Antimicrobial Resistance of Lactic Acid Bacteria from Nono, a Naturally Fermented Milk Product
Abstract
1. Introduction
2. Results
2.1. MIC Determination
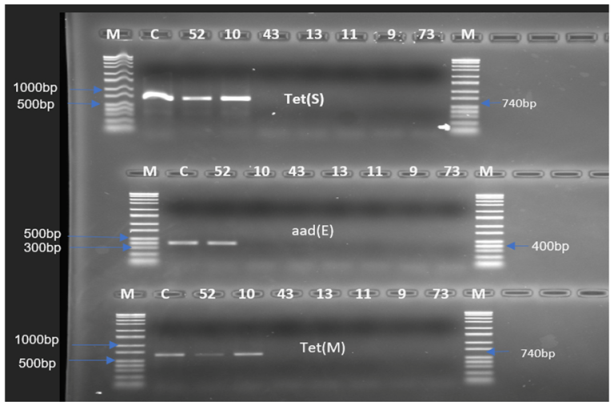
2.2. Determination of Resistance Genes
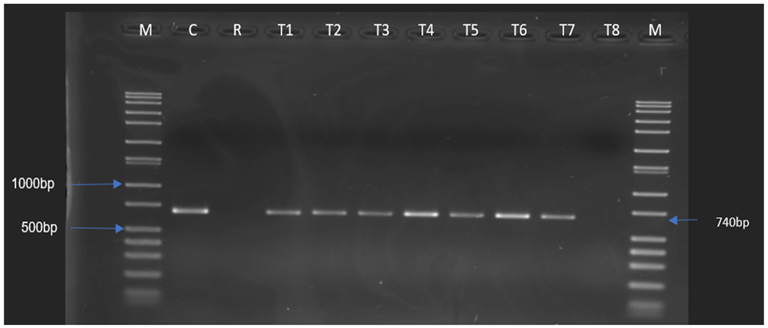
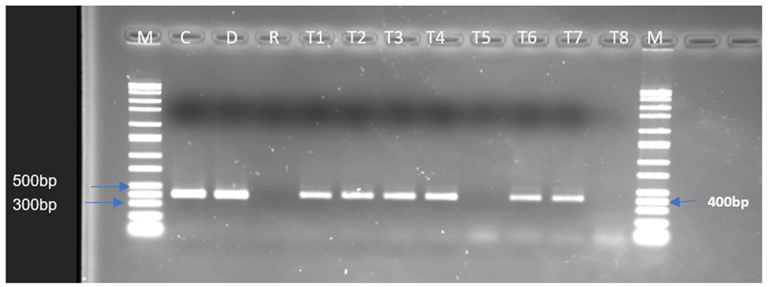
2.3. In Vitro Conjugation Experiments for the Transfer of tet(S), tet(M), and aad(E) Genes
2.4. Determination of the Presence of the Resistance Genes in the Potential Transconjugants
3. Discussion
4. Material and Methods
4.1. Bacterial Isolates
4.2. Determination of Minimal Inhibitory Concentration (MIC) of the Isolates
4.3. Detection of Resistance Genes for Specific Antimicrobials
4.4. Screening Transferability of Resistance Genes (In Vitro Conjugation)
4.5. Confirmation of the Transconjugants Using MIC Determination and PCR
4.6. Determination of the Presence and Location of the Tetracycline and Streptomycin Genes in the Potential Transconjugants
4.7. Determination of the Presence of Transposons
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Health Organisation. Antimicrobial Resistance. 2021. Available online: https://www.who.int/news-room/fact-sheets/detail/antimicrobial-resistance (accessed on 20 July 2022).
- Murray, C.J.; Ikuta, K.S.; Sharara, F.; Swetschinski, L.; Aguilar, G.R.; Gray, A.; Han, C.; Bisignano, C.; Rao, P.; Wool, E.; et al. Global burden of bacterial antimicrobial resistance in 2019: A systematic analysis. Lancet 2022, 399, 629–655. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, J. Tackling Drug-Resistant Infections Globally: Final Reports and Recommendations. The Review on Antimicrobial Resistance. 2016. Available online: https://amr-review.org/sites/default/files/160525_Final%20paper_with%20cover.pdf (accessed on 20 July 2022).
