Antifungal and Antibiofilm Activity of Colombian Essential Oils against Different Candida Strains
Abstract
1. Introduction
2. Results
2.1. Essential Oils
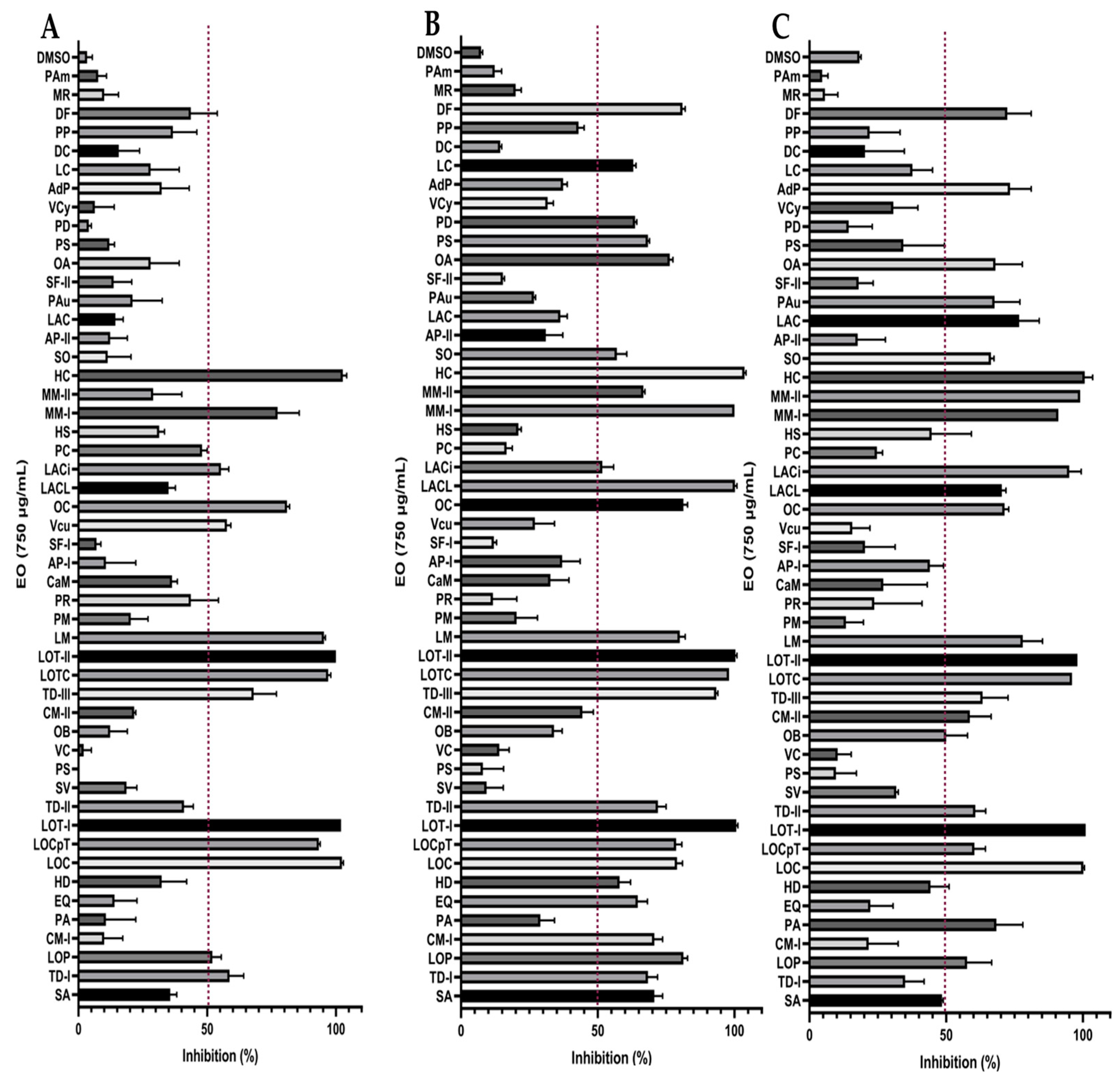
2.2. Determination of Antifungal Activity
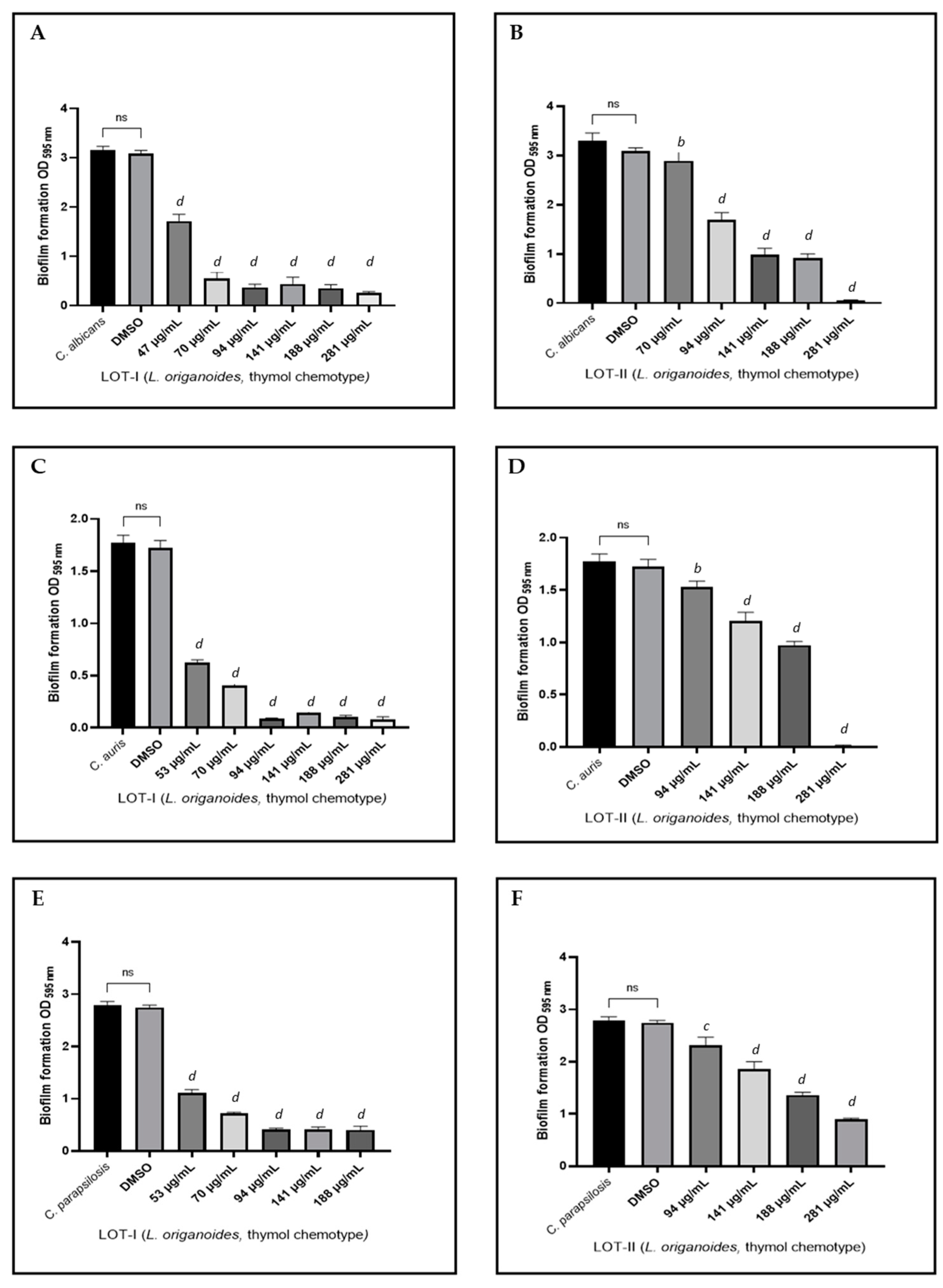
2.3. Effect on Biofilm Formation
2.4. Scanning Electron Microscope (SEM) Analysis
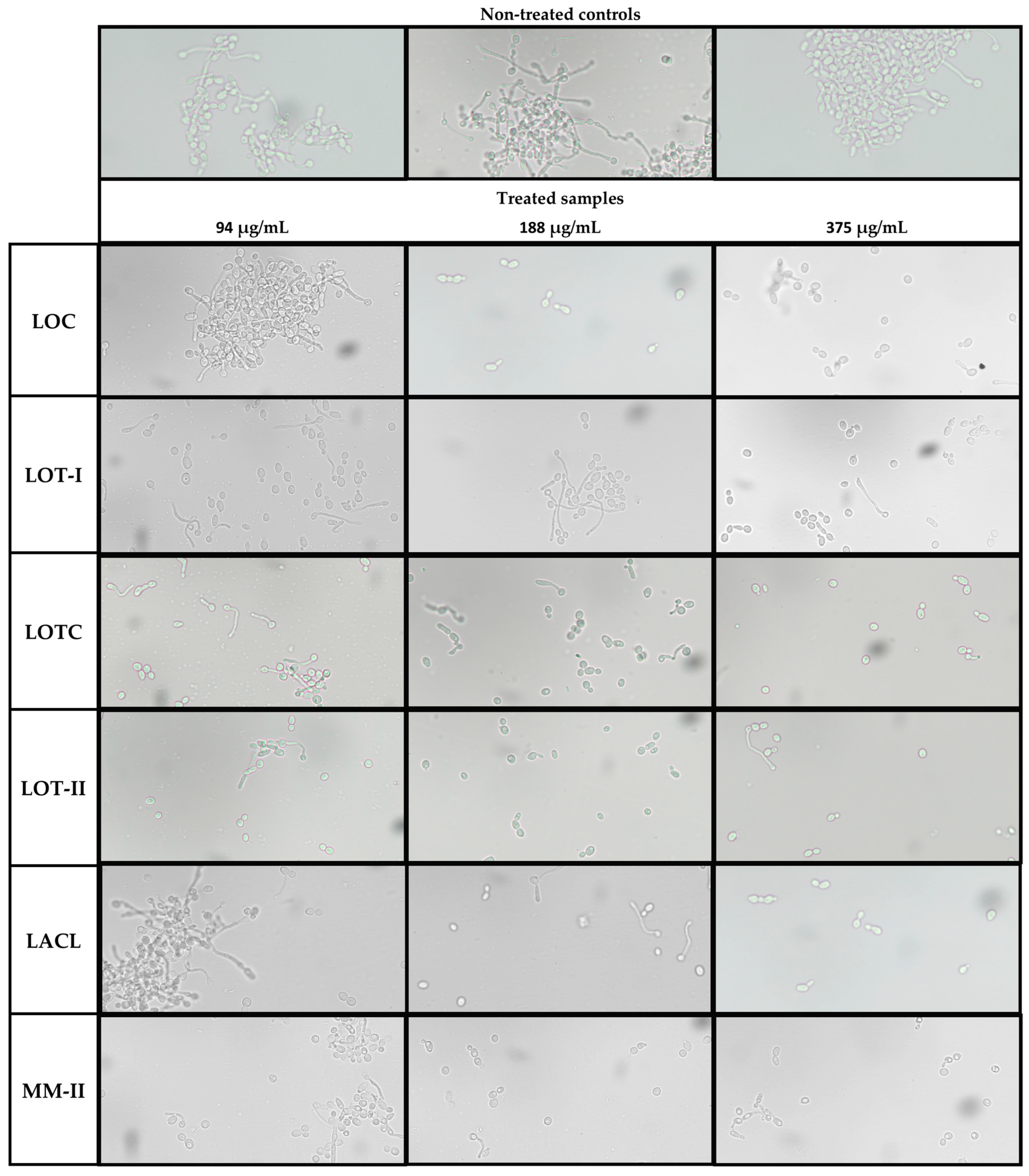
2.5. Inhibition of Hyphal Formation in C. albicans
3. Discussion
4. Materials and Methods
4.1. Plant Material
4.2. Essential Oils
4.3. Microorganisms
4.4. Determination of Antifungal Activity of EOs
4.5. Effect of EOs on Biofilm Formation
4.6. Scanning Electron Microscope Analysis
4.7. Inhibition of Hyphal Formation in C. albicans by EOs
4.8. Data Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Guinea, J. Global Trends in the Distribution of Candida Species Causing Candidemia. Clin. Microbiol. Infect. 2014, 20, 5–10. [Google Scholar] [CrossRef] [PubMed]
- CDC Antibiotic Resistance Threats in the United States, 2019; Centers for Disease Control and Prevention: Atlanta, GA, USA, 2019.
- Fan, F.; Liu, Y.; Liu, Y.; Lv, R.; Sun, W.; Ding, W.; Cai, Y.; Li, W.; Liu, X.; Qu, W. Candida albicans Biofilms: Antifungal Resistance, Immune Evasion, and Emerging Therapeutic Strategies. Int. J. Antimicrob. Agents 2022, 60, 106673. [Google Scholar] [CrossRef]
- World Health Organization (WHO). WHO Fungal Priority Pathogens List to Guide Research, Development and Public Health Action; WHO: Geneva, Switzerland, 2022. [Google Scholar]
- del Pozo, J.L. Biofilm-Related Disease. Expert Rev. Anti-Infect. Ther. 2018, 16, 51–65. [Google Scholar] [CrossRef] [PubMed]
- Tumbarello, M.; Posteraro, B.; Trecarichi, E.M.; Fiori, B.; Rossi, M.; Porta, R.; de Gaetano Donati, K.; la Sorda, M.; Spanu, T.; Fadda, G.; et al. Biofilm Production by Candida Species and Inadequate Antifungal Therapy as Predictors of Mortality for Patients with Candidemia. J. Clin. Microbiol. 2007, 45, 1843–1850. [Google Scholar] [CrossRef] [PubMed]
- Dominguez, E.G.; Andes, D.R. Candida Biofilm Tolerance: Comparison of Planktonic and Biofilm Resistance Mechanisms. In Candida albicans: Cellular and Molecular Biology; Springer International Publishing: Cham, Switzerland, 2017; pp. 77–92. [Google Scholar]
- Chen, H.; Zhou, X.; Ren, B.; Cheng, L. The Regulation of Hyphae Growth in Candida albicans. Virulence 2020, 11, 337–348. [Google Scholar] [CrossRef]
