Post-Stroke Infections: Insights from Big Data Using Clinical Data Warehouse (CDW)
Abstract
1. Introduction
2. Results
2.1. Baseline Characteristics
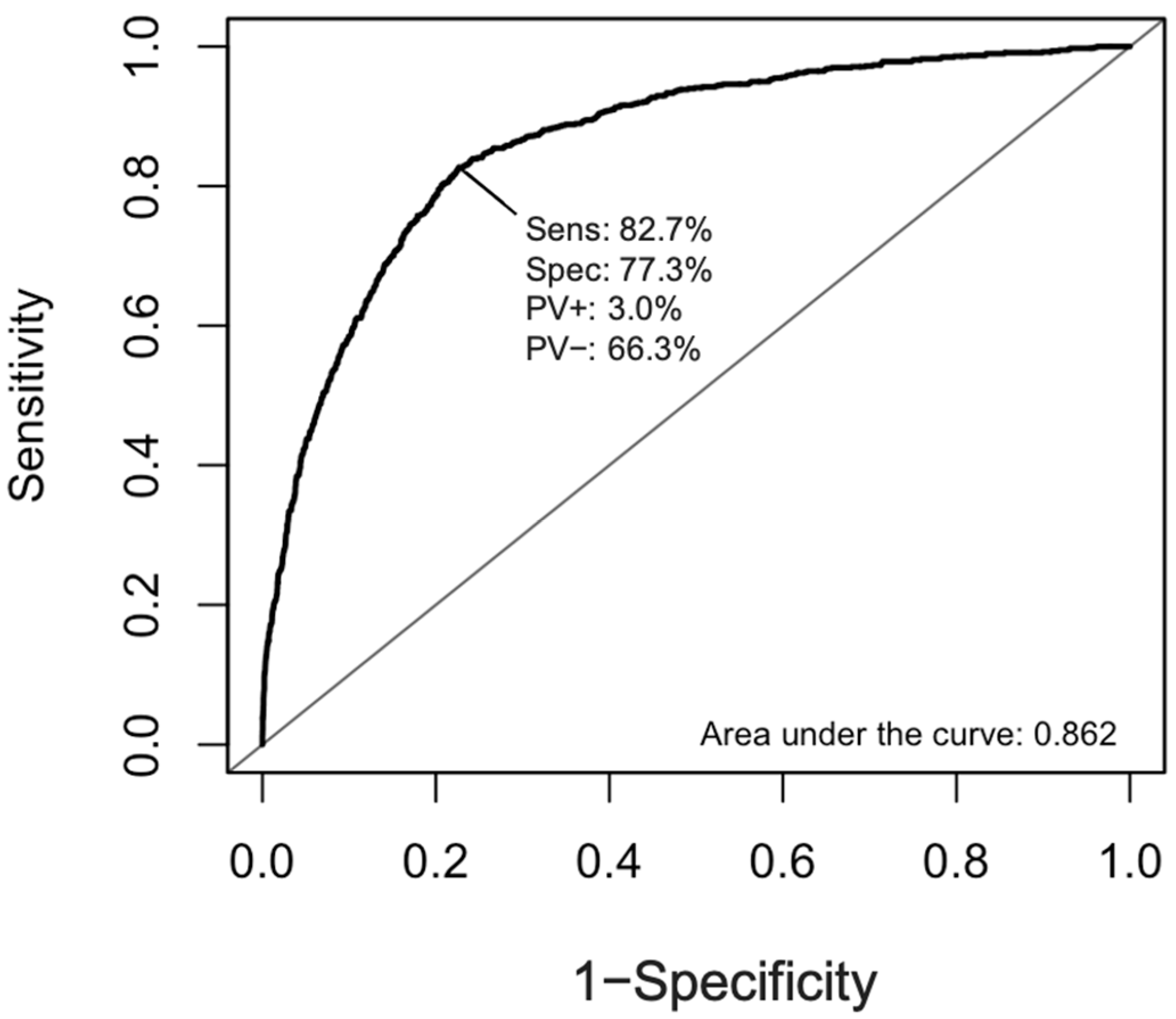
2.2. Clinical Variables Associated with Post-Stroke Infection
3. Discussion
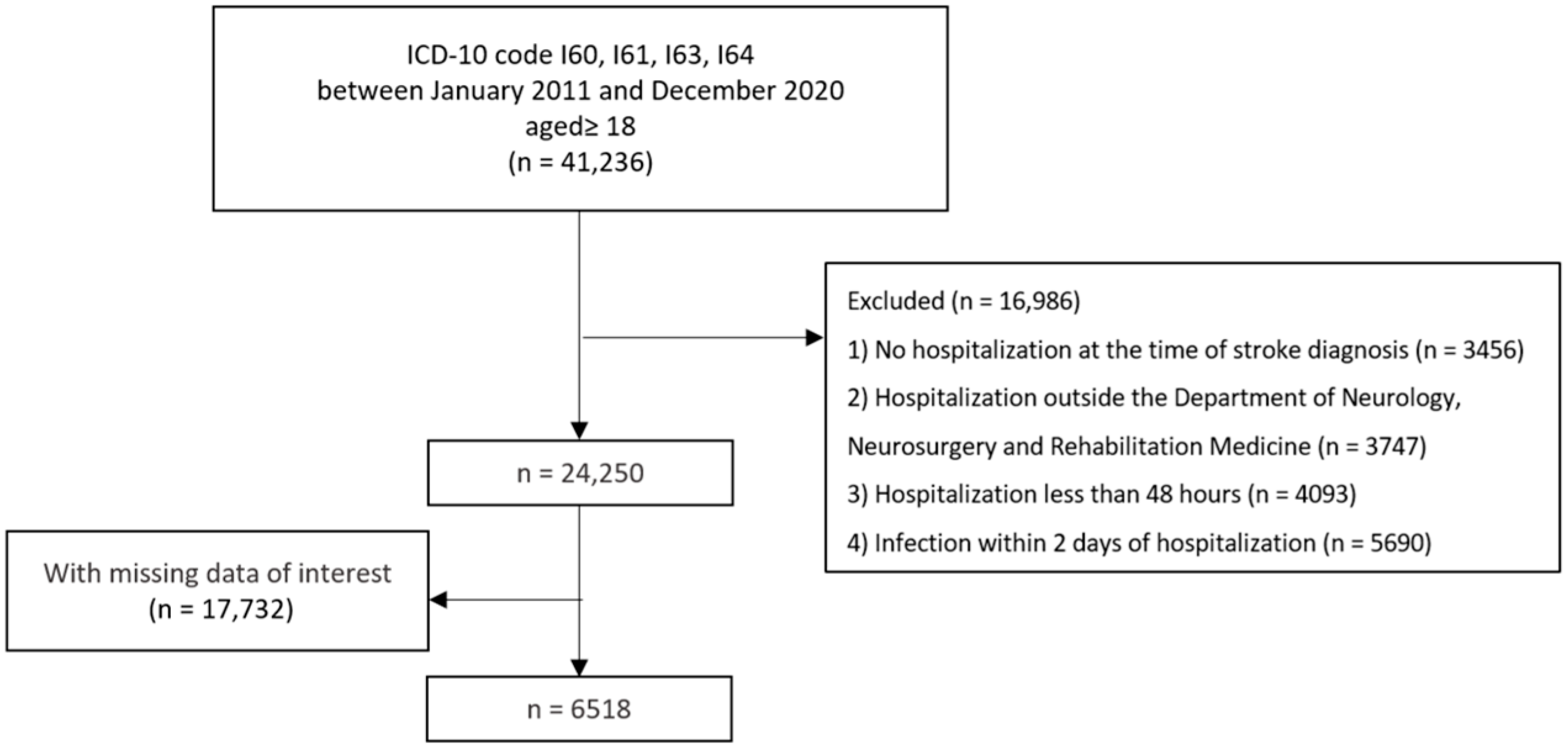
4. Materials and Methods
4.1. Study Design and Participants
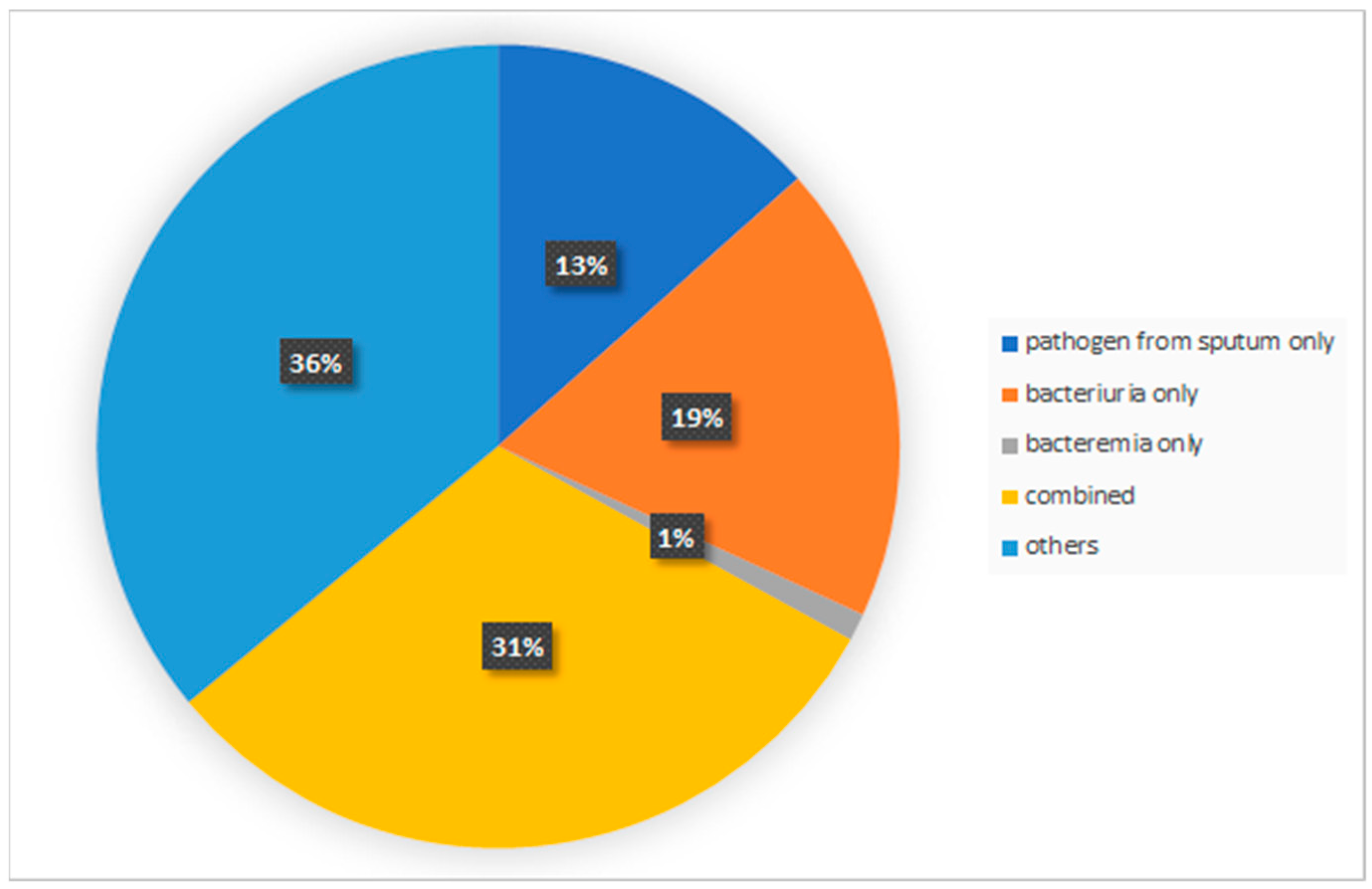
4.2. Identification of Post-Stroke Infection
4.3. Clinical Characteristics and Stroke Severity Variables
4.4. Statistical Analyses
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Westendorp, W.F.; Dames, C.; Nederkoorn, P.J.; Meisel, A. Immunodepression, Infections, and Functional Outcome in Ischemic Stroke. Stroke 2022, 53, 1438–1448. [Google Scholar] [CrossRef] [PubMed]
- Chamorro, A.; Meisel, A.; Planas, A.M.; Urra, X.; van de Beek, D.; Veltkamp, R. The immunology of acute stroke. Nat. Rev. Neurol. 2012, 8, 401–410. [Google Scholar] [CrossRef] [PubMed]
- Gill, D.; Veltkamp, R. Dynamics of T cell responses after stroke. Curr. Opin. Pharmacol. 2016, 26, 26–32. [Google Scholar] [CrossRef] [PubMed]
- Meisel, C.; Meisel, A. Suppressing immunosuppression after stroke. N. Engl. J. Med. 2011, 365, 2134–2136. [Google Scholar] [CrossRef]
- Westendorp, W.F.; Nederkoorn, P.J.; Vermeij, J.-D.; Dijkgraaf, M.G.; de Beek, D.v. Post-stroke infection: A systematic review and meta-analysis. BMC Neurol. 2011, 11, 110. [Google Scholar] [CrossRef]
- Westendorp, W.F.; Vermeij, J.D.; Hilkens, N.A.; Brouwer, M.C.; Algra, A.; van der Worp, H.B.; Dippel, D.W.; van de Beek, D.; Nederkoorn, P.J. Development and internal validation of a prediction rule for post-stroke infection and post-stroke pneumonia in acute stroke patients. Eur. Stroke J. 2018, 3, 136–144. [Google Scholar] [CrossRef]
- Koennecke, H.-C.; Belz, W.; Berfelde, D.; Endres, M.; Fitzek, S.; Hamilton, F.; Kreitsch, P.; Mackert, B.-M.; Nabavi, D.G.; Nolte, C.H.; et al. Factors influencing in-hospital mortality and morbidity in patients treated on a stroke unit. Neurology 2011, 77, 965–972. [Google Scholar] [CrossRef]
- Vermeij, F.H.; Scholte op Reimer, W.J.; de Man, P.; van Oostenbrugge, R.J.; Franke, C.L.; de Jong, G.; de Kort, P.L.; Dippel, D.W.; Netherlands Stroke Survey, I. Stroke-associated infection is an independent risk factor for poor outcome after acute ischemic stroke: Data from the Netherlands Stroke Survey. Cerebrovasc. Dis. 2009, 27, 465–471. [Google Scholar] [CrossRef]
- Elkind, M.S.V.; Boehme, A.K.; Smith, C.J.; Meisel, A.; Buckwalter, M.S. Infection as a Stroke Risk Factor and Determinant of Outcome After Stroke. Stroke 2020, 51, 3156–3168. [Google Scholar] [CrossRef]
- Muscari, A.; Puddu, G.M.; Conte, C.; Falcone, R.; Kolce, B.; Lega, M.V.; Zoli, M. Clinical predictors of fever in stroke patients: Relevance of nasogastric tube. Acta Neurol. Scand. 2015, 132, 196–202. [Google Scholar] [CrossRef]
- Wastfelt, M.; Cao, Y.; Strom, J.O. Predictors of post-stroke fever and infections: A systematic review and meta-analysis. BMC Neurol. 2018, 18, 49. [Google Scholar] [CrossRef] [PubMed]
