Abstract
Myrcianthes discolor, an aromatic native tree from southern Ecuador, was collected to determine the chemical composition and the biological activity of its essential oil (EO). The EO was obtained by steam-distillation and analyzed by gas chromatography coupled to a mass and a FID detector (GC-MS and GC-FID) and a non-polar DB5-MS column. Enantioselective GC-MS analysis was performed in a chiral capillary column. The antimicrobial, antioxidant, and anticholinesterase potency of the EO was carried out by the broth microdilution method, radical scavenging assays using 2,2′-azino-bis-3-ethylbenzthiazoline-6-sulphonic acid (ABTS) and 1,1-diphenyl-2-picrylhydrazyl (DPPH) radical, and by measuring the inhibition of the acetylcholinesterase (AChE) enzyme. A total of 58 chemical compounds were identified, corresponding to 94.80% of the EO composition. Sesquiterpenes hydrocarbons represented more than 75% of the composition. The main compounds detected were E-caryophyllene with 29.40 ± 0.21%, bicyclogermacrene with 7.45 ± 0.16%, β-elemene with 6.93 ± 0.499%, α-cubebene with 6.06 ± 0.053%, α-humulene with 3.96 ± 0.023%, and δ-cadinene with 3.02 ± 0.002%. The enantiomeric analysis revealed the occurrence of two pairs of pure enantiomers, (−)-β-pinene and (−)-α-phellandrene. The EO exerted a strong inhibitory effect against AChE with an IC50 value of 6.68 ± 1.07 µg/mL and a moderate antiradical effect with a SC50 value of 144.93 ± 0.17 µg/mL for the ABTS radical and a weak or null effect for DPPH (3599.6 ± 0.32 µg/mL). In addition, a strong antibacterial effect against Enterococcus faecium was observed with a MIC of 62.5 μg/mL and Enterococcus faecalis with a MIC of 125 μg/mL. To the best of our knowledge, this is the first report of the chemical composition and biological profile of the EO of M. discolor, and its strong inhibitory effect over AChE and against two Gram-positive pathogenic bacteria, which encourage us to propose further studies to validate its pharmacological potential.
1. Introduction
Alzheimer’s disease (AD) is the most common cause of dementia, and it accounts for more than 75% of the reported cases. The pathophysiological process involves synapsis degeneration, mainly characterized by cholinergic neurotransmitter system dysfunction. These characteristics make it possible to face the problem by inhibiting the responsible enzyme of hydrolyzing the neurotransmitter acetylcholine. Galanthamine, huperzine, and other natural derivatives occurring in plants are used for the treatment of AD dementia phase; besides, several plants, including the Galanthus genus where the galantamine comes from, produces a bunch of metabolites such as alkaloids, terpenes, polyphenols, and others that have been assessed for their natural AChE inhibitory potential, becoming potential candidates for the discovery of AD alternative treatments [1]. The substrate of AChE, acetylcholine (ACh), is a monoamine (ACh) that plays a fundamental role in central cholinergic neurotransmission, regulating extrapyramidal motor function, cognitive functions, pain perception, and memory [2]. The enzyme acetylcholinesterase (AChE) terminates the effect of ACh at the synapse, regulating its concentration [3]; however, when there is abnormal cholinergic activity, AChE catalyzes in excess the hydrolysis or deactivation of ACh in the cholinergic terminals, causing a decrease/deficit of this neurotransmitter, leading to neurodegenerative diseases [4].
Synthetic AChE inhibitors such as donepezil, rivastigmine, and tacrine are indeed available to treat this type of disease, but they have certain disadvantages, such as gastrointestinal side effects or high costs considering that they are continuous medications [5]. Therefore, there is a great interest in essential oils, which through trials show a potent AChE inhibitory effect [4], in order to develop an effective natural alternative treatment.
Essential oils are a source of secondary metabolites with numerous biological molecules with antibacterial, antifungal, antiseptic, antioxidant, and inhibitory properties of the enzyme acetylcholinesterase (AChE) [1,6,7,8,9,10], of great importance both in the scientific community and in the health area, as possible precursor agents of new phytopharmaceuticals for the treatment of various conditions [11,12]. Species of the genus Myrcianthes, belonging to the Myrtaceae family, are wild trees or shrubs, which contain essential oils in their leaves, flowers, or fruits, which have been used within a long tradition to alleviate or treat health problems, and in many other cases, to flavor or season foods and beverages [13].
Myrcianthes discolor (Kunth) McVaugh is a native species distributed in the Andes of Ecuador, Peru, Colombia, and Bolivia. It grows favorably in a humid tropical climate, on mountain slopes, between 2900 and 3000 m a.s.l. [14]. There are no extensive reports about its traditional uses, but according to Bussman et al. [15], the fresh whole plant is used to treat inflammation and it was also found to inhibit the growth of Sthapylococcus aureus. Its fruit is edible and due to the natural water resistance of its wood is used for building irrigation channels and mill wheels [16]. Despite the lack of information of this species, it is vital for our research group to continue investigating the native flora of Ecuador to find new alternatives for common and emerging diseases. For that reason, our aim was to determine the chemical composition of the essential oil of M. discolor and to validate its pharmacological properties through in vitro available assays commonly reported in many publications, such as antioxidant, anticholinesterase, and antimicrobial assays.
