Rapid Screening and Comparison of Chimeric Lysins for Antibacterial Activity against Staphylococcus aureus Strains
Abstract
1. Introduction
2. Results
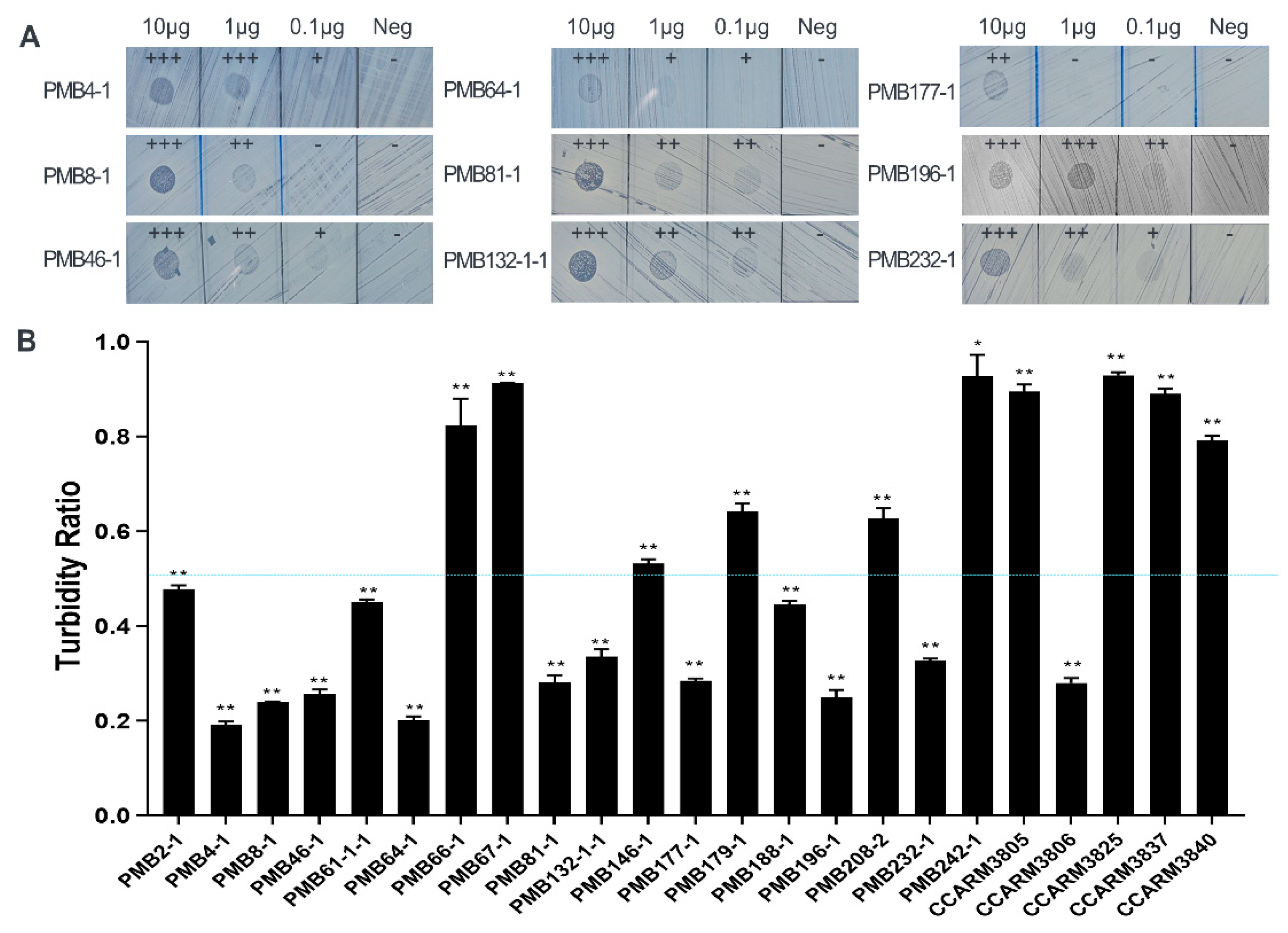
2.1. Rapid Antibacterial Activity Screening and Comparison of Chimeric Lysin Candidates
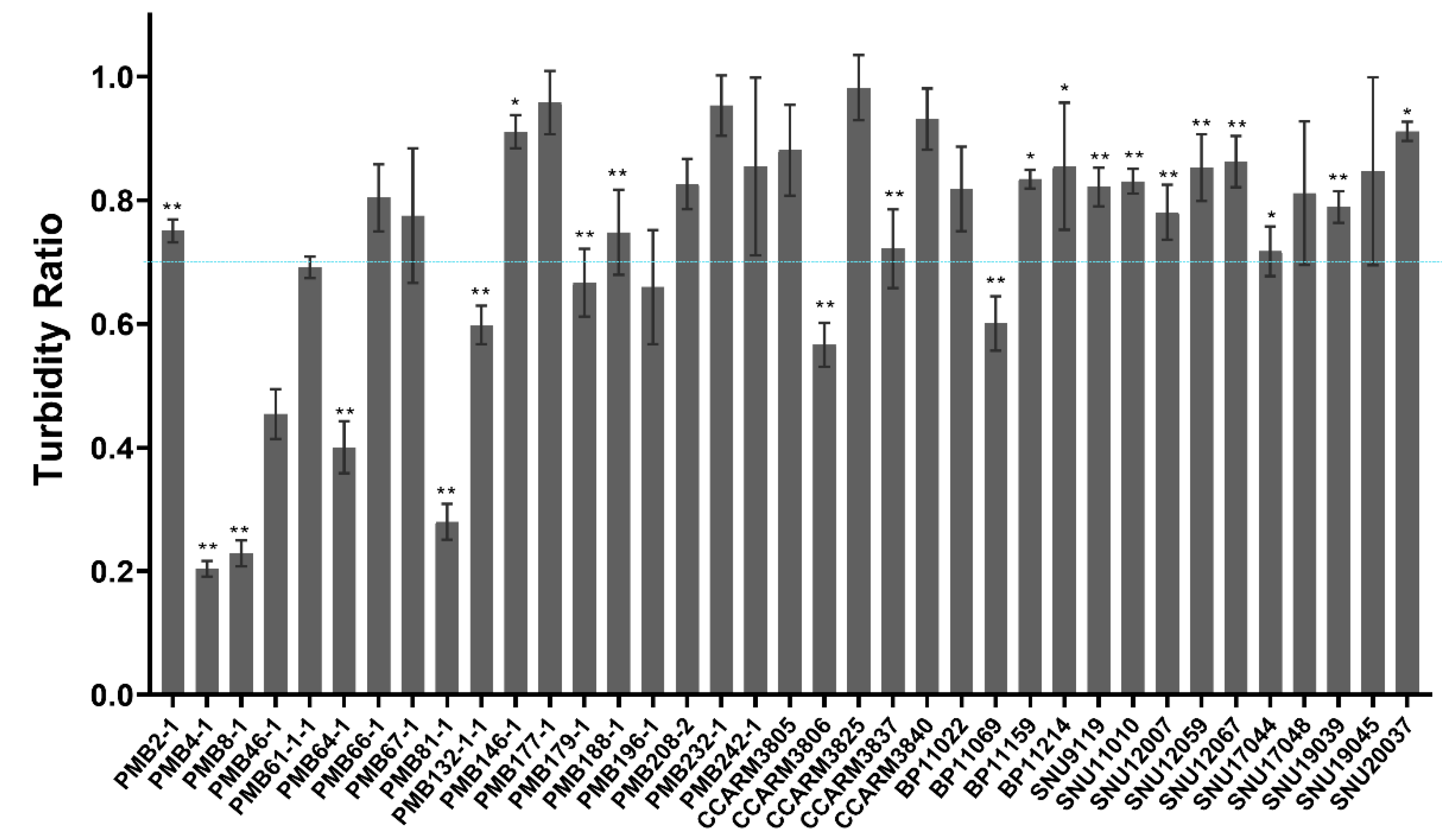
2.2. Comparison of Susceptibility of Various S. aureus Strains to ALS2 Using Cell-Free Expression System
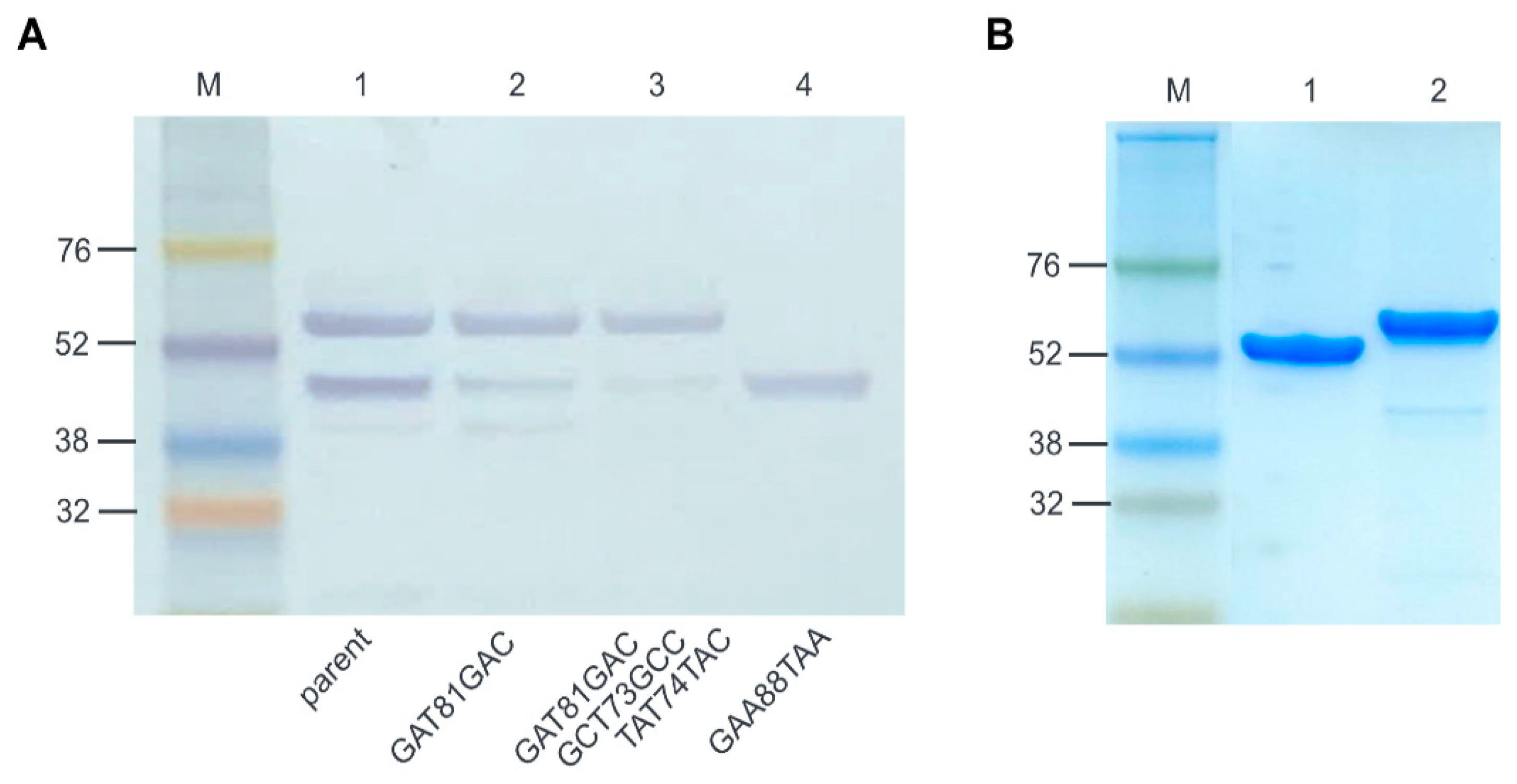
2.3. Optimization of E. coli Expression of ALS2
2.4. Comparison of Susceptibility of Bovine and Human S. aureus Strains to E. coli-Expressed and Highly Purified ALS2
2.5. Accordance of Bacterial Susceptibility to ALS2 Expressed in Cell-Free and E. coli Expression Systems
3. Discussion
4. Materials and Methods
4.1. Bacterial Strains
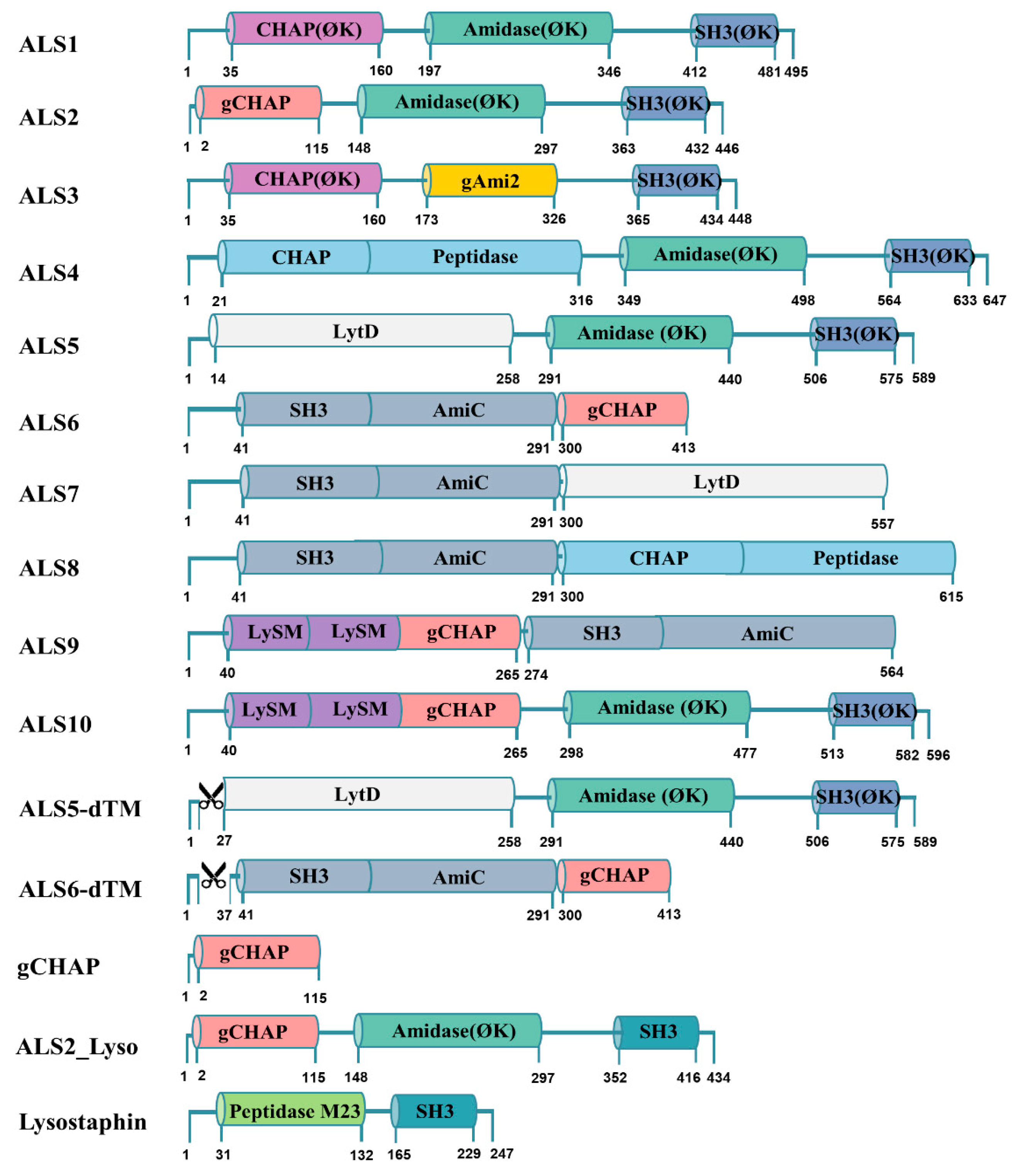
4.2. Bioinformatics
4.3. Design and Construction of Chimeric Lysin Gene for Cell-Free Expression
4.4. Site-Directed Mutagenesis
4.5. Cell-Free Protein Synthesis
4.6. Antibacterial Activity Tests of Chimeric Lysins
4.7. E. coli Expression and Protein Purification
4.8. SDS-PAGE and Western Blotting
4.9. Statistical Analyses
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Burton, J.L.; Erskine, R.J. Immunity and mastitis Some new ideas for an old disease. Vet. Clin. NorthAm. Food Anim. Pract. 2003, 19, 1–45. [Google Scholar] [CrossRef]
- Le Loir, Y.; Baron, F.; Gautier, M. Staphylococcus aureus and food poisoning. Genet. Mol. Res. 2003, 2, 63–76. [Google Scholar] [PubMed]
- McNamee, P.T.; Smyth, J.A. Bacterial chondronecrosis with osteomyelitis (‘femoral head necrosis’) of broiler chickens: A review. Avian Pathol. 2000, 29, 477–495. [Google Scholar] [CrossRef] [PubMed]