- Silva, S.; Negri, M.; Henriques, M.; Oliveira, R.; Williams, D.W.; Azeredo, J. Adherence and Biofilm Formation of Non-Candida albicans Candida Species. Trends Microbiol. 2011, 19, 241–247. [Google Scholar] [CrossRef]
- Powers, C.; Osier, J.; McFeeters, R.; Brazell, C.; Olsen, E.; Moriarity, D.; Satyal, P.; Setzer, W. Antifungal and Cytotoxic Activities of Sixty Commercially-Available Essential Oils. Molecules 2018, 23, 1549. [Google Scholar] [CrossRef]
- Ribeiro, R.; Fernandes, L.; Costa, R.; Cavaleiro, C.; Salgueiro, L.; Henriques, M.; Rodrigues, M.E. Comparing the Effect of Thymus spp. Essential Oils on Candida auris. Ind. Crops Prod. 2022, 178, 114667. [Google Scholar] [CrossRef]
- Córdoba, S.; Vivot, W.; Szusz, W.; Albo, G. Antifungal Activity of Essential Oils Against Candida Species Isolated from Clinical Samples. Mycopathologia 2019, 184, 615–623. [Google Scholar] [CrossRef]
- Imane, N.I.; Fouzia, H.; Azzahra, L.F.; Ahmed, E.; Ismail, G.; Idrissa, D.; Mohamed, K.H.; Sirine, F.; L’Houcine, O.; Noureddine, B. Chemical Composition, Antibacterial and Antioxidant Activities of Some Essential Oils against Multidrug Resistant Bacteria. Eur. J. Integr. Med. 2020, 35, 101074. [Google Scholar] [CrossRef]
- Sounouvou, H.T.; Toukourou, H.; Catteau, L.; Toukourou, F.; Evrard, B.; van Bambeke, F.; Gbaguidi, F.; Quetin-Leclercq, J. Antimicrobial Potentials of Essential Oils Extracted from West African Aromatic Plants on Common Skin Infections. Sci. Afr. 2021, 11, e00706. [Google Scholar] [CrossRef]
- Sobrinho, A.C.N.; de Souza, E.B.; Rocha, M.F.G.; Albuquerque, M.R.J.R.; Bandeira, P.N.; dos Santos, H.S.; de Paula Cavalcante, C.S.; Oliveira, S.S.; Aragão, P.R.; de Morais, S.M.; et al. Chemical Composition, Antioxidant, Antifungal and Hemolytic Activities of Essential Oil from Baccharis trinervis (Lam.) Pers. (Asteraceae). Ind. Crops Prod. 2016, 84, 108–115. [Google Scholar] [CrossRef]
- Nazzaro, F.; Fratianni, F.; Coppola, R.; de Feo, V. Essential Oils and Antifungal Activity. Pharmaceuticals 2017, 10, 86. [Google Scholar] [CrossRef] [PubMed]
- Stashenko, E.; René Martínez, J. Study of Essential Oils Obtained from Tropical Plants Grown in Colombia. In Essential Oils—Oils of Nature; IntechOpen: London, UK, 2020. [Google Scholar]
- Cáceres, M.; Hidalgo, W.; Stashenko, E.; Torres, R.; Ortiz, C. Essential Oils of Aromatic Plants with Antibacterial, Anti-Biofilm and Anti-Quorum Sensing Activities against Pathogenic Bacteria. Antibiotics 2020, 9, 147. [Google Scholar] [CrossRef]
