacy, B.E.; Quigley, E.M.M.; Moayyedi, P. Systematic review with meta-analysis: The efficacy of prebiotics, probiotics, synbiotics and antibiotics in irritable bowel syndrome. Aliment. Pharmacol. Ther. 2018, 48, 1044–1060. [Google Scholar] [CrossRef] [PubMed]
- Yeun, Y.; Lee, J. Effect of a double-coated probiotic formulation on functional constipation in the elderly: A randomized, double blind, controlled study. Arch. Pharmacal Res. 2014, 38, 1345–1350. [Google Scholar] [CrossRef] [PubMed]
- Ojetti, V.; Ianiro, G.; Tortora, A.; D‘angelo, G.; Di Rienzo, T.A.; Bibbò, S.; Migneco, A.; Gasbarrini, A. The Effect of Lactobacillus reuteri Supplementation in Adults with Chronic Functional Constipation: A Randomized, Double-Blind, Placebo-Controlled Trial*. J. Gastrointest. Liver Dis. 2014, 23, 387–391. [Google Scholar] [CrossRef] [PubMed]
- Tursi, A.; Brandimarte, G.; Elisei, W.; Picchio, M.; Forti, G.; Pianese, G.; Rodino, S.; D’Amico, T.; Sacca, N.; Portincasa, P.; et al. Randomised clinical trial: Mesalazine and/or probiotics in maintaining remission of symptomatic uncomplicated diverticular disease-a double-blind, randomised, placebo-controlled study. Aliment. Pharmacol. Ther. 2013, 38, 741–751. [Google Scholar] [CrossRef]
- Endo, H.; Higurashi, T.; Hosono, K.; Sakai, E.; Sekino, Y.; Iida, H.; Sakamoto, Y.; Koide, T.; Takahashi, H.; Yoneda, M.; et al. Efficacy of Lactobacillus casei treatment on small bowel injury in chronic low-dose aspirin users: A pilot randomized controlled study. J. Gastroenterol. 2011, 46, 894–905. [Google Scholar] [CrossRef]
- Nguyen, N.; Zhang, B.; Holubar, S.D.; Pardi, D.S.; Singh, S. Treatment and prevention of pouchitis after ileal pouch-anal anastomosis for chronic ulcerative colitis. Cochrane Database Syst. Rev. 2019, 2019, CD001176. [Google Scholar] [CrossRef]
- Gionchetti, P.; Rizzello, F.; Morselli, C.; Poggioli, G.; Tambasco, R.; Calabrese, C.; Brigidi, P.; Vitali, B.; Straforini, G.; Campieri, M. High-Dose Probiotics for the Treatment of Active Pouchitis. Dis. Colon Rectum 2007, 50, 2075–2084. [Google Scholar] [CrossRef]
- Bibiloni, R.; Fedorak, R.N.; Tannock, G.W.; Madsen, K.L.; Gionchetti, P.; Campieri, M.; De Simone, C.; Sartor, R.B. VSL#3 Probiotic-Mixture Induces Remission in Patients with Active Ulcerative Colitis. Am. J. Gastroenterol. 2005, 100, 1539–1546. [Google Scholar] [CrossRef]
- Rembacken, B.; Snelling, A.; Hawkey, P.; Chalmers, D.; Axon, A. Non-pathogenic Escherichia coli versus mesalazine for the treatment of ulcerative colitis: A randomised trial. Lancet 1999, 354, 635–639. [Google Scholar] [CrossRef]
- Kruis, W.; Fric, P.; Pokrotnieks, J.; Lukas, M.; Fixa, B.; Kaščák, M.; A Kamm, M.; Weismueller, J.; Beglinger, C.; Stolte, M.; et al. Maintaining remission of ulcerative colitis with the probiotic Escherichia coli Nissle 1917 is as effective as with standard mesalazine. Gut 2004, 53, 1617–1623. [Google Scholar] [CrossRef]
- Ponziani, F.R.; Scaldaferri, F.; Petito, V.; Paroni Sterbini, F.; Pecere, S.; Lopetuso, L.R.; Palladini, A.; Gerardi, V.; Masucci, L.; Pompili, M.; et al. The Role of Antibiotics in Gut Microbiota Modulation: The Eubiotic Effects of Rifaximin. Dig. Dis. 2016, 34, 269–278. [Google Scholar] [CrossRef] [PubMed]
- Ponziani, F.R.; Zocco, M.A.; D’aversa, F.; Pompili, M.; Gasbarrini, A. Eubiotic properties of rifaximin: Disruption of the traditional concepts in gut microbiota modulation. World J. Gastroenterol. 2017, 23, 4491–4499. [Google Scholar] [CrossRef] [PubMed]
- Powers, J. Antimicrobial drug development–The past, the present, and the future. Clin. Microbiol. Infect. 2004, 10, 23–31. [Google Scholar] [CrossRef] [PubMed]
- Van Zyl, K.N.; Matukane, S.R.; Hamman, B.L.; Whitelaw, A.C.; Newton-Foot, M. Effect of antibiotics on the human microbiome: A systematic review. Int. J. Antimicrob. Agents 2021, 59, 106502. [Google Scholar] [CrossRef] [PubMed]
- Gough, E.K. The impact of mass drug administration of antibiotics on the gut microbiota of target populations. Infect. Dis. Poverty 2022, 11, 76. [Google Scholar] [CrossRef]
- Ianiro, G.; Tilg, H.; Gasbarrini, A. Antibiotics as deep modulators of gut microbiota: Between good and evil. Gut 2016, 65, 1906–1915. [Google Scholar] [CrossRef]
- Ng, K.M.; Ferreyra, J.A.; Higginbottom, S.K.; Lynch, J.B.; Kashyap, P.C.; Gopinath, S.; Naidu, N.; Choudhury, B.; Weimer, B.C.; Monack, D.M.; et al. Microbiota-liberated host sugars facilitate post-antibiotic expansion of enteric pathogens. Nature 2013, 502, 96–99. [Google Scholar] [CrossRef]
- Ferreyra, J.A.; Wu, K.J.; Hryckowian, A.J.; Bouley, D.M.; Weimer, B.C.; Sonnenburg, J.L. Gut microbiota-produced succinate promotes C. Difficile infection after antibiotic treatment or motility disturbance. Cell Host Microbe 2014, 16, 770–777. [Google Scholar] [CrossRef]
- Karami, N.; Hannoun, C.; Adlerberth, I.; Wold, A.E. Colonization dynamics of ampicillin-resistant Escherichia coli in the infantile colonic microbiota. J. Antimicrob. Chemother. 2008, 62, 703–708. [Google Scholar] [CrossRef]
- Ponziani, F.R.; Gerardi, V.; Pecere, S.; D’aversa, F.; Lopetuso, L.; Zocco, M.A.; Pompili, M.; Gasbarrini, A. Effect of rifaximin on gut microbiota composition in advanced liver disease and its complications. World J. Gastroenterol. 2015, 21, 12322–12333. [Google Scholar] [CrossRef]
- Darkoh, C.; Lichtenberger, L.M.; Ajami, N.; Dial, E.J.; Jiang, Z.-D.; DuPont, H.L. Bile acids improve the antimicrobial effect of rifaximin. Antimicrob. Agents Chemother. 2010, 54, 3618–3624. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, G.; Honikel, K.O.; Knüsel, F.; Nüesch, J. The specific inhibition of the DNA-directed RNA synthesis by rifamycin. Biochim. Biophys. Acta (BBA)-Nucleic Acids Protein Synth. 1967, 145, 843–844. [Google Scholar] [CrossRef]
