Abstract
While the relevance of inter-ethnic differences to the pharmacokinetic variabilities of antimicrobials has been reported in studies recruiting healthy subjects, differences in antimicrobial pharmacokinetics between Asian and non-Asian patients with severe pathologic conditions require further investigation. For the purpose of describing the potential differences in antimicrobial pharmacokinetics between Asian and non-Asian populations, a systematic review was performed using six journal databases and six theses/dissertation databases (PROSPERO record CRD42018090054). The pharmacokinetic data of healthy volunteers and non-critically ill and critically ill patients were reviewed. Thirty studies on meropenem, imipenem, doripenem, linezolid, and vancomycin were included in the final descriptive summaries. In studies recruiting hospitalised patients, inconsistent differences in the volume of distribution (Vd) and drug clearance (CL) of the studied antimicrobials between Asian and non-Asian patients were observed. Additionally, factors other than ethnicity, such as demographic (e.g., age) or clinical (e.g., sepsis) factors, were suggested to better characterise these pharmacokinetic differences. Inconsistent differences in pharmacokinetic parameters between Asian and non-Asian subjects/patients may suggest that ethnicity is not an important predictor to characterise interindividual pharmacokinetic differences between meropenem, imipenem, doripenem, linezolid, and vancomycin. Therefore, the dosing regimens of these antimicrobials should be adjusted according to patients’ demographic or clinical characteristics that can better describe pharmacokinetic differences.
1. Introduction
The recent surge in multi-drug-resistant pathogens, combined with the shortage of new antimicrobials, has created the need to optimise the use of current antimicrobials, particularly in countries with limited resources [,,,]. Applying pharmacokinetic/pharmacodynamic (PK/PD) principles to guide antimicrobial dosing can increase the likelihood of achieving optimal PK/PD exposures, which have been associated with therapeutic success and may limit the emergence of antimicrobial resistance [,,,]. However, difficulties arise when attempting to optimise antimicrobial dosing in special patient populations that are critically ill.
Key antimicrobial pharmacokinetic (PK) parameters of critically ill patients, particularly those with sepsis, may differ from those of non-critically ill patients [,,,,]. Critically ill patients commonly demonstrate extreme physiological changes that can alter antimicrobial PK and exposures [,,]. The volume of distribution (Vd) and drug clearance (CL) are important PK parameters that determine dosing requirements, and both may be dramatically altered during critical illness [,,]. Despite profound physiological and PK differences, critically ill patients in the ICU typically receive conventional antimicrobial dosing, which may likely lead to suboptimal antimicrobial exposure and therapeutic failure in this patient population. Therefore, antimicrobial dosing adjustment is needed to ensure that the optimal PK/PD exposures are achieved.
In addition to the acute illness-mediated changes in PK, inter-ethnic differences may also contribute to differences in PK parameters. The influence of ethnicity on drug PK has been reported for cyclosporine [], methadone [], efavirenz [], tacrolimus [], warfarin [], nifedipine [], midazolam [], and mycophenolic acid []. Inter-ethnic PK differences may stem from differences in (1) body composition [,,] or protein binding capacity [,]; (2) metabolic capacity due to genetic polymorphism in CYP450 [,]; and (3) the drug elimination process due to the variability in genes responsible for drug transporters, particularly those for biliary excretion [,,].
The clinical relevance of inter-ethnic differences to antimicrobial PK variability was reviewed by Tsai et al. in 2015 []. However, the relevance of inter-ethnic PK differences in patients with severe pathologic conditions (e.g., critically ill patients with sepsis) has not been reviewed thus far; Tsai et al. only included studies that recruited healthy volunteers in their systematic review. In addition, the systematic review mostly included studies of orally administered antimicrobials, whereas critically ill patients in the ICU almost always receive intravenous antimicrobials [].
Asia is the region with the largest population and diverse ethnic groups []. Describing the PK of antimicrobials in the Asian population is important to determine dosing requirements for this population. Numerous countries in this region rely heavily on product information to guide antimicrobial dosing, which can potentially be flawed for critically ill patients [,,]. Furthermore, dosing recommendations from the product information were mainly derived from dose-finding studies that mainly included subjects from non-Asian populations [,]. These doses may not always provide the same exposure for Asian patients due to potential inter-ethnic PK differences [].
The aim of this systematic review is to describe potential differences in antimicrobial PK between Asian and non-Asian populations with reference to data from healthy volunteers and non-critically ill and critically ill patients.
2. Results
2.1. Study Selection
The initial literature search from the journal and theses/dissertation databases identified 12,494 and 185 records, respectively. After removing duplicate records (n = 1307) and irrelevant records (n = 11,595), 1084 records were assessed in accordance with the inclusion and exclusion criteria. Only 102 and 249 records in Asian and non-Asian populations, respectively, were further selected for a full review. Of these, 15 Asian and 15 non-Asian PK studies were included in the final review [,,,,,,,,,,,,,,,,,,,,,,,,,,,,,]. The full process of study selection in this systematic review is described in Figure 1. The Asian PK studies were from seven countries, including Japan (n = 5), Thailand (n = 3), South Korea (n = 2), India (n = 2), China (n = 2), and Malaysia (n = 1). The population PK analysis was performed in 22 out of 30 studies.
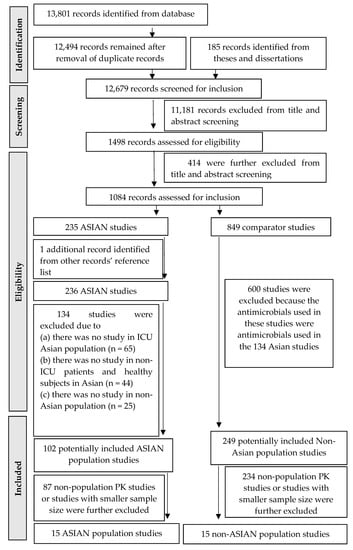
Figure 1.
Diagram for selecting studies.
The quality of studies included in this systematic review was assessed in accordance with the ClinPK checklist (Supplementary Table S1). All items required in the title/abstract and background sections are well reported by at least 90% of studies except for one requirement, which is to “report the PK parameters of the studied antimicrobials known in the literature” (63%). In the methods section, limited studies (33.3%) provided information on potential drug interactions with the studied antimicrobials. Only 11 (36.7%) studies clearly described the specific body weight descriptor used to determine the dose of antimicrobials or to calculate the PK parameters. In the results section, three requirements are reported by at least 90% of studies: (1) reporting the variables that may influence PK variabilities (93.3%); (2) including the measures of precision for the reported PK parameters (90%); and (3) the applicability of study findings (100%).
Studies included in the final PK comparison were on meropenem [,,,,,], imipenem [,,,,,], doripenem [,,,,,], linezolid [,,,,,], and vancomycin [,,,,,] (Table 1). Population PK studies in this systematic review identified body weight as a significant determinant of Vd for hydrophilic antimicrobials, including meropenem [,], imipenem [], doripenem [,,], linezolid [,], and vancomycin [,,]. Renal function was found to be a significant predictor of CL for all studied antimicrobials included in the systematic review [,,,,,,,,,,,,,,]. Serum creatinine (SeCr) was found as a significant covariate of CL in one study for meropenem []. Other covariates that have been reported to also influence the Vd and CL of these antimicrobials are listed in Table 2.

Table 1.
Pharmacokinetic studies of antimicrobials meeting inclusion criteria.

Table 2.
Additional significant covariates of pharmacokinetic parameters of antimicrobials.
2.2. Pharmacokinetic Differences between Asian and Non-Asian Population Groups
Table 3 summarises the antimicrobial PK parameters between Asian and non-Asian populations. The influence of ethnicity on primary PK parameters (i.e., Vd and CL) for each antimicrobial is further discussed below. Table 4 summarises the observed inter-ethnicity differences in Vd and CL for each antimicrobial.

Table 3.
Pharmacokinetic differences between Asian and Non-Asian population groups.

Table 4.
