Abstract
Phenotypic adaptation has been associated with persistent, therapy-resistant Staphylococcus aureus infections. Recently, we described within-host evolution towards a Sigma factor B (SigB)-deficient phenotype in a non-human host, a naturally infected dairy cow with chronic, persistent mastitis. However, to our knowledge, the prevalence of SigB deficiency among clinical S. aureus isolates remains unknown. In this study, we screened a collection of bovine mastitis isolates for phenotypic traits typical for SigB deficiency: decreased carotenoid pigmentation, increased proteolysis, secretion of α-hemolysin and exoproteins. Overall, 8 out of 77 (10.4%) isolates of our bovine mastitis collection exhibited the SigB-deficient phenotype. These isolates were assigned to various clonal complexes (CC8, CC9, CC97, CC151, CC3666). We further demonstrated a strong positive correlation between asp23-expression (a marker of SigB activity) and carotenoid pigmentation (r = 0.6359, p = 0.0008), underlining the role of pigmentation as a valuable predictor of the functional status of SigB. Sequencing of the sigB operon (mazEF-rsbUVW-sigB) indicated the phosphatase domain of the RsbU protein as a primary target of mutations leading to SigB deficiency. Indeed, by exchanging single nucleotides in rsbU, we could either induce SigB deficiency or restore the SigB phenotype, demonstrating the pivotal role of RsbU for SigB functionality. The data presented highlight the clinical relevance of SigB deficiency, and future studies are needed to exploit its role in staphylococcal infections.
1. Introduction
Chronic, persistent Staphylococcus aureus infections are difficult to treat with antibiotics and often recur. Although the role of S. aureus virulence factors in acute infections is well established, bacterial factors contributing to persistence are far less understood. The combined effects of insufficient host clearance mechanisms, bacterial immune evasion and ineffective antibiotic therapies are thought to allow the pathogen to survive for prolonged periods in the host [1]. In particular, intracellular survival by switching to the small-colony variant (SCV) phenotype is suggested to be a common reservoir for persistent infections in humans and bovines [2,3]. This mechanism of intracellular survival and SCV formation requires the upregulation of Sigma factor B (SigB), a major stress regulator in S. aureus [4]. However, frequent isolation of SigB-deficient strains during S. aureus infections suggests an advantage of this phenotype in certain niches [5,6,7]. We recently hypothesized that strains lacking SigB expression might better adapt to the extracellular niche, enabling long-term persistence in chronic mastitis [8]. SigB deficiency is typically associated with high production of proteases and toxins [6,9], suggesting that in bovine mastitis, elevated proteolysis may provide new sources of nutrients, promoting survival in the bovine mammary gland [8]. Moreover, the increased secretion of α-hemolysin (α-toxin, Hla) associated with a more cytotoxic phenotype may improve the capacity to penetrate and disseminate the udder tissue, thus supporting adaptation to the extracellular microenvironment in the mammary gland [10].
The S. aureus genome encodes several known sigma factors with different promotor specificities: the housekeeping sigma factor A (SigA) and at least three alternative sigma factors SigB, SigH and SigS [11]. Sigma factors are necessary to initiate transcription, as they bind to the RNA polymerase holoenzyme, guiding it to the promoters of the target gene. In S. aureus, the sigma factor SigB is a major stress regulator known to respond to changing environmental conditions, as it can be activated by alkaline shock, salt stress and temperature shift and is induced during the stationary phase [11]. SigB transcription is regulated by the hexa-cistronic sigB operon: mazE-mazF-rsbU-rsbV-rsbW-sigB [12]. SigA drives the transcription of the whole sigB operon, incorporating a SigB-dependent positive-feedback loop that leads to the transcription of rsbVW-sigB. Moreover, the sigA-dependent promoter PmazE was demonstrated to contribute to SigB activity by producing a mazEF-rsbUVW-sigB mRNA [13,14]. At the posttranslational level, multiple protein-protein interactions, including a partner-switching mechanism, regulate SigB activity in S. aureus. The anti-sigma factor RsbW holds SigB in an inactive complex, whereas the anti-anti-sigma factor RsbV antagonises RsbW, releasing SigB. The released sigma factor can subsequently interact with RNA polymerase and promote gene transcription. RsbV can only act as an anti-anti-sigma factor when non-phosphorylated, and phosphorylation is regulated by the phosphatase RsbU. In addition, RsbU is involved in passing stress stimuli to SigB and, in contrast to Bacillus subtilis, might be consistently active at a high level in S. aureus without any stimulation [15,16].
SigB regulates the expression of more than 200 genes that control, amongst others, cell-surface and secreted virulence factors such as pigments, toxins and proteolytic enzymes [17]. The golden (“aurum”)-coloured surface pigment results from triterpenoid carotenoids, which act as antioxidants and neutralize reactive oxygen species (ROS), promoting intracellular survival in phagocytes. By interfering with neutrophil killing, they likely promote virulence [18,19]. Staphyloxanthin (STX) is the primary pigment of S. aureus produced by the biosynthetic operon crtOPQMN [20,21]. While SigB directly binds to the crtOPQMN operon for pigment production, the SigB regulon is also linked to multiple global regulatory networks, most notably the accessory gene regulator (agr) quorum-sensing system with its effector molecule RNA III and the transcriptional regulator staphylococcus accessory regulator A (SarA) [22,23]. SigB induces the expression of SarA and inhibits the expression of agr/RNAIII, thereby suppressing the secretion of toxins, incl. α-hemolysin and staphylococcal proteases [17,24]. SigB deficiency has been shown to be associated with genetic alterations within the sigB-locus, resulting in higher RNA III and lower SarA levels. SigB-deficient strains are also reported to secrete higher amounts of proteins often associated with a typical exoprotein pattern [5,8,25]. Collectively, the following phenotypic traits were described as typical for SigB deficiency: (i) reduced pigmentation (white or grey colony colour), (ii) increased proteolysis, (iii) increased α-hemolysin secretion, and (iv) increased protein secretion [5,6,8,9,25]. Since the expression of the alkaline shock protein 23 (Asp23) is solely SigB-dependent, it is often used as an indicator of SigB activity at the transcriptional level [9,26].