- Pimentel, M.; Lembo, A.; Chey, W.D.; Zakko, S.; Ringel, Y.; Yu, J.; Mareya, S.M.; Shaw, A.L.; Bortey, E.; Forbes, W.P. Rifaximin Therapy for Patients with Irritable Bowel Syndrome without Constipation ABSTRACT. N. Engl. J. Med. 2011, 364, 22–32. [Google Scholar] [CrossRef]
- Bajaj, J.S.; Heuman, D.M.; Wade, J.B.; Gibson, D.P.; Saeian, K.; Wegelin, J.A.; Hafeezullah, M.; Bell, D.E.; Sterling, R.K.; Stravitz, R.T.; et al. Rifaximin improves driving simulator performance in a randomized trial of patients with minimal hepatic encephalo-pathy. Gastroenterology 2011, 140, 478–487.e1. [Google Scholar] [CrossRef] [PubMed]
- Cuomo, R.; Barbara, G.; Annibale, B. Rifaximin and diverticular disease: Position paper of the Italian Society of Gastroenterology (SIGE). Dig. Liver Dis. 2017, 49, 595–603. [Google Scholar] [CrossRef]
- Mencarelli, A.; Renga, B.; Palladino, G.; Claudio, D.; Ricci, P.; Distrutti, E.; Barbanti, M.; Baldelli, F.; Fiorucci, S. Inhibition of NF-κB by a PXR-dependent pathway mediates counter-regulatory activities of rifaximin on innate immunity in intestinal epithelial cells. Eur. J. Pharmacol. 2011, 668, 317–324. [Google Scholar] [CrossRef]
- Mencarelli, A.; Migliorati, M.; Barbanti, M.; Cipriani, S.; Palladino, G.; Distrutti, E.; Renga, B.; Fiorucci, S. Pregnane-X-receptor mediates the anti-inflammatory activities of rifaximin on detoxification pathways in intestinal epithelial cells. Biochem. Pharmacol. 2010, 80, 1700–1707. [Google Scholar] [CrossRef]
- Brown, E.L.; Xue, Q.; Jiang, Z.-D.; Xu, Y.; DuPont, H.L. Pretreatment of epithelial cells with rifaximin alters bacterial attachment and internalization profiles. Antimicrob. Agents Chemother. 2010, 54, 388–396. [Google Scholar] [CrossRef]
- Amaya, E.; Caceres, M.; Fang, H.; Ramirez, A.T.; Palmgren, A.C.; Nord, C.E.; Weintraub, A. Extended-spectrum β-lactamase-producing Klebsiella pneumoniae in a Neonatal Intensive Care Unit in León, Nicaragua. Int. J. Antimicrob. Agents 2009, 33, 386–387. [Google Scholar] [CrossRef]
- Bajaj, J.S.; Heuman, D.M.; Sanyal, A.J.; Hylemon, P.B.; Sterling, R.K.; Stravitz, R.T.; Fuchs, M.; Ridlon, J.M.; Daita, K.; Monteith, P.; et al. Modulation of the Metabiome by Rifaximin in Patients with Cirrhosis and Minimal Hepatic Encephalopathy. PLoS ONE 2013, 8, e60042. [Google Scholar] [CrossRef]
- Ponziani, F.; Scaldaferri, F.; de Siena, M.; Mangiola, F.; Matteo, M.; Pecere, S.; Petito, V.; Sterbini, F.P.; Lopetuso, L.; Masucci, L.; et al. Increased Faecalibacterium abundance is associated with clinical improvement in patients receiving rifaximin treatment. Benef. Microbes 2020, 11, 519–525. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Jin, Y.; Zhou, W.; Xiao, T.; Wu, Z.; Su, J.; Gao, H.; Shen, P.; Zheng, B.; Luo, Q.; et al. Rifaximin Modulates the Gut Microbiota to Prevent Hepatic Encephalopathy in Liver Cirrhosis Without Impacting the Resistome. Front. Cell. Infect. Microbiol. 2022, 11, 1427. [Google Scholar] [CrossRef] [PubMed]
- Brigidi, P.; Swennen, E.; Rizzello, F.; Bozzolasco, M.; Matteuzzi, D. Effects of Rifaximin Administration on the Intestinal Microbiota in Patients with Ulcerative Colitis. J. Chemother. 2002, 14, 290–295. [Google Scholar] [CrossRef]
- Omar, N.N.; Mosbah, R.A.; Sarawi, W.S.; Rashed, M.M.; Badr, A.M. Rifaximin Protects against Malathion-Induced Rat Testicular Toxicity: A Possible Clue on Modulating Gut Microbiome and Inhibition of Oxidative Stress by Mitophagy. Molecules 2022, 27, 4069. [Google Scholar] [CrossRef]
- Li, H.; Xiang, Y.; Zhu, Z.; Wang, W.; Jiang, Z.; Zhao, M.; Cheng, S.; Pan, F.; Liu, D.; Ho, R.C.M.; et al. Rifaximin-mediated gut microbiota regulation modulates the function of microglia and protects against CUMS-induced depression-like behaviors in adolescent rat. J. Neuroinflammation 2021, 18, 254. [Google Scholar] [CrossRef] [PubMed]
- Dupraz, L.; Magniez, A.; Rolhion, N.; Richard, M.L.; Da Costa, G.; Touch, S.; Mayeur, C.; Planchais, J.; Agus, A.; Danne, C.; et al. Gut microbiota-derived short-chain fatty acids regulate IL-17 production by mouse and human intestinal γδ T cells. Cell Rep. 2021, 36, 109332. [Google Scholar] [CrossRef]
- Gao, Z.; Yin, J.; Zhang, J.; Ward, R.E.; Martin, R.J.; Lefevre, M.; Cefalu, W.T.; Ye, J. Butyrate improves insulin sensitivity and increases energy expenditure in mice. Diabetes 2009, 58, 1509–1517. [Google Scholar] [CrossRef]
- Yang, L.; Liu, B.; Zheng, J.; Huang, J.; Zhao, Q.; Liu, J.; Su, Z.; Wang, M.; Cui, Z.; Wang, T.; et al. Rifaximin alters intestinal microbiota and prevents progression of ankylosing spondylitis in mice. Front. Cell. Infect. Microbiol. 2019, 9, 44. [Google Scholar] [CrossRef]
- Sánchez-Rabaneda, F.; Jáuregui, O.; Casals, I.; Andrés-Lacueva, C.; Izquierdo-Pulido, M.; Lamuela-Raventós, R.M. Liquid chromatographic/electrospray ionization tandem mass spectrometric study of the phenolic composition of cocoa (Theobroma cacao). J. Mass Spectrom. 2003, 38, 35–42. [Google Scholar] [CrossRef]
- Zareie, M.; Johnson-Henry, K.; Jury, J.; Yang, P.-C.; Ngan, B.-Y.; McKay, D.M.; Soderholm, J.D.; Perdue, M.H.; Sherman, P.M. Probiotics prevent bacterial translocation and improve intestinal barrier function in rats following chronic psychological stress. Gut 2006, 55, 1553–1560. [Google Scholar] [CrossRef]
- Thomas, C.M.; Hong, T.; Van Pijkeren, J.P.; Hemarajata, P.; Trinh, D.V.; Hu, W.; Britton, R.A.; Kalkum, M.; Versalovic, J. Histamine Derived from Probiotic Lactobacillus reuteri Suppresses TNF via Modulation of PKA and ERK Signaling. PLoS ONE 2012, 7, e31951. [Google Scholar] [CrossRef] [PubMed]
- Llopis, M.; Antolin, M.; Carol, M.; Borruel, N.; Casellas, F.; Martinez, C.; Espín-Basany, E.; Guarner, F.; Malagelada, J.R. Lactobacillus casei downregulates commensals’ inflammatory signals in Crohn’s disease mucosa. Inflamm. Bowel Dis. 2009, 15, 275–283. [Google Scholar] [CrossRef] [PubMed]
