Carvacrol—A Natural Phenolic Compound with Antimicrobial Properties
Abstract
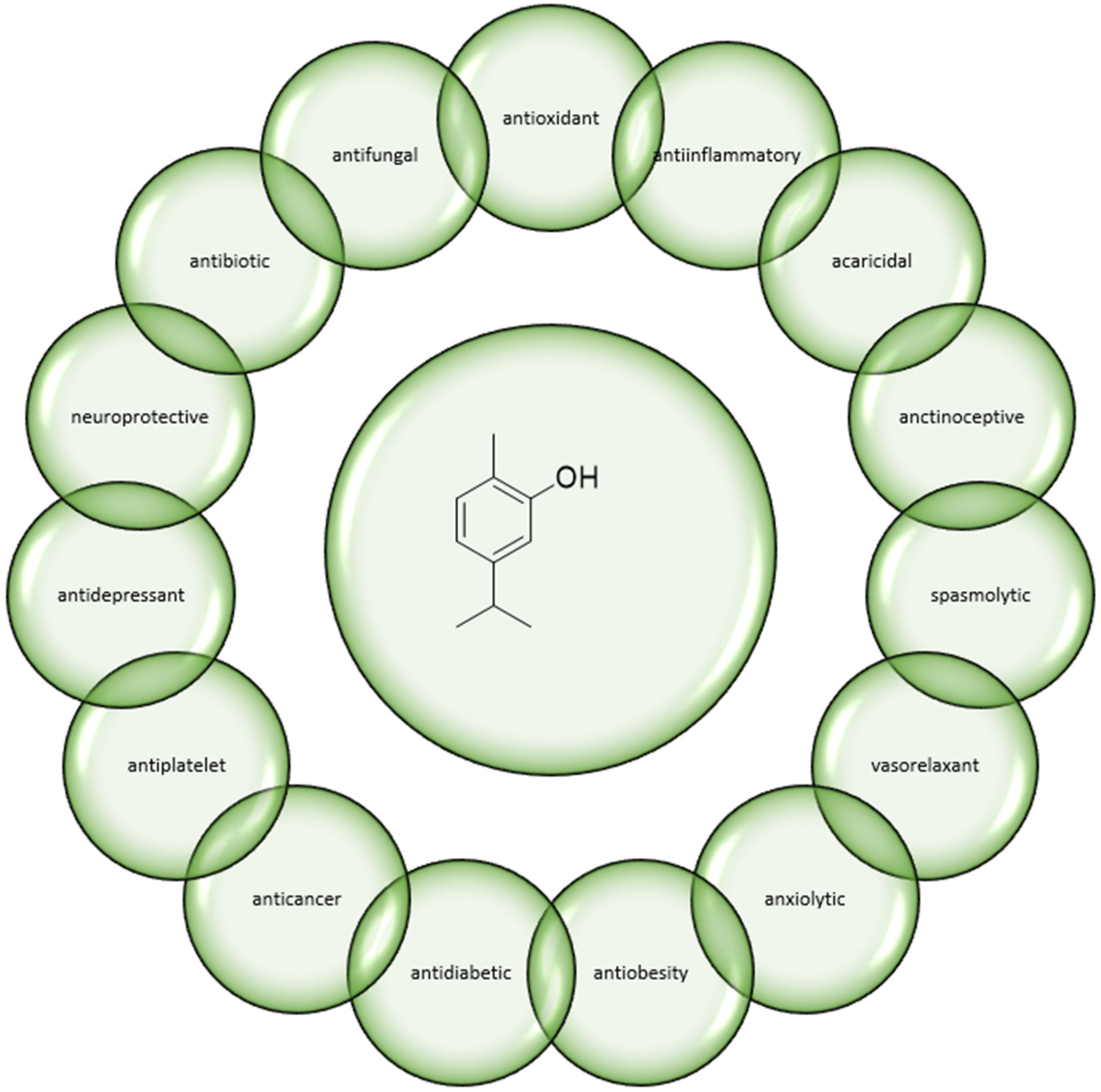
1. Introduction
2. Antimicrobial Activity of Carvacrol
| Microorganism | Determination Method | MIC and/or MFC Value | References |
|---|---|---|---|
| Aspergillus flavus ATCC 204304 | Serial dilution method in liquid medium | MIC: 0.098 mg/mL MFC: 0.098 mg/mL | [48] |
| Candida albicans | Serial dilution method in liquid medium | MFC: 256 mg/L | [37] |
| Candida albicans SC5314 | Microdilution | MIC: 250 mg/L | [38] |
| Candida albicans SC5314 | Microdilution | MIC: 247 μg/mL | [36] |
| Candida auris | Microdilution | MIC: 125 μg/mL | [38] |
| Candida dubliniensis | Serial dilution method in liquid medium | MFC: 161.3 mg/L | [37] |
| Candida glabrata | Serial dilution method in liquid medium | MFC: 238.9 mg/L | [37] |
| Candida krusei | Serial dilution method in liquid medium | MFC: 256 mg/L | [37] |
| Penicillium sp. isolated from soybeans | Serial dilution method in liquid medium | MIC: 0.098 mg/mL MFC: 0.98 mg/mL | [48] |
| Saccharomyces cerevisiae | Serial dilution method in liquid medium | MIC: <500 mg/L | [40] |
| Bacterial strains | |||
| Bacillus cereus ATCC 14579 | Double dilution method in liquid medium | MIC: 0.2 mg/mL | [61] |
| Dickeya zeae MS1 | Serial dilution method in liquid medium | MIC: 0.1 mg/mL | [67] |
| Enterococcus faecalis isolated from French cheese | Serial dilution method in liquid medium | MIC: 0.625 mg/mL | [62] |
| Enterococcus hirae ATCC 10541 | Serial dilution method in liquid medium | MIC: 312.5 μg/mL | [68] |
| Escherichia coli ATCC 25922 | Microdilution | MIC: 0.225 mg/mL | [60] |
| Escherichia coli KBN10P03335 | Serial dilution method in liquid medium | MIC: 150 μg/mL MBC: 300 μg/mL | [66] |
| Gardnerella sp. UM241 | Microdilution | MIC: 0.08 μL/mL | [69] |
| Klebsiella pneumoniae CTX-M-8, OXA-48, KPC | Serial dilution method in liquid medium | MIC: 130 mg/L | [65] |
| Pseudomonas aeruginosa CIP 103467 | Serial dilution method in liquid medium | MIC: 1.25 mg/mL | [62] |
| Pseudomonas aeruginosa ATCC 15442 | Serial dilution method in liquid medium | MIC: 625 μg/mL | [68] |
| Pseudomonas fluorescens ATCC 13525 | Double dilution method in liquid medium | MIC: 0.5 mg/mL | [64] |
| Shewanella putrefaciens ATCC 49138 | Double dilution method in liquid medium | MIC: 0.5 mg/mL | [64] |
| Staphylococcus epidermidis ATCC 12228 | Serial dilution method in liquid medium | MIC: <500 mg/L | [40] |
| Staphylococcus aureus ATCC 25923 | Microdilution | MIC: 0.45 mg/mL | [60] |
| Staphylococcus aureus ATCC 6538 | Double dilution method in liquid medium | MIC: 0.125 mg/mL | [64] |
| Staphylococcus aureus ATCC 6538 | Serial dilution method in liquid medium | MIC: 78 μg/mL | [68] |
| Streptococcus pyogenes ATCC 19615, ATCC 49399 | Serial dilution method in liquid medium | MBIC: 125 μg/mL MBEC: 250 μg/mL | [59] |
| Vibrio parahaemolyticus ATCC 17802 | Double dilution method in liquid medium | MIC: 0.5 mg/mL | [64] |
3. Antioxidant and Anti-Inflammatory Activity of Carvacrol
| Animal/Model | Doses of Carvacrol | Main Results | Reference |
|---|---|---|---|
| In vitro tests (ABTS, DPPH, FRAP, TEAC) | From 50 to 1000 ppm | Antioxidant activity | [81] |
| Guinea pigs exposed to cigarette smoke | 120 and 240 μg/mL | Malondialdehyde↓ | [82] |
| Inhalation of smoke in rats | Nanoparticles of carvacrol in form of SLN | Malondialdehyde↓ | [84] |
| Induction of diabetes in rats | 75 mg/kg for 8 weeks | SOD↓, GPx↓, Bax↓, Bcl-2↑, malondialdehyde↓ | [86] |
| Mice | 50–100 mg/kg | COX-2↓, IL-1β↓, PGE2↓, IL-10↑ | [11] |
| C57BL/6 mice | 25, 50, or 100 mg/kg | IL-1β↓, TNF-α↓, CAT↑, SOD↑, GPx↑ | [89] |
| Model of streptococcal pharyngitis (HTonEpiCs) | 4–125 µg/mL | IL-6↓, IL-8↓, ENA-78↓, GCP-2↓, HBD-2↓, PGE2↓, COX-2↓ | [91] |
| LPS-stimulated cell line J774A.1 | 0.008% and 0.016% | COX-2↓ | [93] |
| Ovine COX-2 activity assay | IC50 = 0.8 μM | Prostaglandin E2↓ | [94] |
| Male Sprague–Dawley rats | 20 mg/kg | Nrf2↑ | [96] |
| HL-1 cardiomyocytes exposed to LPS-G | 6.25–50 µM | IL-1β↓, TLR4↓, NFκ-B↓, NALP3↓ | [102] |
| Mouse splenocytes | 75–300 µg/mL | Gene expression of IL-4↓, IL-17↓, IFN-γ↓, FOXP3↓ | [103] |
| RAW264.7 cells | 0.2 mM | Hsp70↑ | [109] |
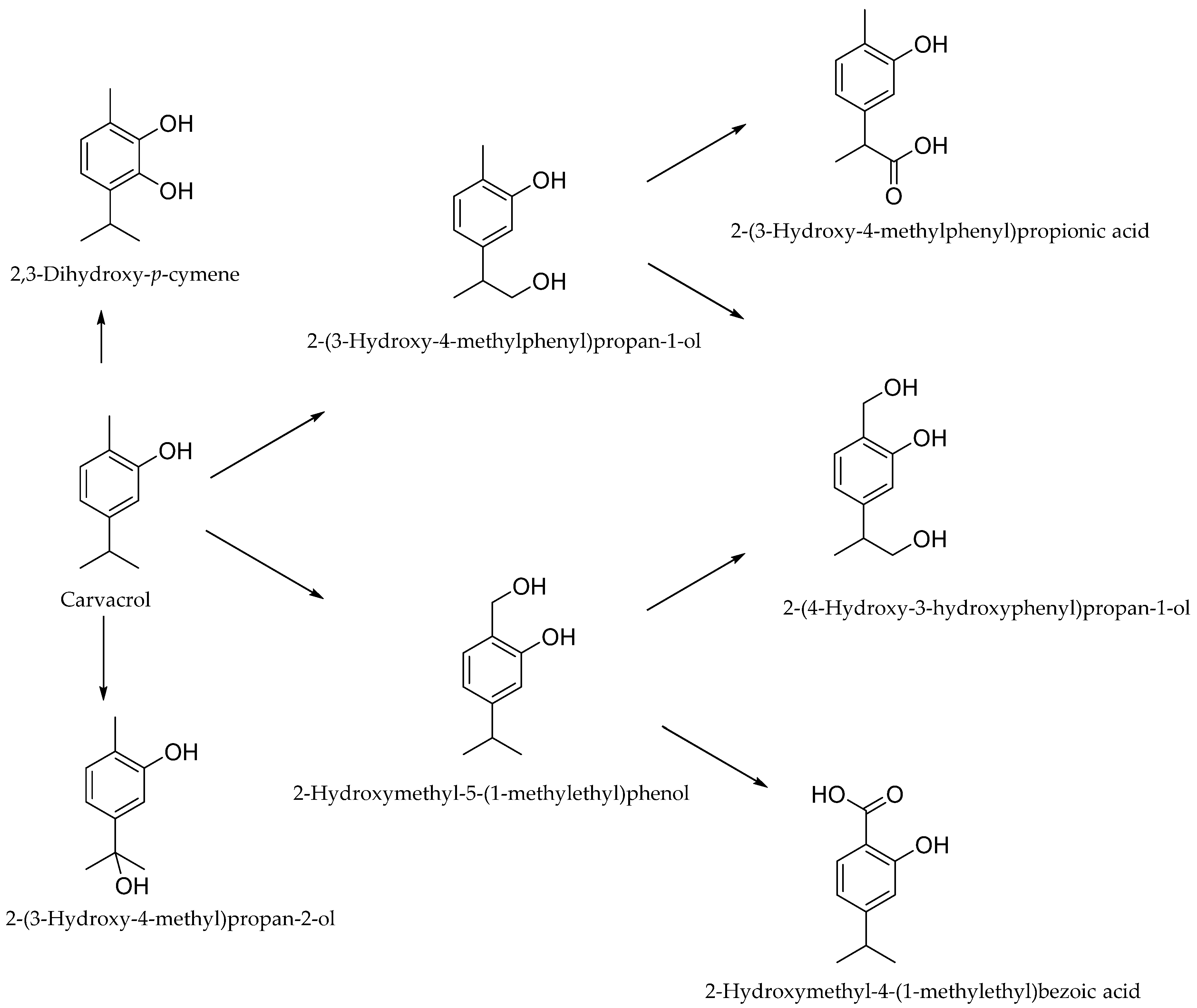
4. The Metabolism of Carvacrol
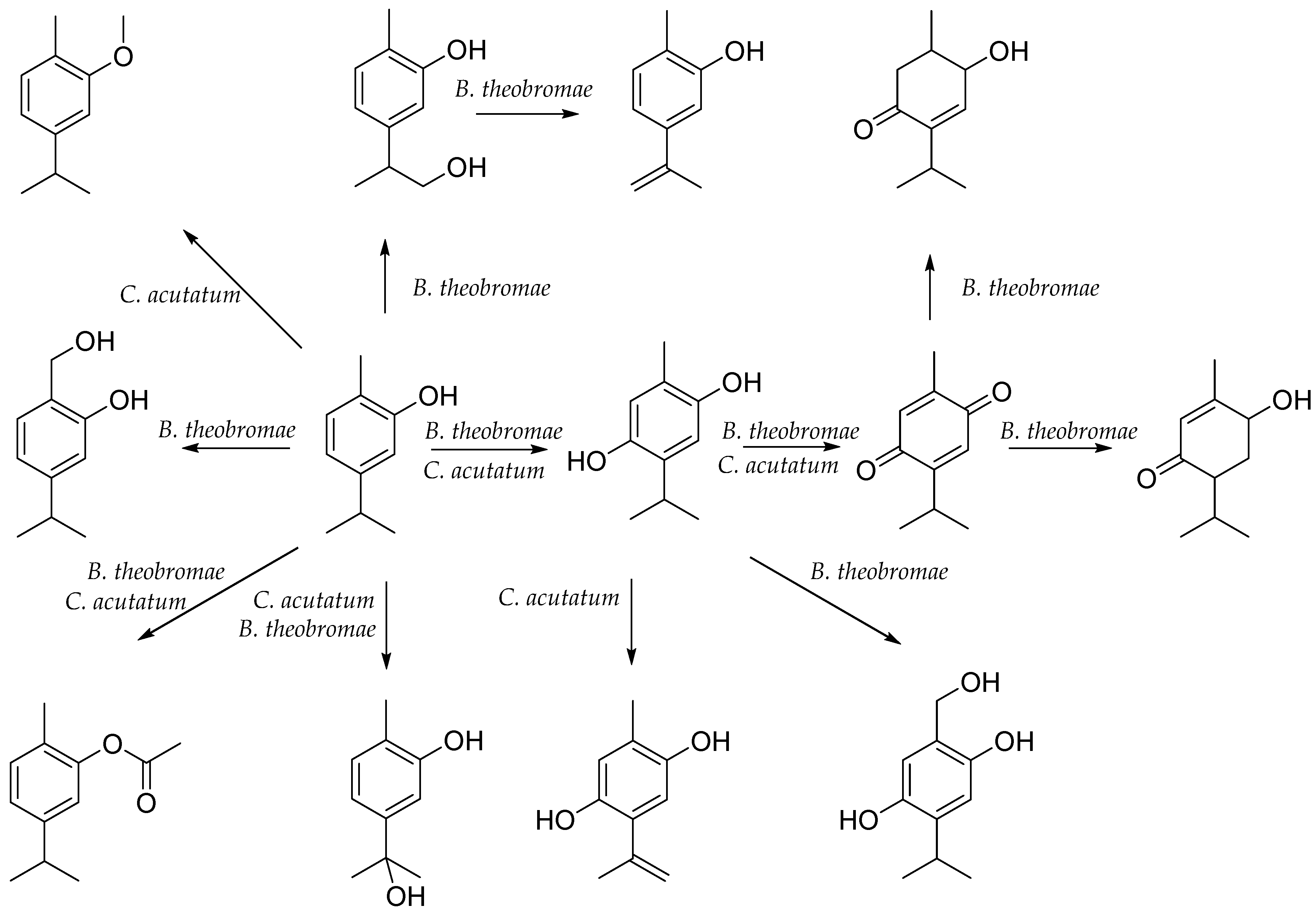
5. Biotransformation of Carvacrol
6. Future Fields of Carvacrol Research