2. Results
2.1. Essential Oil Yield
The essential oil extracted from fresh leaves by steam distillation presented a slightly yellowish viscous appearance and a yield of around 0.075 ± 0.010%.
2.2. Qualitative and Quantitative Analyses
The essential oil of M. discolor was analyzed by gas chromatography coupled to mass spectrometry (GC-MS) and gas chromatography coupled to flame ionization (GC-FID), using a DB5-MS non-polar column. A total of 58 volatile compounds were identified, corresponding to 94.80% of the total EO of M. discolor. Figure 1 shows the GC-MS chromatogram of the EO of M. discolor with the retention time of the majority compounds displayed on the X-axis and their relative abundance on the Y-axis.
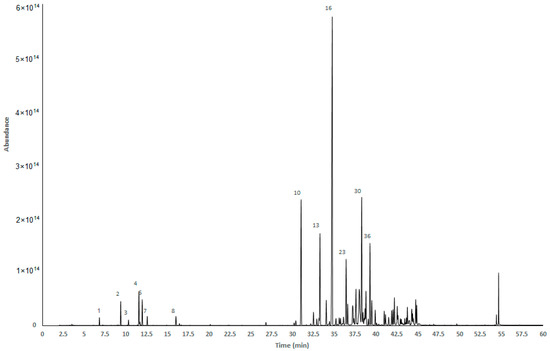
Figure 1.
GC-MS chromatogram of Myrcianthes discolor EO, through a 5%-phenyl-methylpolysiloxane capillary column.
Sesquiterpenes hydrocarbon accounts for 75.42% of the total composition, with E-caryophyllene (29.40 ± 0.217%), bicyclogermacrene (7.45 ± 0. 162%), β-elemene (6.93 ± 0.499%), and α-cubebene (6.06 ± 0.053%) as the major compounds. Oxygenated sesquiterpenes were the second representative group with the highest percentage (11.13%) and hydrocarbonated monoterpenes represented 7.42%. Monoterpenes such as limonene (2.63 ± 0.015%), β-pinene (1.74 ± 0.010%), and Z-β-ocimene (1.69 ± 0.020%) were found in a lower percentage. Table 1 shows the compounds identified according to their elution on the DB5-MS column, and the calculated linear retention index and linear retention as reported in literature [17] are included in this depiction.

Table 1.
Chemical compounds present in the essential oil of the leaves of Myrcianthes discolor.
2.3. Enantioselective Analysis
The enantiomeric composition of the essential oil was analyzed with an enantioselective capillary column 2,3-diethyl-6-tert-butyldimethylsilyl-β-cyclodextrin. Three chiral compounds were identified: (1S,5S)-(−)-β-pinene, (R)-(−)-α-phellandrene, found pure with an e.e. of 100%, and the enantiomeric pair (4S)-(−)-limonene and (4R)-(+) limonene in a racemic mixture (e.e. 17.028%) (Table 2).

Table 2.
Enantioselective analysis of the M. discolor EO.
2.4. Antimicrobial Activity
The antimicrobial activity of the essential oil was determined by the broth microdilution method. The highest antimicrobial inhibitory potential of the M. discolor EO was displayed by the Gram-positive bacteria E. faecium ATCC® 27270, with a MIC value of 62.5 µg/mL, and E. faecalis ATCC® 19433 with a MIC of 125 µg/mL. The remaining microorganisms exhibited a weak or absent inhibitory potential at concentrations above 1000 µg/mL. Ampicillin, ciprofloxacin, and amphotericin B were used as positive controls and dimethyl sulfoxide as a negative control. The results of antimicrobial activity are shown in Table 3.

Table 3.
Minimum inhibitory concentration calculated for the Myrcianthes discolor essential oil against seven human pathogenic microorganisms.
2.5. Antioxidant Capacity
The antioxidant capacity of the EO of M. discolor exhibited weak activity with a value of 144.93 ± 0.1754 and 3599.6 ± 0.324 µg/mL, respectively. Trolox was used as a positive reference control (Table 4).

Table 4.
Antioxidant activity of the essential oil of Myrcianthes discolor.
2.6. Anticholinesterase Activity
The EO from the leaves of M. discolor exhibited a potent inhibitory effect over acetylcholinesterase (AChE), with an IC50 value of 6.68 ± 1.07 µg/mL (Figure 2). Donepezil was used as a positive control with an IC50 of 12.40 ± 1.35 µg/mL (Table 5).