- Godínez-Oviedo, A.; Tamplin, M.L.; Bowman, J.P.; Hernández-Iturriaga, M. Salmonella enterica in Mexico 2000–2017: Epidemiology, Antimicrobial Resistance, and Prevalence in Food. Foodborne Pathog. Dis. 2020, 17, 98–118. [Google Scholar] [CrossRef] [PubMed]
- Oloso, N.O.; Fagbo, S.; Garbati, M.; Olonitola, S.O.; Awosanya, E.J.; Aworh, M.K.; Adamu, H.; Odetokun, I.A.; Fasina, F.O. Antimicrobial Resistance in Food Animals and the Environment in Nigeria: A Review. Int. J. Environ. Res. Public Health 2018, 15, 1284. [Google Scholar] [CrossRef] [PubMed]
- Sosnowski, M.; Lachtara, B.; Wieczorek, K.; Osek, J. Antimicrobial resistance and genotypic characteristics of Listeria monocytogenes isolated from food in Poland. Int. J. Food Microbiol. 2019, 289, 1–6. [Google Scholar] [CrossRef]
- Anyogu, A.; Olukorede, A.; Anumudu, C.; Onyeaka, H.; Areo, E.; Adewale, O.; Odimba, J.N.; Nwaiwu, O. Microorganisms and food safety risks associated with indigenous fermented foods from Africa. Food Control 2021, 129, 108227. [Google Scholar] [CrossRef]
- Jans, C.; Meile, L.; Kaindi, D.M.W.; Kogi-Makua, W.; Lamuka, P.; Renault, P.; Kreikemever, B.; Lacroix, C.; Hattendorf, J.; Zinsstag, J.; et al. African fermented dairy products—Overview of predominant technologically important microorganisms focusing on African Streptococcus infantarius variants and potential future applications for enhanced food safety and security. Int. J. Food Microbiol. 2017, 250, 27–36. [Google Scholar] [CrossRef]
- Gänzle, M. Food fermentations for improved digestibility of plant foods—An essential ex-situ digestion step in agricultural societies? Curr. Opin. Food Sci. 2020, 32, 124–132. [Google Scholar] [CrossRef]
- Samet-Bali, O.; Felfoul, I.; Lajnaf, R.; Attia, H.; Ayadi, M.A. Hygienic quality of “rayeb”, a traditional Tunisian fermented cow’s milk. Int. Food Res. J. 2016, 23, 366–369. [Google Scholar]
- Fagbemigun, O.; Cho, G.S.; Rösch, N.; Brinks, E.; Schrader, K.; Bockelmann, W.; Oguntoyinbo, F.A.; Franz, C.M.A.P. Isolation and Characterization of Potential Starter Cultures from the Nigerian Fermented Milk Product nono. Microorganisms 2021, 9, 640. [Google Scholar] [CrossRef]
- Owusu-Kwarteng, J.; Wuni, A.; Akabanda, F.; Tano-Debrah, K.; Jespersen, L. Prevalence, virulence factor genes and antibiotic resistance of Bacillus cereus sensu lato isolated from dairy farms and traditional dairy products. BMC Microbiol. 2017, 17, 65. [Google Scholar] [CrossRef]
- Walsh, A.M.; Crispie, F.; Daari, K.; O’Sullivan, O.; Martin, J.C.; Arthur, C.T.; Claesson, M.J.; Scott, K.P.; Cotter, P.D. Strain-Level metagenomic analysis of the fermented dairy beverage nunu highlights potential food safety risks. Appl. Environ. Microbiol. 2017, 83, e01144-17. [Google Scholar] [CrossRef]
- Akabanda, F.; Owusu-Kwarteng, J.; Tano-Debrah, K.; Glover, R.L.; Nielsen, D.S.; Jespersen, L. Taxonomic and molecular characterisation of lactic acid bacteria and yeasts in nunu, a Ghanaian fermented milk product. Food Microbiol. 2013, 34, 277–283. [Google Scholar] [CrossRef]
- Bayili, G.R.; Johansen, P.; Nielsen, D.S.; Sawadogo-Lingani, H.; Ouedraogo, G.A.; Diawara, B.; Jespersen, L. Identification of the predominant microbiota during the production of lait caill´e, a spontaneously fermented milk product made in Burkina Faso. World J. Microbiol. Biotechnol. 2019, 35, 100. [Google Scholar] [CrossRef]
- Moonga, H.B.; Schoustra, S.E.; Heuvel, J.V.D.; Linnemann, A.R.; Samad, S.; Shindano, J.; Smid, E.J. Composition and diversity of natural bacterial communities in mabisi, a traditionally fermented milk. Front. Microbiol. 2020, 11, 1816. [Google Scholar] [CrossRef]
- Nyambane, B.; Thari, W.M.; Wangoh, J.; Njage, P.M. Lactic acid bacteria and yeasts involved in the fermentation of amabere amaruranu, a Kenyan fermented milk. Food Sci. Nutr. 2015, 2, 692–699. [Google Scholar] [CrossRef]
- Obioha, P.I.; Ouoba, L.I.I.; Anyogu, A.; Awamaria, B.; Atchia, S.; Ojimelukwe, P.C.; Sutherland, J.P.; Ghoddusi, H.B. Identification and characterisation of the lactic acid bacteria associated with the traditional fermentation of dairy fermented product. Braz. J. Microbiol. 2021, 52, 869–881. [Google Scholar] [CrossRef]
- Food and Drug Administration. Microorganisms and Microbial-Derived Ingredients Used in Food (Partial List). 2018. Available online: https://www.fda.gov/food/generally-recognized-safe-gras/microorganisms-microbial-derived-ingredients-used-food-partial-list (accessed on 20 July 2022).