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yadav, G.; Kamble, S.B. Synthesis of carvacrol by Friedel–Crafts alkylation of o-cresol with isopropanol using superacidic catalyst UDCaT-5. J. Chem. Technol. Biotechnol. 2009, 84, 1499–1508. [Google Scholar] [CrossRef]
- Marinelli, L.; di Stefano, A.; Cacciatore, I. Carvacrol and its derivatives as antibacterial agents. Phytochem. Rev. 2018, 17, 903–921. [Google Scholar] [CrossRef]
- Gholami-Ahangaran, M.; Ahmadi-Dastgerdi, A.; Azizi, S.; Basiratpour, A.; Zokaei, M.; Derakhshan, M. Thymol and carvacrol supplementation in poultry health and performance. Vet. Med. Sci. 2022, 8, 267–288. [Google Scholar] [CrossRef]
- Hao, Y.; Guo, X.; Yang, R.; Yan, Y.; Sun, M.; Li, H.; Bai, H.; Cui, H.; Li, J.; Shi, L. Unraveling the Biosynthesis of Carvacrol in Different Tissues of Origanum vulgare. Int. J. Mol. Sci. 2022, 23, 13231. [Google Scholar] [CrossRef] [PubMed]
- Friedman, M. Chemistry and multibeneficial bioactivities of carvacrol (4-isopropyl-2-methylphenol), a component of essential oils produced by aromatic plants and spices. J. Agric. Food Chem. 2014, 62, 7652–7670. [Google Scholar] [CrossRef]
- Ramak, P.; Kazempour Osaloo, S.; Ebrahimzadeh, H.; Sharifi, M.; Behmanesh, M. Inhibition of the mevalonate pathway enhances carvacrol biosynthesis and DXR gene expression in shoot cultures of Satureja khuzistanica Jamzad. J. Plant Physiol. 2013, 170, 1187–1193. [Google Scholar] [CrossRef]
- Krause, S.; Liao, P.; Crocoll, C.; Boachon, B.; Forster, C.; Leidecker, F.; Wiese, N.; Zhaoe, D.; Joshua, C.; Wood, J.C.; et al. The biosynthesis of thymol, carvacrol, and thymohydroquinone in Lamiaceae proceeds via cytochrome P450s and a short-chain dehydrogenase. Proc. Natl. Acad. Sci. USA 2021, 118, e2110092118. [Google Scholar] [CrossRef]
- Crocoll, C.; Asbach, J.; Novak, J.; Gershenzon, J.; Degenhardt, J. Terpene synthases of oregano (Origanum vulgare L.) and their roles in the pathway and regulation of terpene biosynthesis. Plant Mol. Biol. 2010, 73, 587–603. [Google Scholar] [CrossRef]
- Sun, M.; Zhang, Y.; Zhu, L.; Liu, N.; Bai, H.; Sun, G.; Zhang, J.; Shi, L. Chromosome-level assembly and analysis of the Thymus genome provide insights into glandular secretory trichome formation and monoterpenoid biosynthesis in thyme. Plant Community 2022, 3, 100413. [Google Scholar] [CrossRef]
- Tohidi, B.; Rahimmalek, M.; Arzani, A.; Trindade, H. Sequencing and variation of terpene synthase gene (TPS2) as the major gene in biosynthesis for thymol in different Thymus Species. Phytochemistry 2020, 169, 112126. [Google Scholar] [CrossRef]
- Lima, A.S.; Schimmel, J.; Lukas, B.; Novak, J.; Barroso, J.G.; Figueiredo, A.C.; Pedro, L.G.; Degenhardt, J.; Trindade, H. Genomic characterization, molecular cloning and expression analysis of two terpene synthases from Thymus caespititius (Lamiaceae). Planta 2013, 238, 191–204. [Google Scholar] [CrossRef] [PubMed]
- Vrbková, E.; Šímová, A.; Vyskocilová, E.; Lhotka, M.; Cervený, L. Acid Treated Montmorillonite—Eco-Friendly Clay as Catalyst in Carvone Isomerization to Carvacrol. Reactions 2021, 2, 486–498. [Google Scholar] [CrossRef]
- Phillips, M. The sulfonation of para-cymene. J. Am. Chem. Soc. 1924, 46, 686–694. [Google Scholar] [CrossRef]
- Ritter, J.J.; Ginsburg, D. Preparation of chlorination of alpha-pinene with tert-butyl hypochlorite. J. Am. Chem. Soc. 1950, 72, 2381–2384. [Google Scholar] [CrossRef]
- De Vincenzi, M.; Stammati, A.; de Vincenzi, A.; Silano, M. Constituents of aromatic plants: Carvacrol. Fitoterapia 2004, 75, 801–804. [Google Scholar] [CrossRef]
- Melo, C.I.; Bogel-Łukasik, R.; Bogel-Łukasik, E. Combination of supercritical carbon dioxide and ionic liquid in a novel assembly of carvacrol. J. Supercrit. Fluids 2012, 61, 191–198. [Google Scholar] [CrossRef]
- Salehi, B.; Mishra, A.P.; Shukla, I.; Sharifi-Rad, M.; Contreras, M.D.M.; Segura-Carretero, A.; Fathi, H.; Nasri Nasrabadi, N.; Kobarfard, F.; Sharifi-Rad, J. Thymol, thyme, and other plant sources: Health and potential uses. Phytother. Res. 2018, 32, 1688–1706. [Google Scholar] [CrossRef]
- Xu, H.; Delling, M.; Jun, J.C.; Clapham, D.E. Oregano, thyme and clove-derived flavors and skin sensitizers activate specific TRP channels. Nat. Neurosci. 2006, 9, 628–635. [Google Scholar] [CrossRef]
- Park, B.S.; Choi, W.S.; Kim, J.H.; Kim, K.H.; Lee, S.E. Monoterpenes from thyme (Thymus vulgaris) as potential mosquito repellents. J. Am. Mosq. Control Assoc. 2005, 21, 80–83. [Google Scholar] [CrossRef]