The relevance of inter-ethnic differences to the pharmacokinetic parameters of each antimicrobial.
2.2.1. Carbapenems
Meropenem
Inter-ethnic differences in Vd for meropenem between Asian and non-Asian patients were not observed. Studies in healthy subjects reported a relatively comparable meropenem Vd between Asian and non-Asian subjects. The reported mean Vd of meropenem in Asians and non-Asians was 0.16–0.20 L/kg and 0.18–0.19 L/kg (Table 3), respectively, depending on the dose administered [,]. Additionally, inconsistent findings between ICU and non-ICU studies may suggest that factors other than ethnicity could be driving these Vd differences [,,,]. In non-ICU patients, the mean Vd of meropenem in the central compartment was 4 times larger in Asian patients when compared with non-Asian patients (0.64 L/kg versus 0.15 L/kg; Table 3) [,]. However, in ICU patients, the mean Vd of meropenem in the central compartment was 2 times larger in non-Asian than in Asian patients (0.34 L/kg versus 0.16 L/kg; Table 3) [,].
Likewise, inter-ethnic differences in CL for meropenem between Asian and non-Asian patients were also not observed. Studies recruiting healthy subjects [,], non-ICU patients [,], and ICU patients (450, 451) reported comparable mean CL of meropenem between Asians and non-Asians (Table 3). The difference in the mean CL of meropenem between Asians and non-Asians among healthy subjects was up to 0.04 L/h/kg, which is similar to those of ICU studies [,,,]. In non-ICU studies, however, the difference was 0.01 L/h/kg.
Imipenem
Inter-ethnic differences in Vd for imipenem appear to be unlikely, as only ICU studies showed distinct Vd differences between Asian and non-Asian patients. In ICU studies, the mean Vd of imipenem in Asian and non-Asian patients was 0.5 L/kg and 0.38 L/kg, respectively (Table 3 and Table 4) [,]. In healthy subjects, the difference in the means of imipenem Vd between Asians and non-Asians was around 0.03–0.06 L/kg, depending on the dose administered, whilst it was 0.02–0.03 L/kg in non-ICU studies (Table 3) [,,,].
The available data do not suggest inter-ethnic differences in imipenem CL between Asian and non-Asian populations. Even though ICU studies highlighted that the mean CL of imipenem among Asian patients was double that among non-Asian patients (0.39 L/h/kg versus 0.16 L/h/kg; Table 3) [,], this observation was not seen in studies conducted in healthy subjects [,] and non-ICU patients [,]. The mean CL of imipenem was relatively comparable between Asians and non-Asians in studies of healthy subjects and non-ICU patients.
Doripenem
The difference in Vd for doripenem between healthy Asian and non-Asian subjects may suggest an inter-ethnic influence [,]. The mean total Vd of doripenem was larger in Asian healthy subjects when compared with non-Asian healthy subjects (0.31 L/kg versus 0.21 L/kg; Table 3). In addition to this observation, a remarkable difference was found in the mean Vd in the central compartment between Asian and non-Asian healthy subjects (0.26 L/kg versus 0.13 L/kg; Table 3). However, both non-ICU and ICU studies reported that the mean Vd of doripenem was comparable between Asian and non-Asian patients. The differences in the mean Vd of doripenem between Asians and non-Asians were 0.09 L/kg and 0.07 L/kg in non-ICU and ICU studies, respectively (Table 3) [,,,].
In contrast to Vd, inter-ethnic CL differences for doripenem might not be supported. Even though the mean CL of doripenem in Asian healthy subjects was 0.15 L/h/kg higher than that of non-Asian subjects (Table 3), caution should be exercised to support inter-ethnic differences because the study in non-Asian healthy subjects did not report detailed patient characteristics, including age and renal function []. It is also worth mentioning that both studies recruited healthy volunteers with different age ranges, which may have influenced this observation [,]. The non-ICU and ICU studies consistently suggested that non-Asian patients had faster doripenem CL when compared with Asian patients [,,,]. This could be related to the fact that the mean value of renal function in non-Asian patients, in both non-ICU and ICU settings, was higher than that in Asian patients.
2.2.2. Oxazolidinones
Linezolid is the only oxazolidinone studied in this systematic review. The oral bioavailability of linezolid was reported in one study, which reported a value of approximately 93% []. Some studies included in our systematic review demonstrated that linezolid was eliminated through both non-renal and renal routes []. However, almost all of the included studies in this systematic review reported linezolid CL as the total CL rather than as renal and non-renal CL separately [,,,,].
Linezolid
Inter-ethnic differences were not found for the Vd of linezolid. When Asians and non-Asians were compared, healthy subjects [,] and non-ICU patients [,] showed a similar mean value of Vd. In healthy subjects, the mean Vd of linezolid in Asians and non-Asians was reported as 0.65–0.67 L/kg and 0.58–0.61 L/kg (Table 3), respectively, depending on the route of administration [,]. From non-ICU studies, the reported mean Vd of linezolid in Asian and non-Asian patients was 0.59 L/kg and 0.58 L/kg, respectively [,]. However, ICU studies showed a remarkable difference in the mean Vd of linezolid in the central compartment between Asian and non-Asian patients (0.34 L/kg versus 0.19 L/kg; Table 3) [,]. This difference may be due to factors other than inter-ethnic differences.
Similar to Vd, inter-ethnic differences in linezolid CL were not observed. All studies involving healthy subjects and non-ICU and ICU patients reported a relatively similar mean CL of linezolid between Asian and non-Asian populations (Table 3) [,,,,,].
2.2.3. Glycopeptide
Vancomycin
The studies included in this systematic review did not demonstrate a pattern of inter-ethnic Vd differences between Asian and non-Asian populations for vancomycin. Studies of healthy subjects [,] showed a relatively comparable Vd between Asians and non-Asians. It is worth mentioning that comparisons of Vd in studies of healthy subjects could only be performed for the Vd in the central compartment because the peripheral compartment value was not reported in non-Asian studies []. The difference in the mean Vd of vancomycin in the central compartment between Asian and non-Asian healthy subjects was between 0.07 and 0.1 L/kg, depending on the dose of vancomycin [,]. In non-ICU studies, the mean Vd of vancomycin in the central compartment in Asian patients was more than 2 times larger than that in non-Asian patients (0.73 L/kg and 0.28 L/kg; Table 3) [,], while in ICU studies, the mean Vd of vancomycin in the central compartment was 4 times larger in non-Asian than in Asian patients (1.53 L/kg versus 0.40 L/kg; Table 3). Inconsistent findings between non-ICU and ICU studies may indicate the influence of factors other than ethnicity [,].
Inter-ethnic differences were also not observed for vancomycin CL. Based on studies of healthy subjects, non-ICU patients, and ICU patients, the mean CL of vancomycin was relatively comparable between Asian and non-Asian populations [,,,,,]. The mean CL differences between Asians and non-Asians were 0.02 L/h/kg, 0.03–0.02 L/h/kg (depending on the site of infection), and 0.01 L/h/kg in healthy subjects, non-ICU patients, and ICU patients, respectively (Table 3) [,,,,,].
3. Discussion
In this systematic review, we reviewed inter-ethnic PK differences for select antimicrobials between adult Asian and non-Asian populations, including healthy subjects and ICU, and non-ICU patients [,,,,,,,,,,,,,,,,,,,,,,,,,,,,,]. We found limited evidence to support the hypothesis that Asian subjects differ from non-Asian subjects in the PK disposition of carbapenems, vancomycin, and linezolid.
We found studies recruiting healthy subjects, non-ICU patients, and ICU patients on meropenem, imipenem, and doripenem in Asian and non-Asian populations. Of the three carbapenems, inter-ethnic differences could only be suggested for the Vd of doripenem. The Vd differences between meropenem and imipenem were only observed in studies involving hospitalised patients (ICU and non-ICU), and the findings were inconclusive to show which ethnic groups had a larger Vd. Therefore, factors other than ethnicity, such as demographic (e.g., age) or clinical (e.g., sepsis) factors, are suggested to contribute to the Vd differences between meropenem and imipenem [,,,].