Recently, we described the first within-host evolution towards a SigB-deficient phenotype in a naturally infected dairy cow with chronic, persistent mastitis [8]. We demonstrated that a dominant initial variant (IN) was replaced by a host-adapted, SigB-deficient variant (HA), which carried a single nucleotide polymorphism (SNP) in rsbU(G368A) within the sigB-locus. We assumed that the resulting glycine/aspartate substitution (G122D) within the C-terminal phosphatase domain causes a loss in the RsbV-P-specific phosphatase activity of RsbU, rendering SigB dysfunctional. In the present study, we aimed to determine the prevalence of SigB deficiency among S. aureus bovine mastitis isolates, which is, to our knowledge, unknown so far. Here, we employed several phenotypic assays to search for isolates presenting the SigB-deficient phenotype and evaluated the functional status of SigB by asp23-transcriptional expression. To determine the genetic basis of SigB deficiency, we further constructed isogenic mutants introducing or reverting the SigB-deficient phenotype.
2. Results
2.1. Screening for the SigB-Deficient Phenotype in Bovine Mastitis Isolates
2.1.1. Measurement of Extracted Pigments
The spectra of SigB-functional reference strains (SH1000, 6850 and IN) showed a characteristic spectral profile of carotenoid pigmentation with peaks at ~440 nm and ~470 nm [27]. Those peaks were absent in the SigB-deficient reference strains (SH1000ΔsigB, 8325-4, 6850ΔsigB and HA). The baseline was adjusted as a straight line through the values at 390 and 520 nm to determine carotenoid pigment production (Supplementary Figure S1). Three standard deviations (SD) above the mean area under the curve (AUC) of the SigB-deficient reference strains (SH1000ΔsigB, 6850ΔsigB, 8325-4 and HA) served as a cut-off value (AUCc = −0.2048) for strains not producing pigments. Pigment production of the 77 mastitis-derived isolates revealed AUC values ranging from −1.150 to 3.947, with a mean value of 0.6998 (Figure 1). Isolates were differentiated into non-pigmented (14.3%, 11/77), intermediate-pigmented (24.7%, 19/77), and fully pigmented strains (61.0%, 47/77). Fully pigmented isolates were considered to have fully functional SigB activity and were excluded from further analyses searching for SigB deficiency.
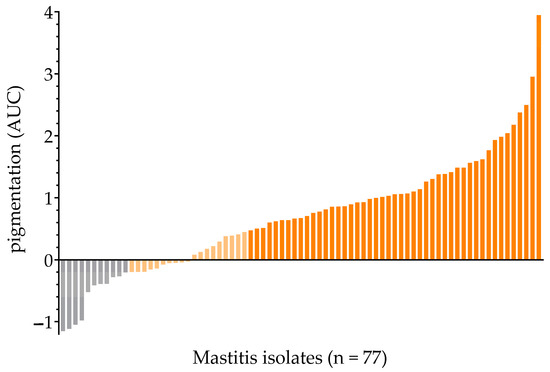
Figure 1.
Mastitis isolates were ordered by increased pigmentation (AUC value). (i) non-pigmented (grey), (ii) intermediate-pigmented (light-yellow) and (iii) fully pigmented (orange).
2.1.2. Proteolytic Activity, Immune-Based Detection of α-Hemolysin Secretion and Protein Secretion Profiling
Most non- and intermediate-pigmented mastitis isolates (27/30) showed proteolytic activity, except isolates ID49, ID67 and ID73 (Figure 2A). Eight out of 30 isolates secreted variable amounts of α-hemolysin: ID17, ID18, ID31, ID37, ID48, ID54, ID56 and ID67 (Figure 2B). When comparing silver-stained protein band patterns, we detected increased protein secretion specific for the SigB-deficient phenotype for ID17, ID18, ID37, ID48, ID54 and ID67 (Figure 2C). Interestingly, although isolate ID67 was not proteolytically active, it secreted high levels of α-hemolysin and exoproteins, suggesting a SigB-deficient phenotype.
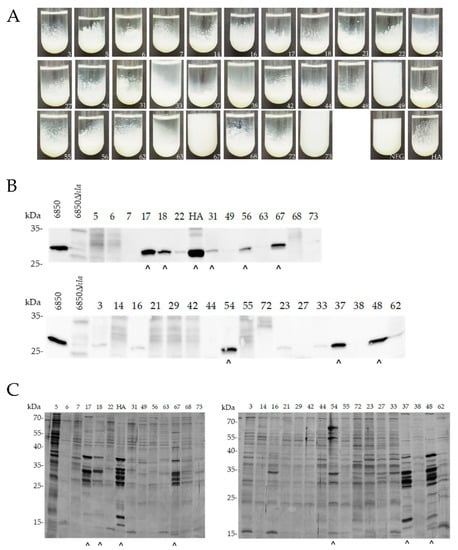
Figure 2.
Phenotypic screening of SigB deficiency of non-/intermediate-pigmented mastitis-derived isolates (n = 30). (A) Proteolysis in skim milk. Isolates were grown in 1% skim milk and proteolysis was assessed after 24 h. (B,C) Western blot against Hla (B) and SDS-PAGE of bacterial secretion pattern (C) were performed from supernatants derived from equal numbers of S. aureus cells harvested at OD600 = 5. HA, reference strain; NEG, negative control. (^) Strains, positive for Hla secretion and increased protein secretion.
Overall, based on the phenotypic assays, the following eight isolates (n = 8/77; 10.4%) were considered SigB-deficient: ID17, ID18, ID31, ID37, ID48, ID54, ID56 and ID67 (Table 1). Notably, these isolates were distributed across various clonal complexes (CC8, CC9, CC97, CC151, CC3666).

Table 1.
Characteristics of isolates presenting the SigB-deficient phenotype.
2.1.3. Asp23-Transcriptional Expression (RT-qPCR)
Since SigB positively controls asp23 transcription, we expected low levels of asp23 expression (reverse transcription-quantitative polymerase chain reaction, RT-qPCR) for all the SigB-deficient phenotypes (n = 8). We detected an average asp23 mRNA expression level relative to housekeeping genes of 69.8, ranging from 1.0 to 176.0, while the SigB-functional reference strain SH1000 had a relative expression of 266.0 (Supplementary Figure S2). Most relative asp23 mRNA expression levels were below 100, indicating minor SigB activity. Interestingly, relative expression levels between 100 and 200 were found for two isolates (ID17 and ID67), indicating moderate SigB activity.
2.2. Positive Correlation between asp23-Transcriptional Expression and Pigmentation
As asp23 gene transcription indicates the functional status of SigB [9], it was interesting to establish whether asp23-transcriptional expression (RT-qPCR) levels correlate with staphylococcal pigmentation (extracted pigments, AUC). Pearson correlation analysis revealed a strong positive correlation between asp23-expression and carotenoid pigmentation (r = 0.6359, p = 0.0008; Figure 3). Notably, many isolates (5/11) were not assigned to the SigB-deficient phenotype because no increased secretion of α-hemolysin and exoproteins could be detected, although they expressed low levels of asp23-mRNA and pigments and showed proteolytic activity.