- Jevons, M.P. “Celbenin”-resistant staphylococci. Br. Med. J. 1961, 1, 124. [Google Scholar] [CrossRef]
- Hiramatsu, K.; Cui, L.; Kuroda, M.; Ito, T. The emergence and evolution of methicillin-resistant Staphylococcus aureus. Trends Microbiol. 2001, 9, 486–493. [Google Scholar] [CrossRef]
- Ko, D.-S.; Kim, N.-H.; Kim, E.-K.; Ha, E.-J.; Ro, Y.-H.; Kim, D.; Choi, K.-S.; Kwon, H.-J. Comparative genomics of bovine mastitis-origin Staphylococcus aureus strains classified into prevalent human genotypes. Res. Vet. Sci. 2021, 139, 67–77. [Google Scholar] [CrossRef]
- Guinane, C.M.; Ben Zakour, N.L.; Tormo-Mas, M.A.; Weinert, L.A.; Lowder, B.V.; Cartwright, R.A.; Smyth, D.S.; Smyth, C.J.; Lindsay, J.A.; Gould, K.A. Evolutionary genomics of Staphylococcus aureus reveals insights into the origin and molecular basis of ruminant host adaptation. Genome Biol. Evol. 2010, 2, 454–466. [Google Scholar] [CrossRef]
- Matuszewska, M.; Murray, G.G.R.; Harrison, E.M.; Holmes, M.A.; Weinert, L.A. The Evolutionary Genomics of Host Specificity in Staphylococcus aureus. Trends Microbiol. 2020, 28, 465–477. [Google Scholar] [CrossRef] [PubMed]
- Lowder, B.V.; Guinane, C.M.; Ben Zakour, N.L.; Weinert, L.A.; Conway-Morris, A.; Cartwright, R.A.; Simpson, A.J.; Rambaut, A.; Nübel, U.; Fitzgerald, J.R. Recent human-to-poultry host jump, adaptation, and pandemic spread of Staphylococcus aureus. Proc. Natl. Acad. Sci. USA 2009, 106, 19545–19550. [Google Scholar] [CrossRef]
- Weinert, L.A.; Welch, J.J.; Suchard, M.A.; Lemey, P.; Rambaut, A.; Fitzgerald, J.R. Molecular dating of human-to-bovid host jumps by Staphylococcus aureus reveals an association with the spread of domestication. Biol. Lett. 2012, 8, 829–832. [Google Scholar] [CrossRef]