In non-ICU studies, the mean Vd of meropenem in the central compartment was 4 times larger in Asian patients when compared with non-Asian patients (0.64 L/kg versus 0.15 L/kg) [,]. However, it could be possible that the larger Vd in non-ICU Asian patients was more likely to be related to age differences rather than ethnicity. Patients included in the Asian study were older compared to the non-Asian study (71.5 ± 13.5 versus 39.6 ± 18.2 years old). Mattioli et al., in their study, highlighted that the Vd of meropenem tended to be larger among older patients, specifically after 61 years old, which supports our findings []. Similar to the non-ICU studies, some caution should be exercised when interpreting the Vd differences observed in ICU studies. In studies involving ICU patients, the mean Vd of meropenem was 2 times larger in non-Asian than in Asian patients (0.34 L/kg versus 0.16 L/kg) [,]. The mean age of ICU patients in the non-Asian study [] was 63.26 years old, while the Asian study (450) did not report the age of participants. If the Asian study predominantly recruited patients with a younger age, then a lower Vd should be expected.
Similar to meropenem, Vd differences for imipenem may not indicate an inter-ethnic influence since the threshold supporting a “distinct difference” (≥0.1 L/kg) was only observed in ICU studies [,]. The mean Vd of imipenem in Asian ICU patients was larger than that in non-Asian ICU patients, and this could be attributed to several possible factors. First, serum albumin concentrations could contribute to the imipenem Vd differences, as serum albumin was found as a significant covariate influencing the Vd of imipenem [,,,]. Unfortunately, the serum albumin data were not reported in the Asian ICU study, and further analysis to rule out the influence of serum albumin could not be performed []. Second, the Vd of hydrophilic antimicrobials in critically ill patients, including imipenem, is subjected to extravascular fluid changes []. The third spacing phenomenon due to capillary leakage syndrome, which is commonly reported in critically ill patients with sepsis, might also contribute to the increase in Vd for imipenem []. In addition, the aggressive fluid treatment to overcome hypotension during septic shock in critically ill patients could further contribute to Vd increases [,,,]. Eighteen out of fifty-one patients in the non-Asian ICU study were patients with septic shock. However, no information was available regarding disease severity (including septic shock) in the Asian ICU study. If the number of patients with septic shock was higher in the Asian ICU study, then this could have contributed to the larger Vd.
Possible inter-ethnic Vd differences can only be suggested for doripenem, as a larger Vd in healthy Asian subjects was observed when compared with non-Asian subjects [,]. One plausible factor contributing to this finding is that the extracellular water volume per kg body weight is larger in Asian subjects [,]. Findings in the other two carbapenems also showed that Vd per kg of weight was higher in Asian than non-in Asian healthy subjects, even though the difference might not reach the threshold defined in our study [,,,]. The mean Vd of meropenem and imipenem in Asian healthy subjects was 0.01 L/kg and 0.02–0.04 L/kg higher when compared to non-Asian healthy subjects, respectively [,,,].
We did not observe inter-ethnic differences in CL for doripenem, even though the difference in the means of CL between Asian [] and non-Asian healthy subjects (0.15 L/h/kg) [] surpassed the threshold to indicate a “distinct difference” (≥0.1 L/h/kg) []. The main reason for this is due to the absence of data regarding the central tendency (such as mean and median values) for renal function in the non-Asian study []. Comparable renal function could not be assumed because the non-Asian study included older healthy subjects (up to 65 years old), while the Asian study recruited healthy subjects aged ≤ 30 years old. It is worth mentioning that, in addition to ethnicity, the renal blood flow and glomerular filtration rate are also influenced by age [,,,,]. Compared to subjects aged below 40 years old, significant reductions in renal blood flow and glomerular filtration were found in subjects aged above 55 years old [,]. Therefore, lower doripenem CL in the non-Asian study could also be related to the recruitment of subjects with a wide range of ages.
For non-carbapenem antimicrobials, including linezolid and vancomycin, studies recruiting hospitalised patients showed remarkable Vd differences [,,,]. The absence of inter-ethnic PK differences for linezolid and vancomycin among healthy subjects was also emphasised in a previous systematic review by Tsai et al. []. In ICU studies, considerable Vd differences for linezolid were observed in the central compartment rather than in the peripheral compartment [,]. The reported mean Vd in the central compartment in Asian and non-Asian ICU studies was 0.34 L/kg and 0.19 L/kg, respectively, while the reported mean Vd in the peripheral compartment was 0.39 L/kg and 0.34 L/kg, respectively [,]. It should be emphasised that the Asian ICU study was conducted specifically among patients with sepsis and septic shock, while the majority of patients in the non-Asian study were pneumonia patients. The number of patients with sepsis in the non-Asian study, however, was unknown. As a hydrophilic antimicrobial [], even though some authors also classified linezolid as an antimicrobial with moderately lipophilic properties [,], the Vd of linezolid in the central compartment may potentially increase due to aggressive fluid therapy to overcome the consequences of sepsis and septic shock [,]. Thus, the larger Vd for linezolid in the Asian study when compared to the non-Asian study could be more related to the severity of illness rather than inter-ethnic differences.
Based on the ICU studies, the mean Vd of vancomycin in Asian patients was approximately half that in non-Asian patients (0.40 L/kg versus 1.53 L/kg) [,]. Similar to what we suggested for linezolid, the different mean Vd of vancomycin reported in ICU studies could be more related to the severity of illness rather than the influence of ethnicity. One population PK study in our systematic review emphasised that the Vd of vancomycin in patients with sepsis and pneumonia was larger than in those with other types of infections []. It is worth noting that approximately 20% of patients in the Asian study were diagnosed with conditions other than pneumonia or sepsis, while patients in the non-Asian study exclusively recruited patients with sepsis. The severity of illness might also contribute to the difference in the mean Vd of vancomycin in non-ICU studies. However, no non-ICU studies in either Asian or non-Asian patients reported detailed patient and clinical characteristics, including the patients’ diagnoses [,]. In addition to the severity of illness, different times of collecting blood samples might also contribute to the larger Vd in Asians in the non-ICU study []. The blood samples in the non-ICU study [] were collected after the fifth dose of vancomycin, indicating a steady-state condition. In the comparator study, the time of sample collection was not reported [].
There are several limitations in this systematic review. Firstly, we included only one study to represent and compare the Asian and non-Asian populations. Therefore, we might not be able to adequately explain the PK variability for each antimicrobial in all Asian and non-Asian populations. Secondly, detailed information about ethnicity might not always be available in non-Asian studies, and it could be possible that the non-Asian population in the reviewed studies included Caucasian, Hispanic, and African-American subjects. Moreover, in the era of rapid globalisation, it is also possible that the non-Asian studies in our systematic review included some percentage of Asian subjects. However, such detailed information about ethnicity might not always be possible to gather from each study. Therefore, the findings might be different if comparisons were made specifically between two distinct ethnicities, for example, between Indonesians and African-American subjects. Thirdly, it is important to note that we included studies with different PK compartment models, and this could limit the interpretation of PK differences, particularly Vd. Many factors, including the number of samples, the time of blood sampling, and the PK analysis method, could contribute to the different reported PK models; it is indeed challenging to find studies with such comparable factors. In one compartment, the distribution of antibiotics was assumed to be rapid and homogeneous among all organs, which might not always be true for all antibiotics, especially for lipophilic antibiotics. On the other hand, in the two-compartment model, the distribution of antibiotics intravascularly and among organs with equilibrium was represented as Vc, and the distribution among organs with late equilibrium was represented as Vp. Although the interpretation of inter-ethnic Vd differences in the present systematic review should be made with caution, comparing the Vd of the one-compartment model with the Vc of the two-compartment model could be considered a reasonable approach for hydrophilic antibiotics, as these antibiotics were not distributed to the intra-cellular compartment. Furthermore, effort to make a rigorous PK comparison was made by considering not only the PK compartment model but also other factors influencing the PK parameters. Finally, it should be noted that limited inter-ethnic PK differences in our systematic review were observed for antimicrobials that undergo minimal hepatic metabolism via the CYP450 system. As human physiology is indeed influenced by ethnicity, particularly CYP-mediated drug metabolism, PK differences should be anticipated among patients with different ethnicities [,,,].
