Should Airway Interstitial Fluid Be Used to Evaluate the Pharmacokinetics of Macrolide Antibiotics for Dose Regimen Determination in Respiratory Infection?
Abstract
1. Introduction
2. ISF Concentrations of Macrolide Antibiotics in the Lower Respiratory Tract
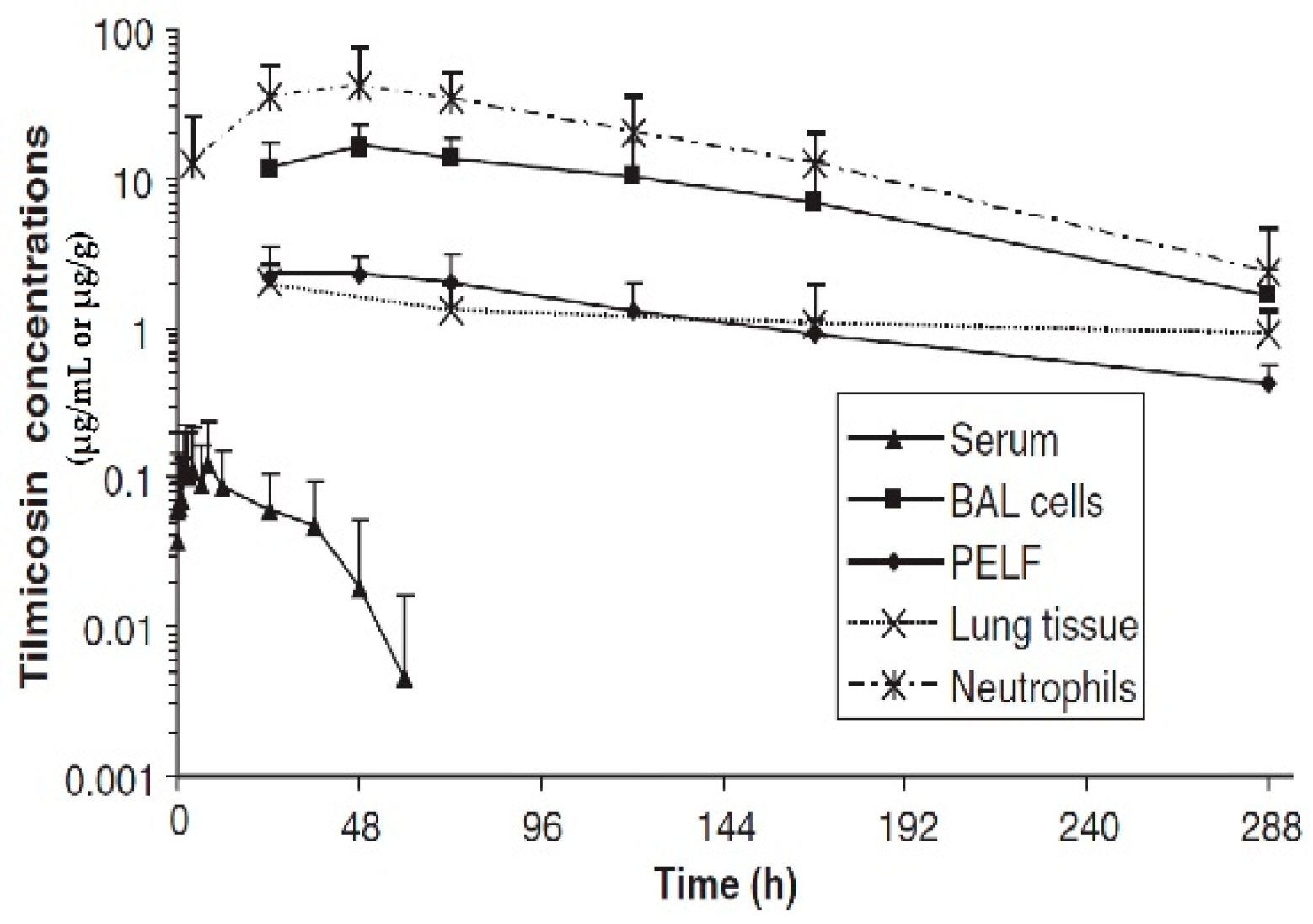
3. Concentrations of Macrolide Antibiotics in Plasma/Serum, Airway Fluid, and Tissues
4. Approaches for Airway ISF Collection
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| BF | bronchial fluid |
| BAL | bronchoalveolar lavage cells |
| ISF | interstitial fluid |
| MIC | minimum inhibitory concentrations |
| PELF | pulmonary epithelial lining fluid |
| PK | pharmacokinetics |
| PD | pharmacodynamics |
References
- Spagnolo, P.; Fabbri, L.M.; Bush, A. Long-term macrolide treatment for chronic respiratory disease. Eur. Respir. J. 2013, 42, 239–251. [Google Scholar] [CrossRef]
- Mazzei, T.; Mini, E.; Novelli, A.; Periti, P. Chemistry and mode of action of macrolides. J. Antimicrob. Chemother. 1993, 31 (Suppl. C), 1–9. [Google Scholar] [CrossRef]
- Bearden, D.T.; Rodvold, K.A. Penetration of macrolides into pulmonary sites of infection. Infect. Med. 1999, 16, 480A–484A. [Google Scholar]
- Drusano, G.L. Infection site concentrations: Their therapeutic importance and the macrolide and macrolide-like lass of antibiotics. Pharmacotherapy 2005, 25, 150S–158S. [Google Scholar] [CrossRef]
- Barza, M. Anatomical barriers for antimicrobial agents. Eur. J. Clin. Microbiol. Infect. Dis. 1993, 12 (Suppl. S1), S31–S35. [Google Scholar] [CrossRef]
- Barza, M. Pharmacokinetics of antibiotics in shallow and deep compartments. J. Antimicrob. Chemother. 1993, 31 (Suppl. D), 17–27. [Google Scholar] [CrossRef] [PubMed]
- Landersdorfer, C.B.; Nation, R.L. Limitations of antibiotic MIC-based PK-PD metrics: Looking back to move forward. Front. Pharmacol. 2021, 12, 3024. [Google Scholar] [CrossRef] [PubMed]
- Matzneller, P.; Krasniqi, S.; Kinzig, M.; Sörgel, F.; Hüttner, S.; Lackner, E.; Müller, M.; Zeitlinger, M. Blood, Tissue, and intracellular concentrations of azithromycin during and after end of therapy. Int. J. Antimicrob. Agents. 2013, 57, 1736–1742. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Müller, M.; Derendorf, H. Rational dosing of antibiotics: The use of plasma concentrations versus tissue concentrations. Int. J. Antimicrob. Agents 2002, 19, 285–290. [Google Scholar] [CrossRef]
- Toutain, P.L.; Bousquet-Melou, A. Free drug fraction vs. free drug concentration: A matter of frequent confusion. J. Vet. Pharmacol. Ther. 2002, 25, 460–463. [Google Scholar] [CrossRef]