- Masiero, S.; Pierobon, R.; Previato, C.; Gomiero, E. Pneumonia in stroke patients with oropharyngeal dysphagia: A six-month follow-up study. Neurol. Sci. 2008, 29, 139–145. [Google Scholar] [CrossRef] [PubMed]
- Szylinska, A.; Kotfis, K.; Bott-Olejnik, M.; Wankowicz, P.; Rotter, I. Post-Stroke Outcomes of Patients with Chronic Obstructive Pulmonary Disease. Brain Sci. 2022, 12, 106. [Google Scholar] [CrossRef] [PubMed]
- Sluggett, J.K.; Lalic, S.; Hosking, S.M.; Ritchie, B.; McLoughlin, J.; Shortt, T.; Robson, L.; Cooper, T.; Cairns, K.A.; Ilomaki, J.; et al. Root cause analysis to identify medication and non-medication strategies to prevent infection-related hospitalizations from australian residential aged care services. Int. J. Environ. Res. Public Health 2020, 17, 3282. [Google Scholar] [CrossRef]
- Sellars, C.; Bowie, L.; Bagg, J.; Sweeney, M.P.; Miller, H.; Tilston, J.; Langhorne, P.; Stott, D.J. Risk factors for chest infection in acute stroke: A prospective cohort study. Stroke 2007, 38, 2284–2291. [Google Scholar] [CrossRef]
- Ding, R.; Logemann, J.A. Pneumonia in stroke patients: A retrospective study. Dysphagia 2000, 15, 51–57. [Google Scholar] [CrossRef]
- Kammersgaard, L.P.; Jorgensen, H.S.; Reith, J.; Nakayama, H.; Houth, J.G.; Weber, U.J.; Pedersen, P.M.; Olsen, T.S. Early infection and prognosis after acute stroke: The Copenhagen Stroke Study. J. Stroke Cerebrovasc. Dis. 2001, 10, 217–221. [Google Scholar] [CrossRef]
- Harms, H.; Grittner, U.; Droge, H.; Meisel, A. Predicting post-stroke pneumonia: The PANTHERIS score. Acta Neurol. Scand. 2013, 128, 178–184. [Google Scholar] [CrossRef]
- Colbert, J.F.; Traystman, R.J.; Poisson, S.N.; Herson, P.S.; Ginde, A.A. Sex-related differences in the risk of hospital-acquired sepsis and pneumonia post acute ischemic stroke. J. Stroke Cerebrovasc. Dis. 2016, 25, 2399–2404. [Google Scholar] [CrossRef]
- Pertsch, N.J.; Tang, O.Y.; Seicean, A.; Toms, S.A.; Weil, R.J. Sepsis after elective neurosurgery: Incidence, outcomes, and predictive factors. J. Clin. Neurosci. 2020, 78, 53–59. [Google Scholar] [CrossRef]
- Zhang, D.; Zhuo, H.; Yang, G.; Huang, H.; Li, C.; Wang, X.; Zhao, S.; Moliterno, J.; Zhang, Y. Postoperative pneumonia after craniotomy: Incidence, risk factors and prediction with a nomogram. J. Hosp. Infect. 2020, 105, 167–175. [Google Scholar] [CrossRef] [PubMed]
- Chughtai, M.; Gwam, C.U.; Mohamed, N.; Khlopas, A.; Newman, J.M.; Khan, R.; Nadhim, A.; Shaffiy, S.; Mont, M.A. The Epidemiology and Risk Factors for Postoperative Pneumonia. J. Clin. Med. Res. 2017, 9, 466–475. [Google Scholar] [CrossRef] [PubMed]
- Zhan, R.; Zhu, Y.; Shen, Y.; Shen, J.; Tong, Y.; Yu, H.; Wen, L. Post-operative central nervous system infections after cranial surgery in China: Incidence, causative agents, and risk factors in 1,470 patients. Eur. J. Clin. Microbiol. Infect. Dis. 2014, 33, 861–866. [Google Scholar] [CrossRef] [PubMed]
- Busl, K.M. Nosocomial Infections in the Neurointensive Care Unit. Neurosurg. Clin. N. Am. 2018, 29, 299–314. [Google Scholar] [CrossRef]
- Al-Jishi, A.; Saluja, R.S.; Al-Jehani, H.; Lamoureux, J.; Maleki, M.; Marcoux, J. Primary or secondary decompressive craniectomy: Different indication and outcome. Can. J. Neurol. Sci. 2011, 38, 612–620. [Google Scholar] [CrossRef] [PubMed]