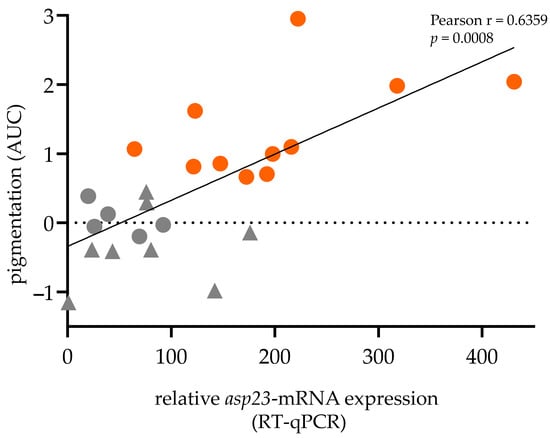
Figure 3.
Asp23-transcriptional expression positively correlates with staphylococcal pigment production. Pearson correlation coefficient (Pearson r), correlation p-value, and best-fit line (linear) shown. Orange: Fully pigmented isolates (n = 11), which are considered SigB-functional; Grey: Non- and intermediate-pigmented isolates (n = 13) including isolates presenting the SigB-deficient phenotype (n = 8; Triangle).
2.3. Sequence Analysis of the sigB Operon and Construction of Mutants to Confirm the SigB-Deficient Phenotype
To search for the genetic basis for SigB dysfunction, we sequenced the sigB operon (mazEF-rsbUVW-sigB) of all isolates exhibiting the SigB-deficient phenotype (n = 8). We did not identify substitution mutations or insertion/deletion mutations creating premature stops of translation. However, we have determined several synonymous and non-synonymous nucleotide substitutions by comparing the sequences of the sigB operon with known SigB-functional strains (Table 1). In particular, we noticed changes in the rsbU gene. We identified the SNP rsbU(G395A → S132N) in strain ID31 and rsbU(G431T → G144V) in strain ID56, which both were located within the phosphatase domain that has been shown to cause SigB dysfunction when inactivated [9]. For isolate ID18, we obtained three synonymous nucleotide substitutions in mazF, rsbW and sigB, and one SNP in the non-coding region between the genes rsbV and rsbU, the latter known to bind the feed-forward sigB-dependent PB promoter [13,14]. However, for the isolates ID17, ID37, ID48, ID54 and ID67, we could not track possible genetic differences in the sigB operon, although presenting the SigB-deficient phenotype.
We further constructed isogenic mutants introducing or reverting the SigB-deficient phenotype, serving as a proof-of-concept to determine the causal relationship of SigB deficiency. Indeed, the revertant ID56:rsbU(T431G) of the SigB-deficient isolate ID56 displayed increased pigmentation accompanied by increased asp23-transcriptional expression (RT-qPCR), both typical for higher SigB activity (Figure 4A). In addition, exchanging the SNP from G to A at position 368 in rsbU in the SigB-functional strains–IN and the human reference strain Newman–induced the SigB-deficient phenotype as shown by abrogating pigment production and asp23 expression (Figure 4B). Thus, we show in two examples that only a single nucleotide substitution within the phosphatase domain of rsbU caused the SigB-deficient phenotype.
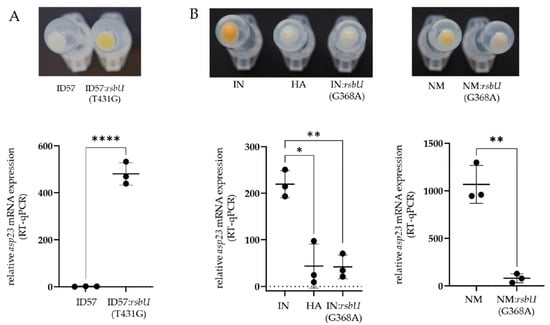
Figure 4.
Proof of the causal relationship of SigB deficiency based on a single nucleotide mutation in rsbU. Changes in bacterial surface pigmentation and asp23-mRNA expression (RT-qPCR) were shown, which are indicators of SigB activity. (A) Restoring the SigB-functional phenotype by exchanging the single nucleotide rsbU(T431G) in the SigB-deficient bovine mastitis isolate ID57. (B) Induction of the SigB-deficient phenotype by exchanging the single nucleotide rsbU(G368A) in the SigB-functional initial (IN) bovine mastitis isolate and the SigB-functional human reference strain Newman (NM); (* p < 0.05, ** p < 0.01, **** p < 0.001).
3. Discussion
In this study, we report a prevalence of 10.4% (n = 77) of bovine mastitis isolates exhibiting the SigB-deficient phenotype. To our knowledge, this is the first time that the prevalence of SigB deficiency among S. aureus isolates has been studied. Sequencing of the sigB operon (mazEF-rsbUVW-sigB) from each of the eight SigB-deficient isolates revealed several single nucleotide substitutions potentially causing the SigB-deficient phenotype. We identified the phosphatase domain of the RsbU protein as a repeated target of mutations, as two SigB-deficient strains had non-synonymous SNPs in this part of the rsbU gene (G395A → S132N; G431T → G144V). Notably, we could confirm the causal relationship of the single SNP in rsbU(G431T), as well as the SNP in rsbU(G368A) from our previous study demonstrating within-host evolution towards the host-adapted, SigB-deficient variant (HA) [8]. Consequently, a loss in the RsbV-P-specific phosphatase activity of RsbU can be assumed, which is a crucial step in regulating SigB via a partner-switching mechanism, and activation of SigB is abolished. In line with this, we obtained reduced levels of asp23 expression and pigmentation for both SigB-deficient bovine isolates compared to their isogenic SigB-functional counterpart. Mutations in the rsbU gene causing SigB deficiency were known from human staphylococcal infections. Most prominently, the laboratory prototype strain NCTC8325 (RN1) was shown to harbour an 11-base-pair (bp) deletion and the high-protease-producing prototype strain V8 to include the insertion of a 1073 bp IS element in rsbU [5,6,7]. Moreover, an 18-bp deletion in rsbU was detected in an isolate derived from a chronic cystic fibrosis infection, and a stop codon (TGA) at AA252 was found in the clinical isolate KS26 [5,6,7,28]. Mutations in rsbU (IS256 insertion, early stop codon occurrence, substitutions A230T and A276D) were identified after exposing S. aureus human clinical isolates to phototoxic conditions (photoantimicrobial chemotherapy-PACT), suggesting a role of the RsbU-dependent SigB activity against reactive oxygen species (ROS) [29]. Our findings from bovine mastitis infections indicate that genetic variation in the rsbU gene is important independent of the genetic background or the host. However, in many isolates, we could not identify genetic features within the sigB operon that may cause the SigB-deficient phenotype. We assume that the SigB-deficient phenotype may be caused by many different underlying mutations. This requires care when inferring the causative genotype-phenotype relationship at the genetic and (post-)transcriptional level.