- Seong, W.-J.; Kim, J.-H.; Kwon, H.-J. Comparison of complete rpoB gene sequence typing and multi-locus sequence typing for phylogenetic analysis of Staphylococcus aureus. J. Gen. Appl. Microbiol. 2013, 59, 335–343. [Google Scholar] [CrossRef] [PubMed]
- Ko, D.-S.; Seong, W.-J.; Kim, D.; Kim, E.-K.; Kim, N.-H.; Lee, C.-Y.; Kim, J.-H.; Kwon, H.-J. Molecular prophage typing of Staphylococcus aureus isolates from bovine mastitis. J. Vet. Sci. 2018, 19, 771–781. [Google Scholar] [CrossRef]
- Ko, D.-S.; Kim, D.; Kim, E.-K.; Kim, J.-H.; Kwon, H.-J. Evolution of a major bovine mastitic genotype (rpoB sequence type 10-2) of Staphylococcus aureus in cows. J. Microbiol. 2019, 57, 587–596. [Google Scholar] [CrossRef] [PubMed]
- Wood, J.M. Bacterial responses to osmotic challenges. J. Gen. Physiol. 2015, 145, 381–388. [Google Scholar] [CrossRef]
- Loskill, P.; Pereira, P.M.; Jung, P.; Bischoff, M.; Herrmann, M.; Pinho, M.G.; Jacobs, K. Reduction of the peptidoglycan crosslinking causes a decrease in stiffness of the Staphylococcus aureus cell envelope. Biophys. J. 2014, 107, 1082–1089. [Google Scholar] [CrossRef] [PubMed]
- Matias, V.R.; Beveridge, T.J. Native cell wall organization shown by cryo-electron microscopy confirms the existence of a periplasmic space in Staphylococcus aureus. J. Bacteriol. 2006, 188, 1011–1021. [Google Scholar] [CrossRef]
- Vollmer, W.; Blanot, D.; De Pedro, M.A. Peptidoglycan structure and architecture. FEMS Microbiol. Rev. 2008, 32, 149–167. [Google Scholar] [CrossRef]