- Mutai, H.; Furukawa, T.; Araki, K.; Misawa, K.; Hanihara, T. Factors associated with functional recovery and home discharge in stroke patients admitted to a convalescent rehabilitation ward. Geriatr. Gerontol. Int. 2012, 12, 215–222. [Google Scholar] [CrossRef]
- Winstein, C.J.; Stein, J.; Arena, R.; Bates, B.; Cherney, L.R.; Cramer, S.C.; Deruyter, F.; Eng, J.J.; Fisher, B.; Harvey, R.L.; et al. Guidelines for Adult Stroke Rehabilitation and Recovery: A Guideline for Healthcare Professionals From the American Heart Association/American Stroke Association. Stroke 2016, 47, e98–e169. [Google Scholar] [CrossRef]
- Bernhardt, J.; English, C.; Johnson, L.; Cumming, T.B. Early mobilization after stroke: Early adoption but limited evidence. Stroke 2015, 46, 1141–1146. [Google Scholar] [CrossRef]
- Schweickert, W.D.; Pohlman, M.C.; Pohlman, A.S.; Nigos, C.; Pawlik, A.J.; Esbrook, C.L.; Spears, L.; Miller, M.; Franczyk, M.; Deprizio, D.; et al. Early physical and occupational therapy in mechanically ventilated, critically ill patients: A randomised controlled trial. Lancet 2009, 373, 1874–1882. [Google Scholar] [CrossRef]
- Needham, D.M.; Korupolu, R.; Zanni, J.M.; Pradhan, P.; Colantuoni, E.; Palmer, J.B.; Brower, R.G.; Fan, E. Early physical medicine and rehabilitation for patients with acute respiratory failure: A quality improvement project. Arch. Phys. Med. Rehabil. 2010, 91, 536–542. [Google Scholar] [CrossRef]
- DeHoff, G.; Lau, W. Medical management of cerebral edema in large hemispheric infarcts. Front. Neurol. 2022, 13, 857640. [Google Scholar] [CrossRef] [PubMed]
- Sandercock, P.A.; Soane, T. Corticosteroids for acute ischaemic stroke. Cochrane Database Syst. Rev. 2011, 2011, CD000064. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Ahmet, A.; Ward, L.; Krishnamoorthy, P.; Mandelcorn, E.D.; Leigh, R.; Brown, J.P.; Cohen, A.; Kim, H. A practical guide to the monitoring and management of the complications of systemic corticosteroid therapy. Allergy Asthma Clin. Immunol. 2013, 9, 30. [Google Scholar] [CrossRef] [PubMed]
- Evans, L.; Rhodes, A.; Alhazzani, W.; Antonelli, M.; Coopersmith, C.M.; French, C.; Machado, F.R.; McIntyre, L.; Ostermann, M.; Prescott, H.C.; et al. Surviving sepsis campaign: International guidelines for management of sepsis and septic shock 2021. Intensive Care Med. 2021, 47, 1181–1247. [Google Scholar] [CrossRef] [PubMed]
- Herzig, S.J.; Howell, M.D.; Ngo, L.H.; Marcantonio, E.R. Acid-suppressive medication use and the risk for hospital-acquired pneumonia. JAMA 2009, 301, 2120–2128. [Google Scholar] [CrossRef] [PubMed]
- Herzig, S.J.; Doughty, C.; Lahoti, S.; Marchina, S.; Sanan, N.; Feng, W.; Kumar, S. Acid-suppressive medication use in acute stroke and hospital-acquired pneumonia. Ann. Neurol. 2014, 76, 712–718. [Google Scholar] [CrossRef]
- Song, T.J.; Kim, J. Risk of post-stroke pneumonia with proton pump inhibitors, H2 receptor antagonists and mucoprotective agents: A retrospective nationwide cohort study. PLoS ONE 2019, 14, e0216750. [Google Scholar] [CrossRef]