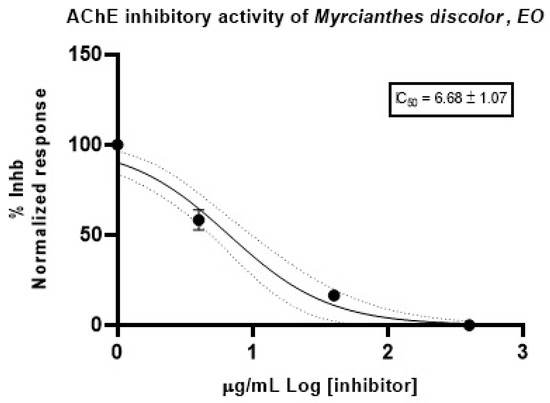
Figure 2.
Inhibitory effect plot of the Myrcianthes discolor EO, against acetylcholinesterase.

Table 5.
Anticholinesterase activity of the essential oil of Myrcianthes discolor.
3. Discussion
The average EO yield was 0.075%, obtained from the leaves of M. discolor, which was very low compared to the EOs of M. fragrans with 0.28% [18], M. pungens with 0.19% [19], M. mollis with 0.2%, and M. myrsinoides with 0.3% [20] or M. leucoxyla with 0.1% [21]. On the one hand, there is relatively little information about the essential oil yield of species from this genus, but according to the literature, the average yield oscillates around 0.1% to 0.3% when obtained from leaves [18,19,20,21,22]. On the other hand, Barra [23] mentioned that endogenous and exogenous factors affect essential oil yields and could even lead to ecotypes or chemotypes in the same plant species, and such factors can involve individual genetic variability, variation among different parts of the plants or phenological stages, and modifications due to the environment. Gupta and Ganjewala [24] suggest that EO can vary due to different factors, such as harvesting conditions, i.e., soil type, cycle in which the plant was found, and part of the plant harvested, or distillation conditions, such as the extraction method or equipment used.
According to the results, it was observed that the EO of M. discolor was mostly composed of sesquiterpene hydrocarbons (75.42%) such as E-caryophyllene (29.40 ± 0.217%), bicyclogermacrene (7.45 ± 0.162%), β-elemene (6.93 ± 0.499%), α-cubebene (6.06 ± 0.053%), α-humulene (3.96 ± 0.023%), and δ-cadinene (3.02 ± 0.002%). Monoterpene hydrocarbons were also found in a lower percentage (7.42%), such as limonene (2.63 ± 0.015%), β-pinene (1.74 ± 0.010%), and Z-β-ocimene (1.69 ± 0.020%). In comparison with studies of related species of the genus Myrcianthes, in M. myrsinoides (collected in Ecuador), the isomer Z-caryophyllene (16.6%) was reported as the main compound, and the monoterpene hydrocarbons limonene (5.3%), β-pinene (1.5%), and α-pinene (2.5%) in a lower percentage [20]. On the other hand, from the leaves of M. leuxycola collected in Colombia, 14 out of 33 compounds were monoterpenes, with 1,8-cineole as the major compound (6.3%) [21]. Other species such as M. fragrans were composed mainly by the oxygenated monoterpenes geranial (31.1%) and neral (23.6%) [18], M. cisplatensis by hydrocarbonated monoterpenes such as 1,8-cineole (53.8%) and α-pinene (16.6%) [25], M. osteomeloides and M. pseudomato by hydrocarbonated monoterpenes such as 1,8-cineole (55.7% and 24.4%) and α-pinene (17.9% and 17.1%), respectively [26], and M. pungens by hydrocarbonated sesquiterpenes such as β-caryophyllene (11.7%) and 1,8-cineole (10.1%) [19].
E-caryophyllene, the main compound of M. discolor, is a common sesquiterpene found in essential oils from several species and it has been found to exert some biological activities such as anticancer or analgesic properties [27]. It can cause tracheal smooth-muscle relaxation through voltage-dependent Ca2+ channels [28], and Dahham et al. [29] reported a good scavenging radical profile with IC50 values of 1.25 and 3.23 µM for DPPH and FRAP assays, respectively, and a more pronounced antibacterial activity against Gram-positive bacteria than Gram-negative, with MIC values ranging from 3 to 9 µM. Additionally, the EO exhibited a moderate AChE inhibitory activity with 32% inhibition at a 0.06 mM concentration [30].
According to the enantioselective analysis, two pairs of pure enantiomers and an almost racemic mixture of limonene (+) and (−) isomers were found. It is worth mentioning that this is the first report of the enantiomeric composition of the EO of M. discolor, which can be compared with the report of Montalván et al. [20] on the species M. myrsinoides, containing the pure chiral compound (+)-limonene with an e.e. of 100% and the enantiomeric pair (+)-β-pinene and (−)-β-pinene, with an enantiomeric excess of 20.8% (−)-β-pinene.