- Tyagi, J.L.; Sharma, M.; Gulati, K.; Kairamkonda, M.; Kumar, D.; Poluri, K.M. Engineering of a T7 Bacteriophage Endolysin Variant with Enhanced Amidase Activity. Biochemistry 2023, 62, 330–344. [Google Scholar] [CrossRef]
- Schmelcher, M.; Donovan, D.M.; Loessner, M.J. Bacteriophage endolysins as novel antimicrobials. Future Microbiol 2012, 7, 1147–1171. [Google Scholar] [CrossRef]
- Ho, M.K.Y.; Zhang, P.; Chen, X.; Xia, J.; Leung, S.S.Y. Bacteriophage endolysins against gram-positive bacteria, an overview on the clinical development and recent advances on the delivery and formulation strategies. Crit. Rev. Microbiol. 2022, 48, 303–326. [Google Scholar] [CrossRef]
- Oliveira, H.; Melo, L.D.; Santos, S.B.; Nóbrega, F.L.; Ferreira, E.C.; Cerca, N.; Azeredo, J.; Kluskens, L.D. Molecular aspects and comparative genomics of bacteriophage endolysins. J. Virol. 2013, 87, 4558–4570. [Google Scholar] [CrossRef]
- O’flaherty, S.; Coffey, A.; Meaney, W.; Fitzgerald, G.; Ross, R.P. The recombinant phage lysin LysK has a broad spectrum of lytic activity against clinically relevant staphylococci, including methicillin-resistant Staphylococcus aureus. J. Bacteriol. 2005, 187, 7161–7164. [Google Scholar] [CrossRef] [PubMed]
- O’Flaherty, S.; Coffey, A.; Edwards, R.; Meaney, W.; Fitzgerald, G.F.; Ross, R.P. Genome of staphylococcal phage K: A new lineage of Myoviridae infecting gram-positive bacteria with a low G+C content. J. Bacteriol. 2004, 186, 2862–2871. [Google Scholar] [CrossRef] [PubMed]
- Low, L.Y.; Yang, C.; Perego, M.; Osterman, A.; Liddington, R. Role of net charge on catalytic domain and influence of cell wall binding domain on bactericidal activity, specificity, and host range of phage lysins. J. Biol. Chem. 2011, 286, 34391–34403. [Google Scholar] [CrossRef]