- Gómez-Sequeda, N.; Cáceres, M.; Stashenko, E.E.; Hidalgo, W.; Ortiz, C. Antimicrobial and Antibiofilm Activities of Essential Oils against Escherichia coli O157:H7 and Methicillin-Resistant Staphylococcus aureus (MRSA). Antibiotics 2020, 9, 730. [Google Scholar] [CrossRef] [PubMed]
- Martínez, A.; Manrique-Moreno, M.; Klaiss-Luna, M.C.; Stashenko, E.; Zafra, G.; Ortiz, C. Effect of Essential Oils on Growth Inhibition, Biofilm Formation and Membrane Integrity of Escherichia coli and Staphylococcus aureus. Antibiotics 2021, 10, 1474. [Google Scholar] [CrossRef]
- Zapata-Zapata, C.; Loaiza-Oliva, M.; Martínez-Pabón, M.C.; Stashenko, E.E.; Mesa-Arango, A.C. In Vitro Activity of Essential Oils Distilled from Colombian Plants against Candida auris and Other Candida Species with Different Antifungal Susceptibility Profiles. Molecules 2022, 27, 6837. [Google Scholar] [CrossRef]
- Ambrosio, C.M.S.; Ikeda, N.Y.; Miano, A.C.; Saldaña, E.; Moreno, A.M.; Stashenko, E.; Contreras-Castillo, C.J.; da Gloria, E.M. Unraveling the Selective Antibacterial Activity and Chemical Composition of Citrus Essential Oils. Sci. Rep. 2019, 9, 17719. [Google Scholar] [CrossRef] [PubMed]
- Arendrup, M.C.; Patterson, T.F. Multidrug-Resistant Candida: Epidemiology, Molecular Mechanisms, and Treatment. J. Infect. Dis. 2017, 216, S445–S451. [Google Scholar] [CrossRef]
- Macias-Paz, I.U.; Pérez-Hernández, S.; Tavera-Tapia, A.; Luna-Arias, J.P.; Guerra-Cárdenas, J.E.; Reyna-Beltrán, E. Candida albicans the Main Opportunistic Pathogenic Fungus in Humans. Rev. Argent. Microbiol. 2022, in press. [Google Scholar] [CrossRef]
- Tsui, C.; Kong, E.F.; Jabra-Rizk, M.A. Pathogenesis of Candida albicans Biofilm. Pathog. Dis. 2016, 74, ftw018. [Google Scholar] [CrossRef] [PubMed]
- Panáček, A.; Kolář, M.; Večeřová, R.; Prucek, R.; Soukupová, J.; Kryštof, V.; Hamal, P.; Zbořil, R.; Kvítek, L. Antifungal Activity of Silver Nanoparticles against Candida spp. Biomaterials 2009, 30, 6333–6340. [Google Scholar] [CrossRef] [PubMed]
- Zottich, U.; de Oliveira, I.S.; Fereira, I.G.; Cerni, F.A.; Karla de Castro Figueiredo, B.; Arantes, E.C.; Gomes, V.M.; Dias, G.B.; Pucca, M.B. Antifungal Activity of Rhopalurus crassicauda Venom against Candida spp. Toxicon X 2022, 14, 100120. [Google Scholar] [CrossRef] [PubMed]