- Azizi, Z.; Majlessi, N.; Choopani, S.; Naghdi, N. Neuroprotective effects of carvacrol against Alzheimer’s disease and other neurodegenerative diseases: A review. Avicenna J. Phytomed. 2022, 12, 371. [Google Scholar]
- Pelvan, E.; Karaoğlu, Ö.; Fırat, E.Ö.; Kalyon, K.B.; Ros, E.; Alasalvar, C. Immunomodulatory effects of selected medicinal herbs and their essential oils: A comprehensive review. J. Funct. Foods 2022, 94, 105108. [Google Scholar] [CrossRef]
- Chen, Y.; Ba, L.; Huang, W.; Liu, Y.; Pan, H.; Mingyao, E.; Shi, P.; Wang, Y.; Li, S.; Qi, H. Role of carvacrol in cardioprotection against myocardial ischemia/reperfusion injury in rats through activation of MAPK/ERK and Akt/eNOS signaling pathways. Eur. J. Pharmacol. 2017, 796, 90–100. [Google Scholar] [CrossRef] [PubMed]
- Sampaio, L.A.; Pina, L.T.S.; Serafini, M.R.; Tavares, D.D.S.; Guimaraes, A.G. Antitumor effects of carvacrol and thymol: A systematic review. Front. Pharmacol. 2021, 12, 702487. [Google Scholar] [CrossRef] [PubMed]
- Bouhtit, F.; Najar, M.; Moussa Agha, D.; Melki, R.; Najimi, M.; Sadki, K.; Boukhatem, N.; Bron, D.; Meuleman, N.; Hamal, A.; et al. New Anti-Leukemic Effect of Carvacrol and Thymol Combination through Synergistic Induction of Different Cell Death Pathways. Molecules 2021, 26, 410. [Google Scholar] [CrossRef]
- Ahmad, A.; Saeed, M.; Ansari, I.A. Molecular insights on chemopreventive and anticancer potential of carvacrol: Implications from solid carcinomas. J. Food Biochem. 2021, 45, e14010. [Google Scholar] [CrossRef]
- Guimarães, A.G.; Silva, F.V.; Xaviera, M.A.; Santos, M.R.V.; Rita, C.M.; Oliveira, R.C.M.; Oliveira, M.G.B.; Oliveira, A.P.; de Souza, C.C.; Quintans-Júnior, L.J. Orofacial Analgesic-Like Activity of Carvacrol in Rodents. Z. Naturforsch. 2012, 67, 481–485. [Google Scholar] [CrossRef]
- Quintans-Junior, L.J.; Guimaraes, A.G.; Araujo, B.E.S.; Oliveira, G.F.; Santana, M.T.; Moreira, F.V.; Santos, M.R.V.; Cavalcanti, S.C.H.; Junior, W.D.L.; Botelho, M.A. Carvacrol, (−)-borneol and citral reduce convulsant activity in rodents. Afr. J. Biotechnol. 2010, 9, 6566–6572. [Google Scholar]
- Anaeigoudari, A. Hepato-and reno-protective effects of thymoquinone, crocin, and carvacrol: A comprehensive review. Asian Pac J. Trop. Biomed. 2022, 12, 185. [Google Scholar] [CrossRef]
- Khazdair, M.R.; Ghorani, V.; Boskabady, M.H. Experimental and clinical evidence on the effect of carvacrol on respiratory, allergic, and immunologic disorders: A comprehensive review. BioFactors 2022, 48, 779–794. [Google Scholar] [CrossRef]
- Imran, M.; Aslam, M.; Alsagaby, S.A.; Saeed, F.; Ahmad, I.; Afzaal, M.; Arshad, M.U.; Abdelgawad, M.A.; El-Ghorab, A.H.; Khames, A.; et al. Therapeutic application of carvacrol: A comprehensive review. Food Sci. Nutr. 2022, 10, 3544–3561. [Google Scholar] [CrossRef]
- Wang, P.; Wu, Y. A review on colloidal delivery vehicles using carvacrol as a model bioactive compound. Food Hydrocoll. 2021, 120, 106922. [Google Scholar] [CrossRef]
- Domingos, E.L.; Vilhena, R.O.; Santos, J.M.; Fachi, M.M.; Böger, B.; Adam, L.M.; Tonin, F.S.; Pontarolo, R. Comparative efficacy and safety of systemic antifungal agents for candidemia: A systematic review with network meta-analysis and multicriteria acceptability analyses. Int. J. Antimicrob. Agents 2022, 60, 106614. [Google Scholar] [CrossRef] [PubMed]
- Nobile, C.J.; Johnson, A.D. Candida albicans biofilms and human disease. Annu. Rev. Microbiol. 2015, 69, 71. [Google Scholar] [CrossRef] [PubMed]
- Lepak, A.; Andes, D. Fungal sepsis: Optimizing antifungal therapy in the critical care setting. Crit Care Clin. 2011, 27, 123–147. [Google Scholar] [CrossRef]
- Jafri, H.; Ahmad, I. Thymus vulgaris essential oil and thymol inhibit biofilms and interact synergistically with antifungal drugs against drug resistant strains of Candida albicans and Candida tropicalis. J. Mycol. Med. 2020, 30, 100911. [Google Scholar] [CrossRef] [PubMed]
- Niu, C.; Wang, C.; Yang, Y.; Chen, R.; Zhang, J.; Chen, H.; Zhuge, Y.; Li, J.; Cheng, J.; Xu, K. Carvacrol induces Candida albicans apoptosis associated with Ca2+/calcineurin pathway. Front. Cell. Infect. Microbiol. 2020, 10, 192. [Google Scholar] [CrossRef]
- Miranda-Cadena, K.; Marcos-Arias, C.; Mateo, E.; Aguirre-Urizar, J.M.; Quindós, G.; Eraso, E. In vitro activities of carvacrol, cinnamaldehyde and thymol against Candida biofilms. Biomed. Pharmacother. 2021, 143, 112218. [Google Scholar] [CrossRef]