- Fowoyo, P.T.; Ogunbanwo, S.T. Antimicrobial resistance in coagulase-negative staphylococci from Nigerian traditional fermented foods. Ann. Clin. Microbiol. Antimicrob. 2017, 16, 4. [Google Scholar] [CrossRef]
- Oguntoyinbo, F.A.; Okueso, O. Prevalence, distribution, and antibiotic resistance pattern among enterococci species in two traditional fermented dairy foods. Ann. Microbiol. 2013, 63, 755–761. [Google Scholar] [CrossRef]
- Ouoba, L.I.I.; Mbozo, A.B.V.; Anyogu, A.; Obioha, P.L.; Lingani-Sawadogo, H.; Sutherland, J.P.; Jespersen, L.; Ghoddusi, H.B. Environmental heterogeneity of Staphylococcus species from alkaline fermented foods and associated toxins and antimicrobial resistance genetic elements. Int. J. Food Microbiol. 2019, 311, 108356. [Google Scholar] [CrossRef]
- Castellone, V.; Bancalari, E.; Rubert, J.; Gatti, M.; Neviani, E.; Bottari, B. Eating Fermented: Health Benefits of LAB-Fermented Foods. Foods 2021, 10, 2639. [Google Scholar] [CrossRef]
- Ma, Q.; Fu, Y.; Sun, H.; Huang, Y.; Li, L.; Yu, Q.; Dinnyes, A.; Sun, Q. Antimicrobial resistance of Lactobacillus spp. from fermented foods and human gut. LWT 2018, 86, 201–208. [Google Scholar]
- Founou, L.L.; Founou, R.C.; Essack, S.Y. Antibiotic Resistance in the Food Chain: A Developing Country-Perspective. Front. Microbiol. 2017, 7, 1881. [Google Scholar] [CrossRef]
- Vignaroli, C.; Zandri, G.; Aquilanti, L.; Pasquaroli, S.; Biavasco, F. Multidrug-resistant enterococci in animal meat and faeces and co-transfer of resistance from an Enterococcus durans to a human Enterococcus faecium. Curr. Microbiol. 2011, 62, 1438–1447. [Google Scholar] [CrossRef] [PubMed]
- Jahan, M.; Zhanel, G.G.; Sparling RHolley, R.A. Horizontal transfer of antibiotic resistance from Enterococcus faecium of fermented meat origin to clinical isolates of E. faecium and Enterococcus faecalis. Int. J. Food Microbiol. 2015, 199, 78–85. [Google Scholar] [CrossRef] [PubMed]
- Ouoba, L.I.I.; Lei, V.; Jensen, L.B. Resistance of potential probiotic lactic acid bacteria and Bifidobacteria of African and European origin to antimicrobials: Determination and transferability of the resistance genes to other bacteria. Int. J. Food Microbiol. 2008, 121, 217–224. [Google Scholar] [CrossRef]
- EFSA-FEEDAP. Guidance on the assessment of bacterial susceptibility to antimicrobials of human and veterinary importance. EFSA J. 2012, 10, 2740. [Google Scholar]
- EUCAST. Breakpoint Tables for Interpretation of MICs and Zone Diameters. 2018. Available online: http://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/Breakpoint_tables/v_8.1_Breakpoint_Tables.pdf (accessed on 2 July 2022).