4. Materials and Methods
This systematic review was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) []. The 27-item PRISMA checklist of this systematic review can be found in Supplementary Materials (Tables S2 and S3).
4.1. Search Strategy
Six journal databases (Pubmed, Embase, Web of Science, Scopus, CINAHL, and Joanna Briggs Institute (JBI) EBP Database) and six theses/dissertation databases (Networked Digital Library of Theses and Dissertations, Open Access Theses and Dissertations, Hong Kong University Theses Online, China Doctoral Dissertations Full-Text Database, and National University of Singapore Theses, Online Union Catalogue of Indian Universities) were searched from inception to September 2017. The search was updated in February 2021. ES developed a list of search terms with additional inputs from other co-authors. There were two stages of the literature search in this study.
The first stage aimed to identify all PK studies in the Asian population. Boolean operators “AND” and “OR” of several pre-defined search terms were used in the first stage of the literature search (Supplementary Table S4). The search terms used in all databases can be found in the PROSPERO record (registration number CRD42018090054). The second stage aimed to identify PK studies in the non-Asian population, which were used as comparator studies. No restriction was applied in either stage of the literature search. The cited references of relevant articles were checked to find additional PK studies in both Asian and non-Asian populations.
4.2. Study Selection
Titles and abstracts from the search were combined and screened in EndNote X9 by ES. All duplicates and irrelevant studies were removed from the database, and the remaining titles and abstracts were reviewed in accordance with the inclusion criteria. Full-text articles of all potentially eligible studies were retrieved and further assessed based on the inclusion criteria. Any uncertainty regarding study inclusion was solved by discussion with co-authors (MOC and MHAA).
Studies that reported Vd and/or CL and recruited subjects ≥18 years old were included in this systematic review. For each antimicrobial, PK studies of healthy subjects and non-critically ill and critically ill patients from both Asian and non-Asian populations were selected. The study with the largest sample size that applied a population PK approach [,,] was selected if more than one study was available. A comparison of PK parameters was not made for an antimicrobial if we could not find at least one published study for each population of interest. Studies were excluded if they met at least one of the following criteria: (1) only recruited patients on RRT and/or extracorporeal membrane oxygenation (ECMO); (2) specifically conducted in burn patients; (3) presented PK parameters of a nebulised antimicrobial; (4) available only as an abstract of a poster presentation; (5) was a case report including only one patient; and (6) published in languages other than English.
4.3. Data Extraction and Quality Assessment of Pharmacokinetic Studies
Data extraction was performed by ES, and the following information was extracted from each study: (1) study design (population PK or non-population PK study); (2) number of subjects, (3) number of PK compartments; (4) subject/patient characteristics (age in years, body weight in kg, and CLCr in mL/min or mL/min/1.73 m2); (5) antimicrobial dosing regimen; (6) PK sampling information (after single dose, multiple doses, or both); and (7) PK parameters. The following PK parameters (abbreviation; unit) were extracted: (1) CL (L/h), (2) t1/2 (h), (3) Vc (L), and Vp (L); (4) intercompartmental clearance (Q; h−1); (5) KCP (h−1) and KPC (h−1). In addition to these PK parameters, the interindividual variability (IIV) for each PK parameter was extracted for population PK studies. Relevant adjustments were performed if the PK parameters were presented in different units. Another author (MHAA) performed a random check on 10% of the manuscripts to ensure the accuracy of data extraction.
The quality of the included studies was reviewed according to the consensus-based checklist for reporting PK studies []. The ClinPK checklist consisted of 24 items, which evaluated the robustness of a PK report in six (6) domains: (1) title/abstract (2 items); (2) background (3 items); (3) methods (10 items); (4) results (6 items); (5) discussion/conclusion (2 items); and (6) other information (1 item).
4.4. Pharmacokinetic Comparison Analysis
Patient characteristics and PK parameters are presented as means (with or without standard deviation, SD) and ranges. Original data presented as medians were converted to means by using the equation developed by Luo et al. (2018) []. The Cockcroft–Gault equation was used to calculate the CLCr if a study provided the SeCr concentration []. Comparisons of Vd and CL between Asian and non-Asian populations were performed as per kg body weight. Values of 60 kg and 70 kg were used as adjustments in Asian and non-Asian populations, respectively, for a study without body weight data [,,,].
In this systematic review, a difference in Vd or CL for an antimicrobial was classified as “were observed”, meaning influenced by ethnicity, if these differences were (1) consistently found in studies of healthy subjects, non-ICU patients, and ICU patients or (2) observed in studies recruiting healthy subjects with relatively comparable patient demographic characteristics. We set a difference value of ≥0.1 for both Vd and CL as the threshold to define inter-ethnic differences, as also used in a previous systematic review [].
5. Conclusions
Inconsistent differences in the PK of carbapenems, vancomycin, and linezolid between Asian and non-Asian subjects/patients in this systematic review indicate that ethnicity might not be a good proxy to adjust the dosing regimens for the studied antimicrobials. Moreover, the findings among studies of healthy subjects further suggested that the PK disposition of carbapenems, vancomycin, and linezolid in Asian subjects were relatively comparable to that in non-Asian subjects. Thus, dose adjustments for these antimicrobials should be made according to patients’ demographic or clinical characteristics that can better describe PK differences. A better understanding of significant covariates explaining the PK variabilities of antimicrobials could be helpful to identify which patients will benefit the most from the implementation of therapeutic drug monitoring (TDM). Finally, knowledge on the distribution of the minimum inhibitory concentrations (MICs) of important pathogens in a specific area would further maximise the attainment of PK/PD exposures associated with therapeutic success.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/antibiotics12050803/s1. Table S1: PRISMA items checklist for systematic review of inter-ethnic pharmacokinetic differences between Asian and non-Asian adult populations. Table S2: PRISMA checklist for abstract for systematic review of inter-ethnic pharmacokinetic differences between Asian and non-Asian adult populations. Table S3: Search terms in systematic review of inter-ethnic pharmacokinetic differences between Asian and non-Asian adult populations. Table S4: Reporting quality of pharmacokinetic studies included in the final analysis.