- Becker, S.C.; Foster-Frey, J.; Donovan, D.M. The phage K lytic enzyme LysK and lysostaphin act synergistically to kill MRSA. FEMS Microbiol. Lett. 2008, 287, 185–191. [Google Scholar] [CrossRef]
- Leonard, A.C.; Goncheva, M.I.; Gilbert, S.E.; Shareefdeen, H.; Petrie, L.E.; Thompson, L.K.; Khursigara, C.M.; Heinrichs, D.E.; Cox, G. Autolysin-mediated peptidoglycan hydrolysis is required for the surface display of Staphylococcus aureus cell wall-anchored proteins. Proc. Natl. Acad. Sci. USA 2023, 120, e2301414120. [Google Scholar] [CrossRef]
- Rupp, M.E.; Fey, P.D.; Heilmann, C.; Götz, F. Characterization of the importance of Staphylococcus epidermidis autolysin and polysaccharide intercellular adhesin in the pathogenesis of intravascular catheter-associated infection in a rat model. J. Infect. Dis. 2001, 183, 1038–1042. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Ma, S.X.; St. John, A.; Torres, V.J. The Major Autolysin Atl Regulates the Virulence of Staphylococcus aureus by Controlling the Sorting of LukAB. Infect. Immun. 2022, 90, e0005622. [Google Scholar] [CrossRef] [PubMed]
- Farrand, A.J.; Haley, K.P.; Lareau, N.M.; Heilbronner, S.; McLean, J.A.; Foster, T.; Skaar, E.P. An iron-regulated autolysin remodels the cell wall to facilitate heme acquisition in Staphylococcus lugdunensis. Infect. Immun. 2015, 83, 3578–3589. [Google Scholar] [CrossRef]
- Layec, S.; Decaris, B.; Leblond-Bourget, N. Diversity of Firmicutes peptidoglycan hydrolases and specificities of those involved in daughter cell separation. Res. Microbiol. 2008, 159, 507–515. [Google Scholar] [CrossRef]