- Klayraung, S.; Viernstein, H.; Sirithunyalug, J.; Okonogi, S. Probiotic Properties of Lactobacilli Isolated from Thai Traditional Food. Sci. Pharm. 2008, 76, 485–503. [Google Scholar] [CrossRef]
- Florez, A.B.; Campedelli, I.; Delgado, S.; Alegría, A.; Salvetti, E.; Felis, G.E.; Mayo, B.; Torriani, S. Antibiotic Susceptibility Profiles of Dairy Leuconostoc, Analysis of the Genetic Basis of Atypical Resistances and Transfer of Genes In Vitro and in a Food Matrix. PLoS ONE 2016, 11, e0145203. [Google Scholar] [CrossRef]
- Obafemi, Y.D.; Oranusi, U.S.; Ajanaku, O.K.; Akinduti, A.P.; Leech, J.; Cotter, D.P. African fermented foods: Overview, emerging benefits, and novel approaches to microbiome profiling. NPJ Sci. Food 2022, 6, 15. [Google Scholar] [CrossRef]
- Adimpong, D.B.; Nielsen, D.S.; Sørensen, K.I.; Derkx, M.F.P.; Jespersen, L. Genotypic characterization and safety assessment of lactic acid bacteria from indigenous African fermented food products. BMC Microbiol. 2012, 12, 75. [Google Scholar] [CrossRef]
- Farahmand, N.; Ouoba, L.I.I.; Naghizadeh Raeisi, S.; Sutherland, J.; Ghoddusi, H.B. Probiotic Lactobacilli in Fermented Dairy Products: Selective Detection, Enumeration and Identification Scheme. Microorganisms 2021, 9, 1600. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Yu, T. Characterization and transfer of antimicrobial resistance in lactic acid bacteria from fermented dairy products in China. J. Infect. Dev. Ctries 2019, 13, 137–148. [Google Scholar] [CrossRef] [PubMed]
- Zhou, N.; Zhang, J.X.; Fan, M.T.; Wang, J.; Guo, G.; Wei, X.Y. Antibiotic resistance of lactic acid bacteria isolated from Chinese yogurts. J. Dairy Sci. 2012, 95, 4775–4783. [Google Scholar] [CrossRef]
- Belletti, N.; Gatti, M.; Bottari, B.; Neviani, E.; Tabanelli, G.; Gardini, F. Antibiotic resistance of lactobacilli isolated from two Italian hard cheeses. J. Food Prot. 2009, 72, 2162–2169. [Google Scholar] [CrossRef] [PubMed]
- Delgado, S.; Flórez, A.B.; Mayo, B. Antibiotic susceptibility of Lactobacillus and Bifidobacterium species from the human gastrointestinal tract. Curr. Microbiol. 2005, 50, 202–207. [Google Scholar] [CrossRef]
- Erginkaya, Z.; Turhan, E.U.; Tatli, D. Determination of antibiotic resistance of lactic acid bacteria isolated from traditional Turkish fermented dairy products. Iran. J. Vet. Res. 2018, 19, 53–56. [Google Scholar]
- Adimpong, D.B.; Sørensen, K.I.; Thorsen, L.; Stuer-Lauridsen, B.; Abdelgadir, W.S.; Nielsen, D.S.; Derkx, P.M.; Jespersen, L. Antimicrobial susceptibility of Bacillus strains isolated from primary starters for African traditional bread production and characterization of the bacitracin operon and bacitracin biosynthesis. Appl. Environ. Microbiol. 2012, 78, 7903–7914. [Google Scholar] [CrossRef]
- Cox, G.; Wright, G.D. Intrinsic antibiotic resistance: Mechanisms, origins, challenges and solutions. Int. J. Med. Microbiol. 2013, 303, 287–292. [Google Scholar] [CrossRef]
- Davies, J.; Davies, D. Origins and evolution of antibiotic resistance. Microbiol. Mol. Biol. Rev. 2010, 74, 417–433. [Google Scholar] [CrossRef]