- Ismail, M.; Srivastava, V.; Marimani, M.; Ahmad, A. Carvacrol modulates the expression and activity of antioxidant enzymes in Candida auris. Res. Microbiol. 2022, 173, 103916. [Google Scholar] [CrossRef]
- Nóbrega, J.R.; Sousa, P.M.S.; de Lira Mota, K.S.; Cordeiro, L.V.; de Andrade Júnior, F.P.; de Oliveira, W.A. Antifungal activity of carvacrol and antifungal agent combinations against non-albicans Candida species. Sci. Plena 2019, 15, 1–7. [Google Scholar] [CrossRef]
- Mauriello, E.; Ferrari, G.; Donsì, F. Effect of formulation on properties, stability, carvacrol release and antimicrobial activity of carvacrol emulsions. Colloids Surf. B Biointerfaces 2021, 197, 111424. [Google Scholar] [CrossRef]
- Fouad, A.M.; Ruan, D.; El-Senousey, H.K.; Chen, W.; Jiang, S.; Zheng, C. Harmful effects and control strategies of aflatoxin b1 produced by Aspergillus flavus and Aspergillus parasiticus strains on poultry. Toxins 2019, 11, 176. [Google Scholar] [CrossRef] [PubMed]
- Thompson, D.P. Influence of pH on the fungitoxic activity of naturally occurring compounds. J. Food Protect. 1990, 53, 428–429. [Google Scholar] [CrossRef] [PubMed]
- Qu, C.; Li, Z.; Wang, X. UHPLC-HRMS-Based Untargeted Lipidomics Reveal Mechanism of Antifungal Activity of Carvacrol against Aspergillus flavus. Foods 2022, 11, 93. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, M.L. The multifunctional fungal ergosterol. MBio 2018, 9, e01755-18. [Google Scholar] [CrossRef]
- Alcazar-Fuoli, L.; Mellado, E. Ergosterol biosynthesis in Aspergillus fumigatus: Its relevance as an antifungal target and role in antifungal drug resistance. Front. Microbiol. 2012, 3, 439. [Google Scholar] [CrossRef]
- Cruz, R.; Wuest, W.M. Beyond ergosterol: Strategies for combatting antifungal resistance in Aspergillus fumigatus and Candida auris. Tetrahedron 2023, 133, 133268. [Google Scholar] [CrossRef]
- Rodriguez-Cuenca, S.; Pellegrinelli, V.; Campbell, M.; Oresic, M.; Vidal-Puig, A. Sphingolipids and glycerophospholipids—The “ying and yang” of lipotoxicity in metabolic diseases. Prog. Lipid Res. 2017, 66, 14–29. [Google Scholar] [CrossRef]
- Fonseca, L.M.; Souza, E.J.D.; Radünz, M.; Gandra, E.A.; da Rosa Zavareze, E.; Dias, A.R.G. Suitability of starch/carvacrol nanofibers as biopreservatives for minimizing the fungal spoilage of bread. Carbohydr. Polym. 2021, 252, 117166. [Google Scholar] [CrossRef]
- Liu, Q.; Qiao, K.; Zhang, S. Potential of a small molecule carvacrol in management of vegetable diseases. Molecules 2019, 24, 1932. [Google Scholar] [CrossRef]
- Wang, K.; Jiang, S.; Pu, T.; Fan, L.; Su, F.; Ye, M. Antifungal activity of phenolic monoterpenes and structure-related compounds against plant pathogenic fungi. Nat. Prod. Res. 2019, 33, 1423–1430. [Google Scholar] [CrossRef]
- Roca-Couso, R.; Flores-Félix, J.D.; Rivas, R. Mechanisms of action of microbial biocontrol agents against Botrytis cinerea. J. Fungi 2021, 7, 1045. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Ma, S.; Du, S.; Chen, S.; Sun, H. Antifungal activity of thymol and carvacrol against postharvest pathogens Botrytis cinerea. J. Food Sci. Technol. 2019, 56, 2611–2620. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Zhang, J.; Hu, C.; Deng, L.; Ritenour, M.A. Use of carvacrol and thymol in shellac coating to control stem-end rot on ‘Ruby Red’ grapefruit and maintain fruit quality during simulated storage and marketing. Sci. Hortic. 2020, 272, 109606. [Google Scholar] [CrossRef]
- Xiao, L.; Kang, S.; Lapu, M.; Jiang, P.; Wang, X.; Liu, D.; Li, J.; Liu, M. Preparation and characterization of chitosan/pullulan film loading carvacrol for targeted antibacterial packaging of chilled meat. Int. J. Biol. Macromol. 2022, 211, 140–149. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Nazifi, S.; Hakimian, A.; Firuznia, R.; Ghasemi, H. “Built to Last”: Plant-based Eco-friendly Durable Antibacterial Coatings. ACS Appl. Mater. Interfaces 2022, 14, 43681–43689. [Google Scholar] [CrossRef]
- Walczak, M.; Michalska-Sionkowska, M.; Olkiewicz, D.; Tarnawska, P.; Warżyńska, O. Potential of carvacrol and thymol in reducing biofilm formation on technical surfaces. Molecules 2021, 26, 2723. [Google Scholar] [CrossRef] [PubMed]