Regarding the biological activity, the EO of M. discolor exhibited significant antimicrobial activity against the Gram-positive bacteria of the genus Enterococcus, with MIC values less than 125 μg/mL and no activity against the other microorganisms studied at the highest dose tested of 1000 µg/mL. There are no investigations of the antimicrobial activity of the same species, but in comparison with other studies of species of the same genus, Araujo et al. [31] reported that the EO of M. myrsinoides presents moderate activity against B. cereus with a MIC between 100 and 200 μg/mL, and B. subtilis and S. epidermidis with a MIC of 200–400 μg/mL. Furthermore, Almeida et al. [19] indicate that the highest antimicrobial activity of M. pungens essential oil was against S. aureus, with a MIC of 78.12 μg/mL. According to Van Vuuren and Holl [32], the antimicrobial effect of the EO exerted for E. faecium and E. faecalis can be classified as a strong activity; meanwhile, for the rest of the microorganisms, there is no antimicrobial effect. According to these criteria, the antimicrobial activity of the EO of M. discolor can be qualified as good, being a potential source of biological molecules to be studied, which can give rise to new phytopharmaceuticals. We can observe that the species of the same genus possess similar qualities.
Regarding to the antioxidant capacity, the EO of M. discolor through the ABTS test displayed a better antiradical capacity with a SC50 value of 144.93 ± 0.17. According to Almeida et al. [19], the EO of M. pungens leaves has a good antioxidant activity through the DPPH assay, with a value of 24.47 ± 2.03 mg/mL, in contrast to our study where the scavenging capacity through this assay was poor. Selestino et al. [33] mention that EOs rich in sesquiterpenes hydrocarbons, as in the present study, have a low antioxidant activity, which may explain the low activity of the EO of M. discolor.
This is the first report of the anticholinesterase activity of the M. discolor EO, which exhibited a potent inhibitory effect on the acetylcholinesterase (AChE) enzyme, with an IC50 value of 6.68 ± 1.07 µg/mL, which is much higher compared to the value of the M. myrsinoides essential oil that has an AChE inhibitory activity of 78.6 μg/mL [21]. This can be explained by the presence of bioactive compounds such as trans-caryophyllene, as suggested by Bonesi et al. [30], and β-pinene, α-humulene, and limonene, with moderate anticholinesterase activity, as suggested by Hung et al. [34].
4. Materials and Methods
4.1. Plant Material
The leaves of M. discolor in the flowering stage were collected in November and December 2021, on the Loja-Chuquiribamba Road, in the province of Loja, Ecuador, at an altitude of 2560 m a.s. l, with coordinates: 3°56′52″ S, 79°16′11″ W. The plant material was collected under a permit from the Ministry of Environment of Ecuador (MAE-DNB-CM-2016-0048) and identified by Dr. Nixon Cumbicus, and the herbarium sample was deposited in the UTPL Herbarium, with voucher number 14549.
4.2. Essential Oil Distillation
The essential oil was obtained from fresh leaves by steam distillation, using a Clevenger-type stainless-steel distiller. The process was carried out for a period of 3 h in each distillation; then, anhydrous sodium sulfate was added to eliminate water residues. It was then stored in sealed amber vials, labeled, and refrigerated at −4 °C for analysis.
4.3. Essential Oil Yield
The EO yield, to check how efficient a process is, was calculated by considering the ratio of the volume of EO obtained (v/w) to the amount in grams of plant matter collected.
4.4. Chemical Profiling
Analysis of the EO was performed by gas chromatography coupled to mass spectrometry (GC-MS), and with a flame ionization detector (GC-FID), to qualitatively and quantitatively determine the chemical compounds of M. discolor.
4.4.1. GC-MS (Qualitative Analysis)
Chemical analysis of the Myrcianthes discolor EO was carried out as suggested by Cartuche et al. [35] with slight modifications, as follows: Samples were prepared by dissolving 990 μL of dichloromethane and 10 μL of EO to reach a 1% (v/v) concentration. The mass spectrometer was operated in SCAN mode (scan range 40–350 m/z), with the electron ionization source set to 70 eV, using an Agilent DB-5ms non-polar column, series 122–5532 (5% phenylmethylpolysiloxane, 30 m-long, 0.25 mm internal diameter, and 0.25 μm film thickness). Then, 1 μL of sample was injected, under the following conditions: split mode (split ratio 40:1), using helium as a carrier gas, at a constant flow rate of 1 mL/min. With this column, the thermal program was based on an initial oven temperature at 60 °C for 5 min, followed by a first thermal gradient of 2 °C/min up to 200 °C. Finally, a new gradient of 15 °C/min was applied up to 250 °C, maintaining the final temperature for 5 min. The analysis was performed on a Thermo Scientific Trace 1310 series chromatograph, coupled to a Thermo Scientific ISQ 7000 series mass spectrometer, equipped with a “Chromeleon XPS Software” data system.