- Marchina, S.; Doros, G.; Modak, J.; Helenius, J.; Aycock, D.M.; Kumar, S. Acid-suppressive medications and risk of pneumonia in acute stroke patients: A systematic review and meta-analysis. J. Neurol. Sci. 2019, 400, 122–128. [Google Scholar] [CrossRef]
- Ubagai, T.; Koshibu, Y.; Koshio, O.; Nakaki, T.; Ono, Y. Downregulation of immunomodulator gene expression in LPS-stimulated human polymorphonuclear leukocytes by the proton pump inhibitor lansoprazole. J. Infect. Chemother. 2009, 15, 374–379. [Google Scholar] [CrossRef]
- Capodicasa, E.; De Bellis, F.; Pelli, M.A. Effect of lansoprazole on human leukocyte function. Immunopharmacol. Immunotoxicol. 1999, 21, 357–377. [Google Scholar] [CrossRef]
- Hofbauer, R.; Losert, H.; Gmeiner, B.; Wagner, O.; Kapiotis, S.; Frass, M.; Kaye, A.D. Inhibitory effect of omeprazole on transmigration of leukocytes through endothelial cell monolayers and leukocyte adhesion. Microvasc. Res. 2000, 59, 169–171. [Google Scholar] [CrossRef] [PubMed]
- Ye, Z.; Reintam Blaser, A.; Lytvyn, L.; Wang, Y.; Guyatt, G.H.; Mikita, J.S.; Roberts, J.; Agoritsas, T.; Bertschy, S.; Boroli, F.; et al. Gastrointestinal bleeding prophylaxis for critically ill patients: A clinical practice guideline. BMJ 2020, 368, l6722. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.K.; Chen, P.H.; Pan, C.H.; Su, S.S.; Tsai, S.Y.; Chen, C.C.; Kuo, C.J. Antipsychotic medications and the progression of upper respiratory infection to pneumonia in patients with schizophrenia. Schizophr. Res. 2020, 222, 327–334. [Google Scholar] [CrossRef] [PubMed]
- Chekani, F.; Holmes, H.M.; Johnson, M.L.; Chen, H.; Sherer, J.T.; Aparasu, R.R. Risk of pneumonia associated with atypical antipsychotic use in nursing home residents with Parkinson's disease. J. Psychiatr. Res. 2019, 117, 116–121. [Google Scholar] [CrossRef]
- Kalra, L.; Irshad, S.; Hodsoll, J.; Simpson, M.; Gulliford, M.; Smithard, D.; Patel, A.; Rebollo-Mesa, I.; Investigators, S.-I. Prophylactic antibiotics after acute stroke for reducing pneumonia in patients with dysphagia (STROKE-INF): A prospective, cluster-randomised, open-label, masked endpoint, controlled clinical trial. Lancet 2015, 386, 1835–1844. [Google Scholar] [CrossRef]
- Westendorp, W.F.; Vermeij, J.D.; Zock, E.; Hooijenga, I.J.; Kruyt, N.D.; Bosboom, H.J.; Kwa, V.I.; Weisfelt, M.; Remmers, M.J.; ten Houten, R.; et al. The Preventive Antibiotics in Stroke Study (PASS): A pragmatic randomised open-label masked endpoint clinical trial. Lancet 2015, 385, 1519–1526. [Google Scholar] [CrossRef]
- Westendorp, W.F.; Vermeij, J.D.; Vermeij, F.; Den Hertog, H.M.; Dippel, D.W.; van de Beek, D.; Nederkoorn, P.J. Antibiotic therapy for preventing infections in patients with acute stroke. Cochrane Database Syst. Rev. 2012, 1, CD008530. [Google Scholar] [CrossRef]
- Valles, J.; Peredo, R.; Burgueno, M.J.; Rodrigues de Freitas, A.P.; Millan, S.; Espasa, M.; Martin-Loeches, I.; Ferrer, R.; Suarez, D.; Artigas, A. Efficacy of single-dose antibiotic against early-onset pneumonia in comatose patients who are ventilated. Chest 2013, 143, 1219–1225. [Google Scholar] [CrossRef]