- Kajimura, J.; Fujiwara, T.; Yamada, S.; Suzawa, Y.; Nishida, T.; Oyamada, Y.; Hayashi, I.; Yamagishi, J.i.; Komatsuzawa, H.; Sugai, M. Identification and molecular characterization of an N-acetylmuramyl-l-alanine amidase Sle1 involved in cell separation of Staphylococcus aureus. Mol. Microbiol. 2005, 58, 1087–1101. [Google Scholar] [CrossRef] [PubMed]
- Biswas, R.; Voggu, L.; Simon, U.K.; Hentschel, P.; Thumm, G.; Götz, F. Activity of the major staphylococcal autolysin Atl. FEMS Microbiol. Lett. 2006, 259, 260–268. [Google Scholar] [CrossRef] [PubMed]
- Fujimura, T.; Murakami, K. Staphylococcus aureus clinical isolate with high-level methicillin resistance with an lytH mutation caused by IS 1182 insertion. Antimicrob. Agents Chemother. 2008, 52, 643–647. [Google Scholar] [CrossRef]
- Osipovitch, D.C.; Therrien, S.; Griswold, K.E. Discovery of novel S. aureus autolysins and molecular engineering to enhance bacteriolytic activity. Appl. Microbiol. Biotechnol. 2015, 99, 6315–6326. [Google Scholar] [CrossRef]
- Ning, H.; Lin, H.; Wang, J.; He, X.; Lv, X.; Ju, L. Characterizations of the endolysin Lys84 and its domains from phage qdsa002 with high activities against Staphylococcus aureus and its biofilms. Enzym. Microb. Technol. 2021, 148, 109809. [Google Scholar] [CrossRef]
- Son, B.; Kong, M.; Cha, Y.; Bai, J.; Ryu, S. Simultaneous Control of Staphylococcus aureus and Bacillus cereus Using a Hybrid Endolysin LysB4EAD-LysSA11. Antibiotics 2020, 9, 906. [Google Scholar] [CrossRef] [PubMed]