About 14% of the isolates from our mastitis strain collection were non-pigmented. However, studies on the prevalence of non-pigmented S. aureus are scarce. A survey conducted in the Shanghai region revealed that 41% (54/132) of isolates were non-pigmented, including clinical and food-related samples [30]. While pigment-producing strains can be considered SigB-functional, reduced pigmentation may be due to SigB deficiency or other factors. For example, knock-out mutations of crtNM genes in the STX-producing operon, of the two-gene regulatory operon-yjbIH and of genes that are part of the de novo purine biosynthesis pathway have been reported to reduce pigment production [30,31,32,33]. The strong positive correlation between asp23-expression (SigB activity) and pigmentation of isolates in our mastitis collection was therefore intriguing and suggested only minor SigB-independent regulatory perturbations on staphylococcal pigmentation. Based on the correlation analysis, it can therefore be assumed that many isolates that produce no amount, or intermediate amounts, of carotenoid pigments (approx. 40%) were likely SigB-dysfunctional or at least SigB-compromised. However, it remains unclear why they do not show all the typical features of the SigB-deficient phenotype.
The co-occurrence of high production of proteases and a-hemolysin has been reported for many phylogenetically unrelated SigB-deficient strains [6,8,9]. Given the substantial proteolytic activity observed in the isolates in our bovine mastitis collection, we anticipate the secretion of proteases at a higher level associated with a slower transition towards the expression of toxins (including α-hemolysin). SarA is known to increase the expression of the agr/RNAIII system and to repress gene expression of proteases independently of agr [11]. SigB directly inhibits expression of the agr/RNAIII system but induces expression of sarA [24]. The lower SigB activity may help counterbalance the high expression of agr/RNAIII and decrease the expression of α-hemolysin in the specific context of these isolates. Moreover, low SarA levels increase the expression and secretion of proteases that were shown to degrade secreted α-hemolysin, possibly contributing to the loss of the α-hemolysin phenotype [34,35]. Furthermore, we recently did not detect secretion of proteases but strong α-hemolysin secretion of a SigB-deficient strain under growth-limiting conditions, indicative of differential regulation of the two virulence factors downstream of the sigB operon [10]. One could even speculate that the expression of proteases is evolutionarily favoured over toxins in bovine-adapted S. aureus, as milk proteins are readily accessible as a nutritional source in the mammary gland. Nevertheless, regulatory cross-talks might exist between the major regulators sigB, agr/RNAIII and sarA, which fine-tune S. aureus gene expression and secretion of proteolytic and cytotoxic virulence factors under certain niche-specific conditions. Thus, repression of SigB could better adapt the pathogen to the extracellular microenvironment. In contrast, SigB activation allows intracellular survival, and the continuous switch between the two lifestyles could then further promote long-lasting, persistent infections.
This work highlights the clinical relevance of SigB deficiency in the pathogenesis of S. aureus infections. To our knowledge, this is the first report evaluating the prevalence of SigB deficiency among S. aureus isolates. We demonstrate that carotenoid pigmentation correlates with asp23 expression, underpinning the role of pigmentation as a valuable predictor of the functional status of SigB. Further, we discuss that SigB activity might be fine-tuned rather than completely on or off, allowing an adaptive shift between intracellular and extracellular niches. However, future studies are needed to fully understand the niche-specific adaptation dynamics within the host to clarify the selection pressure acting on SigB during long-lasting, persistent infections.
4. Materials and Methods
4.1. S. aureus Strains and Growth Conditions
Seventy-seven S. aureus isolates were obtained from bovine mastitis milk samples of various geographic origins (Austria, n = 44; Argentina, n = 18; Rwanda, n = 13; others, n = 2) [36,37,38,39,40,41]. The isogenic SigB-functional, initial (IN) and SigB-deficient, host-adapted (HA) bovine isolates were used as a reference for the phenotypic discrimination of pigment production, proteolytic activity, exoproteome pattern analysis and antibody-based detection of a-hemolysin (Hla) (-toxin) secretion [8]. S. aureus 6850Δhla (kindly provided by L. Tuchscherr de Hauschopp, Institute of Medical Microbiology, University Hospital of Jena, Germany) and wild-type 6850 [42] were used as assay control strains to test for detection of α-hemolysin secretion. The rsbU-repaired SH1000 (SigB-functional) [43], the wild-type 8325-4 (SigB-deficient, 11 bp deletion in rsbU gene) [44] and the isogenic mutant SH1000ΔsigB (SigB-deficient) [4], as well as strain 6850 (SigB-functional) [42] and its isogenic ΔsigB mutant (SigB-deficient) [45], were used as SigB-functional and SigB-deficient reference strains to determine S. aureus carotenoid pigmentation. S. aureus SH1000 and SH1000ΔsigB were included as assay control and reference for mRNA expression studies of asp23 (RT-qPCR). All S. aureus isolates were stored in glycerol stocks at −80 °C until used and were initially grown on tryptic soy agar (TSA) (Thermo Fisher Scientific, Oxoid, Hampshire, UK) at 37 °C, supplemented with erythromycin (5 μg/mL) (Carl Roth, Karlsruhe, Germany) where needed.