- Sebaa, S.; Boucherit-Otmani, Z.; Courtois, P. Effects of Tyrosol and Farnesol on Candida albicans Biofilm. Mol. Med. Rep. 2019, 19, 3201–3209. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Kim, Y.G.; Lee, J.H. Inhibition of Candida albicans Biofilm and Hyphae Formation by Biocompatible Oligomers. Lett. Appl. Microbiol. 2018, 67, 123–129. [Google Scholar] [CrossRef] [PubMed]
- Patel, M.; Srivastava, V.; Ahmad, A. Dodonaea Viscosa Var Angustifolia Derived 5,6,8-Trihydroxy-7,4′ Dimethoxy Flavone Inhibits Ergosterol Synthesis and the Production of Hyphae and Biofilm in Candida albicans. J. Ethnopharmacol. 2020, 259, 112965. [Google Scholar] [CrossRef]
- Mangalagiri, N.P.; Panditi, S.K.; Jeevigunta, N.L.L. Antimicrobial Activity of Essential Plant Oils and Their Major Components. Heliyon 2021, 7, e06835. [Google Scholar] [CrossRef]
- Puvača, N.; Milenković, J.; Galonja Coghill, T.; Bursić, V.; Petrović, A.; Tanasković, S.; Pelić, M.; Ljubojević Pelić, D.; Miljković, T. Antimicrobial Activity of Selected Essential Oils against Selected Pathogenic Bacteria: In Vitro Study. Antibiotics 2021, 10, 546. [Google Scholar] [CrossRef]
- Farisa Banu, S.; Rubini, D.; Shanmugavelan, P.; Murugan, R.; Gowrishankar, S.; Karutha Pandian, S.; Nithyanand, P. Effects of Patchouli and Cinnamon Essential Oils on Biofilm and Hyphae Formation by Candida Species. J. Mycol. Med. 2018, 28, 332–339. [Google Scholar] [CrossRef]
- Barbiéri Holetz, F.; Lorena Pessini, G.; Rogério Sanches, N.; Aparício Garcia Cortez, D.; Vataru Nakamura, C.; Prado Dias Filho, B. Screening of Some Plants Used in the Brazilian Folk Medicine for the Treatment of Infectious Diseases. Mem. Inst. Oswaldo Cruz 2002, 97, 1027–1031. [Google Scholar] [CrossRef]
- León-Méndez, G.; Pájaro-Castro, N.; Pájaro-Castro, E.; Torrenegra- Alarcón, M.; Herrera-Barros, A. Essential Oils as a Source of Bioactive Molecules. Rev. Colomb. Cienc. Químico-Farm. 2019, 48, 80–93. [Google Scholar] [CrossRef]
- Nostro, A.; Roccaro, A.S.; Bisignano, G.; Marino, A.; Cannatelli, M.A.; Pizzimenti, F.C.; Cioni, P.L.; Procopio, F.; Blanco, A.R. Effects of Oregano, Carvacrol and Thymol on Staphylococcus aureus and Staphylococcus epidermidis Biofilms. J. Med. Microbiol. 2007, 56, 519–523. [Google Scholar] [CrossRef]
- Jafri, H.; Ahmad, I. Thymus vulgaris Essential Oil and Thymol Inhibit Biofilms and Interact Synergistically with Antifungal Drugs against Drug Resistant Strains of Candida albicans and Candida tropicalis. J. Mycol. Med. 2020, 30, 100911. [Google Scholar] [CrossRef]