Author Contributions
Conceptualisation, E.S., M.O.C., J.A.R. and M.H.A.-A.; Methodology, E.S. and M.H.A.-A.; Screening and Study Selection, E.S., M.O.C. and M.H.A.-A.; Data Extraction, E.S. and M.H.A.-A.; Writing—original draft preparation, E.S.; Writing—review and editing, M.O.C., J.A.R. and M.H.A.-A.; Supervision, M.O.C., J.A.R. and M.H.A.-A. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Acknowledgments
J.A. Roberts would like to acknowledge funding from the Australian National Health and Medical Research Council for a Centre of Research Excellence (APP1099452) and a Practitioner Fellowship (APP1117065), as well as an Advancing Queensland Clinical Fellowship. All other authors: none to declare.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
| CL | clearance |
| CLCr | creatinine clearance |
| CLnon-ren | non-renal clearance |
| CLren | clearance renal |
| Cmax | maximum concentration in a dosing interval |
| Cmax,ss | maximum concentration at pharmacokinetic steady state |
| Cmin | minimum concentration in a dosing interval |
| CYP450 | cytochrome P450 |
| ECMO | extracorporeal membrane oxygenation |
| ICU | intensive care unit |
| IIV | interindividual variability |
| IV | intravenous |
| KCP | the rate constant from the central compartment to the peripheral compartment |
| Ke | elimination rate constant |
| KPC | the rate constant from the peripheral compartment to the central compartment |
| L | litre |
| L/h | litre per hour |
| L/kg | litre per kilogram body weight |
| L/h/kg | litre per hour per kilogram body weight |
| MIC | minimum inhibitory concentration |
| PK/PD | pharmacokinetic/pharmacodynamic |
| PK | pharmacokinetic |
| Q | intercompartmental clearance |
| RRT | renal replacement therapy |
| SeCr | serum creatinine |
| t1/2 | half-life |
| TDM | therapeutic drug monitoring |
| Vc | volume of distribution in central compartment |
| Vd | volume of distribution |
| Vdss | volume of distribution at steady state |
| Vd-tot | volume of distribution total |
| Vp | volume of distribution in peripheral compartment |
References
- Aslam, B.; Wang, W.; Arshad, M.I.; Khurshid, M.; Muzammil, S.; Rasool, M.H.; Nisar, M.A.; Alvi, R.F.; Aslam, M.A.; Qamar, M.U.; et al. Antibiotic resistance: A rundown of a global crisis. Infect. Drug Resist. 2018, 11, 1645–1658. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Global Antimicrobial Resistance Surveillance System (GLASS) Report: Early Implementation 2020; World Health Organization: Geneva, Switzerland, 2020. [Google Scholar]
- Lepore, C.; Silver, L.; Theuretzbacher, U.; Thomas, J.; Visi, D. The Small-Molecule Antibiotics Pipeline: 2014–2018. Nat. Rev. Drug Discov. 2019, 18, 739. [Google Scholar] [CrossRef] [PubMed]
- Center for Disease Dynamics, Economics & Policy. Access Barriers to Antibiotics; Center for Disease Dynamics, Economics & Policy: Washington, DC, USA, 2019. [Google Scholar]
- Tam, V.H.; Chang, K.T.; Zhou, J.; Ledesma, K.R.; Phe, K.; Gao, S.; Van Bambeke, F.; Sánchez-Díaz, A.M.; Zamorano, L.; Oliver, A.; et al. Determining β-lactam exposure threshold to suppress resistance development in Gram-negative bacteria. J. Antimicrob. Chemother. 2017, 72, 1421–1428. [Google Scholar] [CrossRef]
- Bowker, K.E.; Noel, A.R.; Tomaselli, S.G.; Elliott, H.; MacGowan, A.P. Pharmacodynamics of the antibacterial effect and emergence of resistance to doripenem in Pseudomonas aeruginosa and Acinetobacter baumannii in an in vitro pharmacodynamic model. Antimicrob. Agents Chemother. 2012, 56, 5009–5015. [Google Scholar] [CrossRef]
- MacVane, S.H.; Kuti, J.L.; Nicolau, D.P. Clinical pharmacodynamics of antipseudomonal cephalosporins in patients with ventilator-associated pneumonia. Antimicrob. Agents Chemother. 2014, 58, 1359–1364. [Google Scholar] [CrossRef] [PubMed]
- Muller, A.E.; Punt, N.; Mouton, J.W. Exposure to ceftobiprole is associated with microbiological eradication and clinical cure in patients with nosocomial pneumonia. Antimicrob. Agents Chemother. 2014, 58, 2512–2519. [Google Scholar] [CrossRef] [PubMed]
- Roberts, J.A.; Abdul-Aziz, M.H.; Lipman, J.; Mouton, J.W.; Vinks, A.A.; Felton, T.W.; Hope, W.W.; Farkas, A.; Neely, M.N.; Schentag, J.J.; et al. Individualised antibiotic dosing for patients who are critically ill: Challenges and potential solutions. Lancet Infect. Dis. 2014, 14, 498–509. [Google Scholar] [CrossRef] [PubMed]
- Slaviero, K.A.; Clarke, S.J.; Rivory, L.P. Inflammatory response: An unrecognised source of variability in the pharmacokinetics and pharmacodynamics of cancer chemotherapy. Lancet Oncol. 2003, 4, 224–232. [Google Scholar] [CrossRef] [PubMed]
- Hochepied, T.; Berger, F.G.; Baumann, H.; Libert, C. Alpha(1)-acid glycoprotein: An acute phase protein with inflammatory and immunomodulating properties. Cytokine Growth Factor Rev. 2003, 14, 25–34. [Google Scholar] [CrossRef]
- Ulldemolins, M.; Roberts, J.A.; Lipman, J.; Rello, J. Antibiotic dosing in multiple organ dysfunction syndrome. Chest 2011, 139, 1210–1220. [Google Scholar] [CrossRef]
- Udy, A.A.; Baptista, J.P.; Lim, N.L.; Joynt, G.M.; Jarrett, P.; Wockner, L.; Boots, R.J.; Lipman, J. Augmented renal clearance in the ICU: Results of a multicenter observational study of renal function in critically ill patients with normal plasma creatinine concentrations. Crit. Care Med. 2014, 42, 520–527. [Google Scholar] [CrossRef] [PubMed]
- Inoue, S.; Howgate, E.M.; Rowland-Yeo, K.; Shimada, T.; Yamazaki, H.; Tucker, G.T.; Rostami-Hodjegan, A. Prediction of in vivo drug clearance from in vitro data. II: Potential inter-ethnic differences. Xenobiotica 2006, 36, 499–513. [Google Scholar] [CrossRef] [PubMed]
- Bart, G.; Lenz, S.; Straka, R.J.; Brundage, R.C. Ethnic and genetic factors in methadone pharmacokinetics: A population pharmacokinetic study. Drug Alcohol Depend. 2014, 145, 185–193. [Google Scholar] [CrossRef] [PubMed]
- Ngaimisi, E.; Habtewold, A.; Minzi, O.; Makonnen, E.; Mugusi, S.; Amogne, W.; Yimer, G.; Riedel, K.D.; Janabi, M.; Aderaye, G.; et al. Importance of ethnicity, CYP2B6 and ABCB1 genotype for efavirenz pharmaco-kinetics and treatment outcomes: A parallel-group prospective cohort study in two sub-saharan Africa populations. PLoS ONE 2013, 8, e67946. [Google Scholar] [CrossRef] [PubMed]
- Andrews, L.M.; De Winter, B.C.; Van Gelder, T.; Hesselink, D.A. Consideration of the ethnic prevalence of genotypes in the clinical use of tacrolimus. Pharmacogenomics 2016, 17, 1737–1740. [Google Scholar] [CrossRef] [PubMed]