4.2. Visualization and Determination of Carotenoid Pigmentation
To visualise S. aureus carotenoid pigmentation, one millilitre of overnight culture was precipitated by centrifugation (10,000× g for 1 min; Eppendorf, Hamburg, Germany) at the bottom of the tube. To quantify S. aureus carotenoid pigmentation (incl. STX and intermediate carotenoids), the absorbance of methanol-extracted pigments was measured using an adapted protocol [46]. Overnight cultures were inoculated in 5 mL TSB (Thermo Fisher Scientific, Oxoid, Hampshire, UK) in glass tubes and incubated at 37 °C while shaking at 120 rpm. After 18 h, OD600 was measured (BioSpectrometer, Eppendorf, Hamburg, Germany), and samples were normalised to cell mass (8 mL at OD600 = 1). Next, cultures were centrifuged at 10,000× g for 1 min to harvest the cells. The bacterial cell pellets were resuspended in 800 μL methanol (Carl Roth, Karlsruhe, Germany) and incubated at 55 °C for 3 min to extract the pigments. After removing the cell debris by centrifugation, the supernatant was transferred into new tubes, and 300 μL per sample was added in duplicates to a 96-well plate (Greiner Bio-One, Kremsmünster, Austria). The extracted pigments were quantified by measuring the OD using the SpectraMax M3 (MolecularDevices, San Jose, CA, USA) at an interval of 300 to 750 nm in 1 nm steps. Carotenoid pigmentation was calculated using the area under the curve (AUC) at an OD ranging from 390 to 520 nm, with the baseline adjusted as a line through the OD values at 390 and 520 nm (OriginPro 2022, OriginLab, Northampton, MA, USA) (Supplementary Figure S1). We calculated the cut-off value (AUCc) based on the average mean and standard deviation (SD) of the four SigB-deficient reference strains (SH1000ΔsigB, 6850ΔsigB, 8325-4 and HA), AUCc = average AUC + 3 x average SD (Supplementary Figure S1). The isolates were further divided into the following categories based on the calculated average SD value of the negative controls: (i) no pigmentation, AUC ≤ AUCc, (ii) intermediate pigmentation, AUC ≤ AUCc + 3 × SD, and (iii) full pigmentation, AUCc + 3 × SD < AUC (adapted from [47]). Each sample was measured in technical duplicates on at least two separate days.
4.3. Proteolytic Activity
Staphylococcus aureus proteolysis of non- and intermediate-pigmented (n = 30) mastitis isolates was conducted, as reported earlier [8]. Briefly, 1% skim milk broth was prepared using 10 g/L skim milk powder (Thermo Fisher Scientific) in MQ-water (Merck Millipore, Darmstadt, Germany). The solution was autoclaved for 5 min at 121 °C and stored at 4 °C until use. To assess proteolytic activity, 15 μL of a resuspended pellet derived from a 1 mL TSB overnight culture (5000× g for 2 min) was added to 3 mL 1% skim milk broth and cultivated at 37 °C without shaking. Results were recorded after 24 h.
4.4. α-Hemolysin (Hla) Western Blot and Analysis of Exoproteome Pattern
We tested non- and intermediate-pigmented isolates for increased α-hemolysin secretion and increased protein secretion, both typical phenotypic features of SigB deficiency [9,23]. Sterile filtrated bacterial supernatants were used to determine the amount of secreted α-hemolysin by western blotting and to analyse the bacterial exoproteome pattern. Overnight cultures were inoculated in 3 mL TSB and incubated at 37 °C. After 18 h, the OD600 was measured, and the samples were diluted to OD = 0.05/mL in 50 mL TSB and grown in 250 mL Erlenmeyer flasks at 37 °C at 120 rpm to OD600 = 5. The cultures were centrifuged at 5000× g at 4 °C for 10 min. The supernatant was sterile filtrated using 0.22 μm Millex-filters (Merck Millipore) and kept frozen at −80 °C until use. α-hemolysin western blots were performed according to Mayer et al., 2021 [10], using a combination of the anti-α-hemolysin antibody [8B7]—N-terminal (#ab190467) (Abcam, Cambridge, UK) diluted 1:3000, and the peroxidase-conjugated AffiniPure Goat Anti- Mouse IgG (H + L) antibody (#115-035-062) (Dianova, Hamburg, Germany) diluted 1:20,000. Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and silver staining were performed to visualise the bacterial exoproteome pattern using a 12% separation gel [48].
4.5. Reverse-Transcription (RT)-qPCR
Isolates exhibiting the SigB-deficient phenotype were tested for asp23-expression (RT-qPCR) to assess the level of SigB activity [26]. Total RNA extraction and asp23-RT-qPCR were performed on bacterial strains grown at 37 °C in TSB (120 rpm and aerobe) according to Marbach et al., 2019 [8] using the indicated primers for asp23 [4] normalised to the geometric mean of three reference genes (rpoD, rho and dnaN). Relative expression levels were calculated using the REST method [49]. For experiments validating SNPs (Section 2.3.), strains were grown until OD600 = 5 in three independent experiments with technical duplicates. For screening SigB activity (Section 2.1.3 and Section 2.2), we used a sample of 24 isolates from our mastitis strain collection, exhibiting all pigmentation phenotypes, including the eight isolates presenting the SigB-deficient phenotype. Strains were grown until OD600 = 3 in two independent experiments with technical duplicates. Mean relative expression and standard deviations were calculated from independently grown samples and their technical duplicates.
4.6. Genetic Manipulation
Genetic manipulation of S. aureus was carried out using the thermosensitive plasmid pIMAY [50] and engineered E. coli strains to simplify the transformation of S. aureus isolates [51]. For the introduction of rsbU(G368A) in various strains, the rsbU(G368A) allele was amplified using the chromosomal DNA of the host-adapted isolate (HA) and cloned into pIMAY. The recombinant plasmid was used to transform the initial isolate (IN) and S. aureus Newman (NM). Allelic replacement was used to exchange plasmid and chromosomal alleles [50]. Successful replacement was verified by Sanger sequencing. To exchange nucleotide rsbU:431T in strain ID57 to rsbU:431G, chromosomal DNA of ID57 was used, and two primer pairs (AB and CD) were used to amplify two fragments of rsbU. The desired point mutation was integrated into complementary primers B and C. The two fragments were fused by spliced extension overlap PCR (using primers A and D), and the created mutant allele was cloned into pIMAY. The recombinant plasmid was used to transform S. aureus ID57, followed by allelic replacement as described above. Oligonucleotides used for genetic manipulation in this study were summarized in Supplementary Table S1.
4.7. Sequencing of the SigB-Locus and Molecular Strain Typing
The sigB-locus (mazEF-rsbUVW-sigB) was amplified using the following primers: SigB-A_for, GCAGTGTTAATACTGCTTC and SigB-B_rev, GTTAATGAAGGAACGGAGG, [52]; rsbU_for, GAGAAATACACTGACGAAG [53] and rsbU_rev, CACAGCTTCACTAACTGCAA [53] and rsbU_RS_for, ACAGCAAAGCTCATTGTGCC (this study) and rsbU_RS_rev, AGGGAAGTGAGGAGGCAACT (this study); mazEF_for, AACCAAAGCCTTTAACGATTTCT (this study) and mazEF_rev, AGACGTTTGCCGCGAATCTA (this study). The following cycling conditions were used: initial denaturation at 98 °C/30 s, followed by 30 cycles of denaturation at 98 °C/10 s, annealing at 54 °C (sigB), 57 °C (rsbU), 59 °C (mazEF) and 60 °C (rsbU_RS) for 30 s and extension at 72 °C/60 s followed by a final extension at 72 °C/7 min. Genomic information of known SigB-functional strains was retrieved from the NCBI website (SH1000, 6850, COL, Newman, USA300_FPR3757, RF122) or in-house sequencing (IN) and used to analyse the sigB-locus. Selected isolates were genotyped using MLST as previously described [37].