- Toutain, P.L.; del Castillo, J.R.E.; Bousquet-Mélou, A. The pharmacokinetic–pharmacodynamic approach to a rational regimen for antibiotics. Res. Vet. Sci. 2002, 73, 105–114. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, D.; Schmidt, S.; Derendorf, H. Importance of relating efficacy measures to unbound drug concentrations for anti-infective agents. Clin. Microbiol. Rev. 2013, 26, 274–288. [Google Scholar] [CrossRef]
- Rose, M.; Menge, M.; Bohland, C.; Zschiesche, E.; Wilhelm, C.; Kilp, S.; Metz, W.; Allan, M.; Ropke, R.; Nurnberger, M. Pharmacokinetics of tildipirosin in porcine plasma, lung tissue, and bronchial fluid and effects of test conditions on in vitro activity against reference strains and field isolates of Actinobacillus pleuropneumoniae. J. Vet. Pharmacol. Ther. 2013, 36, 140–153. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.A.; Letendre, L.T.; Banav, N.; Fischer, J.; Somerville, B. Pharmacokinetics of gamithromycin in cattle with comparison of plasma and lung tissue concentrations and plasma antibacterial activity. J. Vet. Pharmacol. Ther. 2010, 33, 227–237. [Google Scholar] [CrossRef]
- Giguere, S.; Tessman, R.K. Rational dosing of antimicrobial agents for bovine respiratory disease: The use of plasma versus tissue concentrations in predicting efficacy. Int. J. Appl. Res. Vet. M 2009, 9, 342–355. [Google Scholar]
- Ball, P.; Baquero, F.; Cars, O.; File, T.; Garau, J.; Klugman, K.; Low, D.E.; Rubinstein, E.; Wise, R. Antibiotic therapy of community respiratory tract infections: Strategies for optimal outcomes and minimized resistance emergence. J. Antimicrob. Chemother. 2002, 49, 31–40. [Google Scholar] [CrossRef] [PubMed]
- Togami, K.; Chono, S.; Morimoto, K. Distribution characteristics of clarithromycin and azithromycin, macrolide antimicrobial agents used for treatment of respiratory infections, in lung epithelial lining fluid and alveolar macrophages. Biopharm. Drug Dispos. 2011, 32, 389–397. [Google Scholar] [CrossRef]
- Di, L.; Kerns, E.H. Chapter 37—Pharmacokinetic Methods. In Drug-Like Properties, 2nd ed.; Di, L., Kerns, E.H., Eds.; Academic Press: Boston, MA, USA, 2016; pp. 455–461. [Google Scholar]
- Maglio, D.; Capitano, B.; Banevicius, M.A.; Geng, Q.; Nightingale, C.H.; Nicolau, D.P. Differential efficacy of clarithromycin in lung versus thigh infection models. Chemotherapy 2004, 50, 63–66. [Google Scholar] [CrossRef]