- Ma, Q.; Pei, Z.; Fang, Z.; Wang, H.; Zhu, J.; Lee, Y.K.; Zhang, H.; Zhao, J.; Lu, W.; Chen, W. Evaluation of Tetracycline Resistance and Determination of the Tentative Microbiological Cutoff Values in Lactic Acid Bacterial Species. Microorganisms 2021, 9, 2128. [Google Scholar] [CrossRef]
- Capita, R.; Alonso-Calleja, C. Antibiotic-resistant bacteria: A challenge for the food industry. Crit. Rev. Food Sci. Nutr. 2013, 53, 11–48. [Google Scholar] [CrossRef]
- Flórez, A.B.; Vázquez, L.; Rodríguez, J.; Mayo, B. Directed Recovery and Molecular Characterization of Antibiotic Resistance Plasmids from Cheese Bacteria. Int. J. Mol. Sci. 2021, 22, 7801. [Google Scholar] [CrossRef]
- Kumar, S.B.; Arnipalli, S.R.; Ziouzenkova, O. Antibiotics in Food Chain: The Consequences for Antibiotic Resistance. Antibiotics 2020, 9, 688. [Google Scholar] [CrossRef]
- Thumu, S.C.; Halami, P.M. Presence of erythromycin and tetracycline resistance genes in lactic acid bacteria from fermented foods of Indian origin. Antonie Van Leeuwenhoek 2012, 102, 541–551. [Google Scholar] [CrossRef]
- Gaglio, R.; Couto, N.; Marques, C.; de Fatima Silva Lopes, M.; Moschetti, G.; Pomba, C.; Settanni, L. Evaluation of antimicrobial resistance and virulence of enterococci from equipment surfaces, raw materials, and traditional cheeses. Int. J. Food Microbiol. 2016, 236, 107–114. [Google Scholar] [CrossRef]
- Li, B.; Yang, Y.; Ma, L.; Ju, F.; Guo, F.; Tiedje, J.M.; Zhang, T. Metagenomic and network analysis reveal wide distribution and co-occurrence of environmental antibiotic resistance genes. ISME J. 2015, 9, 2490–2502. [Google Scholar] [CrossRef]
- Choi, J.; Rieke, E.L.; Moorman, T.B.; Soupir, M.L.; Allen, H.K.; Smith, S.D.; Howe, A. Practical implications of erythromycin resistance gene diversity on surveillance and monitoring of resistance. FEMS Microbiol. Ecol. 2018, 94, fiy006. [Google Scholar] [CrossRef]
- Davis, M.A.; Besser, T.E.; Orfe, L.H.; Baker, K.N.; Lanier, A.S.; Broschat, S.L.; New, D.; Call, D.R. Genotypic-phenotypic discrepancies between antibiotic resistance characteristics of Escherichia coli isolates from calves in management settings with high and low antibiotic use. Appl. Environ. Microbiol. 2011, 77, 3293–3299. [Google Scholar] [CrossRef]
- Gevers, D.; Danielsen, M.; Huys, G.; Swings, J. Molecular characterization of tet(M) genes in Lactobacillus isolates from different types of fermented dry sausage. Appl. Environ. Microbiol. 2003, 69, 1270–1275. [Google Scholar] [CrossRef]
- O’Connor, E.B.O.; O’Sullivan, O.; Stanton, C.; Danielson, M.; Simpson, P.J.; Callanan, M.J.; Ross, R.P.; Hill, C. pEOC01: A plasmid from Pediococcus acidilactici which encodes an identical streptomycin resistance (aadE) gene to that found in Campylobacter jejuni. Plasmid 2007, 58, 115–126. [Google Scholar] [CrossRef]
- Huys, G.; D’Haene, K.; Collard, J.; Swing, J. Prevalence and molecular characterisation of tetracycline resistance in Enterococcus isolates from food. Appl. Environ. Microbiol. 2004, 70, 1555–1562. [Google Scholar] [CrossRef] [PubMed]