- Belato, K.K.; de Oliveira, J.R.; de Oliveira, F.S.; de Oliveira, L.D.; Camargo, S.E.A. Cytotoxicity and Genotoxicity of Thymol Verified in Murine Macrophages (RAW 264.7) after Antimicrobial Analysis in Candida albicans, Staphylococcus aureus, and Streptococcus mutans. J. Funct. Foods 2018, 40, 455–460. [Google Scholar] [CrossRef]
- Miladi, H.; Zmantar, T.; Kouidhi, B.; al Qurashi, Y.M.A.; Bakhrouf, A.; Chaabouni, Y.; Mahdouani, K.; Chaieb, K. Synergistic Effect of Eugenol, Carvacrol, Thymol, p-Cymene and γ-Terpinene on Inhibition of Drug Resistance and Biofilm Formation of Oral Bacteria. Microb. Pathog. 2017, 112, 156–163. [Google Scholar] [CrossRef]
- Rathod, N.B.; Kulawik, P.; Ozogul, F.; Regenstein, J.M.; Ozogul, Y. Biological Activity of Plant-Based Carvacrol and Thymol and Their Impact on Human Health and Food Quality. Trends Food Sci. Technol. 2021, 116, 733–748. [Google Scholar] [CrossRef]
- Balahbib, A.; el Omari, N.; el Hachlafi, N.; Lakhdar, F.; el Menyiy, N.; Salhi, N.; Mrabti, H.N.; Bakrim, S.; Zengin, G.; Bouyahya, A. Health Beneficial and Pharmacological Properties of P-Cymene. Food Chem. Toxicol. 2021, 153, 112259. [Google Scholar] [CrossRef]
- Vasconcelos, S.E.C.B.; Melo, H.M.; Cavalcante, T.T.A.; Júnior, F.E.A.C.; de Carvalho, M.G.; Menezes, F.G.R.; de Sousa, O.V.; Costa, R.A. Plectranthus Amboinicus Essential Oil and Carvacrol Bioactive against Planktonic and Biofilm of Oxacillin- and Vancomycin-Resistant Staphylococcus Aureus. BMC Complement. Altern. Med. 2017, 17, 462. [Google Scholar] [CrossRef]
- Gucwa, K.; Milewski, S.; Dymerski, T.; Szweda, P. Investigation of the Antifungal Activity and Mode of Action of Thymus vulgaris, Citrus limonum, Pelargonium graveolens, Cinnamomum cassia, Ocimum basilicum, and Eugenia caryophyllus Essential Oils. Molecules 2018, 23, 1116. [Google Scholar] [CrossRef] [PubMed]
- Karpiński, T.M.; Ożarowski, M.; Seremak-Mrozikiewicz, A.; Wolski, H.; Adamczak, A. Plant Preparations and Compounds with Activities against Biofilms Formed by Candida spp. J. Fungi 2021, 7, 360. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, L.; Ribeiro, R.; Costa, R.; Henriques, M.; Rodrigues, M.E. Essential Oils as a Good Weapon against Drug-Resistant Candida auris. Antibiotics 2022, 11, 977. [Google Scholar] [CrossRef] [PubMed]