- Patel, V.C.; Lee, S.; McPhail, M.J.; Da Silva, K.; Guilly, S.; Zamalloa, A.; Witherden, E.; Støy, S.; Vijay, G.K.M.; Pons, N.; et al. Rifaximin-α reduces gut-derived inflammation and mucin degradation in cirrhosis and encephalopathy: RIFSYS randomised controlled trial. J. Hepatol. 2021, 76, 332–342. [Google Scholar] [CrossRef]
- Woodhouse, C.; Singanayagam, A.; Patel, V.C. Modulating the gut–liver axis and the pivotal role of the faecal microbiome in cirrhosis. Clin. Med. J. R. Coll. Physicians Lond. 2020, 20, 493–500. [Google Scholar] [CrossRef]
- Alexeev, E.E.; Lanis, J.M.; Kao, D.J.; Campbell, E.L.; Kelly, C.J.; Battista, K.D.; Gerich, M.E.; Jenkins, B.R.; Walk, S.T.; Kominsky, D.J.; et al. Microbiota-Derived Indole Metabolites Promote Human and Murine Intestinal Homeostasis through Regulation of Interleukin-10 Receptor. Am. J. Pathol. 2018, 188, 1183–1194. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Yang, Y.; Sun, S.; Xie, Y.; Pan, C.; Li, M.; Li, C.; Liu, Y.; Xu, Z.; Liu, W.; et al. Indolepropionic acid reduces obesity-induced metabolic dysfunction through colonic barrier restoration mediated via tuft cell-derived IL-25. FEBS J. 2022, 289, 5985–6004. [Google Scholar] [CrossRef]
- Sinicropi, M.S.; Iacopetta, D.; Ceramella, J.; Catalano, A.; Mariconda, A.; Pellegrino, M.; Saturnino, C.; Longo, P.; Aquaro, S. Triclosan: A Small Molecule with Controversial Roles. Antibiotics 2022, 11, 735. [Google Scholar] [CrossRef]
- Shrestha, P.; Zhang, Y.; Chen, W.-J.; Wong, T.-Y. Triclosan: Antimicrobial mechanisms, antibiotics interactions, clinical applications, and human health. J. Environ. Sci. Health Part C 2020, 38, 245–268. [Google Scholar] [CrossRef]
- Heath, R.J.; Rubin, J.R.; Holland, D.R.; Zhang, E.; Snow, M.E.; Rock, C.O. Mechanism of Triclosan Inhibition of Bacterial Fatty Acid Synthesis. J. Biol. Chem. 1999, 274, 11110–11114. [Google Scholar] [CrossRef]
- Abbott, A. Italian scientists under investigation after olive-tree deaths. Nature 2015. [Google Scholar] [CrossRef]
- Poole, A.C.; Pischel, L.; Ley, C.; Suh, G.; Goodrich, J.K.; Haggerty, T.D.; Ley, R.E.; Parsonnet, J. Crossover Control Study of the Effect of Personal Care Products Containing Triclosan on the Microbiome. mSphere 2016, 1, e00056-15. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.A.; Moon, H.; Lee, K.; Rhee, M.S. Bactericidal effects of triclosan in soap both in vitro and in vivo. J. Antimicrob. Chemother. 2015, 70, 3345–3352. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Guo, Y.; Ye, H.; Zhang, J.; Ke, Y. Perinatal Triclosan exposure in the rat induces long-term disturbances in metabolism and gut microbiota in adulthood and old age. Environ. Res. 2019, 182, 109004. [Google Scholar] [CrossRef]
- Giuliano, C.A.; Rybak, M.J. Efficacy of Triclosan as an Antimicrobial Hand Soap and Its Potential Impact on Antimicrobial Resistance: A Focused Review. Pharmacother. J. Hum. Pharmacol. Drug Ther. 2015, 35, 328–336. [Google Scholar] [CrossRef]
- Sun, D.; Zuo, C.; Huang, W.; Wang, J.; Zhang, Z. Triclosan targeting of gut microbiome ameliorates hepatic steatosis in high fat diet-fed mice. J. Antibiot. 2022, 75, 341–353. [Google Scholar] [CrossRef] [PubMed]
- Bever, C.S.; Rand, A.A.; Nording, M.; Taft, D.; Kalanetra, K.M.; Mills, D.A.; Breck, M.A.; Smilowitz, J.T.; German, J.B.; Hammock, B.D. Effects of triclosan in breast milk on the infant fecal microbiome. Chemosphere 2018, 203, 467–473. [Google Scholar] [CrossRef] [PubMed]
- Van Dyke, T.E.; Bartold, P.M.; Reynolds, E.C. The Nexus Between Periodontal Inflammation and Dysbiosis. Front. Immunol. 2020, 11, 511. [Google Scholar] [CrossRef]
- Arimatsu, K.; Yamada, H.; Miyazawa, H.; Minagawa, T.; Nakajima, M.; Ryder, M.I.; Gotoh, K.; Motooka, D.; Nakamura, S.; Iida, T.; et al. Oral pathobiont induces systemic inflammation and metabolic changes associated with alteration of gut microbiota. Sci. Rep. 2015, 4, 4828. [Google Scholar] [CrossRef]
- Olsen, I.; Yamazaki, K. Can oral bacteria affect the microbiome of the gut? J. Oral Microbiol. 2019, 11, 1586422. [Google Scholar] [CrossRef]
- Komazaki, R.; Katagiri, S.; Takahashi, H.; Maekawa, S.; Shiba, T.; Takeuchi, Y.; Kitajima, Y.; Ohtsu, A.; Udagawa, S.; Sasaki, N.; et al. Periodontal pathogenic bacteria, Aggregatibacter actinomycetemcomitans affect non-alcoholic fatty liver disease by altering gut microbiota and glucose metabolism. Sci. Rep. 2017, 7, 13950. [Google Scholar] [CrossRef]
- Bajaj, J.S.; Matin, P.; White, M.B.; Fagan, A.; Deeb, J.G.; Acharya, C.; Dalmet, S.S.; Sikaroodi, M.; Gillevet, P.M.; Sahingur, S.E. Periodontal therapy favorably modulates the oral-gut-hepatic axis in cirrhosis. Am. J. Physiol. Gastrointest. Liver Physiol. 2018, 315, G824–G837. [Google Scholar] [CrossRef] [PubMed]
- Davies, R.M. The clinical efficacy of triclosan/copolymer and other common therapeutic approaches to periodontal health. Clin. Microbiol. Infect. 2007, 13, 25–29. [Google Scholar] [CrossRef] [PubMed]
- Wallet, M.A.; Calderon, N.L.; Alonso, T.R.; Choe, C.S.; Catalfamo, D.L.; Lalane, C.J.; Neiva, K.G.; Panagakos, F.; Wallet, S.M. Triclosan alters antimicrobial and inflammatory responses of epithelial cells. Oral Dis. 2012, 19, 296–302. [Google Scholar] [CrossRef]
- Meade, K.G.; O’Farrelly, C. β-Defensins: Farming the Microbiome for Homeostasis and Health. Front. Immunol. 2019, 9, 3072. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.-Y.; Chang, M.-C.; Chen, C.-S.; Lin, H.-C.; Tsai, H.-P.; Yang, C.-C.; Yang, C.-H.; Lin, C.-M. Topoisomerase i inhibitor evodiamine acts as an antibacterial agent against drug-resistant Klebsiella pneumoniae. Planta Medica 2012, 79, 27–29. [Google Scholar] [CrossRef]
- Zhu, L.-Q.; Zhang, L.; Zhang, J.; Chang, G.-L.; Liu, G.; Yu, D.-D.; Yu, X.-M.; Zhao, M.-S.; Ye, B. Evodiamine inhibits high-fat diet-induced colitis-associated cancer in mice through regulating the gut microbiota. J. Integr. Med. 2020, 19, 56–65. [Google Scholar] [CrossRef]