4.4.2. GC-FID (Quantitative Analysis)
The analysis technique to quantify the chemical compounds of the EO was performed using an Agilent 6890N series gas chromatograph coupled to a flame ionization detector (FID), employing the same column used for the qualitative analysis (DB5-MS), under the same injection conditions. Quantitative results were recorded as mean values and standard deviation, over three replicates.
The percentage values refer to the weight of the analytes with respect to the mass of the total EO, without using a correction factor.
4.4.3. Enantioselective Analysis
Enantioselective analysis was performed according to the same equipment described above and an enantioselective capillary column (2,3-diethyl-6-tert butyldimethylsilyl-β-cyclodextrin, 25 m-long, 0.25 mm internal diameter, and 0.25 μm film thickness). The thermal program was based on an initial oven temperature of 60 °C for 5 min, followed by a thermal gradient of 2 °C/min up to 220 °C, which was finally maintained for 2 min. The homologous series of n-alkanes (C9–C25) was also injected, which allowed the calculation of the linear retention indices of the stereoisomers.
4.5. Antimicrobial Activity
Antimicrobial activity was measured according to the methodology suggested by Cartuche et al. [35]. The values of minimum inhibitory concentration and antimicrobial activity were determined using the broth microdilution technique, with three Gram-positive bacteria (E. faecalis ATCC® 19433, E. faecium ATCC® 27270, S. aureus ATCC® 25923), two Gram-negative bacteria (E. coli (O157:H7) ATCC® 43888, P. aeruginosa ATCC® 10145), and two fungi (C. albicans ATCC® 10231, A. niger ATCC® 6275) as microbial test models. The double serial dilution method was used to achieve concentrations ranging from 4000 to 31.25 µg/mL, and a final inoculum concentration of 5 × 105 cfu/mL for bacteria, 2.5 × 105 cfu/mL for yeasts, and 5 × 104 spores/mL were used for sporulated fungi. Mueller Hinton II (MH II) for bacteria and Sabouraud broth for fungi were used as test media.
Commercial antimicrobial agents were used as positive controls as follows: ampicillin solution, 1 mg/mL, for E. faecalis, E. faecium, and S. aureus, ciprofloxacin solution, 1 mg/mL, for P. aeruginosa and E. coli, and finally, amphotericin B solution, 250 µg/mL, for C. albicans and A. niger.
4.6. Antioxidant Capacity
4.6.1. The 2,2-Diphenyl-1-picrylhydrazyl Radical Scavenging Assay
The DPPH radical scavenging assay was developed according to the methodology proposed by Cartuche et al. [35], using the free radical 2,2-diphenyl-1-picrylhydrazyl (DPPH-). A working solution was prepared by dissolving 24 mg of DPPH in 100 mL of methanol and stabilized in an EPOCH 2 microplate reader (BIOTEK, Winooski, VT, USA) at 515 nm until an absorbance of 1.1 ± 0.01 was reached. The antiradical reaction between EO and free radicals was performed at different concentrations of EO (1, 0.5, and 0.25 mg/mL). Then, 270 µL of the DPPH-adjusted working solution and 30 µL of the EO sample were placed in a 96-microwell plate. The reaction was monitored at 515 nm for 60 min at room temperature. Trolox and methanol were used as a positive control and blank control, respectively. The results were expressed as SC50 (sweep concentration of the radical at 50%). Measurements were performed in triplicate.
4.6.2. The 2,2-Azinobis-3-ethylbenzothiazoline-6-sulfonic Acid Radical Scavenging Assay
The antioxidant power measured against the ABTS-+ cation (2,2′-azinobis-3-ethylbenzothiazoline-6-sulfonic acid) was determined as reported by Cartuche et al. [35], with slight modifications, as described. Briefly, the assay began with the preparation of a stock solution of the radical by reacting equal volumes of ABTS (7.4 µM) and potassium persulfate (2.6 µM) for 12 h, with stirring. The standard solution was prepared by dissolution in methanol to an absorbance of 1.1 ± 0.02, measured at 734 nm on an EPOCH 2 microplate reader (BIOTEK, Winooski, VT, USA). The antiradical reaction was evaluated for 1 h in the dark at room temperature by plating 270 µL of the adjusted ABTS working solution and 30 µL of the M. discolor EO at different concentrations (1, 0.5, and 0.25 mg/mL). Trolox and methanol were used as a positive control and blank control, respectively. The results were expressed as SC50 (sweep concentration of the radical at 50%). Measurements were performed in triplicate.
4.7. Anticholinesterase Assay
AChE inhibition was measured using the spectrophotometric method developed by Andrade et al. [36]. Briefly, the reaction mixture contained 40 μL of Tris Buffer, 20 μL of the analyzed sample solution, 20 μL of acetylthiocholine (ATCh, 15 mM PBS, pH 7.4), and 100 μL of DTNB (3 Mm, Tris Buffer). Preincubation was carried out for 3 min at 25 °C, with continuous shaking. Finally, the addition of 20 μL of 0.5 U/mL AChE initiated the reaction and the amount of product released was monitored on an EPOCH 2 microplate reader (BIOTEK 1) at 405 nm, at 25 °C for 60 min.