- Parker, R.A.; Gabriel, K.T.; Graham, K.D.; Butts, B.K.; Cornelison, C.T. Antifungal Activity of Select Essential Oils against Candida auris and Their Interactions with Antifungal Drugs. Pathogens 2022, 11, 821. [Google Scholar] [CrossRef]
- Choonharuangdej, S.; Srithavaj, T.; Thummawanit, S. Fungicidal and Inhibitory Efficacy of Cinnamon and Lemongrass Essential Oils on Candida albicans Biofilm Established on Acrylic Resin: An In Vitro Study. J. Prosthet. Dent. 2021, 125, 707.e1–707.e6. [Google Scholar] [CrossRef]
- Jackson, S.; Coulthwaite, L.; Loewy, Z.; Scallan, A.; Verran, J. Biofilm Development by Blastospores and Hyphae of Candida albicans on Abraded Denture Acrylic Resin Surfaces. J. Prosthet. Dent. 2014, 112, 988–993. [Google Scholar] [CrossRef]
- Agarwalla, S.V.; Ellepola, K.; Silikas, N.; Castro Neto, A.H.; Seneviratne, C.J.; Rosa, V. Persistent Inhibition of Candida albicans Biofilm and Hyphae Growth on Titanium by Graphene Nanocoating. Dent. Mater. 2021, 37, 370–377. [Google Scholar] [CrossRef] [PubMed]
- Duque, J.E.; Urbina, D.L.; Vesga, L.C.; Ortiz-Rodríguez, L.A.; Vanegas, T.S.; Stashenko, E.E.; Mendez-Sanchez, S.C. Insecticidal Activity of Essential Oils from American Native Plants against Aedes Aegypti (Diptera: Culicidae): An Introduction to Their Possible Mechanism of Action. Sci. Rep. 2023, 13, 2989. [Google Scholar] [CrossRef] [PubMed]
- Shirazi, M.T.; Gholami, H.; Kavoosi, G.; Rowshan, V.; Tafsiry, A. Chemical Composition, Antioxidant, Antimicrobial and Cytotoxic Activities of Tagetes minuta and Ocimum basilicum Essential Oils. Food Sci. Nutr. 2014, 2, 146–155. [Google Scholar] [CrossRef]
- Chen, Y.; Luo, Q.; Li, S.; Li, C.; Liao, S.; Yang, X.; Zhou, R.; Zhu, Y.; Teng, L.; Chen, H.; et al. Antiviral Activity against Porcine Epidemic Diarrhea Virus of Pogostemon cablin Polysaccharide. J. Ethnopharmacol. 2020, 259, 113009. [Google Scholar] [CrossRef]
- M27-A2-CLSI; Reference Method for Broth Dilution Antifungal Susceptibility Testing of Yeasts. Clinical Laboratory Standards Institute: Berwyn, PA, USA, 2002.
- Arendrup, M.C.; Meletiadis, J.; Mouton, J.W.; Lagrou, K.; Hamal, P.; Guinea, J. EUCAST-AFST Method for the Determination of Broth Dilution Minimum Inhibitory Concentrations of Antifungal Agents for Yeasts. In EUCAST Antifungal MIC Method for Yeasts; V 7.3.2; EUCAST: Copenhagen, Denmark, 2020; pp. 1–21. Available online: https://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/AFST/Files/EUCAST_E_Def_7.3.2_Yeast_testing_definitive_revised_2020.pdf (accessed on 23 February 2023).