- Bachtold, K.A.; Alcorn, J.M.; Boison, J.O.; Matus, J.L.; Woodbury, M.R. Pharmacokinetics and lung and muscle concentrations of tulathromycin following subcutaneous administration in white-tailed deer (Odocoileus virginianus). J. Vet. Pharmacol. Ther. 2016, 39, 292–298. [Google Scholar] [CrossRef]
- Romanet, J.; Smith, G.W.; Leavens, T.L.; Baynes, R.E.; Wetzlich, S.E.; Riviere, J.E.; Tell, L.A. Pharmacokinetics and tissue elimination of tulathromycin following subcutaneous administration in meat goats. Am. J. Vet. Res. 2012, 73, 1634–1640. [Google Scholar] [CrossRef]
- Kobuchi, S.; Kabata, T.; Maeda, K.; Ito, Y.; Sakaeda, T. Pharmacokinetics of macrolide antibiotics and transport into the interstitial fluid: Comparison among erythromycin, clarithromycin, and azithromycin. Antibiotics 2020, 9, 199. [Google Scholar] [CrossRef]
- Okamoto, H.; Miyazaki, S.; Tateda, K.; Ishii, Y.; Yamaguchi, K. In vivo efficacy of telithromycin (HMR3647) against Streptococcus pneumoniae and Haemophilus influenzae. Antimicrob. Agents Chemother. 2001, 45, 3250–3252. [Google Scholar] [CrossRef] [PubMed]
- Tulkens, P.M. Intracellular distribution and activity of antibiotics. Eur. J. Clin. Microbiol. Infect. Dis. 1991, 10, 100–106. [Google Scholar] [CrossRef] [PubMed]
- Nyberg, K.; Johansson, U.; Johansson, A.; Camner, P. Phagolysosomal pH in alveolar macrophages. Environ. Health Perspect. 1992, 97, 149–152. [Google Scholar] [CrossRef] [PubMed]
- McDonald, P.J.; Pruul, H. Phagocyte uptake and transport of azithromycin. Eur. J. Clin. Microbiol. Infect. Dis. 1991, 10, 828–833. [Google Scholar] [CrossRef] [PubMed]
- Togami, K.; Chono, S.; Morimoto, K. Subcellular distribution of azithromycin and clarithromycin in rat alveolar macrophages (NR8383) in vitro. Biol. Pharm. Bull. 2013, 36, 1494–1499. [Google Scholar] [CrossRef] [PubMed]
- Villarino, N.; Martín-Jiménez, T. Pharmacokinetics of macrolides in foals. J. Vet. Pharmacol. Ther. 2013, 36, 1–13. [Google Scholar] [CrossRef]
- Ng, A.W.; Bidani, A.; Heming, T.A. Innate Host Defense of the lung: Effects of lung-lining fluid pH. Lung 2004, 182, 297–317. [Google Scholar] [CrossRef]
- Bodem, C.R.; Lampton, L.M.; Miller, D.P.; Tarka, E.F.; Everett, E.D. Endobronchial, pH Relevance of aminoglycoside activity in gram-negative bacillary pneumonia. Am. Rev. Respir. Dis. 1983, 127, 39–41. [Google Scholar] [CrossRef]
- Van Bambeke, F.; Barcia-Macay, M.; Lemaire, S.; Tulkens, P.M. Cellular pharmacodynamics and pharmacokinetics of antibiotics: Current views and perspectives. Curr. Opin. Drug Discov. Devel. 2006, 9, 218–230. [Google Scholar]