The EO of M. discolor was prepared by dissolving 10 mg in 1 mL of MeOH. Four further dilutions (10× factor dilution) were included to obtain final concentrations of 1000, 100, and 10 μg/mL. All compounds were assayed at a maximum concentration of 250 μM. Progression curves were calculated from the absorbance according to a standard curve of DTNB and L-GSH at different molar concentrations to measure the rate, expressed as mM/min of product released. As a non-selective protic solvent, MeOH was selected to dissolve the samples and was used as a negative control at a maximum concentration of 10% in the final volume of the mixture, without affecting the enzymatic reaction. Donepezil hydrochloride was used as a positive control with a calculated IC50 value of 12.40 ± 1.35 nM.
4.8. Statistical Analysis
Data from antioxidant and cholinesterase assays were analyzed through non-linear regression analysis: the log concentration vs. normalized response-variable slope model, by means of the Graph Pad Prism v8.0.1 package (GraphPad, San Diego, CA, USA). SC50 and IC50 parameters were calculated according to this model. MIC interpretation was in accordance with international standards, such as those suggested by the Clinical and Laboratory Standards Institute, USA, and measured by visual inspection of growth, and due to the method of dilution employed, there were no standard deviations to show. Chemical composition data (%) represented the mean ± SD of three different injections.
5. Conclusions
This is the first report of the chemical and biological profile of the essential oil of Myrcianthes discolor, which displayed a good antibacterial and strong anticholinesterase effect. Despite its poor antioxidant capacity, similar to other related species from the genus, the information provided in this study encouraged us to propose future scientific research, looking for possible interactions and synergistic or additive effects exerted by the main compounds found in this essential oil. It is uncommon to find an EO that possesses good biological anticholinesterase activity such as that exerted by M. discolor, and a possible application could be in a pharmaceutical formulation as an adjuvant for the treatment of AD.
Author Contributions
Conceptualization, D.R. and V.M.; methodology, D.R., V.M., E.V., L.C. and N.C.; formal analysis, D.R. and V.M.; investigation, D.R.; data curation, V.M.; writing—original draft preparation, D.R.; writing—review and editing, D.R., E.V., V.M., L.C. and N.C.; supervision, V.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
We are very grateful to Universidad Técnica Particular de Loja (UTPL) for supporting this project and the Ecuadorian Environmental Ministry (MAE) for the permission granted.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Dos Santos, T.C.; Gomes, T.M.; Pinto, B.A.S.; Camara, A.L.; Paes, A.M.A. Naturally Occurring Acetylcholinesterase Inhibitors and Their Potential Use for Alzheimer’s Disease Therapy. Front. Pharmacol. 2018, 9, 1192. [Google Scholar] [CrossRef] [PubMed]
- Trang, A.; Khandhar, P.B. Physiology, Acetylcholinesterase. Updated 2022 May 8. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2022. Available online: https://www.ncbi.nlm.nih.gov/books/NBK539735/ (accessed on 22 March 2023).
- Sánchez, G.; Salceda, R. Polyfunctional Enzymes: The Case of Acethylcholinesterase. Biochem. Educ. Mag. 2008, 27, 44–51. [Google Scholar]
- Owokotomo, I.A.; Ekundayo, O.; Abayomi, T.G.; Chukwuka, A.V. In-Vitro Anti-Cholinesterase Activity of Essential Oil from Four Tropical Medicinal Plants. Elsevier 2015, 2, 850–857. [Google Scholar] [CrossRef] [PubMed]
- Schulz, V. Ginkgo Extract or Cholinesterase Inhibitors in Patients with Dementia: What Clinical Trials and Guidelines Fail to Consider. Phytomedicine 2003, 10, 74–79. [Google Scholar] [CrossRef]