- Pierce, C.G.; Uppuluri, P.; Tristan, A.R.; Wormley, F.L.; Mowat, E.; Ramage, G.; Lopez-Ribot, J.L. A Simple and Reproducible 96-Well Plate-Based Method for the Formation of Fungal Biofilms and Its Application to Antifungal Susceptibility Testing. Nat. Protoc. 2008, 3, 1494–1500. [Google Scholar] [CrossRef]
- O’Toole, G.A. Microtiter Dish Biofilm Formation Assay. J. Vis. Exp. 2011, e2437. [Google Scholar] [CrossRef]
- Haque, F.; Alfatah, M.; Ganesan, K.; Bhattacharyya, M.S. Inhibitory Effect of Sophorolipid on Candida albicans Biofilm Formation and Hyphal Growth. Sci. Rep. 2016, 6, 23575. [Google Scholar] [CrossRef]
- Tsang, P.W.K.; Bandara, H.M.H.N.; Fong, W.P. Purpurin Suppresses Candida albicans Biofilm Formation and Hyphal Development. PLoS ONE 2012, 7, e50866. [Google Scholar] [CrossRef] [PubMed]
- Akoglu, H. User’s Guide to Correlation Coefficients. Turk. J. Emerg. Med. 2018, 18, 91–93. [Google Scholar] [CrossRef] [PubMed]

| Plant Code | Plant Species | Vouche Number | Major EO Compounds |
|---|---|---|---|
| TD-I | Turnera diffusa | UIS Herbarium 22037 | Dehydrofukinone (25.4%), aristolochene (17.9%), valencene (7.4%), β-selinene (5.2%), trans-β-caryophyllene (4.0%), β-elemene (4.0%), premnaspirodiene (3.7%), guaiol (3.5%), germacra-4,5,10-trien-1-α-ol (3.5%), and caryophyllene oxide (3.3%). |
| EQ | Elaphandra quinquenervis | COL 587094 | Germacrene D (20.7%), α-phellandrene (9.1%), α-pinene (6.8%), trans-β-caryophyllene (5.1%), Δ3-carene (4.9%), limonene (4.5%), β-cubebene (3.5%), α-humulene (2.6%), premnaspirodiene (2.6%), and δ-cadinene (2.6%). |
| LOC | Lippia origanoides, carvacrol chemotype | UIS Herbarium 22034 | Carvacrol (35%), p-cymene (14.4%), thymol (8.0%), γ-terpinene (5.3%), trans-β-caryophyllene (4.4%), β-myrcene (2.4%), carvacryl acetate (2.0%), thymyl methyl ether (1.9%), and α-terpinene (1.7%). |
| LOCpT | Lippia origanoides, β-Caryophyllene-thymol chemotype | UIS Herbarium 22035 | trans-β-Caryophyllene (15.1%), thymol (14%), 1,8-cineole (13%), p-cymene (12.6%), α-humulene (8.1%), α-phellandrene (7.1%), α-eudesmol (2.6%), caryophyllene oxide (2.5%), γ-terpinene (2.4%), and limonene (2.1%). |
| LOT-I | Lippia origanoides, thymol chemotype | COL 587107 | Thymol (75.3%), trans-β-caryophyllene (5.4%), carvacrol (4.9%), α-humulene (3.2%), p-cymene (2.3%), thymyl acetate (1.6%), thymyl methyl ether (1.3%), caryophyllene oxide (1.3%), and trans-β-bergamotene (1.0%). |
| TD-III | Turnera diffusa | Herbarium UIS 22037 | Aristolochene (20.6%), dehydrofukinone (17.3%), p-cymene (5.8%), β-selinene (5.6%), valencene (5.2%), premnaspirodiene (4.2%), caryophyllene oxide (3.6%), trans-β-caryophyllene (2.8%), germacra-4,5,10-trien-1-α-ol (2.4%), and α-selinene. |
| LOTC | Lippia origanoides, thymol-p-cymene chemotype | Herbarium UIS 22039 | Thymol (49.4%), p-cymene (19.1%), γ-terpinene (9.2%), β-myrcene (5.2%), α-terpinene (2.9%), carvacrol (2.7%), thymyl methyl ether (1.8%), trans-β-caryophyllene (1.6%), cis-β-ocimene (1.2%), and limonene (0.9%). |
| LOT-II | Lippia origanoides, thymol chemotype | Herbarium UIS 22036 | Thymol (71.7%), p-cymene (10.5%), carvacrol (4.4%), β-myrcene (2.1%), γ-terpinene (2.0%), caryophyllene oxide (1.6%), thymyl methyl ether (0.9%), trans-β-caryophyllene (0.9%), humulene epoxide II (0.7%), and terpinen-4-ol (0.7%). |
| LM | Lippia micromera | COL 560986 | p-Cymene (26.8%), thymyl methyl ether (26.3%), thymol (17.8%), thymyl acetate (5.7%), γ-terpinene (5.4%), 1,8-cineole (5.1%), α-terpinene (2.0%), β-myrcene (2.0%), trans-β-caryophyllene (1.7%), α-thujene (1.3%), and caryophyllene oxide (0.9%). |