- Wang, M.; Zhou, B.; Cong, W.; Zhang, M.; Li, Z.; Li, Y.; Liang, S.; Chen, K.; Yang, D.; Wu, Z. Amelioration of AOM/DSS-Induced Murine Colitis-Associated Cancer by Evodiamine Intervention is Primarily Associated with Gut Microbiota-Metabolism-Inflammatory Signaling Axis. Front. Pharmacol. 2021, 12, 797605. [Google Scholar] [CrossRef]
- Shen, P.; Zhang, Z.; Zhu, K.; Cao, H.; Liu, J.; Lu, X.; Li, Y.; Jing, Y.; Yuan, X.; Fu, Y.; et al. Evodiamine prevents dextran sulfate sodium-induced murine experimental colitis via the regulation of NF-κB and NLRP3 inflammasome. Biomed. Pharmacother. 2018, 110, 786–795. [Google Scholar] [CrossRef]
- Yang, J.Y.; Kim, J.-B.; Lee, P.; Kim, S.-H. Evodiamine Inhibits Helicobacter pylori Growth and Helicobacter pylori-Induced Inflammation. Int. J. Mol. Sci. 2021, 22, 3385. [Google Scholar] [CrossRef]
- Dai, Y.; Zhu, W.; Zhou, J.; Shen, T. The combination of berberine and evodiamine ameliorates high-fat diet-induced non-alcoholic fatty liver disease associated with modulation of gut microbiota in rats. Braz. J. Med. Biol. Res. 2022, 55, e12096. [Google Scholar] [CrossRef]
- Mesas, C.; Martínez, R.; Doello, K.; Ortiz, R.; López-Jurado, M.; Bermúdez, F.; Quiñonero, F.; Prados, J.; Porres, J.; Melguizo, C. In vivo antitumor activity of Euphorbia lathyris ethanol extract in colon cancer models. Biomed. Pharmacother. 2022, 149, 112883. [Google Scholar] [CrossRef] [PubMed]
- Sforcin, J.M. Biological Properties and Therapeutic Applications of Propolis. Phytother. Res. 2016, 30, 894–905. [Google Scholar] [CrossRef] [PubMed]
- Garzarella, E.U.; Navajas-Porras, B.; Pérez-Burillo, S.; Ullah, H.; Esposito, C.; Santarcangelo, C.; Hinojosa-Nogueira, D.; Pastoriza, S.; Zaccaria, V.; Xiao, J.; et al. Evaluating the effects of a standardized polyphenol mixture extracted from poplar-type propolis on healthy and diseased human gut microbiota. Biomed. Pharmacother. 2022, 148, 112759. [Google Scholar] [CrossRef] [PubMed]
- Xue, M.; Liu, Y.; Xu, H.; Zhou, Z.; Ma, Y.; Sun, T.; Liu, M.; Zhang, H.; Liang, H. Propolis modulates the gut microbiota and improves the intestinal mucosal barrier function in diabetic rats. Biomed. Pharmacother. 2019, 118, 109393. [Google Scholar] [CrossRef] [PubMed]
- Efenberger-Szmechtyk, M.; Nowak, A.; Czyzowska, A. Plant extracts rich in polyphenols: Antibacterial agents and natural preservatives for meat and meat products. Crit. Rev. Food Sci. Nutr. 2020, 61, 149–178. [Google Scholar] [CrossRef]
- Chung, K.-T.; Lu, Z.; Chou, M. Mechanism of inhibition of tannic acid and related compounds on the growth of intestinal bacteria. Food Chem. Toxicol. 1998, 36, 1053–1060. [Google Scholar] [CrossRef]
- Zhao, W.-H.; Hu, Z.-Q.; Hara, Y.; Shimamura, T. Inhibition of Penicillinase by Epigallocatechin Gallate Resulting in Restoration of Antibacterial Activity of Penicillin against Penicillinase-Producing Staphylococcus aureus. Antimicrob. Agents Chemother. 2002, 46, 2266–2268. [Google Scholar] [CrossRef]
- Yi, S.; Wang, W.; Bai, F.; Zhu, J.; Li, J.; Li, X.; Xu, Y.; Sun, T.; He, Y. Antimicrobial effect and membrane-active mechanism of tea polyphenols against Serratia marcescens. World J. Microbiol. Biotechnol. 2013, 30, 451–460. [Google Scholar] [CrossRef]
- Berman, A.Y.; Motechin, R.A.; Wiesenfeld, M.Y.; Holz, M.K. The therapeutic potential of resveratrol: A review of clinical trials. NPJ Precis. Oncol. 2017, 1, 35. [Google Scholar] [CrossRef]
- Zhao, Y.; Jiang, Q. Roles of the Polyphenol–Gut Microbiota Interaction in Alleviating Colitis and Preventing Colitis-Associated Colorectal Cancer. Adv. Nutr. Int. Rev. J. 2021, 12, 546–565. [Google Scholar] [CrossRef]
- Qi, L.; Liang, R.; Duan, J.; Song, S.; Pan, Y.; Liu, H.; Zhu, M.; Li, L. Synergistic antibacterial and anti-biofilm activities of resveratrol and polymyxin B against multidrug-resistant Pseudomonas aeruginosa. J. Antibiot. 2022, 75, 567–575. [Google Scholar] [CrossRef]
- Ruan, X.; Deng, X.; Tan, M.; Wang, Y.; Hu, J.; Sun, Y.; Yu, C.; Zhang, M.; Jiang, N.; Jiang, R. Effect of resveratrol on the biofilm formation and physiological properties of avian pathogenic Escherichia coli. J. Proteom. 2021, 249, 104357. [Google Scholar] [CrossRef] [PubMed]
- Alrafas, H.R.; Busbee, P.B.; Nagarkatti, M.; Nagarkatti, P.S. Resveratrol modulates the gut microbiota to prevent murine colitis development through induction of Tregs and suppression of Th17 cells. J. Leukoc. Biol. 2019, 106, 467–480. [Google Scholar] [CrossRef] [PubMed]
- Alrafas, H.R.; Busbee, P.B.; Nagarkatti, M.; Nagarkatti, P.S. Resveratrol Downregulates miR-31 to Promote T Regulatory Cells during Prevention of TNBS-Induced Colitis. Mol. Nutr. Food Res. 2019, 64, e1900633. [Google Scholar] [CrossRef] [PubMed]
- Rouas, R.; Kazan, H.F.; El Zein, N.; Lewalle, P.; Rothe, F.; Simion, A.; Akl, H.; Mourtada, M.; El Rifai, M.; Burny, A.; et al. Human natural Treg microRNA signature: Role of microRNA-31 and microRNA-21 in FOXP3 expression. Eur. J. Immunol. 2009, 39, 1608–1618. [Google Scholar] [CrossRef]
- aWang, P.; Wang, J.; Li, D.; Ke, W.; Chen, F.; Hu, X. Targeting the gut microbiota with resveratrol: A demonstration of novel evidence for the management of hepatic steatosis. J. Nutr. Biochem. 2020, 81, 108363. [Google Scholar] [CrossRef]
- Most, J.; Penders, J.; Lucchesi, M.; Goossens, G.H.; Blaak, E.E. Gut microbiota composition in relation to the metabolic response to 12-week combined polyphenol supplementation in overweight men and women. Eur. J. Clin. Nutr. 2017, 71, 1040–1045. [Google Scholar] [CrossRef]
- Neveu, V.; Pérez-Jiménez, J.; Vos, F.; Crespy, V.; du Chaffaut, L.; Mennen, L.; Knox, C.; Eisner, R.; Cruz, J.; Wishart, D.; et al. Phenol-Explorer: An online comprehensive database on polyphenol contents in foods. Database 2010, 2010, bap024. [Google Scholar] [CrossRef]