- Ye, C.; Jin, H.; Zhang, R.; Sun, Y.; Wang, Z.; Sun, W.; Sun, W.; Peng, Q.; Liu, R.; Huang, Y. Variability of warfarin dose response associated with CYP2C9 and VKORC1 gene polymorphisms in Chinese patients. J. Int. Med. Res. 2014, 42, 67–76. [Google Scholar] [CrossRef]
- Ahsan, C.H.; Renwick, A.G.; Macklin, B.; Challenor, V.F.; Waller, D.G.; George, C.F. Ethnic differences in the pharmacokinetics of oral nifedipine. Br. J. Clin. Pharmacol. 1991, 31, 399–403. [Google Scholar] [CrossRef]
- Guo, T.; Mao, G.F.; Xia, D.Y.; Su, X.Y.; Zhao, L.S. Pharmacokinetics of midazolam tablet in different Chinese ethnic groups. J. Clin. Pharm. Ther. 2011, 36, 406–411. [Google Scholar] [CrossRef]
- Tornatore, K.M.; Meaney, C.J.; Wilding, G.E.; Chang, S.S.; Gundroo, A.; Cooper, L.M.; Gray, V.; Shin, K.; Fetterly, G.J.; Prey, J.; et al. Influence of sex and race on mycophenolic acid pharmacokinetics in stable African American and Caucasian renal transplant recipients. Clin. Pharmacokinet. 2015, 54, 423–434. [Google Scholar] [CrossRef]
- Staiano, A.E.; Broyles, S.T.; Gupta, A.K.; Katzmarzyk, P.T. Ethnic and sex differences in visceral, subcutaneous, and total body fat in children and adolescents. Obesity 2013, 21, 1251–1255. [Google Scholar] [CrossRef]
- Liu, A.; Byrne, N.M.; Kagawa, M.; Ma, G.; Kijboonchoo, K.; Nasreddine, L.; Koon Poh, B.; Ismail, M.N.; Hills, A.P. Ethnic differences in body fat distribution among Asian pre-pubertal children: A cross-sectional multicentre study. BMC Public Health 2011, 11, 500. [Google Scholar] [CrossRef] [PubMed]
- Deurenberg, P.; Tagliabue, A.; Wang, J.; Wolde-Gebriel, Z. Multi-frequency bioelectrical impedence for the prediction of body water compartments: Validation in different ethnic groups. Asia Pac. J. Clin. Nutr. 1996, 5, 217–221. [Google Scholar] [PubMed]
- Johnson, J.A.; Livingston, T.N. Differences between blacks and whites in plasma protein binding of drugs. Eur. J. Clin. Pharmacol. 1997, 51, 485–488. [Google Scholar] [CrossRef] [PubMed]
- Silva, A.M.; Wang, J.; Pierson, R.N., Jr.; Wang, Z.M.; Heymsfield, S.B.; Sardinha, L.B.; Heshka, S. Extracellular water: Greater expansion with age in African Americans. J. Appl. Physiol. 2005, 99, 261–267. [Google Scholar] [CrossRef]
- Kitada, M. Genetic polymorphism of cytochrome P450 enzymes in Asian populations: Focus on CYP2D6. Int. J. Clin. Pharmacol. Res. 2003, 23, 31–35. [Google Scholar]
- Daniel, L.H. Polymorphisms of cytochrome P450 are potential candidates that could potentially help clinicians on the treatment of cardiovascular diseases among Asian populations. Indian Heart J. 2017, 69, 655–656. [Google Scholar] [CrossRef]
- Cropp, C.D.; Yee, S.W.; Giacomini, K.M. Genetic variation in drug transporters in ethnic populations. Clin. Pharmacol. Ther. 2008, 84, 412–416. [Google Scholar] [CrossRef]
- Tanaka, Y.; Manabe, A.; Fukushima, H.; Suzuki, H.; Nakadate, H.; Kondoh, K.; Nakamura, K.; Koh, K.; Fukushima, T.; Tsuchida, M.; et al. Multidrug resistance protein 4 (MRP4) polymorphisms impact the 6-mercaptopurine dose tolerance during maintenance therapy in Japanese childhood acute lymphoblastic leukemia. Pharm. J. 2015, 15, 380–384. [Google Scholar] [CrossRef]
- Abla, N.; Chinn, L.W.; Nakamura, T.; Liu, L.; Huang, C.C.; Johns, S.J.; Kawamoto, M.; Stryke, D.; Taylor, T.R.; Ferrin, T.E.; et al. The human multidrug resistance protein (MRP4, ABCC4): Functional analysis of a highly polymorphic gene. J. Pharmacol. Exp. Ther. 2008, 325, 859–868. [Google Scholar] [CrossRef]
- Tsai, D.; Jamal, J.A.; Davis, J.S.; Lipman, J.; Roberts, J.A. Interethnic differences in pharmacokinetics of antibacterials. Clin. Pharmacokinet. 2015, 54, 243–260. [Google Scholar] [CrossRef]
- Walton, J.; Harris, A.; Iwabuchi, K. Introduction: Everyday multiculturalism in/across Asia. Ethn. Racial Stud. 2020, 43, 807–815. [Google Scholar] [CrossRef]
- Ishihara, N.; Nishimura, N.; Ikawa, K.; Karino, F.; Miura, K.; Tamaki, H.; Yano, T.; Isobe, T.; Morikawa, N.; Naora, K. Population pharmacokinetic modeling and pharmacodynamic target attainment simulation of piperacillin/tazobactam for dosing optimization in late elderly patients with pneumonia. Antibiotics 2020, 9, 113. [Google Scholar] [CrossRef] [PubMed]
- Cao, G.; Zhang, J.; Wu, X.; Yu, J.; Chen, Y.; Ye, X.; Zhu, D.; Zhang, Y.; Guo, B.; Shi, Y. Pharmacokinetics and pharmacodynamics of levofloxacin injection in healthy Chinese volunteers and dosing regimen optimization. J. Clin. Pharm. Ther. 2013, 38, 394–400. [Google Scholar] [CrossRef] [PubMed]
- Ikawa, K.; Nomura, K.; Morikawa, N.; Ikeda, K.; Ohge, H.; Sueda, T.; Taniwaki, M. Pharmacokinetic-pharmacodynamic target attainment analysis of cefozopran in Japanese adult patients. J. Infect. Chemother. 2008, 14, 130–136. [Google Scholar] [CrossRef] [PubMed]
- Ding, J.; Zhou, Y.; Khan, M.S.; Sy, R.N.; Khosa, F. Representation of sex, race, and ethnicity in pivotal clinical trials for dermatological drugs. Int. J. Womens Dermatol. 2021, 7, 428–434. [Google Scholar] [CrossRef]
- Cwalina, T.B.; Jella, T.K.; Manyak, G.A.; Kuo, A.; Kamath, A.F. Is our science representative? A systematic review of racial and ethnic diversity in orthopaedic clinical trials from 2000 to 2020. Clin. Orthop. Relat. Res. 2022, 480, 848–858. [Google Scholar] [CrossRef]
- Jaruratanasirikul, S.; Sriwiriyajan, S. Comparison of the pharmacodynamics of meropenem in healthy volunteers following administration by intermittent infusion or bolus injection. J. Antimicrob. Chemother. 2003, 52, 518–521. [Google Scholar] [CrossRef]
- Krueger, W.A.; Bulitta, J.; Kinzig-Schippers, M.; Landersdorfer, C.; Holzgrabe, U.; Naber, K.G.; Drusano, G.L.; Sörgel, F. Evaluation by monte carlo simulation of the pharmacokinetics of two doses of meropenem administered intermittently or as a continuous infusion in healthy volunteers. Antimicrob. Agents Chemother. 2005, 49, 1881–1889. [Google Scholar] [CrossRef]
- Muro, T.; Sasaki, T.; Hosaka, N.; Umeda, Y.; Takemoto, S.; Yamamoto, H.; Kamimura, H.; Higuchi, S.; Karube, Y. Population pharmacokinetic analysis of meropenem in Japanese adult patients. J. Clin. Pharm. Ther. 2011, 36, 230–236. [Google Scholar] [CrossRef]
- Li, C.; Kuti, J.L.; Nightingale, C.H.; Nicolau, D.P. Population pharmacokinetic analysis and dosing regimen optimization of meropenem in adult patients. J. Clin. Pharmacol. 2006, 46, 1171–1178. [Google Scholar] [CrossRef]
- Mathew, S.K.; Mathew, B.S.; Neely, M.N.; Naik, G.S.; Prabha, R.; Jacob, G.G.; Fleming, D.H. A nonparametric pharmacokinetic approach to determine the optimal dosing regimen for 30-minute and 3-hour meropenem infusions in critically ill patients. Ther. Drug Monit. 2016, 38, 593–599. [Google Scholar] [CrossRef] [PubMed]