4.8. Statistics
Differences in relative asp23 expression were tested by an unpaired Student t-test (two-tailed) of log-transformed data. Minimum statistical significance was set to p < 0.05. A correlation analysis (Pearson r, correlation p-value) was performed on log-transformed relative asp23-expression levels and the area under the curve (AUC) of carotenoid pigmentation. GraphPad Prism software (San Diego, CA, USA) version 7.0 was used for all statistical comparisons and visualisations.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/antibiotics12040699/s1, Supplementary Figure S1, Determination of carotenoid pigmentation; Supplementary Figure S2, asp23-mRNA expression of mastitis isolates; Supplementary Table S1, Oligonucleotides used for genetic manipulation in this study.
Author Contributions
T.G. conceived and designed the study; A.W., H.M. and D.B. performed the experiments; A.W., H.M., D.B., C.V., S.H. and T.G. carried out data analyses and interpretation; A.W., M.E.-S. and T.G. prepared the original draft; A.W., H.M., C.V., S.H., M.E.-S. and T.G. reviewed and edited the manuscript; S.H. and T.G. procured funding. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Austrian Science Fund under Grant FWF-P29304-B22 (T.G.); Deutsche Forschungsgemeinschaft (DFG in the frame of Germany’s Excellence Strategy) under Grant EXC 2124–390838134 (S.H.); German Center of Infection Research (DZIF) under Grant TTU 08.708_00 (S.H.).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The authors confirm that the data supporting the findings of this study are available within the article and its Supplementary Materials.
Acknowledgments
We thank Daniela Drin and Tatjana Svoboda from our lab (Institute of Microbiology, Vetmeduni Vienna) for their skilful technical assistance in RT-qPCR measurements, sequencing and protein gel-based assays. Open Access Funding by the Austrian Science Fund (FWF).
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Fisher, R.A.; Gollan, B.; Helaine, S. Persistent bacterial infections and persister cells. Nat. Rev. Microbiol. 2017, 15, 453–464. [Google Scholar] [CrossRef] [PubMed]
- Tuchscherr, L.; Löffler, B.; Proctor, R.A. Persistence of Staphylococcus aureus: Multiple Metabolic Pathways Impact the Expression of Virulence Factors in Small-Colony Variants (SCVs). Front. Microb. 2020, 11, 1028. [Google Scholar] [CrossRef] [PubMed]
- Atalla, H.; Gyles, C.; Mallard, B. Staphylococcus aureus small colony variants (SCVs) and their role in disease. Anim. Health Res. Rev. 2011, 12, 33–45. [Google Scholar] [CrossRef]
- Tuchscherr, L.; Bischoff, M.; Lattar, S.M.; Noto Llana, M.; Pförtner, H.; Niemann, S.; Geraci, J.; Van de Vyver, H.; Fraunholz, M.J.; Cheung, A.L.; et al. Sigma Factor SigB Is Crucial to Mediate Staphylococcus aureus Adaptation during Chronic Infections. PLoS Pathog. 2015, 11, e1004870. [Google Scholar] [CrossRef]
- Herbert, S.; Ziebandt, A.K.; Ohlsen, K.; Schäfer, T.; Hecker, M.; Albrecht, D.; Novick, R.; Götz, F. Repair of global regulators in Staphylococcus aureus 8325 and comparative analysis with other clinical isolates. Infect. Immun. 2010, 78, 2877–2889. [Google Scholar] [CrossRef] [PubMed]
- Karlsson-Kanth, A.; Tegmark-Wisell, K.; Arvidson, S.; Oscarsson, J. Natural human isolates of Staphylococcus aureus selected for high production of proteases and alpha-hemolysin are sigmaB deficient. IJMM 2006, 296, 229–236. [Google Scholar] [CrossRef]
- McAdam, P.R.; Holmes, A.; Templeton, K.E.; Fitzgerald, J.R. Adaptive evolution of Staphylococcus aureus during chronic endobronchial infection of a cystic fibrosis patient. PLoS ONE 2011, 6, e24301. [Google Scholar] [CrossRef]
- Marbach, H.; Mayer, K.; Vogl, C.; Lee, J.Y.H.; Monk, I.R.; Sordelli, D.O.; Buzzola, F.R.; Ehling-Schulz, M.; Grunert, T. Within-host evolution of bovine Staphylococcus aureus selects for a SigB-deficient pathotype characterized by reduced virulence but enhanced proteolytic activity and biofilm formation. Sci. Rep. 2019, 9, 13479. [Google Scholar] [CrossRef]
- Giachino, P.; Engelmann, S.; Bischoff, M. Sigma(B) activity depends on RsbU in Staphylococcus aureus. J. Bacteriol. 2001, 183, 1843–1852. [Google Scholar] [CrossRef]
- Mayer, K.; Kucklick, M.; Marbach, H.; Ehling-Schulz, M.; Engelmann, S.; Grunert, T. Within-Host Adaptation of Staphylococcus aureus in a Bovine Mastitis Infection Is Associated with Increased Cytotoxicity. Int. J. Mol. Sci. 2021, 22, 8840. [Google Scholar] [CrossRef]
- Jenul, C.; Horswill, A.R. Regulation of Staphylococcus aureus Virulence. Microbiol. Spectr. 2019, 6, GPP3-0031-2018. [Google Scholar] [CrossRef] [PubMed]
- Senn, M.M.; Giachino, P.; Homerova, D.; Steinhuber, A.; Strassner, J.; Kormanec, J.; Flückiger, U.; Berger-Bächi, B.; Bischoff, M. Molecular analysis and organization of the sigmaB operon in Staphylococcus aureus. J. Bacteriol. 2005, 187, 8006–8019. [Google Scholar] [CrossRef]
- Donegan, N.P.; Cheung, A.L. Regulation of the mazEF toxin-antitoxin module in Staphylococcus aureus and its impact on sigB expression. J. Bacteriol. 2009, 191, 2795–2805. [Google Scholar] [CrossRef] [PubMed]