- Mechmechani, S.; Gharsallaoui, A.; Karam, L.; El Omari, K.; Fadel, A.; Hamze, M.; Chihib, N.E. Pepsin and Trypsin Treatment Combined with Carvacrol: An Efficient Strategy to Fight Pseudomonas aeruginosa and Enterococcus faecalis Biofilms. Microorganisms 2023, 11, 143. [Google Scholar] [CrossRef]
- Avire, N.J.; Whiley, H.; Ross, K. A Review of Streptococcus pyogenes: Public health risk factors, prevention and control. Pathogens 2021, 10, 248. [Google Scholar] [CrossRef]
- Wijesundara, N.M.; Lee, S.F.; Rupasinghe, H.V. Carvacrol inhibits Streptococcus pyogenes biofilms by suppressing the expression of genes associated with quorum-sensing and reducing cell surface hydrophobicity. Microb. Pathog. 2022, 169, 105684. [Google Scholar] [CrossRef]
- Luna, M.; Beltran, O.; Encinas-Basurto, D.A.; Ballesteros-Monrreal, M.G.; Topete, A.; Hassan, N.; López-Mata, M.A.; Reyes-Márquez, V.; Valdez, M.A.; Juarez, J. High antibacterial performance of hydrophobic chitosan-based nanoparticles loaded with Carvacrol. Colloids Surf. B Biointerfaces 2022, 209, 112191. [Google Scholar] [CrossRef]
- Cui, H.; Lu, J.; Li, C.; Rashed, M.M.; Lin, L. Antibacterial and physical effects of cationic starch nanofibers containing carvacrol@ casein nanoparticles against Bacillus cereus in soy products. Int. J. Food Microbiol. 2022, 364, 109530. [Google Scholar] [CrossRef] [PubMed]
- Mechmechani, S.; Gharsallaoui, A.; Fadel, A.; El Omari, K.; Khelissa, S.; Hamze, M.; Chihib, N.E. Microencapsulation of carvacrol as an efficient tool to fight Pseudomonas aeruginosa and Enterococcus faecalis biofilms. PLoS ONE 2022, 17, e0270200. [Google Scholar] [CrossRef]
- Yammine, J.; Gharsallaoui, A.; Fadel, A.; Mechmechani, S.; Karam, L.; Ismail, A.; Chihib, N.E. Enhanced antimicrobial, antibiofilm and ecotoxic activities of nanoencapsulated carvacrol and thymol as compared to their free counterparts. Food Control 2023, 143, 109317. [Google Scholar] [CrossRef]
- Fang, S.; Zhou, Q.; Hu, Y.; Liu, F.; Mei, J.; Xie, J. Antimicrobial carvacrol incorporated in flaxseed gum-sodium alginate active films to improve the quality attributes of Chinese sea bass (Lateolabrax maculatus) during cold storage. Molecules 2019, 24, 3292. [Google Scholar] [CrossRef] [PubMed]
- De Souza, G.H.D.A.; dos Santos Radai, J.A.; Mattos Vaz, M.S.; Esther da Silva, K.; Fraga, T.L.; Barbosa, L.S.; Simionatto, S. In vitro and in vivo antibacterial activity assays of carvacrol: A candidate for development of innovative treatments against KPC-producing Klebsiella pneumoniae. PLoS ONE 2021, 16, e0246003. [Google Scholar] [CrossRef]
- Khan, I.; Bahuguna, A.; Shukla, S.; Aziz, F.; Chauhan, A.K.; Ansari, M.B.; Bajpai, V.K.; Huh, Y.S.; Kang, S.C. Antimicrobial potential of the food-grade additive carvacrol against uropathogenic E. coli based on membrane depolarization, reactive oxygen species generation, and molecular docking analysis. Microb. Pathog. 2020, 142, 104046. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Zhang, J.; Yang, Q.; Sun, D.; Pu, X.; Shen, H.; Li, Q.; Wang, Z.; Lin, B. Antimicrobial activity of natural plant compound carvacrol against soft rot disease agent Dickeya zeae. Curr. Microbiol. 2021, 78, 3453–3463. [Google Scholar] [CrossRef]
- Kasthuri, T.; Swetha, T.K.; Bhaskar, J.P.; Pandian, S.K. Rapid-killing efficacy substantiates the antiseptic property of the synergistic combination of carvacrol and nerol against nosocomial pathogens. Arch. Microbiol. 2022, 204, 590. [Google Scholar] [CrossRef]
- Sousa, L.G.V.; Castro, J.; Cavaleiro, C.; Salgueiro, L.; Tomás, M.; Palmeira-Oliveira, R.; Martinez-Oliveira, J.; Cerca, N. Synergistic Effects of Carvacrol, α-Terpinene, γ-Terpinene, p-Cymene and Linalool against Gardnerella Species. Sci. Rep. 2022, 12, 4417. [Google Scholar] [CrossRef]
- Li, Q.; Yu, S.; Han, J.; Wu, J.; You, L.; Shi, X.; Wang, S. Synergistic antibacterial activity and mechanism of action of nisin/carvacrol combination against Staphylococcus aureus and their application in the infecting pasteurized milk. Food Chem. 2022, 380, 132009. [Google Scholar] [CrossRef]
- Churklam, W.; Chaturongakul, S.; Ngamwongsatit, B.; Aunpad, R. The mechanisms of action of carvacrol and its synergism with nisin against Listeria monocytogenes on sliced bologna sausage. Food Control 2020, 108, 106864. [Google Scholar] [CrossRef]