- Idoate Grijalba, A.I.; Aldaz Pastor, A.; Marquet, P.; Woillard, J.B. Evaluation of a non-parametric modelling for meropenem in critically ill patients using Monte Carlo simulation. Eur. J. Clin. Pharmacol. 2019, 75, 1405–1414. [Google Scholar] [CrossRef]
- Jaruratanasirikul, S.; Raungsri, N.; Punyo, J.; Sriwiriyajan, S. Pharmacokinetics of imipenem in healthy volunteers following administration by 2 h or 0.5 h infusion. J. Antimicrob. Chemother. 2005, 56, 1163–1165. [Google Scholar] [CrossRef] [PubMed]
- Norrby, S.R.; Björnegård, B.; Ferber, F.; Jones, K.H. Pharmacokinetics of imipenem in healthy volunteers. J. Antimicrob. Chemother. 1983, 12 (Suppl. D), 109–124. [Google Scholar] [CrossRef] [PubMed]
- Yoshizawa, K.; Ikawa, K.; Ikeda, K.; Kumon, H.; Ohge, H.; Morikawa, N. Optimisation of imipenem regimens in patients with impaired renal function by pharmacokinetic-pharmacodynamic target attainment analysis of plasma and urinary concentration data. Int. J. Antimicrob. Agents 2012, 40, 427–433. [Google Scholar] [CrossRef]
- Finch, R.G.; Craddock, C.; Kelly, J.; Deaney, N.B. Pharmacokinetic studies of imipenem/cilastatin in elderly patients. J. Antimicrob. Chemother. 1986, 18 (Suppl. E), 103–107. [Google Scholar] [CrossRef]
- Abhilash, B.; Tripathi, C.D.; Gogia, A.R.; Meshram, G.G.; Kumar, M.; Suraj, B. Pharmaco-kinetic/pharmacodynamic profiling of imipenem in patients admitted to an intensive care unit in India: A nonrandomized, cross-sectional, analytical, open-labeled study. Indian J. Crit. Care Med. 2015, 19, 587–592. [Google Scholar] [CrossRef]
- Couffignal, C.; Pajot, O.; Laouénan, C.; Burdet, C.; Foucrier, A.; Wolff, M.; Armand-Lefevre, L.; Mentré, F.; Massias, L. Population pharmacokinetics of imipenem in critically ill patients with suspected ventilator-associated pneumonia and evaluation of dosage regimens. Br. J. Clin. Pharmacol. 2014, 78, 1022–1034. [Google Scholar] [CrossRef]
- Kim, S.W.; Choe, S.; Kim, D.J.; Zang, D.Y.; Lee, D.H. Pharmacokinetics of doripenem in healthy Koreans and Monte Carlo simulations to explore optimal dosage regimens in patients with normal and enhanced renal function. Ther. Drug Monit. 2018, 40, 425–434. [Google Scholar] [CrossRef]
- Bhavnani, S.M.; Hammel, J.P.; Cirincione, B.B.; Wikler, M.A.; Ambrose, P.G. Use of pharmacokinetic-pharmacodynamic target attainment analyses to support phase 2 and 3 dosing strategies for doripenem. Antimicrob. Agents Chemother. 2005, 49, 3944–3947. [Google Scholar] [CrossRef]
- Lee, D.H.; Kim, Y.K.; Jin, K.; Kang, M.J.; Joo, Y.D.; Kim, Y.W.; Moon, Y.S.; Shin, J.G.; Kiem, S. Population pharmacokinetic analysis of doripenem after intravenous infusion in Korean patients with acute infections. Antimicrob. Agents Chemother. 2017, 61, e02185–e021816. [Google Scholar] [CrossRef] [PubMed]
- Bhalodi, A.A.; Keel, R.A.; Quintiliani, R.; Lodise, T.P.; Nicolau, D.P.; Kuti, J.L. Pharmacokinetics of doripenem in infected patients treated within and outside the intensive care unit. Ann. Pharmacother. 2013, 47, 617–627. [Google Scholar] [CrossRef] [PubMed]
- Abdul-Aziz, M.H.; Abd Rahman, A.N.; Mat-Nor, M.B.; Sulaiman, H.; Wallis, S.C.; Lipman, J.; Roberts, J.A.; Staatz, C.E. Population pharmacokinetics of doripenem in critically ill patients with sepsis in a Malaysian intensive care unit. Antimicrob. Agents Chemother. 2015, 60, 206–214. [Google Scholar] [CrossRef] [PubMed]
- Roberts, J.A.; Lipman, J. Optimal doripenem dosing simulations in critically ill nosocomial pneumonia patients with obesity, augmented renal clearance, and decreased bacterial susceptibility. Crit. Care Med. 2013, 41, 489–495. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Zhang, J.; Chen, Y.; Liang, X.; Guo, Y.; Yu, J.; Zhu, D.; Zhang, Y. Optimization of linezolid treatment regimens for Gram-positive bacterial infections based on pharmacokinetic/pharmacodynamic analysis. Future Microbiol. 2017, 12, 39–50. [Google Scholar] [CrossRef] [PubMed]
- Stalker, D.J.; Jungbluth, G.L.; Hopkins, N.K.; Batts, D.H. Pharmacokinetics and tolerance of single- and multiple-dose oral or intravenous linezolid, an oxazolidinone antibiotic, in healthy volunteers. J. Antimicrob. Chemother. 2003, 51, 1239–1246. [Google Scholar] [CrossRef]
- Sasaki, T.; Takane, H.; Ogawa, K.; Isagawa, S.; Hirota, T.; Higuchi, S.; Horii, T.; Otsubo, K.; Ieiri, I. Population pharmacokinetic and pharmacodynamic analysis of linezolid and a hematologic side effect, thrombocytopenia, in Japanese patients. Antimicrob. Agents Chemother. 2011, 55, 1867–1873. [Google Scholar] [CrossRef]
- Crass, R.L.; Cojutti, P.G.; Pai, M.P.; Pea, F. Reappraisal of linezolid dosing in renal impairment to improve safety. Antimicrob. Agents Chemother. 2019, 63, e00605–e00619. [Google Scholar] [CrossRef]
- Ide, T.; Takesue, Y.; Ikawa, K.; Morikawa, N.; Ueda, T.; Takahashi, Y.; Nakajima, K.; Takeda, K.; Nishi, S. Population pharmacokinetics/pharmacodynamics of linezolid in sepsis patients with and without continuous renal replacement therapy. Int. J. Antimicrob. Agents 2018, 51, 745–751. [Google Scholar] [CrossRef]
- Taubert, M.; Zoller, M.; Maier, B.; Frechen, S.; Scharf, C.; Holdt, L.M.; Frey, L.; Vogeser, M.; Fuhr, U.; Zander, J. Predictors of inadequate linezolid concentrations after standard dosing in critically ill patients. Antimicrob. Agents Chemother. 2016, 60, 5254–5261. [Google Scholar] [CrossRef]
- Yamamoto, M.; Kuzuya, T.; Baba, H.; Yamada, K.; Nabeshima, T. Population pharmacokinetic analysis of vancomycin in patients with gram-positive infections and the influence of infectious disease type. J. Clin. Pharm. Ther. 2009, 34, 473–483. [Google Scholar] [CrossRef] [PubMed]
- Healy, D.P.; Polk, R.E.; Garson, M.L.; Rock, D.T.; Comstock, T.J. Comparison of steady-state pharmacokinetics of two dosage regimens of vancomycin in normal volunteers. Antimicrob. Agents Chemother. 1987, 31, 393–397. [Google Scholar] [CrossRef] [PubMed]
- Shen, K.; Yang, M.; Fan, Y.; Liang, X.; Chen, Y.; Wu, J.; Yu, J.; Zhang, H.; Wang, R.; Zhang, F.; et al. Model-based evaluation of the clinical and microbiological efficacy of vancomycin: A prospective study of Chinese adult in-house patients. Clin. Infect. Dis. 2018, 67 (Suppl. 2), S256–S262. [Google Scholar] [CrossRef] [PubMed]
- Sánchez, J.L.; Dominguez, A.R.; Lane, J.R.; Anderson, P.O.; Capparelli, E.V.; Cornejo-Bravo, J.M. Population pharmacokinetics of vancomycin in adult and geriatric patients: Comparison of eleven approaches. Int. J. Clin. Pharmacol. Ther. 2010, 48, 525–533. [Google Scholar] [CrossRef]
- Dedkaew, T.; Cressey, T.R.; Punyawudho, B.; Lucksiri, A. Pharmacokinetics of vancomycin in critically ill patients in Thailand. Int. J. Pharm. Pharm. Sci. 2015, 9, 232–237. [Google Scholar]