- Kim, Y.H.; Heinze, T.M.; Beger, R.; Pothuluri, J.V.; Cerniglia, C.E. A kinetic study on the degradation of erythromycin A in aqueous solution. Int. J. Pharm. 2004, 271, 63–76. [Google Scholar] [CrossRef] [PubMed]
- Nix, D.E.; Goodwin, S.D.; Peloquin, C.A.; Rotella, D.L.; Schentag, J.J. Antibiotic tissue penetration and its relevance: Impact of tissue penetration on infection response. Antimicrob. Agents Chemother. 1991, 35, 1953–1959. [Google Scholar] [CrossRef] [PubMed]
- Periti, P.; Mazzei, T.; Mini, E.; Novelli, A. Clinical pharmacokinetic properties of the macrolide antibiotics. Clin. Pharmacokinet. 1989, 16, 193–214. [Google Scholar] [CrossRef]
- Jacks, S.; Giguère, S.; Gronwall, P.R.; Brown, M.P.; Merritt, K.A. Pharmacokinetics of azithromycin and concentration in body fluids and bronchoalveolar cells in foals. Am. J. Vet. Res. 2001, 62, 1870–1875. [Google Scholar] [CrossRef] [PubMed]
- Womble, A.Y.; Giguère, S.; Lee, E.A.; Vickroy, T.W. Pharmacokinetics of clarithromycin and concentrations in body fluids and bronchoalveolar cells of foals. Am. J. Vet. Res. 2006, 67, 1681–1686. [Google Scholar] [CrossRef]
- Ambrose, P.G.; Bhavnani, S.M.; Ellis-Grosse, E.J.; Drusano, G.L. Pharmacokinetic-pharmacodynamic considerations in the design of hospital-acquired or ventilator-associated bacterial pneumonia studies: Look before you leap! Clin. Infect. Dis. 2010, 51, S103–S110. [Google Scholar] [CrossRef]
- Kanoh, S.; Rubin, B.K. Mechanisms of Action and Clinical Application of Macrolides as Immunomodulatory Medications. Clin. Microbiol. Rev. 2010, 23, 590–615. [Google Scholar] [CrossRef]
- Danesi, R.; Lupetti, A.; Barbara, C.; Ghelardi, E.; Chella, A.; Malizia, T.; Senesi, S.; Alberto Angeletti, C.; Del Tacca, M.; Campa, M. Comparative distribution of azithromycin in lung tissue of patients given oral daily doses of 500 and 1000 mg. J. Antimicrob. Chemother. 2003, 51, 939–945. [Google Scholar] [CrossRef]
- Firth, A.; Prathapan, P. Azithromycin: The First Broad-spectrum Therapeutic. Eur. J. Med. Chem. 2020, 207, 112739. [Google Scholar] [CrossRef]
- Kong, F.Y.; Rupasinghe, T.W.; Simpson, J.A.; Vodstrcil, L.A.; Fairley, C.K.; McConville, M.J.; Hocking, J.S. Pharmacokinetics of a single 1g dose of azithromycin in rectal tissue in men. PLoS ONE 2017, 12, e0174372. [Google Scholar] [CrossRef]
- Zuckerman, J.M. Macrolides and ketolides: Azithromycin, clarithromycin, telithromycin. Infect. Dis. Clin. N. Am. 2004, 18, 621–649. [Google Scholar] [CrossRef] [PubMed]