- Wu, M.; Dong, Q.; Ma, Y.; Yang, S.; Aslam, M.Z.; Liu, Y.; Li, Z. Potential antimicrobial activities of probiotics and their derivatives against Listeria monocytogenes in food field: A review. Food Res. Int. 2022, 160, 111733. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Hu, Y.; Sun, M.L.; Zheng, X.F.; Yang, M.; Rao, S.Q. Synergistic antibacterial effects of carvacrol and ε-polylysine. Qual. Assur. Saf. Crops Foods 2021, 13, 13–23. [Google Scholar] [CrossRef]
- Yoshida, T.; Nagasawa, T. ε-Poly-L-lysine: Microbial production, biodegradation and application potential. Appl. Microbiol. Biotechnol. 2003, 62, 21–26. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.Q.; Han, Q.; Feng, J.L.; Tian, W.L.; Mo, H.Z. Antibacterial characteristics and mechanisms of ɛ-poly-lysine against Escherichia coli and Staphylococcus aureus. Food Control 2014, 43, 22–27. [Google Scholar] [CrossRef]
- Addo, K.A.; Li, H.; Yu, Y.; Xiao, X. Unraveling the mechanism of the synergistic antimicrobial effect of cineole and carvacrol on Escherichia coli O157: H7 inhibition and its application on fresh-cut cucumbers. Food Control 2023, 144, 109339. [Google Scholar] [CrossRef]
- Fan, L.; Ismail, B.B.; Gao, L.; Liu, D. Comparison of high-and low-frequency thermosonication and carvacrol treatments of carrot juice: Microbial inactivation and quality retention. Appl. Food Res. 2022, 2, 100162. [Google Scholar] [CrossRef]
- Liu, Q.; Zhou, Y.H.; Yang, Z.Q. The cytokine storm of severe influenza and development of immunomodulatory therapy. Cell. Mol. Immunol. 2016, 13, 3–10. [Google Scholar] [CrossRef]
- Nedeva, C.; Menassa, J.; Puthalakath, H. Sepsis: Inflammation is a necessary evil. Front. Cell Dev. Biol. 2019, 7, 108. [Google Scholar] [CrossRef]
- Hafezi, B.; Chan, L.; Knapp, J.P.; Karimi, N.; Alizadeh, K.; Mehrani, Y.; Bridle, B.W.; Karimi, K. Cytokine storm syndrome in SARS-CoV-2 infections: A functional role of mast cells. Cells 2021, 10, 1761. [Google Scholar] [CrossRef]
- Al-Mansori, B.; El-Ageeli, W.H.; Alsagheer, S.H.; Ben-Khayal, F.A. Antioxidant Activity-Synergistic Effects of Thymol and Carvacrol. Al-Mukhtar J. Sci. 2020, 35, 185–194. [Google Scholar] [CrossRef]
- Mahtaj, L.G.; Feizpour, A.; Kianmehr, M.; Soukhtanloo, M.; Boskabady, M.H. The effect of carvacrol on systemic inflammation in Guinea pigs model of {COPD} induced by cigarette smoke exposure. Pharmacol. Rep. 2015, 67, 140–145. [Google Scholar] [CrossRef] [PubMed]
- Del Rio, D.; Stewart, A.J.; Pellegrini, N. A review of recent studies on malondialdehyde as toxic molecule and biological marker of oxidative stress. Nutr. Metab. Cardiovasc. Dis. 2005, 15, 316–328. [Google Scholar] [CrossRef]
- Carvalho, F.O.; Silva, R.; Nunes, P.S.; Felipe, F.A.; Ramos, K.P.P.; Ferreira, L.A.S.; Lima, V.N.B.; Shanmugam, S.; Oliveira, A.S.; Guterres, S.S.; et al. Effects of the solid lipid nanoparticle of carvacrol on rodents with lung injury from smoke inhalation. Naunyn-Schmiedeberg’s. Arch. Pharmacol. 2019, 393, 445–455. [Google Scholar] [CrossRef] [PubMed]
- Cicalău, G.; Babes, P.; Calniceanu, H.; Popa, A.; Ciavoi, G.; Iova, G.; Ganea, M.; Scrobotă, I. Anti-Inflammatory and Antioxidant Properties of Carvacrol and Magnolol, in Periodontal Disease and Diabetes Mellitus. Molecules 2021, 26, 6899. [Google Scholar] [CrossRef] [PubMed]
- Shoorei, H.; Khaki, A.; Khaki, A.A.; Hemmati, A.A.; Moghimian, M.; Shokoohi, M. The ameliorative effect of carvacrol on oxidative stress and germ cell apoptosis in testicular tissue of adult diabetic rats. Biomed. Pharmacother. 2019, 111, 568–578. [Google Scholar] [CrossRef]
- Da Silva Lima, M.; Quintans-Júnior, L.J.; de Santana, W.A.; Martins Kaneto, C.; Pereira Soares, M.B.; Villarreal, C.F. Antiinflammatory effects of carvacrol: Evidence for a key role of interleukin-10. Eur. J. Pharm. 2013, 699, 112–117. [Google Scholar] [CrossRef]
- Silva, F.V.; Guimaraes, A.G.; Silva, E.R.; Sousa-Neto, B.P.; Machado, F.D.; Quintans-Junior, L.J.; Arcanjo, D.D.; Oliveira, F.A.; Oliveira, R.C. Anti-inflammatory and anti-ulcer activities of carvacrol, a monoterpene present in the essential oil of oregano. J. Med. Food 2012, 15, 984–991. [Google Scholar] [CrossRef]
- De Santana Souza, M.T.; Teixeira, D.F.; de Oliveira, J.P.; Oliveira, A.S.; Quintans-Junior, L.J.; Correa, C.B.; Camargo, E.A. Protective effect of carvacrol on acetic acid-induced colitis. Biomed. Pharmacother. 2017, 96, 313–319. [Google Scholar] [CrossRef]
- Araruna, M.E.; Serafim, C.; Alves Júnior, E.; Hiruma-Lima, C.; Diniz, M.; Batista, L. Intestinal anti-inflammatory activity of terpenes in experimental models (2010–2020): A review. Molecules 2020, 25, 5430. [Google Scholar] [CrossRef]