- Hummel, A.S.; Hertel, C.; Holzapfel, W.H.; Franz, M.A.P. Antibiotic resistance of lactic acid bacteria starter and probiotic strains. Appl. Environ. Microbiol. 2007, 73, 730–739. [Google Scholar] [CrossRef] [PubMed]
- Lunde, T.M.; Hjerdeh, E.; Al-Haroni, M. Prevalence, diversity and transferability of the Tn916-Tn1545 family ICE in oral streptococci. J. Oral Microbiol. 2021, 13, 1896874. [Google Scholar] [CrossRef] [PubMed]
- Santoro, F.; Vianna MERoberts, A.P. Variation on a theme; an overview of the Tn916/Tn1545 family of mobile genetic elements in the oral and nasopharyngeal streptococci. Front. Microbiol. 2014, 5, 535. [Google Scholar] [CrossRef]
- Brouwer, M.S.M.; Mullany, P.; Roberts, A.P. Characterisation of the conjugative transposon Tn6000 from Enterococcus casseliflavus 664.1H1 (formerly Enterococcus faecium 664.1H1). FEMS Microbiol. Lett. 2010, 309, 71–76. [Google Scholar]
- Hegstad, K.; Mikalsen, T.; Coque, T.M.; Werner, G.; Sundsfjord, A. Mobile genetic elements and their contribution to the emergence of antimicrobial resistant Enterococcus faecalis and Enterococcus faecium. Clin. Microbiol. Infect. 2010, 16, 541–554. [Google Scholar] [CrossRef]
- Naghizadeh-Raeisi, S.; Ghoddusi, H.B.; Boll, E.J.; Farahmand, N.; Stuer-Lauridsen, B.; Johansen, E.; Sutherland, J.P.; Ouoba, L.I.I. Antimicrobial susceptibility of bifidobacteria from probiotic milk products and determination of the genetic basis of tetracycline resistance in Enterococcus species after in vitro conjugation with Bifidobacterium animalis subsp. Lactis. Food Control 2018, 94, 205–211. [Google Scholar] [CrossRef]
- Lancaster, H.; Roberts, A.P.; Bedi, R.; Wilson, M.; Mullany, P. Characterisation of Tn916S, a Tn916-like element containing the tetracycline resistance determinant tet(S). J. Bacteriol. 2004, 186, 4395–4398. [Google Scholar] [CrossRef]
- Gomez, J.E.; Kaufmann-Malaga, B.B.; Wivagg, C.N.; Kim, P.B.; Silvis, M.R.; Renedo, N.; Ioerger, T.R.; Ahmad, R.; Livny, J.; Fishbein, S.; et al. Ribosomal mutations promote the evolution of antibiotic resistance in a multidrug environment. eLife 2017, 6, e20420. [Google Scholar] [CrossRef]
- Lipin, M.Y.; Stepanshina, V.N.; Shemyakin, I.G.; Shinnick, T.M. Association of specific mutation in katG, rpoB, rpsl and rrs gene with spoligotype of multidrug-resistant Mycobacterium tuberculosis isolates in Russia. Clin. Microbiol. Infect. 2007, 13, 620–626. [Google Scholar] [CrossRef]
| Antimicrobial | Isolate/MIC (µg/mL) a | ||||||
|---|---|---|---|---|---|---|---|
| Ent. thailandicus | S. infantarius | Lent. senioris | L. fermentum | Lact. delbrueckii subsp. indicus | Leuc. pseudomesenteriodes | S. thermophilus | |
| Ampicillin | ≤0.12 s | ≤0.12 s | 0.5 s | 0.25 s | ≤0.12 s | 0.25 s | ≤0.12 s |
| Ceftriaxone | <8 s | <8 s | <8 s | <8 s | <8 s | <8 s | <8 s |
| Clindamycin | >2 r | ≤0.12 s | 2 r | ≤0.12 s | 2 r | ≤0.12 s | 2 s |
| Ciprofloxacin | 4 s | 8 r | ≥16 r | ≥16 r | ≥16 r | 8 r | 2 s |
| Daptomycin | >8 r | 8 r | >8 r | >8 r | >8 r | >8 r | 1 s |
| Erythomycin | >4 r | 4 r | >4 r | >4 r | 1 s | 1 s | ≤0.25 s |
| Gatifloxacin | 2 s | 2 s | 8 r | >8 r | 8 r | ≤2 s | ≤1 s |