- Muller-Serieys, C.; Soler, P.; Cantalloube, C.; Lemaitre, F.; Gia, H.P.; Brunner, F.; Andremont, A. Bronchopulmonary disposition of the ketolide telithromycin (HMR 3647). Antimicrob. Agents Chemother. 2001, 45, 3104–3108. [Google Scholar] [CrossRef] [PubMed]
- Menge, M.; Rose, M.; Bohland, C.; Zschiesche, E.; Kilp, S.; Metz, W.; Allan, M.; Ropke, R.; Nurnberger, M. Pharmacokinetics of tildipirosin in bovine plasma, lung tissue, and bronchial fluid (from live, nonanesthetized cattle). J. Vet. Pharmacol. Ther. 2012, 35, 550–559. [Google Scholar] [CrossRef] [PubMed]
- Torres, F.; Santamaria, R.; Jimenez, M.; Menjón, R.; Ibanez, A.; Collell, M.; Azlor, O.; Fraile, L. Pharmacokinetics of tildipirosin in pig tonsils. J. Vet. Pharmacol. Ther. 2016, 39, 199–201. [Google Scholar] [CrossRef]
- Giguere, S.; Huang, R.; Malinski, T.J.; Dorr, P.M.; Tessman, R.K.; Somerville, B.A. Disposition of gamithromycin in plasma, pulmonary epithelial lining fluid, bronchoalveolar cells, and lung tissue in cattle. Am. J. Vet. Res. 2011, 72, 326–330. [Google Scholar] [CrossRef]
- Berghaus, L.J.; Giguère, S.; Sturgill, T.L.; Bade, D.; Malinski, T.J.; Huang, R. Plasma pharmacokinetics, pulmonary distribution, and in vitro activity of gamithromycin in foals. J. Vet. Pharmacol. Ther. 2012, 35, 59–66. [Google Scholar] [CrossRef]
- Cox, S.R.; McLaughlin, C.; Fielder, A.E.; Yancey, M.; Bowersock, T.; Garcia-Tapia, D.; Bryson, L.; Lucas, M.J.; Robinson, J.A.; Nanjiani, I.; et al. Rapid and prolonged distribution of tulathromycin into lung homogenate and pulmonary epithelial lining fluid of holstein calves following a single subcutaneous administration of 2.5 mg/kg body weight. Int. J. Appl. Res. Vet. Med. 2010, 8, 129–137. [Google Scholar] [CrossRef]
- Leventhal, H.R.; McKenzie, H.C.; Estell, K.; Council-Troche, M.; Davis, J.L. Pharmacokinetics and pulmonary distribution of Draxxin® (tulathromycin) in healthy adult horses. J. Vet. Pharmacol. Ther. 2021, 44, 714–723. [Google Scholar] [CrossRef]
- Villarino, N.; Brown, S.A.; Martín-Jiménez, T. Understanding the pharmacokinetics of tulathromycin: A pulmonary perspective. J. Vet. Pharmacol. Ther. 2014, 37, 211–221. [Google Scholar] [CrossRef]
- Villarino, N.; Lesman, S.; Fielder, A.; García-Tapia, D.; Cox, S.; Lucas, M.; Robinson, J.; Brown, S.A.; Martín-Jiménez, T. Pulmonary pharmacokinetics of tulathromycin in swine. Part 2: Intra-airways compartments. J. Vet. Pharmacol. Ther. 2013, 36, 340–349. [Google Scholar] [CrossRef]