- Wijesundara, N.M.; Lee, S.F.; Davidson, R.; Cheng, Z.; Rupasinghe, H.P.V. Carvacrol Suppresses Inflammatory Biomarkers Production by Lipoteichoic Acid- and Peptidoglycan-Stimulated Human Tonsil Epithelial Cells. Nutrients 2022, 14, 503. [Google Scholar] [CrossRef] [PubMed]
- Vecchio, A.J.; Simmons, D.M.; Malkowski, M.G. Structural basis of fatty acid substrate binding to cyclooxygenase-2. J. Biol. Chem. 2010, 285, 22152–22163. [Google Scholar] [CrossRef] [PubMed]
- Jalalvand, M.; Shahsavari, G.; Sheikhian, A.; Ganji, A.; Mosayebi, G. In vitro anti-inflammatory effects of Satureja khuzestanica essential oil compared to carvacrol. Int. J. Basic Sci. Med. 2020, 5, 61–65. [Google Scholar] [CrossRef]
- Landa, P.; Kokoska, L.; Pribylova, M.; Vanek, T.; Marsik, P. In vitro Anti-inflammatory Activity of Carvacrol: Inhibitory Effect on COX-2 Catalyzed Prostaglandin E2 Biosynthesis. Arch. Pharm. Res. 2009, 32, 75–78. [Google Scholar] [CrossRef]
- Hotta, M.; Nakata, R.; Katsukawa, M.; Hori, K.; Takahashi, S.; Inoue, H. Carvacrol, a component of thyme oil, activates PPARα and γ and suppresses COX-2 expression. J. Lipid Res. 2010, 51, 132–139. [Google Scholar] [CrossRef] [PubMed]
- Naeem, K.; Al Kury, L.T.; Nasar, F.; Alattar, A.; Alshaman, R.; Shah, F.A.; Khan, A.; Li, S. Natural dietary supplement, carvacrol, alleviates LPS-induced oxidative stress, neurodegeneration, and depressive-like behaviors via the Nrf2/HO-1 pathway. J. Inflamm. Res. 2021, 14, 1313. [Google Scholar] [CrossRef] [PubMed]
- Basauri, A.; González-Fernández, C.; Fallanza, M.; Bringas, E.; Fernandez-Lopez, R.; Giner, L.; Moncalián, G.; De, L.; Ortiz, I. Biochemical interactions between LPS and LPS-binding molecules. Crit. Rev. Biotechnol. 2020, 40, 292–305. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Zhang, L.; Joo, D.; Sun, S.C. NF-κB signaling in inflammation. Signal Transduct. Target. Ther. 2017, 2, 17023. [Google Scholar] [CrossRef] [PubMed]
- Kelley, N.; Jeltema, D.; Duan, Y.H.; He, Y. The NLRP3 Inflammasome: An Overview of Mechanisms of Activation and Regulation. Int. J. Mol. Sci. 2019, 20, 3328. [Google Scholar] [CrossRef]
- Kawai, T.; Takeuchi, O.; Fujita, T.; Inoue, J.; Muhlradt, P.F.; Sato, S.; Hoshino, K.; Akira, S. Lipopolysaccharide stimulates the MyD88-independent pathway and results in activation of IFN-regulatory factor 3 and the expression of a subset of lipopolysaccharide-inducible genes. J. Immunol. 2001, 167, 5887–5894. [Google Scholar] [CrossRef]
- Pfalzgraff, A.; Weindl, G. Intracellular lipopolysaccharide sensing as a potential therapeutic target for sepsis. Trends Pharmacol. Sci. 2019, 40, 187–197. [Google Scholar] [CrossRef] [PubMed]
- Marconi, G.D.; della Rocca, Y.; Fonticoli, L.; Guarnieri, S.; Carradori, S.; Rajan, T.S.; Pizzicannella, J.; Diomede, F. The Beneficial Effect of Carvacrol in HL-1 Cardiomyocytes Treated with LPS-G: Anti-Inflammatory Pathway Investigations. Antioxidants 2022, 11, 386. [Google Scholar] [CrossRef] [PubMed]
- Kianmehr, M.; Rezaei, A.; Boskabady, M.H. Effect of carvacrol on various cytokines genes expression in splenocytes of asthmatic mice. Iran. J. Basic Med. Sci. 2016, 19, 402–410. [Google Scholar] [PubMed]
- Somensi, N.; Rabelo, T.K.; Guimaraes, A.G.; Quintans-Junior, L.J.; de Souza Araújo, A.A.; Moreira, J.C.F.; Gelain, D.P. Carvacrol suppresses LPS-induced pro-inflammatory activation in RAW 264.7 macrophages through ERK1/2 and NF-κB pathway. Int. Immunopharmacol. 2019, 75, 105743. [Google Scholar] [CrossRef] [PubMed]
- Cui, J.; Chen, Y.; Wang, H.Y.; Wang, R.F. Mechanisms and pathways of innate immune activation and regulation in health and cancer. Hum. Vaccines Immunother. 2014, 10, 3270–3285. [Google Scholar] [CrossRef]
- Guimarães, A.G.; Oliveira, G.F.; Melo, M.S.; Cavalcanti, S.C.; Antoniolli, A.R.; Bonjardim, L.R.; Quintans-Júnior, L.J. Bioassay-guided evaluation of antioxidant and antinociceptive activities of carvacrol. Basic Clin. Pharmacol. Toxicol. 2010, 107, 949–957. [Google Scholar] [CrossRef]
- Wan, Q.; Song, D.; Li, H.; He, M.L. Stress proteins: The biological functions in virus infection, present and challenges for target-based antiviral drug development. Signal Transduct. Target. Ther. 2020, 5, 125. [Google Scholar] [CrossRef]
- Wieten, L.; van der Zee, R.; Goedemans, R.; Sijtsma, J.; Serafini, M.; Lubsen, N.H.; van Eden, W.; Broere, F. Hsp70 expression and induction as a readout for detection of immune modulatory components in food. Cell Stress Chaperones 2010, 15, 25–37. [Google Scholar] [CrossRef]