- Wang, L.; Li, X.; Yang, Z.; Tang, X.; Yuan, Q.; Deng, L.; Sun, X. Semi-recumbent position versus supine position for the prevention of ventilator-associated pneumonia in adults requiring mechanical ventilation. Cochrane Database Syst. Rev. 2016, 2016, CD009946. [Google Scholar] [CrossRef]
- Pozuelo-Carrascosa, D.P.; Herraiz-Adillo, A.; Alvarez-Bueno, C.; Anon, J.M.; Martinez-Vizcaino, V.; Cavero-Redondo, I. Subglottic secretion drainage for preventing ventilator-associated pneumonia: An overview of systematic reviews and an updated meta-analysis. Eur. Respir. Rev. 2020, 29, 190107. [Google Scholar] [CrossRef]
- Allegranzi, B.; Zayed, B.; Bischoff, P.; Kubilay, N.Z.; de Jonge, S.; de Vries, F.; Gomes, S.M.; Gans, S.; Wallert, E.D.; Wu, X.; et al. New WHO recommendations on intraoperative and postoperative measures for surgical site infection prevention: An evidence-based global perspective. Lancet Infect. Dis. 2016, 16, e288–e303. [Google Scholar] [CrossRef]
- Scheeren, T.W.; Wiesenack, C.; Gerlach, H.; Marx, G. Goal-directed intraoperative fluid therapy guided by stroke volume and its variation in high-risk surgical patients: A prospective randomized multicentre study. J. Clin. Monit. Comput. 2013, 27, 225–233. [Google Scholar] [CrossRef] [PubMed]
- Marik, P.E.; Zaloga, G.P. Gastric versus post-pyloric feeding: A systematic review. Crit. Care 2003, 7, R46–R51. [Google Scholar] [CrossRef] [PubMed]
- Gomes, C.A.R., Jr.; Andriolo, R.B.; Bennett, C.; Lustosa, S.A.S.; Matos, D.; Waisberg, D.R.; Waisberg, J. Percutaneous endoscopic gastrostomy versus nasogastric tube feeding for adults with swallowing disturbances. Cochrane Database Syst. Rev. 2015, 2015, CD008096. [Google Scholar] [CrossRef] [PubMed]
- Klompas, M.; Speck, K.; Howell, M.D.; Greene, L.R.; Berenholtz, S.M. Reappraisal of routine oral care with chlorhexidine gluconate for patients receiving mechanical ventilation: Systematic review and meta-analysis. JAMA Intern. Med. 2014, 174, 751–761. [Google Scholar] [CrossRef]
- DiBardino, D.M.; Wunderink, R.G. Aspiration pneumonia: A review of modern trends. J. Crit. Care 2015, 30, 40–48. [Google Scholar] [CrossRef] [PubMed]
- Boos, C.J. Infection and atrial fibrillation: Inflammation begets AF. Eur. Heart J. 2020, 41, 1120–1122. [Google Scholar] [CrossRef] [PubMed]
- Walkey, A.J.; Wiener, R.S.; Ghobrial, J.M.; Curtis, L.H.; Benjamin, E.J. Incident stroke and mortality associated with new-onset atrial fibrillation in patients hospitalized with severe sepsis. JAMA 2011, 306, 2248–2254. [Google Scholar] [CrossRef] [PubMed]
- Di Bella, S.; Luzzati, R.; Principe, L.; Zerbato, V.; Meroni, E.; Giuffre, M.; Croce, L.S.; Merlo, M.; Perotto, M.; Dolso, E.; et al. Aspirin and infection: A narrative review. Biomedicines 2022, 10, 263. [Google Scholar] [CrossRef]
- Kim, T.J.; Lee, J.S.; Kang, M.K.; Nam, K.W.; Lee, C.H.; Mo, H.; Jeong, H.Y.; Yoon, B.W.; Ko, S.B. Clopidogrel may decrease the risk of post-stroke infection after ischaemic stroke. Eur. J. Neurol. 2019, 26, 261–267. [Google Scholar] [CrossRef]
- Miarons, M.; Tomsen, N.; Nascimento, W.; Espin, A.; Lopez-Faixo, D.; Clave, P.; Rofes, L. Increased levels of substance P in patients taking beta-blockers are linked with a protective effect on oropharyngeal dysphagia. Neurogastroenterol. Motil. 2018, 30, e13397. [Google Scholar] [CrossRef]