- Rincón, C.; Castaño, J.; Vázquez, E. Biological Activity of Essential Oils from Acmella ciliata (Kunth) Cass. Rev. Cuba. Plantas Med. 2012, 17, 160–171. [Google Scholar]
- Dohi, S.; Terasaki, M.; Makino, M. Acetylcholinesterase Inhibitory Activity and Chemical Composition of Commercial Essential Oils. J. Agric. Food Chem. 2009, 57, 4313–4318. [Google Scholar] [CrossRef]
- D’agostino, M.; Tesse, N.; Frippiat, J.P.; Machouart, M.; Debourgogne, A. Essential Oils and Their Natural Active Compounds Presenting Antifungal Properties. Molecules 2019, 24, 3713. [Google Scholar] [CrossRef]
- Sharifi-Rad, J.; Sureda, A.; Tenore, G.C.; Daglia, M.; Sharifi-Rad, M.; Valussi, M.; Tundis, R.; Sharifi-Rad, M.; Loizzo, M.R.; Oluwaseun Ademiluyi, A.; et al. Biological Activities of Essential Oils: From Plant Chemoecology to Traditional Healing Systems. Mol. A J. Synth. Chem. Nat. Prod. Chem. 2017, 22, 70. [Google Scholar] [CrossRef]
- Amorati, R.; Foti, M.C.; Valgimigli, L. Antioxidant activity of essential oils. J. Agric. Food Chem. 2013, 61, 10835–10847. [Google Scholar] [CrossRef]
- Ferreira, M.J.; Pinto, D.C.G.A.; Cunha, Â.; Silva, H. Halophytes as Medicinal Plants against Human Infectious Diseases. Appl. Sci. 2022, 12, 7493. [Google Scholar] [CrossRef]
- Seidel, V. Plant-Derived Chemicals: A Source of Inspiration for New Drugs. Plants 2020, 9, 1562. [Google Scholar] [CrossRef] [PubMed]
- Mitra, S.K.; Irenaeus, T.K.S.; Gurung, M.R.; Pathak, P.K. Taxonomy and Importance of Myrtaceae. Acta Horticulturae. 2012, 959, 23–34. [Google Scholar] [CrossRef]
- Kew, R.B.G. Myrcianthes discolor (Kunth) McVaugh. In Plants of the World Online [Database]; Royal Botanic Gardens, Kew: Richmond, UK, 2022; Available online: https://powo.science.kew.org/taxon/urn:lsid:ipni.org:names:166084-2 (accessed on 22 March 2023).
- Bussmann, R.W.; Glenn, A.; Douglas, S. Antibacterial activity of medicinal plants of Northern Perú—Can traditional aplications provide leads for modern science? Indian J. Tradit. Knowl. 2010, 9, 742–753. [Google Scholar]
- De la Torre, L.; Navarrete, H.; Muriel, M.P.; Macía, M.; Balslev, H. Enciclopedia de las Plantas Útiles del Ecuador; Herbario QCA de la Escuela de Ciencias Biológicas de la Pontificia Universidad Católica del Ecuador & Herbario AAU del Departamento de Ciencias Biológicas de la Universidad de Aarhus: Quito, Ecuador, 2008; ISBN 9789978771358. [Google Scholar]
- Adams, R. Identification of Essential Oil Components by Gas Chromatography_mass Spectrometry, 4th ed.; Allured Publishing Corporation: Carol Stream, IL, USA, 2007. [Google Scholar]
- Armijos, C.; Valarezo, E.; Cartuche, L.; Zaragoza, T.; Finzi, P.; Mellerio, G.; Vidari, G. Chemical Composition and Antimicrobial Activity of Myrcianthes fragrans Essential Oil, a Natural Aromatizer of the Traditional Ecuadorian Beverage Colada Morada. J. Ethnopharmacol. 2018, 225, 319–326. [Google Scholar] [CrossRef] [PubMed]
- Almeida, R.; Line, H.; Wanessa, D.C.; Araújo, C.; Iecher, M.; Gonçalves, J.; Barros, N.; Cristiani, Z.; Linde, G.A. Antioxidant and Antibacterial Activity of Myrcianthes pungens Leaf Essential Oil. Bol. Latinoam. Y Caribe Plantas Med. Y Aromat. 2021, 20, 147–161. [Google Scholar] [CrossRef]
- Montalván, M.; Peñafiel, M.A.; Ramírez, J.; Cumbicus, N.; Bec, N.; Larroque, C.; Bicchi, C.; Gilardoni, G. Chemical Composition, Enantiomeric Distribution, and Sensory Evaluation of the Essential Oils Distilled from the Ecuadorian Species Myrcianthes myrsinoides (Kunth) Grifo and Myrcia mollis (Kunth) DC. (Myrtaceae). Plants 2019, 8, 511. [Google Scholar] [CrossRef]
- Ospina, L.M.P.; Muñoz, P.B.; Matulevich, J.; Teherán, A.A.; Villamizar, L.B. Composition and Antimicrobial Activity of the Essential Oils of Three Plant Species from the Sabana of Bogota (Colombia): Myrcianthes leucoxyla, Vallea stipularis and Phyllanthus salviifolius. Nat. Prod. Commun. 2016, 11, 1913–1918. [Google Scholar] [CrossRef]