- Turpin, W.; Dong, M.; Sasson, G.; Garay, J.A.R.; Espin-Garcia, O.; Lee, S.-H.; Neustaeter, A.; Smith, M.I.; Leibovitzh, H.; Guttman, D.S.; et al. Mediterranean-Like Dietary Pattern Associations with Gut Microbiome Composition and Subclinical Gastrointestinal Inflammation. Gastroenterology 2022, 163, 685–698. [Google Scholar] [CrossRef]
- Wang, X.; Qi, Y.; Zheng, H. Dietary Polyphenol, Gut Microbiota, and Health Benefits. Antioxidants 2022, 11, 1212. [Google Scholar] [CrossRef]
- Rodríguez-Morató, J.; Matthan, N.R.; Liu, J.; de la Torre, R.; Chen, C.-Y.O. Cranberries attenuate animal-based diet-induced changes in microbiota composition and functionality: A randomized crossover controlled feeding trial. J. Nutr. Biochem. 2018, 62, 76–86. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Lin, X.; Huang, G.; Zhang, W.; Rao, P.; Ni, L. Prebiotic effects of almonds and almond skins on intestinal microbiota in healthy adult humans. Anaerobe 2013, 26, 1–6. [Google Scholar] [CrossRef]
- Konopelski, P.; Mogilnicka, I. Biological Effects of Indole-3-Propionic Acid, a Gut Microbiota-Derived Metabolite, and Its Precursor Tryptophan in Mammals’ Health and Disease. Int. J. Mol. Sci. 2022, 23, 1222. [Google Scholar] [CrossRef] [PubMed]
- Fang, H.; Fang, M.; Wang, Y.; Zhang, H.; Li, J.; Chen, J.; Wu, Q.; He, L.; Xu, J.; Deng, J.; et al. Indole-3-Propionic Acid as a Potential Therapeutic Agent for Sepsis-Induced Gut Microbiota Disturbance. Microbiol. Spectr. 2022, 10, e00125-22. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.-H.; Xin, F.-Z.; Xue, Y.; Hu, Z.; Han, Y.; Ma, F.; Zhou, D.; Liu, X.-L.; Cui, A.; Liu, Z.; et al. Indole-3-propionic acid inhibits gut dysbiosis and endotoxin leakage to attenuate steatohepatitis in rats. Exp. Mol. Med. 2019, 51, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Xiao, H.-W.; Cui, M.; Li, Y.; Dong, J.-L.; Zhang, S.-Q.; Zhu, C.-C.; Jiang, M.; Zhu, T.; Wang, B.; Wang, H.-C.; et al. Gut microbiota-derived indole 3-propionic acid protects against radiation toxicity via retaining acyl-CoA-binding protein. Microbiome 2020, 8, 69. [Google Scholar] [CrossRef]
- Baunwall, S.M.D.; Lee, M.M.; Eriksen, M.K.; Mullish, B.H.; Marchesi, J.R.; Dahlerup, J.F.; Hvas, C.L. Faecal microbiota transplantation for recurrent Clostridioides difficile infection: An updated systematic review and meta-analysis. Eclinicalmedicine 2020, 29–30, 100642. [Google Scholar] [CrossRef]
- Hvas, C.L.; Dahl Jørgensen, S.M.; Jørgensen, S.P.; Storgaard, M.; Lemming, L.; Hansen, M.M.; Erikstrup, C.; Dahlerup, J.F. Fecal Microbiota Transplantation Is Superior to Fidaxomicin for Treatment of Recurrent Clostridium difficile Infection. Gastroenterology 2019, 156, 1324–1332.e3. [Google Scholar] [CrossRef]
- Cammarota, G.; Masucci, L.; Ianiro, G.; Bibbò, S.; Dinoi, G.; Costamagna, G.; Sanguinetti, M.; Gasbarrini, A. Randomised clinical trial: Faecal microbiota transplantation by colonoscopy vs. vancomycin for the treatment of recurrent Clostridium difficile infection. Aliment. Pharmacol. Ther. 2015, 41, 835–843. [Google Scholar] [CrossRef]
- Baunwall, S.M.D.; Terveer, E.M.; Dahlerup, J.F.; Erikstrup, C.; Arkkila, P.; Vehreschild, M.J.; Ianiro, G.; Gasbarrini, A.; Sokol, H.; Kump, P.K.; et al. The use of Faecal Microbiota Transplantation (FMT) in Europe: A Europe-wide survey. Lancet Reg. Health-Eur. 2021, 9, 100181. [Google Scholar] [CrossRef]
- Marcella, C.; Cui, B.; Kelly, C.R.; Ianiro, G.; Cammarota, G.; Zhang, F. Systematic review: The global incidence of faecal microbi-ota transplantation-related adverse events from 2000 to 2020. Aliment. Pharmacol. Ther. 2021, 53, 33–42. [Google Scholar] [CrossRef] [PubMed]
- Bibbò, S.; Settanni, C.R.; Porcari, S.; Bocchino, E.; Ianiro, G.; Cammarota, G.; Gasbarrini, A. Fecal microbiota transplantation: Screening and selection to choose the optimal donor. J. Clin. Med. 2020, 9, 1757. [Google Scholar] [CrossRef] [PubMed]
- Cammarota, G.; Ianiro, G.; Tilg, H.; Rajilić-Stojanović, M.; Kump, P.; Satokari, R.; Sokol, H.; Arkkila, P.; Pintus, C.; Hart, A.; et al. European consensus conference on faecal microbiota transplantation in clinical practice. In Gut; BMJ Publishing Group: London, UK, 2017; Volume 66, pp. 569–580. [Google Scholar] [CrossRef]
- Ianiro, G.; Porcari, S.; Bibbò, S.; Giambò, F.; Quaranta, G.; Masucci, L.; Sanguinetti, M.; Gasbarrini, A.; Cammarota, G. Donor program for fecal microbiota transplantation: A 3-year experience of a large-volume Italian stool bank. Dig. Liver Dis. 2021, 53, 1428–1432. [Google Scholar] [CrossRef] [PubMed]
- Ianiro, G.; Bibbò, S.; Masucci, L.; Quaranta, G.; Porcari, S.; Settanni, C.R.; Lopetuso, L.R.; Fantoni, M.; Sanguinetti, M.; Gasbarrini, A.; et al. Maintaining standard volumes, efficacy and safety, of fecal microbiota transplantation for C. difficile infection during the COVID-19 pandemic: A prospective cohort study. Dig. Liver Dis. 2020, 52, 1390–1395. [Google Scholar] [CrossRef]
- Ianiro, G.; Mullish, B.H.; Kelly, C.R.; Kassam, Z.; Kuijper, E.J.; Ng, S.C.; Iqbal, T.H.; Allegretti, J.R.; Bibbò, S.; Sokol, H.; et al. Reorganisation of faecal microbiota transplant services during the COVID-19 pandemic. Gut 2020, 69, 1555–1563. [Google Scholar] [CrossRef]
- Cammarota, G.; Ianiro, G.; Kelly, C.R.; Mullish, B.H.; Allegretti, J.R.; Kassam, Z.; Putignani, L.; Fischer, M.; Keller, J.J.; Costello, S.P.; et al. International consensus conference on stool banking for faecal microbiota transplantation in clinical practice. Gut 2019, 68, 2111–2121. [Google Scholar] [CrossRef]
- Kootte, R.S.; Levin, E.; Salojärvi, J.; Smits, L.P.; Hartstra, A.V.; Udayappan, S.D.; Hermes, G.; Bouter, K.E.; Koopen, A.M.; Holst, J.J.; et al. Improvement of Insulin Sensitivity after Lean Donor Feces in Metabolic Syndrome Is Driven by Baseline Intestinal Microbiota Composition. Cell Metab. 2017, 26, 611–619.e6. [Google Scholar] [CrossRef]