- Prass, K.; Braun, J.S.; Dirnagl, U.; Meisel, C.; Meisel, A. Stroke propagates bacterial aspiration to pneumonia in a model of cerebral ischemia. Stroke 2006, 37, 2607–2612. [Google Scholar] [CrossRef]
- Vaezi, M.F.; Yang, Y.X.; Howden, C.W. Complications of proton pump inhibitor therapy. Gastroenterology 2017, 153, 35–48. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Q.; Xu, F.; Jiang, S. The impact of previous hospitalization in the preceding 90 days on the outcome in critically ill patients with gram-negative bloodstream infection. Diagn. Microbiol. Infect. Dis. 2014, 80, 136–140. [Google Scholar] [CrossRef] [PubMed]
- Shin, S.Y.; Kim, W.S.; Lee, J.H. Characteristics desired in clinical data warehouse for biomedical research. Healthc Inform. Res. 2014, 20, 109–116. [Google Scholar] [CrossRef]
- Lee, C.H.; Yoon, H.J. Medical big data: Promise and challenges. Kidney Res. Clin. Pract. 2017, 36, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Dong, P.V.; Cremer, O.L. Limitations of the use of the Glasgow Coma Scale in intensive care patients with non-neurological primary disease: A search for alternatives. Crit. Care 2011, 15, P506. [Google Scholar] [CrossRef]
- Nardi, K.; Milia, P.; Eusebi, P.; Paciaroni, M.; Caso, V.; Agnelli, G. Admission leukocytosis in acute cerebral ischemia: Influence on early outcome. J. Stroke Cerebrovasc. Dis. 2012, 21, 819–824. [Google Scholar] [CrossRef]
- Maharshak, N.; Kassirer, M.; Zeltser, D.; Rotstein, R.; Rogowski, O.; Shapira, I.; Deutsch, V.; Arber, N.; Eldor, A.; Berliner, S. The inflammation meter: Novel technology to detect the presence of infection/inflammation in patients without leukocytosis but with increased leukocyte adhesiveness/aggregation. Acta Haematol. 2000, 104, 16–21. [Google Scholar] [CrossRef]
- Van Decker, S.G.; Bosch, N.; Murphy, J. Catheter-associated urinary tract infection reduction in critical care units: A bundled care model. BMJ Open Qual. 2021, 10, e001534. [Google Scholar] [CrossRef]
- Poisson, S.N.; Johnston, S.C.; Josephson, S.A. Urinary tract infections complicating stroke: Mechanisms, consequences, and possible solutions. Stroke 2010, 41, e180–e184. [Google Scholar] [CrossRef] [PubMed]
- Cazzola, M.; Calzetta, L.; Page, C.; Jardim, J.; Chuchalin, A.G.; Rogliani, P.; Matera, M.G. Influence of N-acetylcysteine on chronic bronchitis or COPD exacerbations: A meta-analysis. Eur. Respir. Rev. 2015, 24, 451–461. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Kuo, S.; Lin, L.; Yang, Y. The efficacy of N-acetylcysteine in chronic obstructive pulmonary disease patients: A meta-analysis. Ther. Adv. Respir. Dis. 2023, 17, 17534666231158563. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.P.; Wen, F.Q.; Bai, C.X.; Wan, H.Y.; Kang, J.; Chen, P.; Yao, W.Z.; Ma, L.J.; Li, X.; Raiteri, L.; et al. Twice daily N-acetylcysteine 600 mg for exacerbations of chronic obstructive pulmonary disease (PANTHEON): A randomised, double-blind placebo-controlled trial. Lancet Respir. Med. 2014, 2, 187–194. [Google Scholar] [CrossRef]