- Villarino, N.; Lesman, S.; Fielder, A.; García-Tapia, D.; Cox, S.; Lucas, M.; Robinson, J.; Brown, S.A.; Martín-Jiménez, T. Pulmonary pharmacokinetics of tulathromycin in swine. Part I: Lung homogenate in healthy pigs and pigs challenged intratracheally with lipopolysaccharide of Escherichia coli. J. Vet. Pharmacol. Ther. 2013, 36, 329–339. [Google Scholar] [CrossRef]
- Womble, A.; Giguère, S.; Murthy, Y.V.S.N.; Cox, C.; Obare, E. Pulmonary disposition of tilmicosin in foals and in vitro activity against Rhodococcus equi and other common equine bacterial pathogens. J. Vet. Pharmacol. Ther. 2006, 29, 561–568. [Google Scholar] [CrossRef]
- Javsicas, L.; Giguère, S.; Womble, A.Y. Disposition of oral telithromycin in foals and in vitro activity of the drug against macrolide-susceptible and macrolide-resistant Rhodococcus equi isolates. J. Vet. Pharmacol. Ther. 2010, 33, 383–388. [Google Scholar] [CrossRef] [PubMed]
- Suarez-Mier, G.; Giguère, S.; Lee, E.A. Pulmonary disposition of erythromycin, azithromycin, and clarithromycin in foals. J. Vet. Pharmacol. Ther. 2007, 30, 109–115. [Google Scholar] [CrossRef] [PubMed]
- Kobuchi, S.; Aoki, M.; Inoue, C.; Murakami, H.; Kuwahara, A.; Nakamura, T.; Yasui, H.; Ito, Y.; Takada, K.; Sakaeda, T. Transport of Azithromycin into extravascular space in rats. Antimicrob. Agents Chemother. 2016, 60, 6823–6827. [Google Scholar] [CrossRef] [PubMed]
- Hunter, R.P.; Burnett, T.J.; Simjee, S.A. Letter to the editor. Brit. Poultry Sci. 2009, 50, 544–545. [Google Scholar] [CrossRef]
- Winther, L. Antimicrobial drug concentrations and sampling techniques in the equine lung. Vet. J. 2012, 193, 326–335. [Google Scholar] [CrossRef]
- Toutain, P.L.; Potter, T.; Pelligand, L.; Lacroix, M.; Illambas, J.; Lees, P. Standard PK/PD concepts can be applied to determine a regimen for a macrolide: The case of tulathromycin in the calf. J. Vet. Pharmacol. Ther. 2017, 40, 16–27. [Google Scholar] [CrossRef]
- Dhanani, J.; Roberts, J.A.; Chew, M.; Lipman, J.; Boots, R.J.; Paterson, D.L.; Fraser, J.F. Antimicrobial chemotherapy and lung microdialysis: A review. Int. J. Antimicrob. Agents 2010, 36, 491–500. [Google Scholar] [CrossRef]
- Mimoz, O.; Dahyot-Fizelier, C. Mini-broncho-alveolar lavage: A simple and promising method for assessment of antibiotic concentration in epithelial lining fluid. Intensive Care Med. 2007, 33, 1495–1497. [Google Scholar] [CrossRef]
- Müller, M.; dela Peña, A.; Derendorf, H. Issues in pharmacokinetics and pharmacodynamics of anti-infective agents: Distribution in tissue. Antimicrob. Agents Chemother. 2004, 48, 1441–1453. [Google Scholar] [CrossRef]