- El-Salhy, M.; Hatlebakk, J.G.; Gilja, O.H.; Kristoffersen, A.B.; Hausken, T. Efficacy of faecal microbiota transplantation for patients with irritable bowel syndrome in a randomised, double-blind, placebo-controlled study. Gut 2019, 69, 859–867. [Google Scholar] [CrossRef]
- Haifer, C.; Paramsothy, S.; O Kaakoush, N.; Saikal, A.; Ghaly, S.; Yang, T.; Luu, L.D.W.; Borody, T.J.; Leong, R.W. Lyophilised oral faecal microbiota transplantation for ulcerative colitis (LOTUS): A randomised, double-blind, placebo-controlled trial. Lancet Gastroenterol. Hepatol. 2021, 7, 141–151. [Google Scholar] [CrossRef]
- Kao, D.; Roach, B.; Silva, M.; Beck, P.; Rioux, K.; Kaplan, G.G.; Chang, H.-J.; Coward, S.; Goodman, K.J.; Xu, H.; et al. Effect of oral capsule–vs. colonoscopy-delivered fecal microbiota transplantation on recurrent Clostridium difficile infection: A randomized clinical trial. JAMA J. Am. Med. Assoc. 2017, 318, 1985–1993. [Google Scholar] [CrossRef]
- Moayyedi, P.; Surette, M.G.; Kim, P.T.; Libertucci, J.; Wolfe, M.; Onischi, C.; Armstrong, D.; Marshall, J.K.; Kassam, Z.; Reinisch, W.; et al. Fecal Microbiota Transplantation Induces Remission in Patients with Active Ulcerative Colitis in a Randomized Controlled Trial. Gastroenterology 2015, 149, 102–109.e6. [Google Scholar] [CrossRef] [PubMed]
- Ianiro, G.; Masucci, L.; Quaranta, G.; Simonelli, C.; Lopetuso, L.R.; Sanguinetti, M.; Gasbarrini, A.; Cammarota, G. Randomised clinical trial: Faecal microbiota transplantation by colonoscopy plus vancomycin for the treatment of severe refractory Clostridium difficile infection-single versus multiple infusions. Aliment. Pharmacol. Ther. 2018, 48, 152–159. [Google Scholar] [CrossRef] [PubMed]
- Ianiro, G.; Maida, M.; Burisch, J.; Simonelli, C.; Hold, G.; Ventimiglia, M.; Gasbarrini, A.; Cammarota, G. Efficacy of different faecal microbiota transplantation protocols for Clostridium difficile infection: A systematic review and meta-analysis. United Eur. Gastroenterol. J. 2018, 6, 1232–1244. [Google Scholar] [CrossRef] [PubMed]
- Cold, F.; Baunwall, S.M.D.; Dahlerup, J.F.; Petersen, A.M.; Hvas, C.L.; Hansen, L.H. Systematic review with meta-analysis: Encapsulated faecal microbiota transplantation–evidence for clinical efficacy. Ther. Adv. Gastroenterol. 2021, 14, 17562848211041004. [Google Scholar] [CrossRef]
- Vaughn, B.P.; Fischer, M.; Kelly, C.R.; Allegretti, J.R.; Graiziger, C.; Thomas, J.; McClure, E.; Kabage, A.J.; Khoruts, A. Effectiveness and Safety of Colonic and Capsule Fecal Microbiota Transplantation for Recurrent Clostridioides difficile Infection. Clin. Gastroenterol. Hepatol. 2023, 21, 1330–1337.e2. [Google Scholar] [CrossRef]
- van Prehn, J.; Reigadas, E.; Vogelzang, E.H.; Bouza, E.; Hristea, A.; Guery, B.; Krutova, M.; Norén, T.; Allerberger, F.; Coia, J.E.; et al. European Society of Clinical Microbiology and Infectious Diseases: 2021 update on the treatment guidance document for Clostridioides difficile infection in adults. Clin. Microbiol. Infect. 2021, 27, S1–S21. [Google Scholar] [CrossRef]
- Ianiro, G.; Murri, R.; Sciumè, G.D.; Impagnatiello, M.; Masucci, L.; Ford, A.C.; Law, G.R.; Tilg, H.; Sanguinetti, M.; Cauda, R.; et al. Incidence of bloodstream infections, length of hospital stay, and survival in patients with recurrent clostridioides difficile infection treated with fecal microbiota transplantation or antibiotics a prospective cohort study. Ann. Intern. Med. 2019, 171, 695. [Google Scholar] [CrossRef]
- Cammarota, G.; Ianiro, G.; Magalini, S.; Gasbarrini, A.; Gui, D. Decrease in Surgery for Clostridium difficile Infection After Starting a Program to Transplant Fecal Microbiota. Ann. Intern. Med. 2015, 163, 487–488. [Google Scholar] [CrossRef]
- Baunwall, S.M.D.; Andreasen, S.E.; Hansen, M.M.; Kelsen, J.; Høyer, K.L.; Rågård, N.; Eriksen, L.L.; Støy, S.; Rubak, T.; Damsgaard, E.M.S.; et al. Faecal microbiota transplantation for first or second Clostridioides difficile infection (EarlyFMT): A randomised, double-blind, placebo-controlled trial. Lancet Gastroenterol. Hepatol. 2022, 7, 1083–1091. [Google Scholar] [CrossRef]
- Tariq, R.M.; Syed, T.; Yadav, D.M.; Prokop, L.J.M.; Singh, S.M.; Loftus, E.V.J.; Pardi, D.S.M.; Khanna, S.M. Outcomes of Fecal Microbiota Transplantation for C. difficile Infection in Inflammatory Bowel Disease. J. Clin. Gastroenterol. 2023, 57, 285–293. [Google Scholar] [CrossRef]
- Cheng, F.; Huang, Z.; Li, Z.; Wei, W. Efficacy and safety of fecal microbiota transplant for recurrent Clostridium difficile infection in inflammatory bowel disease: A systematic review and meta-analysis. Rev. Esp. De Enferm. Dig. 2022, 114, 543–549. [Google Scholar] [CrossRef] [PubMed]
- Settanni, C.R.; Ianiro, G.; Bibbò, S.; Cammarota, G.; Gasbarrini, A. Gut microbiota alteration and modulation in psychiatric disorders: Current evidence on fecal microbiota transplantation. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2021, 109, 110258. [Google Scholar] [CrossRef]
- Zheng, L.; Ji, Y.-Y.; Wen, X.-L.; Duan, S.-L. Fecal microbiota transplantation in the metabolic diseases: Current status and perspectives. World J. Gastroenterol. 2022, 28, 2546–2560. [Google Scholar] [CrossRef] [PubMed]
- Meighani, A.; Alimirah, M.; Ramesh, M.; Salgia, R. Fecal Microbiota Transplantation for Clostridioides Difficile Infection in Patients with Chronic Liver Disease. Int. J. Hepatol. 2020, 2020, 1874570. [Google Scholar] [CrossRef]
- Baruch, E.N.; Youngster, I.; Ben-Betzalel, G.; Ortenberg, R.; Lahat, A.; Katz, L.; Adler, K.; Dick-Necula, D.; Raskin, S.; Bloch, N.; et al. Fecal microbiota transplant promotes response in immunotherapy-refractory melanoma patients. Science 2020, 371, 602–609. [Google Scholar] [CrossRef] [PubMed]