- Wieten, L.; van der Zee, R.; Spiering, R.; Wagenaar-Hilbers, J.; van Kooten, P.; Broere, F.; van Eden, W. A novel heat-shock protein coinducer boosts stress protein Hsp70 to activate T cell regulation of inflammation in autoimmune arthritis. Arthritis Rheum. 2010, 62, 1026–1035. [Google Scholar] [CrossRef]
- Ghorani, V.; Alavinezhad, A.; Rajabi, O.; Mohammadpour, A.H.; Boskabady, M.H. Safety and tolerability of carvacrol in healthy subjects: A phase I clinical study. Drug Chem. Toxicol. 2021, 44, 177–189. [Google Scholar] [CrossRef]
- Wang, Q.; Gong, J.; Huang, X.; Yu, H.; Xue, F. In vitro evaluation of the activity of microencapsulated carvacrol against Escherichia coli with K88 pili. J. Appl. Microbiol. 2009, 107, 1781–1788. [Google Scholar] [CrossRef] [PubMed]
- Dong, R.H.; Fang, Z.Z.; Zhu, L.L.; Ge, G.B.; Cao, Y.F.; Li, X.B.; Hu, C.M.; Yang, L.; Liu, Z.Y. Identification of CYP isoforms involved in the metabolism of thymol and carvacrol in human liver microsomes (HLMs). Pharmazie 2012, 67, 1002–1006. [Google Scholar] [PubMed]
- Aristatile, B.; Al-Assafa, A.H.; Pugalendi, K.V. Carvacrol ameliorates the Ppar-A and cytochrome P450 expression on d-galactosamine induced hepatotoxicity rats. Afr. J. Tradit. Complement. Altern. Med. 2014, 11, 118–123. [Google Scholar] [CrossRef] [PubMed]
- Hagan, E.C.; Hansen, W.H.; Fitzhugh, O.G.; Jenner, P.M.; Jones, W.I.; Taylor, J.M.; Long, E.L.; Nelson, A.A.; Brouwer, J.B. Food flavourings and compounds of related structure. II. Subacute and chronic toxicity. Food Cosmet. Toxicol. 1967, 5, 141–157. [Google Scholar] [CrossRef] [PubMed]
- Andersen, A. Final report on the safety assessment of sodium p-chloro-m-cresol, p-chloro-m-cresol, chlorothymol, mixed cresols, m-cresol, o-cresol, p-cresol, isopropyl cresols, thymol, o-cymen-5-ol, and carvacrol. Int. J. Toxicol. 2006, 25, 29–127. [Google Scholar] [PubMed]
- Livingston, A.E. The comparative toxicity of thymol and carvacrol (isothymol). Public Health Rep. 1921, 36, 1317–1331. [Google Scholar] [CrossRef]
- Schroder, V.; Vollmer, H. Über die Ausscheidung von Thymol, Carvacrol, Eugenol und Guajacol und die Verteilung dieser Sub stanzen im Organismus. Arch. Exp. Pathol. Pharmak. 1932, 168, 331–353. [Google Scholar] [CrossRef]
- Austgulen, L.T.; Solheim, E.; Scheline, R.R. Metabolism in rats of p-cymene derivatives: Carvacrol and thymol. Pharmacol. Toxicol. 1987, 61, 98–102. [Google Scholar] [CrossRef]
- De Alvarenga, J.F.R.; Genaro, B.; Costa, B.L.; Purgatto, E.; Manach, C.; Fiamoncini, J. Monoterpenes: Current knowledge on food source, metabolism, and health effects. Crit. Rev. Food Sci. Nutr. 2021, 13, 1352–1389. [Google Scholar] [CrossRef]
- Herber, R.; Lepage, M.; Pierfitte, M.; Villoutreix, J. Metabolism of some phenol derivatives in Mucor hiemalis. Compt. Rend. Sean. Soc. Biol. Filial. 1972, 166, 1087–1090. [Google Scholar]
- Numpaque, M.A.; Oviedo, L.A.; Gil, J.H.; García, C.M.; Durango, D.L. Thymol and carvacrol: Biotransformation and antifungal activity against the plant pathogenic fungi Colletotrichum acutatum and Botryodiplodia theobromae. Trop. Plant Pathol. 2011, 36, 3–13. [Google Scholar] [CrossRef]
- Noma, Y.; Asakawa, Y. Biotransformation of Monoterpenoids by Microorganisms, Insects, and Mammals. In Handbook of Essential Oils: Science, Technology, and Applications; Başer, K.H.C., Buchbauer, G., Eds.; CRC Press, Taylor and Francis Group: London, UK, 2010. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mączka, W.; Twardawska, M.; Grabarczyk, M.; Wińska, K. Carvacrol—A Natural Phenolic Compound with Antimicrobial Properties. Antibiotics 2023, 12, 824. https://doi.org/10.3390/antibiotics12050824
Mączka W, Twardawska M, Grabarczyk M, Wińska K. Carvacrol—A Natural Phenolic Compound with Antimicrobial Properties. Antibiotics. 2023; 12(5):824. https://doi.org/10.3390/antibiotics12050824
Chicago/Turabian StyleMączka, Wanda, Martyna Twardawska, Małgorzata Grabarczyk, and Katarzyna Wińska. 2023. "Carvacrol—A Natural Phenolic Compound with Antimicrobial Properties" Antibiotics 12, no. 5: 824. https://doi.org/10.3390/antibiotics12050824
APA StyleMączka, W., Twardawska, M., Grabarczyk, M., & Wińska, K. (2023). Carvacrol—A Natural Phenolic Compound with Antimicrobial Properties. Antibiotics, 12(5), 824. https://doi.org/10.3390/antibiotics12050824