| Gentamicin | 64 r | 16 s | 32 r | 32 r | 64 r | 8 s | 16 s |
| Levofloxacin | 8 r | 8 r | >8 r | >8 r | >8 r | 8 r | 2 s |
| Linezolid | 4 s | 4 s | 4 s | 8 r | 2 s | 2 s | 2 s |
| Oxacillin + 2% NaCl | 2 s | 2 s | 2 s | 1 s | ≤0.25 s | 0.5 s | ≤0.25 s |
| Penicillin | ≤0.06 s | ≤0.06 s | 0.5 s | 0.12 s | ≤0.06 s | ≤0.06 s | >8 r |
| Quinupristin/Dalfopristin | 4 s | ≤0.12 s | 4 s | 1 s | 1 s | 0.5 s | 2 s |
| Rifampin | 2 s | ≤0.5 s | ≤0.5 s | ≤0.5 s | ≤0.5 s | ≤0.5 s | ≤0.5 s |
| Streptomycin | 512 r | 64 s | 256 r | 256 r | 32 r | 64 s | >32 s |
| Tetracycline | 64 r | 32 r | 16 r | 16 r | ≤2 s | ≤2 s | ≤2 s |
| Trimethoprim/Sulfamethoxazole | >4/76 s | 2/28 s | >4/76 s | >4/76 s | >4/76 s | >4/76 s | >4/76 s |
| Vancomycin | 2 s | ≤1 s | >128 r | >128 r | ≤1 s | >128 r | 16 r |
| Antimicrobial | Isolate/Resistance Genes | ||||||
|---|---|---|---|---|---|---|---|
| Ent. thailandicus | S. infantarius | Lent. senioris | L. fermentum | Lact. delbrueckii subsp. indicus | Leuc. pseudomesenteriodes | S. thermophilus | |
| Chloramphenicol | - | - | - | - | - | - | - |
| Erythromycin | - | - | - | - | - | - | - |
| Gentamicin | - | - | - | - | - | - | - |
| Kanamycin | - | - | - | - | - | - | - |
| Penicillin | - | - | - | - | - | - | - |
| Streptomycin | aad(E) | - | - | - | - | - | - |
| Tetracycline | tet(S)/tet(M) | tet(S)/tet(M) | - | - | - | - | - |
| Vancomycin | - | - | - | - | - | - | - |
| Isolate | Antimicrobial/MIC µg/mL | ||
|---|---|---|---|
| Tetracycline | Streptomycin | ||
| Donor | Ent. thailandicus 52 | 64 r | 512 r |
| Recipient | Ent. faecalis JH2-2 | <1 s | 512 r |
| Transconjugants | T1 | 8 r | 128 r |
| T2 | 8 r | 128 r | |
| T3 | 8 r | 128 r | |
| T4 | 8 r | 256 r | |
| T5 | 32 r | 256 r | |
| T6 | 32 r | 128 r | |
| T7 | 16 r | 128 r | |
| T8 | 8 r | 128 r | |
| Antimicrobial | Resistance Genes | Sequence (5′–3′) | Annealing Temperature (°C) |
|---|---|---|---|
| Tetracycline | tet(M) | 5′-GTTAAATAGTGTTCTTGGAG-3′ 5′-CTAAGATATGGCTCTAACAA-3′ | 45 °C |
| tet(L) | 5′-GTTGCGCGCTATATTCCAAA-3′ 5′-TTAAGCAAACTCATTCCAGC-3′ | 54 °C | |
| tet(S) | 5′-TGGAACGCCAGAGAGGTATT-3′ 5′-ACATAGACAAGCCGTTGACC-3′ | 55 °C | |
| tet(Q) | 5′-ATGTTCAATATCGGTATCAATGA-3′ 5′-GCGGATATCACCTTGCTTC-3′ | 55 °C | |
| tet(K) | 5′-TTAGGTGAAGGGTTAGGTCC-3′ 5′-GCAAACTCATTCCAGAAGCA-3′ | 55 °C | |
| tet(O) | 5′-GATGGCATACAGGCACAGAC-3′ 5′-CAATATCACCAGAGCAGGCT-3′ | 55 °C | |
| tet(W) | 5′-GCCATCTTGGTGATCTCC-3′ 5′-TGGTCCCCTAATACATCGTT-3′ | 55 °C | |
| Kanamycin | aph(3″)-I | 5′-AACGTCTTGCTCGAGGCCGCG-3′ 5′-GGCAAGATCCTGGTATCGGTCTGCG-3′ | 68 °C |
| aph(3″)-III | 5′-GCCGATGTGGATTGCGAAAA-3′ 5′-GCTTGATCCCCAGTAAGTCA-3′ | 52 °C | |
| Gentamicin | ant(2″)-I | 5′-GGGCGCGTCATGGAGGAGTT-3′ 5′-TATCGCGACCTGAAAGCGGC-3′ | 67 °C |
| aac(6′)aph(2″) | 5′-CCAAGAGCAATAAGGGCATA-3′ 5′-CACTATCATAACCACTACCG-3′ | 48 °C | |
| aac(3″)IV | 5′-GTGTGCTGCTGGTCCACAGC-3′ 5′-AGTTGACCCAGGGCTGTCGC-3′ | 63 °C | |
| Streptomycin | str(A) | 5′-CTTGGTGATAACGGCAATTC-3′ 5′-CCAATCGCAGATAGAAGGC-3′ | 55 °C |
| str(B) | 5′-ATCGTCAAGGGATTGAAACC-3′ 5′-GGATCGTAGAACATATTGGC-3′ | 56 °C | |