- Maselyne, J.; Adriaens, I.; Huybrechts, T.; De Ketelaere, B.; Millet, S.; Vangeyte, J.; Van Nuffel, A.; Saeys, W. Measuring the drinking behaviour of individual pigs housed in group using radio frequency identification (RFID). Animal 2016, 10, 1557–1566. [Google Scholar] [CrossRef]
- Rottbøll, L.A.; Friis, C. Microdialysis as a tool for drug quantification in the bronchioles of anaesthetized pigs. Basic Clin. Pharmacol. Toxicol. 2014, 114, 226–232. [Google Scholar] [CrossRef] [PubMed]
- Chefer, V.I.; Thompson, A.C.; Zapata, A.; Shippenberg, T.S. Overview of brain microdialysis. Curr. Protoc. Neurosci. 2009, 47, 7.1.1–7.1.28. [Google Scholar] [CrossRef] [PubMed]
- Toutain, P.-L.; Pelligand, L.; Lees, P.; Bousquet-Melou, A.; Ferran, A.A.; Turnidge, J. The pharmacokinetic/pharmacodynamic paradigm for antimicrobial drugs in veterinary medicine: Recent advances and critical appraisal. J. Vet. Pharmacol. Ther. 2021, 44, 172–200. [Google Scholar] [CrossRef] [PubMed]
- Hopper, D.L. Automated microsampling technologies and enhancements in the 3Rs. ILAR J. 2016, 57, 166–177. [Google Scholar] [CrossRef]
- Janle, E.M.; Kissinger, P.T. Microdialysis and ultrafiltration. Adv. Food Nutr. Res. 1996, 40, 183–196. [Google Scholar] [CrossRef]
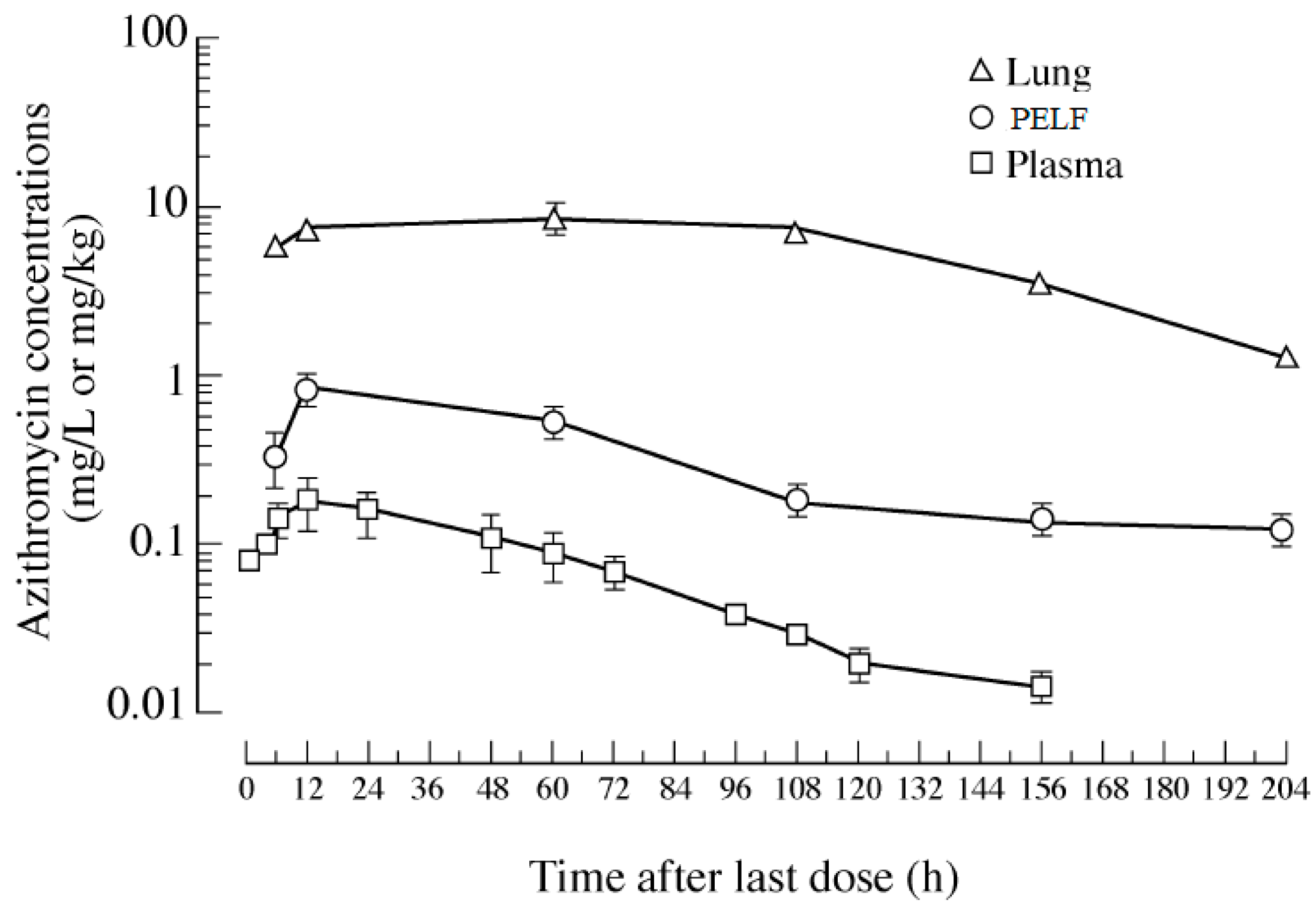
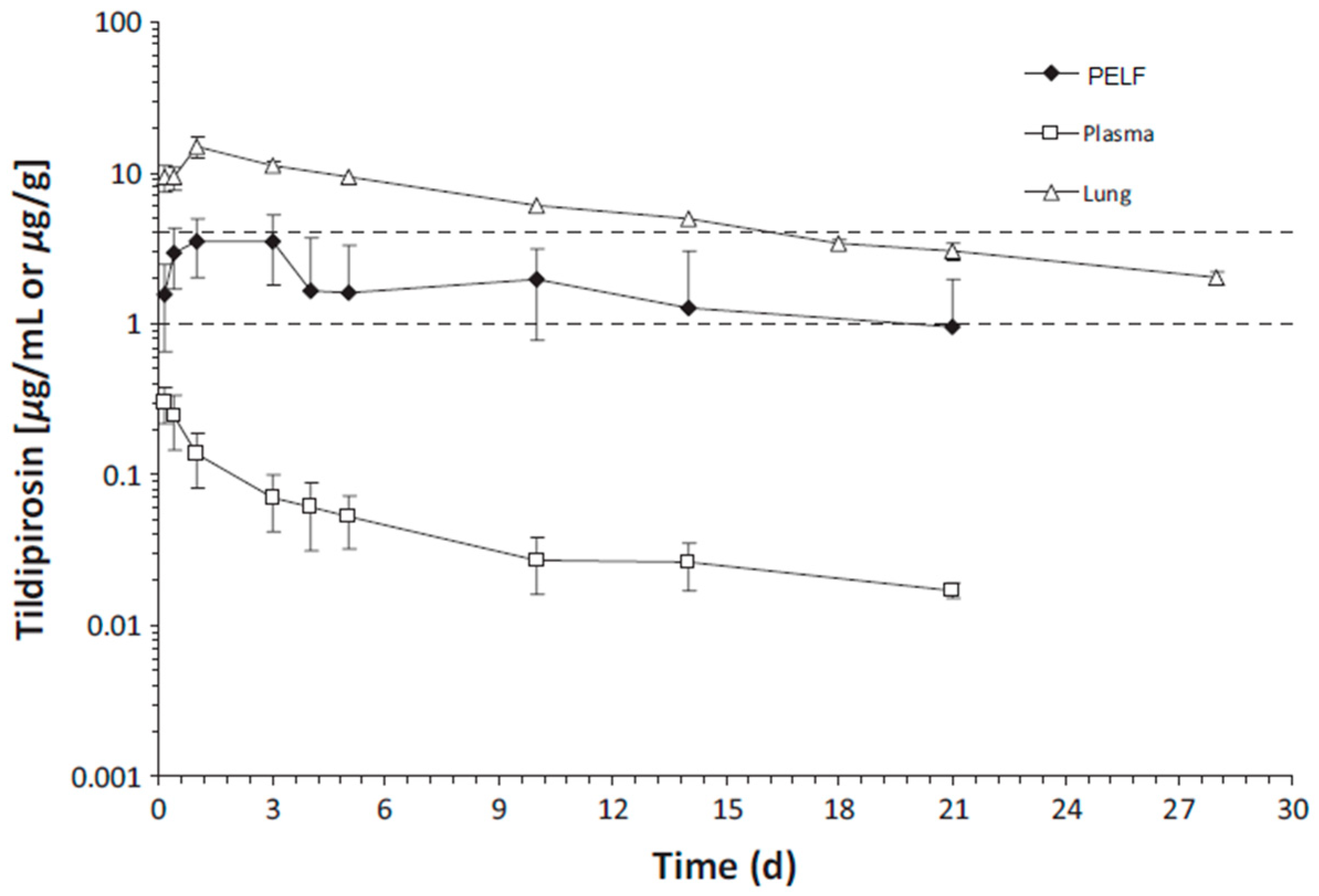

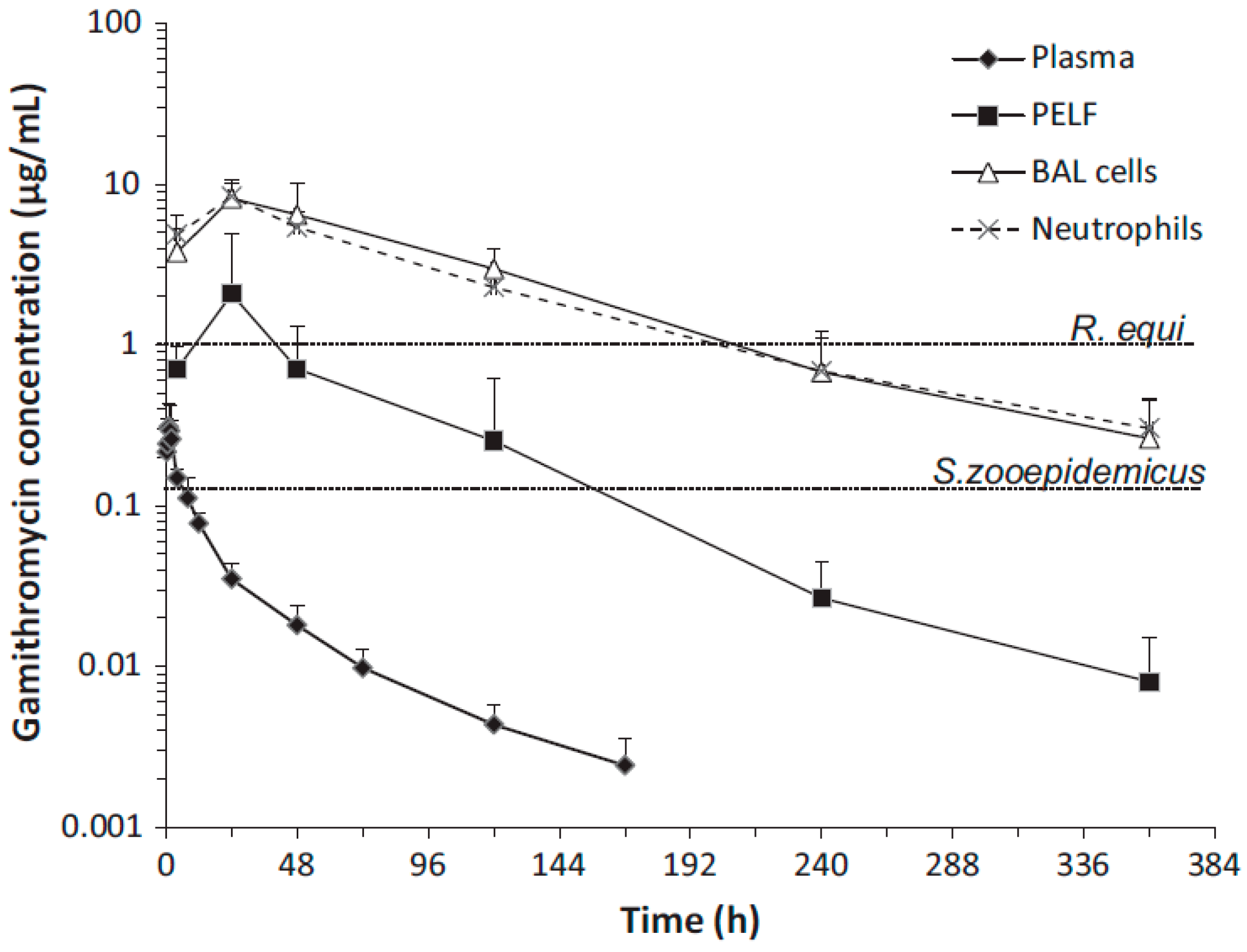
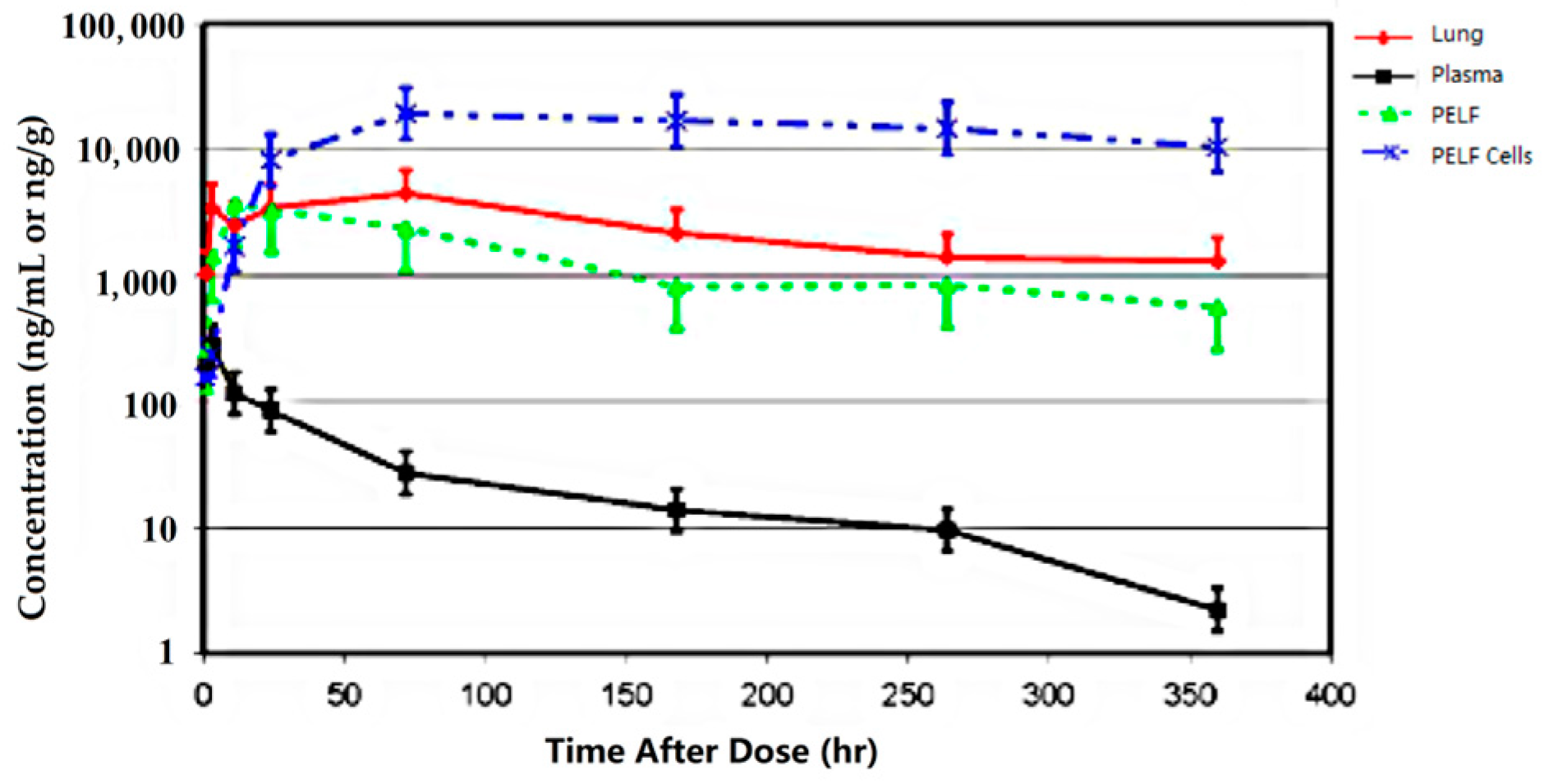
| Drug | Species | Regimen | PK Parameter | Lung | Plasma | PELF | Ratio of Lung/Plasma | Ratio of PELF/Plasma | References |
|---|---|---|---|---|---|---|---|---|---|
| Tidipirosin | Cattle | SC 4 mg/kg BW | PK Parameter (units) | [44] | |||||
| AUClast (µg·h/mL) | 3846.735 | 24.208 | 882.45 | 159 | 37 | ||||
| AUCinf (µg·h/mL) | 4497.563 | 28.973 | 1233.75 | 155 | 43 | ||||
| T1/2 (h) | 242 | 216 | 267 | 1 | 1 | ||||
| Gamithromycin | Cattle | SC 6 mg/kg BW | PK Parameter (units) | [46] | |||||
| AUClast (µg·h/mL) | 2154 | 7.82 | 334 | 275.4v | 42.7 | ||||
| AUCinf (µg·h/mL) | 2235 | 7.95 | 348 | 281.1 | 43.7 | ||||
| Cmax (µg·h/mL) | 27.8 | 0.433 | 4.61 | 64.6 | 3.2 | ||||
| Tmax (h) | 12.01 | 1.00 | 24.0 | 12.01 | 24 | ||||
| T1/2 (h) | 93.0 | 62.0 | - | 1.5 | NR | ||||
| Tulathromycin | Cattle | SC 2.5 mg/kg BW | PK Parameter (units) | [48] | |||||
| AUC0-360 (µg·h/mL) | 867 | 9.26 | 492 | 93.6 | 53.13 | ||||
| Cmax (ng/mL) | 4510 | 277 | 3730 | 16.28 | 35.13 | ||||