- Routy, B.; le Chatelier, E.; DeRosa, L.; Duong, C.P.M.; Alou, M.T.; Daillère, R.; Fluckiger, A.; Messaoudene, M.; Rauber, C.; Roberti, M.P.; et al. Gut microbiome influences efficacy of PD-1–based immunotherapy against epithelial tumors. Science 2018, 359, 91–97. [Google Scholar] [CrossRef]
- Ianiro, G.; Rossi, E.; Thomas, A.M.; Schinzari, G.; Masucci, L.; Quaranta, G.; Settanni, C.R.; Lopetuso, L.R.; Armanini, F.; Blanco-Miguez, A.; et al. Faecal microbiota transplantation for the treatment of diarrhoea induced by tyrosine-kinase inhibitors in patients with metastatic renal cell carcinoma. Nat. Commun. 2020, 11, 4333. [Google Scholar] [CrossRef]
- Kump, P.; Wurm, P.; Gröchenig, H.P.; Wenzl, H.; Petritsch, W.; Halwachs, B.; Wagner, M.; Stadlbauer, V.; Eherer, A.; Hoffmann, K.M.; et al. The taxonomic composition of the donor intestinal microbiota is a major factor influencing the efficacy of faecal microbiota transplantation in therapy refractory ulcerative colitis. Aliment. Pharmacol. Ther. 2017, 47, 67–77. [Google Scholar] [CrossRef]
- Vermeire, S.; Joossens, M.; Verbeke, K.; Wang, J.; Machiels, K.; Sabino, J.; Ferrante, M.; Van Assche, G.; Rutgeerts, P.; Raes, J. Donor Species Richness Determines Faecal Microbiota Transplantation Success in Inflammatory Bowel Disease. J. Crohn’s Colitis 2015, 10, 387–394. [Google Scholar] [CrossRef]
- Gopalakrishnan, V.; Spencer, C.N.; Nezi, L.; Reuben, A.; Andrews, M.C.; Karpinets, T.V.; Prieto, P.A.; Vicente, D.; Hoffman, K.; Wei, S.C.; et al. Gut microbiome modulates response to anti–PD-1 immunotherapy in melanoma patients. Science 2018, 359, 97–103. [Google Scholar] [CrossRef]
- Costello, S.P.; Hughes, P.; Waters, O.; Bryant, R.V.; Vincent, A.D.; Blatchford, P.; Katsikeros, R.; Makanyanga, J.; Campaniello, M.A.; Mavrangelos, C.; et al. Effect of Fecal Microbiota Transplantation on 8-Week Remission in Patients With Ulcerative Colitis: A Randomized Clinical Trial. JAMA 2019, 321, 156–164. [Google Scholar] [CrossRef] [PubMed]
- Podlesny, D.; Durdevic, M.; Paramsothy, S.; Kaakoush, N.O.; Högenauer, C.; Gorkiewicz, G.; Walter, J.; Fricke, W.F. Identification of clinical and ecological determinants of strain engraftment after fecal microbiota transplantation using metagenomics. Cell Rep. Med. 2022, 3, 100711. [Google Scholar] [CrossRef] [PubMed]
- Ianiro, G.; Valerio, L.; Masucci, L.; Pecere, S.; Bibbò, S.; Quaranta, G.; Posteraro, B.; Currò, D.; Sanguinetti, M.; Gasbarrini, A.; et al. Predictors of failure after single faecal microbiota transplantation in patients with recurrent Clostridium difficile infection: Results from a 3-year, single-centre cohort study. Clin. Microbiol. Infect. 2017, 23, 337.e1–337.e3. [Google Scholar] [CrossRef]
- Ianiro, G.; Punčochář, M.; Karcher, N.; Porcari, S.; Armanini, F.; Asnicar, F.; Beghini, F.; Blanco-Míguez, A.; Cumbo, F.; Manghi, P.; et al. Variability of strain engraftment and predictability of microbiome composition after fecal microbiota transplantation across different diseases. Nat. Med. 2022, 28, 1913–1923. [Google Scholar] [CrossRef] [PubMed]
- Rossen, N.G.; Fuentes, S.; van der Spek, M.J.; Tijssen, J.G.; Hartman, J.H.A.; Duflou, A.; Löwenberg, M.; Van Den Brink, G.R.; Mathus-Vliegen, E.M.H.; de Vos, W.M.; et al. Findings From a Randomized Controlled Trial of Fecal Transplantation for Patients with Ulcerative Colitis. Gastroenterology 2015, 149, 110–118.e4. [Google Scholar] [CrossRef] [PubMed]
- Beghini, F.; McIver, L.J.; Blanco-Míguez, A.; Dubois, L.; Asnicar, F.; Maharjan, S.; Mailyan, A.; Manghi, P.; Scholz, M.; Thomas, A.M.; et al. Integrating taxonomic, functional, and strain-level profiling of diverse microbial communities with bioBakery 3. Elife 2021, 10, e65088. [Google Scholar] [CrossRef]
- Feuerstadt, P.; Louie, T.J.; Lashner, B.; Wang, E.E.; Diao, L.; Bryant, J.A.; Sims, M.; Kraft, C.S.; Cohen, S.H.; Berenson, C.S.; et al. SER-109, an Oral Microbiome Therapy for Recurrent Clostridioides difficile Infection. N. Engl. J. Med. 2022, 386, 220–229. [Google Scholar] [CrossRef]
- Khanna, S.; Assi, M.; Lee, C.; Yoho, D.; Louie, T.; Knapple, W.; Aguilar, H.; Garcia-Diaz, J.; Wang, G.P.; Berry, S.M.; et al. Efficacy and Safety of RBX2660 in PUNCH CD3, a Phase III, Randomized, Double-Blind, Placebo-Controlled Trial with a Bayesian Primary Analysis for the Prevention of Recurrent Clostridioides difficile Infection. Drugs 2022, 82, 1527–1538. [Google Scholar] [CrossRef]
- Voorhees, P.; Cruz-Teran, C.; Edelstein, J.; Lai, S.K.; Professor, A. Challenges & Opportunities for Phage-Based in Situ Microbiome Engineering in the Gut. J. Control. Release 2020, 326, 106–119. [Google Scholar]
- Landry, B.P.; Tabor, J.J. Engineering Diagnostic and Therapeutic Gut Bacteria. Microbiol. Spectr. 2017, 5, 331–361. [Google Scholar] [CrossRef]
- Steidler, L.; Hans, W.; Schotte, L.; Neirynck, S.; Obermeier, F.; Falk, W.; Fiers, W.; Remaut, E. Treatment of murine colitis by Lactococcus lactis secreting interleukin-10. Science 2000, 289, 1352–1355. [Google Scholar] [CrossRef]
- Vandenbroucke, K.; De Haard, H.; Beirnaert, E.; Dreier, T.; Lauwereys, M.; Huyck, L.; Van Huysse, J.; Demetter, P.; Steidler, L.; Remaut, E.; et al. Orally administered L. lactis secreting an anti-TNF Nanobody demonstrate efficacy in chronic colitis. Mucosal Immunol. 2010, 3, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Hanson, M.L.; Hixon, J.A.; Li, W.; Felber, B.K.; Anver, M.R.; Stewart, C.A.; Janelsins, B.M.; Datta, S.K.; Shen, W.; McLean, M.H.; et al. Oral Delivery of IL-27 Recombinant Bacteria Attenuates Immune Colitis in Mice. Gastroenterology 2014, 146, 210–221.e13. [Google Scholar] [CrossRef] [PubMed]