- Fey, P.D.; Endres, J.L.; Yajjala, V.K.; Widhelm, T.J.; Boissy, R.J.; Bose, J.L.; Bayles, K.W. A Genetic Resource for Rapid and Comprehensive Phenotype Screening of Nonessential Staphylococcus aureus Genes. mBio 2013, 4, e00537-12. [Google Scholar] [CrossRef] [PubMed]
- Pané-Farré, J.; Jonas, B.; Förstner, K.; Engelmann, S.; Hecker, M. The σB regulon in Staphylococcus aureus and its regulation. IJMM 2006, 296, 237–258. [Google Scholar] [CrossRef]
- Pané-Farré, J.; Jonas, B.; Hardwick, S.W.; Gronau, K.; Lewis, R.J.; Hecker, M.; Engelmann, S. Role of RsbU in Controlling SigB Activity in Staphylococcus aureus following Alkaline Stress. J. Bacteriol. 2009, 191, 2561–2573. [Google Scholar] [CrossRef]
- Bischoff, M.; Dunman, P.; Kormanec, J.; Macapagal, D.; Murphy, E.; Mounts, W.; Berger-Bächi, B.; Projan, S. Microarray-based analysis of the Staphylococcus aureus sigmaB regulon. J. Bacteriol. 2004, 186, 4085–4099. [Google Scholar] [CrossRef]
- Liu, G.Y.; Essex, A.; Buchanan, J.T.; Datta, V.; Hoffman, H.M.; Bastian, J.F.; Fierer, J.; Nizet, V. Staphylococcus aureus golden pigment impairs neutrophil killing and promotes virulence through its antioxidant activity. J. Exp. Med. 2005, 202, 209–215. [Google Scholar] [CrossRef]
- Marshall, J.H.; Wilmoth, G.J. Proposed pathway of triterpenoid carotenoid biosynthesis in Staphylococcus aureus: Evidence from a study of mutants. J. Bacteriol. 1981, 147, 914–919. [Google Scholar] [CrossRef]
- Pelz, A.; Wieland, K.P.; Putzbach, K.; Hentschel, P.; Albert, K.; Götz, F. Structure and biosynthesis of staphyloxanthin from Staphylococcus aureus. JBC 2005, 280, 32493–32498. [Google Scholar] [CrossRef]
- Wieland, B.; Feil, C.; Gloria-Maercker, E.; Thumm, G.; Lechner, M.; Bravo, J.M.; Poralla, K.; Götz, F. Genetic and biochemical analyses of the biosynthesis of the yellow carotenoid 4,4’-diaponeurosporene of Staphylococcus aureus. J. Bacteriol. 1994, 176, 7719–7726. [Google Scholar] [CrossRef]
- Novick, R.P.; Geisinger, E. Quorum sensing in staphylococci. Annu. Rev. Genet. 2008, 42, 541–564. [Google Scholar] [CrossRef] [PubMed]
- Cheung, A.L.; Chien, Y.T.; Bayer, A.S. Hyperproduction of alpha-hemolysin in a sigB mutant is associated with elevated SarA expression in Staphylococcus aureus. Infect. Immun. 1999, 67, 1331–1337. [Google Scholar] [CrossRef] [PubMed]
- Bischoff, M.; Entenza, J.M.; Giachino, P. Influence of a functional sigB operon on the global regulators sar and agr in Staphylococcus aureus. J. Bacteriol. 2001, 183, 5171–5179. [Google Scholar] [CrossRef]
- Karlsson, A.; Saravia-Otten, P.; Tegmark, K.; Morfeldt, E.; Arvidson, S. Decreased Amounts of Cell Wall-Associated Protein A and Fibronectin-Binding Proteins in Staphylococcus aureus sarA Mutants due to Up-Regulation of Extracellular Proteases. Infect. Immun. 2001, 69, 4742–4748. [Google Scholar] [CrossRef]
- Gertz, S.; Engelmann, S.; Schmid, R.; Ohlsen, K.; Hacker, J.; Hecker, M. Regulation of sigmaB-dependent transcription of sigB and asp23 in two different Staphylococcus aureus strains. Mol. Gen. Genet. 1999, 261, 558–566. [Google Scholar] [CrossRef] [PubMed]
- Marshall, J.H.; Wilmoth, G.J. Pigments of Staphylococcus aureus, a series of triterpenoid carotenoids. J. Bacteriol. 1981, 147, 900–913. [Google Scholar] [CrossRef]
- Kullik, I.; Giachino, P.; Fuchs, T. Deletion of the alternative sigma factor sigmaB in Staphylococcus aureus reveals its function as a global regulator of virulence genes. J. Bacteriol. 1998, 180, 4814–4820. [Google Scholar] [CrossRef]
- Kossakowska-Zwierucho, M.; Kaźmierkiewicz, R.; Bielawski, K.P.; Nakonieczna, J. Factors Determining Staphylococcus aureus Susceptibility to Photoantimicrobial Chemotherapy: RsbU Activity, Staphyloxanthin Level, and Membrane Fluidity. Front. Microb. 2016, 7, 1141. [Google Scholar] [CrossRef]
- Zhang, J.; Suo, Y.; Zhang, D.; Jin, F.; Zhao, H.; Shi, C. Genetic and Virulent Difference Between Pigmented and Non-pigmented Staphylococcus aureus. Front. Microb. 2018, 9, 598. [Google Scholar] [CrossRef]
- Lan, L.; Cheng, A.; Dunman, P.M.; Missiakas, D.; He, C. Golden pigment production and virulence gene expression are affected by metabolisms in Staphylococcus aureus. J. Bacteriol. 2010, 192, 3068–3077. [Google Scholar] [CrossRef]
- Austin, C.M.; Garabaglu, S.; Krute, C.N.; Ridder, M.J.; Seawell, N.A.; Markiewicz, M.A.; Boyd, J.M.; Bose, J.L. Contribution of YjbIH to Virulence Factor Expression and Host Colonization in Staphylococcus aureus. Infect. Immun. 2019, 87, e00155-19. [Google Scholar] [CrossRef] [PubMed]
- Paudel, A.; Panthee, S.; Hamamoto, H.; Grunert, T.; Sekimizu, K. YjbH regulates virulence genes expression and oxidative stress resistance in Staphylococcus aureus. Virulence 2021, 12, 470–480. [Google Scholar] [CrossRef]
- Kwak, Y.K.; Högbom, M.; Colque-Navarro, P.; Möllby, R.; Vécsey-Semjén, B. Biological relevance of natural alpha-toxin fragments from Staphylococcus aureus. J. Membr. Biol. 2010, 233, 93–103. [Google Scholar] [CrossRef]
- Zielinska, A.K.; Beenken, K.E.; Mrak, L.N.; Spencer, H.J.; Post, G.R.; Skinner, R.A.; Tackett, A.J.; Horswill, A.R.; Smeltzer, M.S. sarA-mediated repression of protease production plays a key role in the pathogenesis of Staphylococcus aureus USA300 isolates. Mol. Microbiol. 2012, 86, 1183–1196. [Google Scholar] [CrossRef] [PubMed]