| Streptomycin | aad(A) | 5′-ATCCTTCGGCGCGATTTTG-3′ 5′-GCAGCGCAATGACATTCTTG-3′ | 56 °C |
| aad(E) | 5′-ATGGAATTATTCCCACCTGA-3′ 5′-TCAAAACCCCTATTAAAGCC-3′ | 50 °C | |
| Erythromycin | erm(A) | 5′-AAGCGGTAAAACCCCTCTGAG-3′ 5′-TCAAAGCCTGTCGGAATTGG-3′ | 55 °C |
| erm(B) | 5′-CATTTAACGACGAAACTGGC-3′ 5′-GGAACATCTGTGGTATGGCG-3′ | 52 °C | |
| erm(C) | 5′-CAAACCCGTATTCCACGATT-3′ 5′-ATCTTTGAAATCGGCTCAGG-3′ | 48 °C | |
| Vancomycin | vanA | 5′-AACAACTTACGCGGCACT-3′ 5′-AAAGTGCGAAAAACCTTG-3′ | 55 °C |
| vanB | 5′-GATATTCAAAGCTCCGCAGC-3′ 5′-TGATGGATGCGGAAG ATACC-3′ | 55 °C | |
| vanX | 5′-TGCGATTTTGCGCTTCATTG-3′ 5′-ACTTGGGATAATTTCACCGG-3′ | 55 °C | |
| Chloramphenicol | cmlA | 5′-TACTCGGATCCATGCTGGCC-3′ 5′-TCCTCGAAGAGCGCCATTGG-3′ | 65 °C |
| cat501 | 5′-GGATATGAAATTTATCCCTC-3′ 5′-CAATCATACCCTATGAAT-3′ | 47 °C | |
| catA1 | 5′-CGCCTGATGAATGCTCATCCG-3′ 5′-CCTGCCACTCATCGCAGTAC-3′ | 60 °C | |
| Penicillin | blaZ | 5′-CAGTTCACATGCCAAAGAG-3′ 5′-TACACTCTTGGCGGTTTC-3′ | 54 °C |
| Transposon | Tn916-1545 | 5′-GCGTGATTGTATCTCACT-3′ 5′-GACGCTCCTGTTGCTTCT-3′ | 50 °C |
| Tn916 | 5′-GGCTGTCGCTGTAGGATAGAG-3′ 5′-GGGTACTTTTAGGGCTTAGT-3′ | 50 °C |
| Bacteria * | Related Genes |
|---|---|
| Salmonella Rissen 7522486-1 | aph(3″)-I |
| Enterococcus faecalis pEF418 | aad(E) |
| Salmonella enterica #74 | aad(A) |
| Staphylococcus aureus RN422 | erm(C) |
| Enterococcus faecalis JH2-2 Tn1545 | erm(B) |
| Staphylococcus aureus 1206 Tn554 | erm(A) |
| Staphylococcus aureus pSTS9-like | tet(L) |
| Staphylococcus aureus pT181-like | tet(K) |
| Staphylococcus intermedius 2567 | tet(M) |
| Escherichia coli pBT-1 | tet(Q) |
| Listeria monocytogenes BM4210 pIP811 | tet(S) |
| Escherichia coli K2 | ant(2″)-I |
| Enterococcus faecalis JH2-1-5 | aph(3″)-III |
| Escherichia coli TetW | tet(W) |
| Enterococcus faecium BM4147 | vanA |
| Enterococcus faecalis V583 | vanB |
| Enterococcus faecium UW6605 | vanX |
| Enterococcus faecium JH2-2 cat pip 501 | cat501 |
| Escherichia coli K13 aac(3)-IV | |
| No positive control | tet(O), str(A), str(B), aac(6′)aph(2″), cmlA, and catA1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Obioha, P.I.; Anyogu, A.; Awamaria, B.; Ghoddusi, H.B.; Ouoba, L.I.I. Antimicrobial Resistance of Lactic Acid Bacteria from Nono, a Naturally Fermented Milk Product. Antibiotics 2023, 12, 843. https://doi.org/10.3390/antibiotics12050843
Obioha PI, Anyogu A, Awamaria B, Ghoddusi HB, Ouoba LII. Antimicrobial Resistance of Lactic Acid Bacteria from Nono, a Naturally Fermented Milk Product. Antibiotics. 2023; 12(5):843. https://doi.org/10.3390/antibiotics12050843
Chicago/Turabian StyleObioha, Promiselynda I., Amarachukwu Anyogu, Brigitte Awamaria, Hamid B. Ghoddusi, and Labia Irene I. Ouoba. 2023. "Antimicrobial Resistance of Lactic Acid Bacteria from Nono, a Naturally Fermented Milk Product" Antibiotics 12, no. 5: 843. https://doi.org/10.3390/antibiotics12050843
APA StyleObioha, P. I., Anyogu, A., Awamaria, B., Ghoddusi, H. B., & Ouoba, L. I. I. (2023). Antimicrobial Resistance of Lactic Acid Bacteria from Nono, a Naturally Fermented Milk Product. Antibiotics, 12(5), 843. https://doi.org/10.3390/antibiotics12050843