| Tmax (h) | 72 | 3 | 11 | 24 | 3.67 | ||||
| T1/2 (h) | 279 | 64 | 330 | 4.35 | 5.15 | ||||
| Tulathromycin | Pig | IM 2.5 mg/kg BW | PK Parameter (units) | [50,51,52] | |||||
| AUClast (ng·h/mL) | NR | 5670 | 435000 | NR | 76.7 | ||||
| AUCinf (ng·h/mL) | NR | 5650 | 473500 | NR | 83.8 | ||||
| Cmax (ng/mL) | NR | 458 | 5235 | NR | 11.4 | ||||
| T1/2 (h) | NR | 55.82 | 97.65 | NR | 1.75 | ||||
| Azithromycin | human | PO 500 mg/once daily for 3 days | PK Parameter (units) | [39] | |||||
| AUClast (mg·h/L) | 1245.4 | 11.62 | 70.29 | 107.2 | 6.04 | ||||
| Cmax (mg/L) | 8.93 | 0.18 | 0.83 | 5.55 | 4.6 | ||||
| Azithromycin | Foals | IG 10 mg/kg BW | PK Parameter (units) | [55] | |||||
| AUCinf (µg·h/mL) | NR | 7.70 | 247 | NR | 35.2 | ||||
| Cmax (µg/mL) | NR | 0.83 | 10.00 | NR | 5.4 | ||||
| T1/2 (h) | NR | 25.7 | 34.8 | NR | 1.35 | ||||
| Clarithromycin | Foals | IG 10 mg/kg BW | PK Parameter (units) | [55] | |||||
| AUClast (mg·h/L) | NR | 4.76 | 629 | NR | 132.14 | ||||
| Cmax (mg/L) | NR | 0.94 | 48.96 | NR | 52 | ||||
| Tilmicosin | Foals | IM 10 mg/kg BW | PK Parameter (units) | [53] | |||||
| AUCinf (µg·h/mL) | 711 | 5.76 | 461 | 123.4 | 80 | ||||
| Cmax (µg/mL) | 1.90 | 0.19 | 2.91 | 10 | 15.3 | ||||
| T1/2 (h) | 193.3 | 18.4 | 73.1 | 10.5 | 3.97 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.; Zhou, X.; Elazab, S.T.; Park, S.-C.; Hsu, W.H. Should Airway Interstitial Fluid Be Used to Evaluate the Pharmacokinetics of Macrolide Antibiotics for Dose Regimen Determination in Respiratory Infection? Antibiotics 2023, 12, 700. https://doi.org/10.3390/antibiotics12040700
Wang J, Zhou X, Elazab ST, Park S-C, Hsu WH. Should Airway Interstitial Fluid Be Used to Evaluate the Pharmacokinetics of Macrolide Antibiotics for Dose Regimen Determination in Respiratory Infection? Antibiotics. 2023; 12(4):700. https://doi.org/10.3390/antibiotics12040700
Chicago/Turabian StyleWang, Jianzhong, Xueying Zhou, Sara T. Elazab, Seung-Chun Park, and Walter H. Hsu. 2023. "Should Airway Interstitial Fluid Be Used to Evaluate the Pharmacokinetics of Macrolide Antibiotics for Dose Regimen Determination in Respiratory Infection?" Antibiotics 12, no. 4: 700. https://doi.org/10.3390/antibiotics12040700
APA StyleWang, J., Zhou, X., Elazab, S. T., Park, S.-C., & Hsu, W. H. (2023). Should Airway Interstitial Fluid Be Used to Evaluate the Pharmacokinetics of Macrolide Antibiotics for Dose Regimen Determination in Respiratory Infection? Antibiotics, 12(4), 700. https://doi.org/10.3390/antibiotics12040700