- Shigemori, S.; Watanabe, T.; Kudoh, K.; Ihara, M.; Nigar, S.; Yamamoto, Y.; Suda, Y.; Sato, T.; Kitazawa, H.; Shimosato, T. Oral delivery of Lactococcus lactis that secretes bioactive heme oxygenase-1 alleviates development of acute colitis in mice. Microb. Cell Factories 2015, 14, 189. [Google Scholar] [CrossRef]
- Duan, F.F.; Liu, J.H.; March, J.C. Engineered Commensal Bacteria Reprogram Intestinal Cells into Glucose-Responsive Insulin-Secreting Cells for the Treatment of Diabetes. Diabetes 2015, 64, 1794–1803. [Google Scholar] [CrossRef] [PubMed]
- Danino, T.; Prindle, A.; Kwong, G.A.; Skalak, M.; Li, H.; Allen, K.; Hasty, J.; Bhatia, S.N. Programmable probiotics for detection of cancer in urine. Sci. Transl. Med. 2015, 7, 289ra84. [Google Scholar] [CrossRef]
- Daeffler, K.N.; Galley, J.D.; Sheth, R.U.; Ortiz-Velez, L.C.; O Bibb, C.; Shroyer, N.F.; A Britton, R.; Tabor, J.J. Engineering bacterial thiosulfate and tetrathionate sensors for detecting gut inflammation. Mol. Syst. Biol. 2017, 13, 923. [Google Scholar] [CrossRef]
- Zheng, L.-M.; Luo, X.; Feng, M.; Li, Z.; Le, T.; Ittensohn, M.; Trailsmith, M.; Bermudes, D.; Lin, S.; King, I. Tumor Amplified Protein Expression Therapy: Salmonella as a Tumor-Selective Protein Delivery Vector. Oncol. Res. Featur. Preclin. Clin. Cancer Ther. 2001, 12, 127–135. [Google Scholar] [CrossRef]
- Saltzman, D.A.; Heise, C.P.; Hasz, D.E.; Zebede, M.; Kelly, S.M.; Curtiss, R.; Leonard, A.S.; Anderson, P.M. Attenuated Salmonella Typhimurium Containing Interleukin-2 Decreases MC-38 Hepatic Métastases: A Novel Anti-Tumor Agent; Mary Ann Liebert, Inc.: Larchmont, NY, USA, 1996; Volume 11. [Google Scholar]
- Gunn, G.R.; Zubair, A.; Peters, C.; Pan, Z.-K.; Wu, T.-C.; Paterson, Y. Two Listeria monocytogenes Vaccine Vectors That Express Different Molecular Forms of Human Papilloma Virus-16 (HPV-16) E7 Induce Qualitatively Different T Cell Immunity That Correlates with Their Ability to Induce Regression of Established Tumors Immortalized by HPV-16. J. Immunol. 2001, 167, 6471–6479. [Google Scholar] [CrossRef]
- Kortright, K.E.; Chan, B.K.; Koff, J.L.; Turner, P.E. Phage Therapy: A Renewed Approach to Combat Antibiotic-Resistant Bacteria. Cell Host Microbe 2019, 25, 219–232. [Google Scholar] [CrossRef]
- Gordillo Altamirano, F.L.; Barr, J.J. Phage Therapy in the Postantibiotic Era. Clin. Microbiol. Rev. 2019, 32, e00066-18. [Google Scholar] [CrossRef] [PubMed]
- Galtier, M.; de Sordi, L.; Sivignon, A.; De Vallée, A.; Maura, D.; Neut, C.; Rahmouni, O.; Wannerberger, K.; Darfeuille-Michaud, A.; Desreumaux, P.; et al. Bacteriophages targeting adherent invasive Escherichia coli strains as a promising new treatment for Crohn’s disease. J. Crohn’s Colitis 2017, 11, 840–847. [Google Scholar] [CrossRef] [PubMed]
- Duan, Y.; Young, R.; Schnabl, B. Bacteriophages and their potential for treatment of gastrointestinal diseases. Nat. Rev. Gastroenterol. Hepatol. 2021, 19, 135–144. [Google Scholar] [CrossRef]
- Duan, Y.; Llorente, C.; Lang, S.; Brandl, K.; Chu, H.; Jiang, L.; White, R.C.; Clarke, T.H.; Nguyen, K.; Torralba, M.; et al. Bacteriophage targeting of gut bacterium attenuates alcoholic liver disease. Nature 2019, 575, 505–511. [Google Scholar] [CrossRef] [PubMed]
- Ross, A.; Ward, S.; Hyman, P. More Is Better: Selecting for Broad Host Range Bacteriophages. Front. Microbiol. 2016, 7, 1352. [Google Scholar] [CrossRef]
- Ando, H.; Lemire, S.; Pires, D.P.; Lu, T.K. Engineering Modular Viral Scaffolds for Targeted Bacterial Population Editing. Cell Syst. 2015, 1, 187–196. [Google Scholar] [CrossRef]
- Yoichi, M.; Abe, M.; Miyanaga, K.; Unno, H.; Tanji, Y. Alteration of tail fiber protein gp38 enables T2 phage to infect Escherichia coli O157:H7. J. Biotechnol. 2005, 115, 101–107. [Google Scholar] [CrossRef]
- Bertozzi Silva, J.; Storms, Z.; Sauvageau, D. Host receptors for bacteriophage adsorption. FEMS Microbiol Lett. 2016, 363, fnw002. [Google Scholar] [CrossRef]
- Le, S.; He, X.; Tan, Y.; Huang, G.; Zhang, L.; Lux, R.; Shi, W.; Hu, F. Mapping the Tail Fiber as the Receptor Binding Protein Responsible for Differential Host Specificity of Pseudomonas aeruginosa Bacteriophages PaP1 and JG004. PLoS ONE 2013, 8, e68562. [Google Scholar] [CrossRef]
- Chen, M.; Zhang, L.; Abdelgader, S.A.; Yu, L.; Xu, J.; Yao, H.; Lu, C.; Zhang, W. Alterations in gp37 Expand the Host Range of a T4-Like Phage. Appl. Environ. Microbiol. 2017, 83, e01576-17. [Google Scholar] [CrossRef]
- Yu, P.; Mathieu, J.; Li, M.; Dai, Z.; Alvarez, P.J.J. Isolation of Polyvalent Bacteriophages by Sequential Multiple-Host Approaches. Appl. Environ. Microbiol. 2016, 82, 808–815. [Google Scholar] [CrossRef] [PubMed]
- Yosef, I.; Goren, M.G.; Globus, R.; Molshanski-Mor, S.; Qimron, U. Extending the Host Range of Bacteriophage Particles for DNA Transduction. Mol. Cell 2017, 66, 721–728.e3. [Google Scholar] [CrossRef] [PubMed]
- Yehl, K.; Lemire, S.; Yang, A.C.; Ando, H.; Mimee, M.; Torres, M.D.T.; de la Fuente-Nunez, C.; Lu, T.K. Engineering Phage Host-Range and Suppressing Bacterial Resistance through Phage Tail Fiber Mutagenesis. Cell 2019, 179, 459–469.e9. [Google Scholar] [CrossRef] [PubMed]
- Doron, S.; Melamed, S.; Ofir, G.; Leavitt, A.; Lopatina, A.; Keren, M.; Amitai, G.; Sorek, R. Systematic discovery of antiphage defense systems in the microbial pangenome. Science 2018, 359, eaar4120. [Google Scholar] [CrossRef] [PubMed]
- Waller, M.C.; Bober, J.R.; Nair, N.U.; Beisel, C.L. Toward a genetic tool development pipeline for host-associated bacteria. Curr. Opin. Microbiol. 2017, 38, 156–164. [Google Scholar] [CrossRef] [PubMed]
- Tock, M.R.; Dryden, D.T. The biology of restriction and anti-restriction. Curr. Opin. Microbiol. 2005, 8, 466–472. [Google Scholar] [CrossRef]
- Roberts, R.J.; Vincze, T.; Posfai, J.; Macelis, D. REBASE—A database for DNA restriction and modification: Enzymes, genes and genomes. Nucleic Acids Res. 2014, 43, D298–D299. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).