- Roberts, J.A.; Taccone, F.S.; Udy, A.A.; Vincent, J.L.; Jacobs, F.; Lipman, J. Vancomycin dosing in critically ill patients: Robust methods for improved continuous-infusion regimens. Antimicrob. Agents Chemother. 2011, 55, 2704–2709. [Google Scholar] [CrossRef]
- Mattioli, F.; Fucile, C.; Del Bono, V.; Marini, V.; Parisini, A.; Molin, A.; Zuccoli, M.L.; Milano, G.; Danesi, R.; Marchese, A.; et al. Population pharmacokinetics and probability of target attainment of meropenem in critically ill patients. Eur. J. Clin. Pharmacol. 2016, 72, 839–848. [Google Scholar] [CrossRef]
- Ulldemolins, M.; Roberts, J.A.; Rello, J.; Paterson, D.L.; Lipman, J. The effects of hypoalbuminaemia on optimizing antibacterial dosing in critically ill patients. Clin. Pharmacokinet. 2011, 50, 99–110. [Google Scholar] [CrossRef]
- Craig, W.A. The pharmacology of meropenem, a new carbapenem antibiotic. Clin. Infect. Dis. 1997, 24 (Suppl. 2), S266–S275. [Google Scholar] [CrossRef]
- Zhanel, G.G.; Wiebe, R.; Dilay, L.; Thomson, K.; Rubinstein, E.; Hoban, D.J.; Noreddin, A.M.; Karlowsky, J.A. Comparative review of the carbapenems. Drugs 2007, 67, 1027–1052. [Google Scholar] [CrossRef]
- Finfer, S.; Bellomo, R.; McEvoy, S.; Lo, S.K.; Myburgh, J.; Neal, B.; Norton, R. Effect of baseline serum albumin concentration on outcome of resuscitation with albumin or saline in patients in intensive care units: Analysis of data from the saline versus albumin fluid evaluation (SAFE) study. BMJ 2006, 333, 1044. [Google Scholar] [PubMed]
- Udy, A.A.; Roberts, J.A.; Lipman, J. Clinical implications of antibiotic pharmacokinetic principles in the critically ill. Intensive Care Med. 2013, 39, 2070–2082. [Google Scholar] [CrossRef] [PubMed]
- Pea, F.; Viale, P. Bench-to-bedside review: Appropriate antibiotic therapy in severe sepsis and septic shock—Does the dose matter? Crit. Care 2009, 13, 214. [Google Scholar] [CrossRef] [PubMed]
- Price, D.A.; Fisher, N.D.L.; Osei, S.Y.; Lansang, M.C.; Hollenberg, N.K. Renal perfusion and function in healthy African Americans. Kidney Int. 2001, 59, 1037–1043. [Google Scholar] [CrossRef] [PubMed]
- Levey, A.S.; Inker, L.A.; Coresh, J. GFR estimation: From physiology to public health. Am. J. Kidney Dis. 2014, 63, 820–834. [Google Scholar] [CrossRef]
- Hollenberg, N.K.; Rivera, A.; Meinking, T.; Martinez, G.; McCullough, M.; Passan, D.; Preston, M.; Taplin, D.; Vicaria-Clement, M. Age, renal perfusion and function in island-dwelling indigenous Kuna Amerinds of Panama. Nephron 1999, 82, 131–138. [Google Scholar] [CrossRef]
- Hoang, K.; Tan, J.C.; Derby, G.; Blouch, K.L.; Masek, M.; Ma, I.; Lemley, K.V.; Myers, B.D. Determinants of glomerular hypofiltration in aging humans. Kidney Int. 2003, 64, 1417–1424. [Google Scholar] [CrossRef]
- Fuiano, G.; Sund, S.; Mazza, G.; Rosa, M.; Caglioti, A.; Gallo, G.; Natale, G.; Andreucci, M.; Memoli, B.; De Nicola, L.; et al. Renal hemodynamic response to maximal vasodilating stimulus in healthy older subjects. Kidney Int. 2001, 59, 1052–1058. [Google Scholar] [CrossRef]
- Roberts, J.A.; Lipman, J. Pharmacokinetic issues for antibiotics in the critically ill patient. Crit. Care Med. 2009, 37, 840–851. [Google Scholar] [CrossRef]
- Morata, L.; Cuesta, M.; Rojas, J.F.; Rodriguez, S.; Brunet, M.; Casals, G.; Cobos, N.; Hernandez, C.; Martínez, J.A.; Mensa, J.; et al. Risk factors for a low linezolid trough plasma concentration in acute infections. Antimicrob. Agents Chemother. 2013, 57, 1913–1917. [Google Scholar] [CrossRef]
- Pea, F.; Furlanut, M.; Cojutti, P.; Cristini, F.; Zamparini, E.; Franceschi, L.; Viale, P. Therapeutic drug monitoring of linezolid: A retrospective monocentric analysis. Antimicrob. Agents Chemother. 2010, 54, 4605–4610. [Google Scholar] [CrossRef] [PubMed]
- Katip, W.; Jaruratanasirikul, S.; Pattharachayakul, S.; Wongpoowarak, W.; Jitsurong, A.; Lucksiri, A. The pharmacokinetics of vancomycin during the initial loading dose in patients with septic shock. Infect. Drug Resist. 2016, 9, 253–260. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Duffull, S.B.; Wright, D.F.; Winter, H.R. Interpreting population pharmacokinetic-pharmacodynamic analyses—A clinical viewpoint. Br. J. Clin. Pharmacol. 2011, 71, 807–814. [Google Scholar] [CrossRef]
- Wright, D.F.; Winter, H.R.; Duffull, S.B. Understanding the time course of pharmacological effect: A PKPD approach. Br. J. Clin. Pharmacol. 2011, 71, 815–823. [Google Scholar] [CrossRef]
- Mould, D.R.; Upton, R.N. Basic concepts in population modeling, simulation, and model-based drug development-part 2: Introduction to pharmacokinetic modeling methods. CPT Pharmacomet. Syst. Pharmacol. 2013, 2, e38. [Google Scholar] [CrossRef]
- Kanji, S.; Hayes, M.; Ling, A.; Shamseer, L.; Chant, C.; Edwards, D.J.; Edwards, S.; Ensom, M.H.; Foster, D.R.; Hardy, B.; et al. Reporting guidelines for clinical pharmacokinetic studies: The ClinPK Statement. Clin. Pharmacokinet. 2015, 54, 783–795. [Google Scholar] [CrossRef]
- Luo, D.; Wan, X.; Liu, J.; Tong, T. Optimally estimating the sample mean from the sample size, median, mid-range, and/or mid-quartile range. Stat. Methods Med. Res. 2018, 27, 1785–1805. [Google Scholar] [CrossRef]
- Botev, R.; Mallié, J.P.; Couchoud, C.; Schück, O.; Fauvel, J.P.; Wetzels, J.F.; Lee, N.; De Santo, N.G.; Cirillo, M. Estimating glomerular filtration rate: Cockcroft-Gault and Modification of Diet in Renal Disease formulas compared to renal inulin clearance. Clin. J. Am. Soc. Nephrol. 2009, 4, 899–906. [Google Scholar] [CrossRef]
- Walpole, S.C.; Prieto-Merino, D.; Edwards, P.; Cleland, J.; Stevens, G.; Roberts, I. The weight of nations: An estimation of adult human biomass. BMC Public Health 2012, 12, 439. [Google Scholar] [CrossRef]
- Katherina, K.; Sudiarti, T. Body weight prediction model using mid upper arm circumferences and knee height in adult. Indones. J. Public Health Nutr. 2020, 1, 24–32. [Google Scholar] [CrossRef]
- Hayes, D.J.; van Buuren, S.; ter Kuile, F.O.; Stasinopoulos, D.M.; Rigby, R.A.; Terlouw, D.J. Developing regional weight-for-age growth references for malaria-endemic countries to optimize age-based dosing of antimalarials. Bull. World Health Organ. 2015, 93, 74–83. [Google Scholar] [CrossRef] [PubMed]
- Heymsfield, S.B.; Peterson, C.M.; Thomas, D.M.; Heo, M.; Schuna, J.M. Why are there race/ethnic differences in adult body mass indexadiposity relationships? A quantitative critical review. Obes. Rev. 2016, 17, 262–275. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).