- Decramer, M.; Rutten-van Molken, M.; Dekhuijzen, P.N.; Troosters, T.; van Herwaarden, C.; Pellegrino, R.; van Schayck, C.P.; Olivieri, D.; Del Donno, M.; De Backer, W.; et al. Effects of N-acetylcysteine on outcomes in chronic obstructive pulmonary disease (Bronchitis Randomized on NAC Cost-Utility Study, BRONCUS): A randomised placebo-controlled trial. Lancet 2005, 365, 1552–1560. [Google Scholar] [CrossRef]
- Cuzzocrea, S.; Mazzon, E.; Costantino, G.; Serraino, I.; Dugo, L.; Calabro, G.; Cucinotta, G.; De Sarro, A.; Caputi, A.P. Beneficial effects of n-acetylcysteine on ischaemic brain injury. Br. J. Pharmacol. 2000, 130, 1219–1226. [Google Scholar] [CrossRef]
- Hoffer, M.E.; Balaban, C.; Slade, M.D.; Tsao, J.W.; Hoffer, B. Amelioration of acute sequelae of blast induced mild traumatic brain injury by N-acetyl cysteine: A double-blind, placebo controlled study. PLoS ONE 2013, 8, e54163. [Google Scholar] [CrossRef]
- Park, S.J.; Lee, S.J.; Kim, H.; Kim, J.K.; Chun, J.W.; Lee, S.J.; Lee, H.K.; Kim, D.J.; Choi, I.Y. Machine learning prediction of dropping out of outpatients with alcohol use disorders. PLoS ONE 2021, 16, e0255626. [Google Scholar] [CrossRef]
- Das, A.; Kennedy, K.; Spyropoulos, G.; Collignon, P. Administrative data has poor accuracy for surveillance of Staphylococcus aureus bacteraemia. Infect. Dis. Health 2016, 21, 162–168. [Google Scholar] [CrossRef]
- Fan, Y.; Zou, J.; Cao, X.; Wu, Y.; Gao, F.; Xiong, L. Data on antibiotic use for detecting clusters of healthcare-associated infection caused by multidrug-resistant organisms in a hospital in China, 2014 to 2017. J. Hosp. Infect. 2019, 101, 305–312. [Google Scholar] [CrossRef]
- Mandell, L.A.; Niederman, M.S. Chpater 126: Pneumonia. Harrison’s Principles of Internal Medicine, 21e; McGraw Hill: New York, NY, USA, 2022; p. 16. [Google Scholar]
- Ohura, T.; Hase, K.; Nakajima, Y.; Nakayama, T. Validity and reliability of a performance evaluation tool based on the modified Barthel Index for stroke patients. BMC Med. Res. Methodol. 2017, 17, 131. [Google Scholar] [CrossRef] [PubMed]
| Clinical Variables | Total (n = 6518) | Infection (n = 797) | No Infection (n = 5721) | p-Value |
|---|---|---|---|---|
| Age (years), mean ± SD | 67.5 ± 13.2 | 67.9 ± 13.6 | 67.4 ± 13.1 | 0.345 |
| Male, n (%) | 3736 (57.3) | 499 (62.6) | 3237 (56.6) | 0.001 * |
| Hospital length of stay (days), mean ± SD | 19.4 ± 75.2 | 38.9 ± 59.1 | 16.7 ± 76.8 | 0.001 * |
| Brain surgery, n (%) | 523 (8.0) | 250 (31.4) | 273 (4.8) | <0.001 * |
| Mechanical ventilation, n (%) | 71 (1.1) | 63 (7.9) | 8 (0.1) | <0.001 * |
| Enteral tube feeding, n (%) | 1132 (17.4) | 431 (54.1) | 701 (12.3) | <0.001 * |
| MBI, mean ± SD | 47.5 ± 31.5 | 23.4 ± 27.6 | 50.9 ± 30.6 | <0.001 * |
| CCI, mean ± SD | 3.7 ± 1.6 | 4.0 ± 1.7 | 3.7 ± 1.5 | <0.001 * |
| Hypertension, n (%) | 696 (10.7) | 99 (12.4) | 597 (10.4) | 0.101 |
| Atrial fibrillation, n (%) | 277 (4.3) | 46 (5.8) | 231 (4.0) | 0.029 * |
| Medication exposures, n (%) | ||||
| Antipsychotics | 1194 (18.3) | 212 (26.6) | 982 (17.2) | <0.001 * |
| Opioid | 111 (1.7) | 22 (2.8) | 89 (1.6) | 0.021 * |
| Benzodiazepine | 401 (6.2) | 53 (6.6) | 348 (6.1) | 0.585 |
| Anticholinergic | 457 (7.0) | 62 (7.8) | 395 (6.9) | 0.405 |