- Antók, F.I.; Mayrhofer, R.; Marbach, H.; Masengesho, J.C.; Keinprecht, H.; Nyirimbuga, V.; Fischer, O.; Lepuschitz, S.; Ruppitsch, W.; Ehling-Schulz, M.; et al. Characterization of Antibiotic and Biocide Resistance Genes and Virulence Factors of Staphylococcus Species Associated with Bovine Mastitis in Rwanda. Antibiotics 2019, 9, 1. [Google Scholar] [CrossRef] [PubMed]
- Grunert, T.; Stessl, B.; Wolf, F.; Sordelli, D.O.; Buzzola, F.R.; Ehling-Schulz, M. Distinct phenotypic traits of Staphylococcus aureus are associated with persistent, contagious bovine intramammary infections. Sci. Rep. 2018, 8, 15968. [Google Scholar] [CrossRef]
- Grunert, T.; Wenning, M.; Barbagelata, M.S.; Fricker, M.; Sordelli, D.O.; Buzzola, F.R.; Ehling-Schulz, M. Rapid and reliable identification of Staphylococcus aureus capsular serotypes by means of artificial neural network-assisted Fourier transform infrared spectroscopy. J. Clin. Microbiol. 2013, 51, 2261–2266. [Google Scholar] [CrossRef]
- Kummel, J.; Stessl, B.; Gonano, M.; Walcher, G.; Bereuter, O.; Fricker, M.; Grunert, T.; Wagner, M.; Ehling-Schulz, M. Staphylococcus aureus Entrance into the Dairy Chain: Tracking S. aureus from Dairy Cow to Cheese. Front. Microb. 2016, 7, 1603. [Google Scholar] [CrossRef]
- Schabauer, A.; Pinior, B.; Gruber, C.M.; Firth, C.L.; Käsbohrer, A.; Wagner, M.; Rychli, K.; Obritzhauser, W. The relationship between clinical signs and microbiological species, spa type, and antimicrobial resistance in bovine mastitis cases in Austria. Vet. Microbiol. 2018, 227, 52–60. [Google Scholar] [CrossRef]
- Sordelli, D.O.; Buzzola, F.R.; Gomez, M.I.; Steele-Moore, L.; Berg, D.; Gentilini, E.; Catalano, M.; Reitz, A.J.; Tollersrud, T.; Denamiel, G.; et al. Capsule expression by bovine isolates of Staphylococcus aureus from Argentina: Genetic and epidemiologic analyses. J. Clin. Microbiol. 2000, 38, 846–850. [Google Scholar] [CrossRef]
- Balwit, J.M.; van Langevelde, P.; Vann, J.M.; Proctor, R.A. Gentamicin-resistant menadione and hemin auxotrophic Staphylococcus aureus persist within cultured endothelial cells. J. Infect. Dis. 1994, 170, 1033–1037. [Google Scholar] [CrossRef] [PubMed]
- Horsburgh, M.J.; Aish, J.L.; White, I.J.; Shaw, L.; Lithgow, J.K.; Foster, S.J. sigmaB modulates virulence determinant expression and stress resistance: Characterization of a functional rsbU strain derived from Staphylococcus aureus 8325-4. J. Bacteriol. 2002, 184, 5457–5467. [Google Scholar] [CrossRef] [PubMed]
- Novick, R. Properties of a cryptic high-frequency transducing phage in Staphylococcus aureus. Virology 1967, 33, 155–166. [Google Scholar] [CrossRef]
- Lâm, T.T.; Giese, B.; Chikkaballi, D.; Kühn, A.; Wolber, W.; Pané-Farré, J.; Schäfer, D.; Engelmann, S.; Fraunholz, M.; Sinha, B. Phagolysosomal integrity is generally maintained after Staphylococcus aureus invasion of nonprofessional phagocytes but is modulated by strain 6850. Infect. Immun. 2010, 78, 3392–3403. [Google Scholar] [CrossRef] [PubMed]
- Morikawa, K.; Maruyama, A.; Inose, Y.; Higashide, M.; Hayashi, H.; Ohta, T. Overexpression of Sigma Factor, ςB, Urges Staphylococcus aureus to Thicken the Cell Wall and to Resist β-Lactams. BBRC 2001, 288, 385–389. [Google Scholar] [CrossRef]
- Stepanovic, S.; Vukovic, D.; Hola, V.; Di Bonaventura, G.; Djukic, S.; Cirkovic, I.; Ruzicka, F. Quantification of biofilm in microtiter plates: Overview of testing conditions and practical recommendations for assessment of biofilm production by staphylococci. APMIS 2007, 115, 891–899. [Google Scholar] [CrossRef]
- Grunert, T.; Leitner, N.R.; Marchetti-Deschmann, M.; Miller, I.; Wallner, B.; Radwan, M.; Vogl, C.; Kolbe, T.; Kratky, D.; Gemeiner, M.; et al. A comparative proteome analysis links tyrosine kinase 2 (Tyk2) to the regulation of cellular glucose and lipid metabolism in response to poly(I:C). J. Proteom. 2011, 74, 2866–2880. [Google Scholar] [CrossRef]
- Pfaffl, M.W. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001, 29, e45. [Google Scholar] [CrossRef]
- Monk, I.R.; Shah, I.M.; Xu, M.; Tan, M.W.; Foster, T.J. Transforming the untransformable: Application of direct transformation to manipulate genetically Staphylococcus aureus and Staphylococcus epidermidis. mBio 2012, 3, e00277-11. [Google Scholar] [CrossRef]
- Monk, I.R.; Tree, J.J.; Howden, B.P.; Stinear, T.P.; Foster, T.J. Complete Bypass of Restriction Systems for Major Staphylococcus aureus Lineages. mBio 2015, 6, e00308-15. [Google Scholar] [CrossRef] [PubMed]
- Valle, J.; Vergara-Irigaray, M.; Merino, N.; Penadés, J.R.; Lasa, I. sigmaB regulates IS256-mediated Staphylococcus aureus biofilm phenotypic variation. J. Bacteriol. 2007, 189, 2886–2896. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Kim, C.H.; Hacker, J.; Ziebuhr, W.; Lee, B.K.; Cho, S.H. Molecular characterization of regulatory genes associated with biofilm variation in a Staphylococcus aureus strain. J. Microbiol. Biotechnol. 2008, 18, 28–34. [Google Scholar] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).