| OC | Ocimum campechianum | UIS Herbarium 20889 | Eugenol (35.3%), 1,8-cineole (15.6%), β-selinene (11.0%), trans-β-caryophyllene (7.4%), germacrene D (5.6%), α-selinene (4.8%), β-pinene (2.4%), β-elemene (1.9%), and α-humulene (1.5%). |
| LACL | Lippia alba, carvone-limonene chemotype | UIS Herbarium 22031 | Limonene (40.1%), carvone (37.7%), germacrene D (8.1%), β-bourbonene (3.0%), piperitone (1.9%), β-myrcene (0.9%), piperitenone (0.8%), linalool (0.7%), borneol (0.7%), and trans-β-farnesene (0.7%). |
| LACi | Lippia alba, citral chemotype | UIS Herbarium 22032 | Geranial (24.5%), geraniol (19.0%), neral (11.9%), trans-β-caryophyllene (9.1%), germacrene D (4.3%), geranyl acetate (2.8%), α-humulene (2.8%), β-elemene (2.6%), nerol (2.5%), and limonene (2.4%). |
| MM-I | Minthostachys mollis (Benth.) Griseb. | UIS Herbarium 22041 | trans-Piperitone oxide (49.6%), menthone (8.9%), piperitenone oxide (4.8%), trans-β-caryophyllene (4.0%), limonene (3.3%), thymol (2.3%), 6-hydroxycarvotanacetone (2.3%), germacrene D (2.1%), β-pinene (2.0%), linalool (1.9%), and pulegone (1.7%). |
| MM-II | Minthostachys mollis (Benth.) Griseb. | UIS Herbarium 22042 | Menthone (46.1%), pulegone (13.3%), piperitone (12.1%), trans-β-caryophyllene (7.0%), germacrene D (3.8%), iso-menthone (3.5%), bicyclogermacrene (3.4%), α-humulene (1.9%), α-pinene (1.3%), and β-pinene (1.2%). |
| EO | C. albicans ATCC 10231 | C. parapsilosis ATCC 22019 | C. auris CDC B11903 | |||
|---|---|---|---|---|---|---|
| MIC50 (µg/mL) | MFC (µg/mL) | MIC50 (µg/mL) | MFC (µg/mL) | MIC50 (µg/mL) | MFC (µg/mL) | |
| EQ | NA | NA | 375 | NA | NA | NA |
| LOC | 375 | NA | 281 | NA | 281 | NA |
| LOCpT | 563 | 750 | 188 | NA | 750 | NA |
| LOT-I | 281 | 750 | 188 | 750 | 188 | 563 |
| LOTC | 188 | NA | 281 | NA | 188 | 375 |
| LOT-II | 188 | 563 | 141 | 563 | 141 | 375 |
| LM | 750 | NA | 188 | NA | 750 | NA |
| OC | 563 | NA | 563 | NA | 563 | NA |
| LACL | 750 | NA | 750 | NA | 188 | 563 |
| LACi | 750 | NA | 750 | NA | 563 | NA |
| MM-I | 750 | NA | 375 | 750 | 375 | NA |
| HC | 563 | NA | 375 | 750 | 375 | NA |
| Essential Oil | C. albicans ATCC 10231 | C. parapsilosis ATCC 22019 | C. auris CDC B11903 |
|---|---|---|---|
| MBIC50 (µg/mL) | MBIC50 (µg/mL) | MBIC50 (µg/mL) | |
| TD-I | NA | 750 | NA |
| LOC | 281 | 188 | NA |
| LOCpT | 375 | 281 | NA |
| LOT-I | 53 | 53 | 53 |
| CM-II | NA | 750 | NA |
| TD-III | NA | 750 | NA |
| LOTC | 94 | 141 | 141 |
| LOT-II | 188 | 188 | 141 |
| LACL | NA | 750 | NA |
| MM-II | NA | NA | 188 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ruiz-Duran, J.; Torres, R.; Stashenko, E.E.; Ortiz, C. Antifungal and Antibiofilm Activity of Colombian Essential Oils against Different Candida Strains. Antibiotics 2023, 12, 668. https://doi.org/10.3390/antibiotics12040668
Ruiz-Duran J, Torres R, Stashenko EE, Ortiz C. Antifungal and Antibiofilm Activity of Colombian Essential Oils against Different Candida Strains. Antibiotics. 2023; 12(4):668. https://doi.org/10.3390/antibiotics12040668
Chicago/Turabian StyleRuiz-Duran, Jennifer, Rodrigo Torres, Elena E. Stashenko, and Claudia Ortiz. 2023. "Antifungal and Antibiofilm Activity of Colombian Essential Oils against Different Candida Strains" Antibiotics 12, no. 4: 668. https://doi.org/10.3390/antibiotics12040668
APA StyleRuiz-Duran, J., Torres, R., Stashenko, E. E., & Ortiz, C. (2023). Antifungal and Antibiofilm Activity of Colombian Essential Oils against Different Candida Strains. Antibiotics, 12(4), 668. https://doi.org/10.3390/antibiotics12040668