| Dopamine agonist | 349 (5.4) | 88 (11.0) | 261 (4.6) | <0.001 * |
| SSRI/SNRI | 890 (13.7) | 163 (20.5) | 727 (12.7) | <0.001 * |
| Steroid | 310 (4.8) | 86 (10.8) | 224 (3.9) | <0.001 * |
| Acid-suppressant drugs | 4707 (72.2) | 662 (83.1) | 4045 (70.7) | <0.001 * |
| Aspirin | 4753 (73.0) | 484 (60.7) | 4269 (74.6) | <0.001 * |
| Clopidogrel | 4288 (65.8) | 442 (55.5) | 3846 (67.2) | <0.001 * |
| Univariable | Multivariable | |||
|---|---|---|---|---|
| OR (95% CI) | p-Value | OR (95% CI) | p-Value | |
| Age, years | ||||
| Mean ± SD | 1.00 (1.00–1.01) | 0.345 | ||
| Male | 1.29 (1.10–1.50) | 0.001 | 1.79 (1.49–2.16) | <0.001 * |
| Hospital length of stay (days) | ||||
| Mean ± SD | 1.003 (1.002–1.005) | <0.001 | 1.001 (1.000–1.002) | 0.006 * |
| Brain surgery | 9.12 (7.52–11.06) | <0.001 | 7.84 (6.24–9.87) | <0.001 * |
| Mechanical ventilation | 61.29 (31.07–139.13) | <0.001 | 18.11 (8.40–44.04) | <0.001 * |
| Tube feeding | 8.43 (7.19–9.90) | <0.001 | 3.61 (2.95–4.43) | <0.001 * |
| MBI | ||||
| Mean ± SD | 0.97 (0.97–0.97) | <0.001 | 0.98 (0.98–0.98) | <0.001 * |
| Charlson comorbidity index | ||||
| Mean ± SD | 1.12 (1.07–1.17) | <0.001 | 1.02 (0.97–1.09) | 0.427 |
| Hypertension | 1.22 (0.97–1.52) | 0.089 | 1.03 (0.78–1.37) | 0.821 |
| Atrial fibrillation | 1.46 (1.04–2.00) | 0.024 | 0.62 (0.40–0.93) | 0.022 * |
| Other medication exposures | ||||
| Antipsychotic | 1.75 (1.47–2.07) | <0.001 | 0.81 (0.66–1.00) | 0.053 |
| Opioid | 1.80 (1.09–2.83) | 0.015 | 1.41 (0.78–2.46) | 0.235 |
| Benzodiazepine | 1.10 (0.81–1.47) | 0.533 | ||
| Anticholinergic | 1.14 (0.85–1.49) | 0.365 | ||
| Dopamine agonist | 2.60 (2.00–3.33) | <0.001 | 1.33 (0.97–1.82) | 0.074 |
| SSRI/SNRI | 1.77 (1.46–2.13) | <0.001 | 1.20 (0.95–1.51) | 0.124 |
| Steroid | 2.97 (2.28–3.84) | <0.001 | 2.18 (1.57–3.01) | <0.001 * |
| Acid-suppressant drugs | 2.03 (1.68–2.48) | <0.001 | 1.43 (1.15–1.80) | 0.002 * |
| Aspirin | 0.53 (0.45–0.61) | <0.001 | 0.81 (0.63–1.03) | 0.083 |
| Clopidogrel | 0.61 (0.52–0.71) | <0.001 | 0.87 (0.69–1.10) | 0.241 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jung, M.; Park, H.-Y.; Park, G.-Y.; Lee, J.I.; Kim, Y.; Kim, Y.H.; Lim, S.H.; Yoo, Y.J.; Im, S. Post-Stroke Infections: Insights from Big Data Using Clinical Data Warehouse (CDW). Antibiotics 2023, 12, 740. https://doi.org/10.3390/antibiotics12040740
Jung M, Park H-Y, Park G-Y, Lee JI, Kim Y, Kim YH, Lim SH, Yoo YJ, Im S. Post-Stroke Infections: Insights from Big Data Using Clinical Data Warehouse (CDW). Antibiotics. 2023; 12(4):740. https://doi.org/10.3390/antibiotics12040740
Chicago/Turabian StyleJung, Moa, Hae-Yeon Park, Geun-Young Park, Jong In Lee, Youngkook Kim, Yeo Hyung Kim, Seong Hoon Lim, Yeun Jie Yoo, and Sun Im. 2023. "Post-Stroke Infections: Insights from Big Data Using Clinical Data Warehouse (CDW)" Antibiotics 12, no. 4: 740. https://doi.org/10.3390/antibiotics12040740
APA StyleJung, M., Park, H.-Y., Park, G.-Y., Lee, J. I., Kim, Y., Kim, Y. H., Lim, S. H., Yoo, Y. J., & Im, S. (2023). Post-Stroke Infections: Insights from Big Data Using Clinical Data Warehouse (CDW). Antibiotics, 12(4), 740. https://doi.org/10.3390/antibiotics12040740