- Malagón, O.; Vila, R.; Iglesias, J.; Zaragoza, T.; Cañigueral, S. Composition of the Essential Oils of Four Medicinal Plants from Ecuador. Flavour Fragr. J. 2003, 18, 527–531. [Google Scholar] [CrossRef]
- Barra, A. Factors Affecting Chemical Variability of Essential Oils: A Review of Recent Developments. Nat. Prod. Commun. 2009, 4, 1147–1154. [Google Scholar] [CrossRef]
- Gupta, A.; Ganjewala, D. A Study on Developmental Changes in Essential Oil Content and Composition in Cymbopogon flexuosus Cultivar Suvarna. Acta Biol. Szeged. 2015, 59, 119–125. [Google Scholar]
- Lorenzo, D.; Dellacassa, E.; Bonaccorsi, I.; Mondello, L. Uruguayan Essential Oils. Composition of Leaf Oil of Myrcianthes cisplatensis (Camb.) Berg. (‘Guayabo Colorado’) (Myrtaceae). Flavour Fragr. J. 2001, 16, 97–99. [Google Scholar] [CrossRef]
- Lopez, J.; Jean, F.; Gagnon, H.; Collin, G.; Garneau, F.; Pichette, A. Essential Oils from Bolivia. VII. Myrtaceae: Myrcianthes osteomeloides (Rusby) McVaugh and Myrcianthes pseudomato (Legrand) McVaugh. J. Essent. Oil Res. 2011, 17, 64–65. [Google Scholar] [CrossRef]
- Fidyt, K.; Fiedorowicz, A.; Strządała, L.; Szumny, A. β-caryophyllene and β-caryophyllene oxide-natural compounds of anticancer and analgesic properties. Cancer Med. 2016, 5, 007–3017. [Google Scholar] [CrossRef]
- Pinho-da-Silva, L.; Mendes-Maia, P.V.; Teófilo, T.M.; Barbosa, R.; Ceccatto, V.M.; Coelho-de-Souza, A.N.; Santos Cruz, J.; Leal-Cardoso, J.H. trans-Caryophyllene, a Natural Sesquiterpene, Causes Tracheal Smooth Muscle Relaxation Through Blockade of Voltage-Dependent Ca2⁺ Channels. Molecules 2012, 17, 11965–11977. [Google Scholar] [CrossRef]
- Dahham, S.S.; Tabana, Y.M.; Iqbal, M.A.; Ahamed, M.B.; Ezzat, M.O.; Majid, A.S.; Majid, A.M. The Anticancer, Antioxidant and Antimicrobial Properties of the Sesquiterpene β-Caryophyllene from the Essential Oil of Aquilaria crassna. Molecules 2015, 20, 11808–11829. [Google Scholar] [CrossRef] [PubMed]
- Bonesi, M.; Menichini, F.; Tundis, R.; Loizzo, M.R.; Conforti, F.; Passalacqua, N.G.; Statti, G.A.; Menichini, F. Acetylcholinesterase and butyrylcholinesterase inhibitory activity of Pinus species essential oils and their constituents. J. Enzym. Inhib. Med. Chem. 2010, 25, 622–628. [Google Scholar] [CrossRef]
- Araujo, L.; Rondón, M.; Morillo, A.; Páez, E.; Rojas-Fermín, L. Antimicrobial Activity of the Essential Oil of Myrcianthes myrcinoides (Kunth) Grifo (Myrtaceae) Collected in the Venezuelan Andes. Pharmacol. OnLine 2017, 2, 200–204. [Google Scholar]
- Van Vuuren, S.; Holl, D. Antimicrobial natural product research: A review from a South African perspective for the years 2009–2016. J. Ethnopharmacol. 2017, 208, 236–252. [Google Scholar] [CrossRef]
- Selestino, N.; Vittorazzi, C.; Guimarães, A.; Fronza, M.; Coutinho, D.; Scherer, R. Pharmaceutical Biology Effects of β-Caryophyllene and Murraya Paniculata Essential Oil in the Murine Hepatoma Cells and in the Bacteria and Fungi 24-h Time-Kill Curve Studies. Phamaceutical Biol. 2016, 55, 190–197. [Google Scholar] [CrossRef]
- Hung, N.H.; Quan, P.M.; Satyal, P.; Dai, D.N.; Hoa, V.V.; Huy, N.G.; Giang, L.D.; Ha, N.T.; Huong, L.T.; Hien, V.T.; et al. Acetylcholinesterase Inhibitory Activities of Essential Oils from Vietnamese Traditional Medicinal Plants. Molecules 2022, 27, 7092. [Google Scholar] [CrossRef]
- Cartuche, L.; Calva, J.; Valarezo, E.; Chuchuca, N.; Morocho, V. Chemical and Biological Activity Profiling of Hedyosmum strigosum Todzia Essential Oil, an Aromatic Native Shrub from Southern Ecuador. Plants 2022, 11, 2832. [Google Scholar] [CrossRef] [PubMed]
- Andrade, J.M.; Pachar, P.; Trujillo, L.; Cartuche, L. Suillin: A Mixed-Type Acetylcholinesterase Inhibitor from Suillus luteus Which Is Used by Saraguros Indigenous, Southern Ecuador. PLoS ONE 2022, 17, 1–13. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).