- Fujiki, J.; Nakamura, T.; Furusawa, T.; Ohno, H.; Takahashi, H.; Kitana, J.; Usui, M.; Higuchi, H.; Tanji, Y.; Tamura, Y.; et al. Characterization of the Lytic Capability of a LysK-Like Endolysin, Lys-phiSA012, Derived from a Polyvalent Staphylococcus aureus Bacteriophage. Pharmaceuticals 2018, 11, 25. [Google Scholar] [CrossRef]
- Park, J.-M. Development of a Novel Chimeric Lysin(gCHAP-LysK) against Staphylococcus aureus. Master’s Thesis, Seoul National University, Seoul, Republic of Korea, 2019. [Google Scholar]
- Seong, W.-J.; Kim, D.; Kim, E.-K.; Ko, D.-S.; Ro, Y.; Kim, J.-H.; Kwon, H.-J. Molecular identification of coagulase-negative staphylococci by rpoB sequence typing. Korean J. Vet. Res. 2018, 58, 51–55. [Google Scholar] [CrossRef]
- Tang, G.-Q.; Bandwar, R.P.; Patel, S.S. Extended upstream AT sequence increases T7 promoter strength. J. Biol. Chem. 2005, 280, 40707–40713. [Google Scholar] [CrossRef]
- Calvopina-Chavez, D.G.; Gardner, M.A.; Griffitts, J.S. Engineering efficient termination of bacteriophage T7 RNA polymerase transcription. G3 2022, 12, jkac070. [Google Scholar] [CrossRef]
- Komarova, E.S.; Chervontseva, Z.S.; Osterman, I.A.; Evfratov, S.A.; Rubtsova, M.P.; Zatsepin, T.S.; Semashko, T.A.; Kostryukova, E.S.; Bogdanov, A.A.; Gelfand, M.S. Influence of the spacer region between the Shine–Dalgarno box and the start codon for fine-tuning of the translation efficiency in Escherichia coli. Microb. Biotechnol. 2020, 13, 1254–1261. [Google Scholar] [CrossRef]
- Horgan, M.; O’Flynn, G.; Garry, J.; Cooney, J.; Coffey, A.; Fitzgerald, G.F.; Ross, R.P.; McAuliffe, O. Phage lysin LysK can be truncated to its CHAP domain and retain lytic activity against live antibiotic-resistant staphylococci. Appl. Environ. Microbiol. 2009, 75, 872–874. [Google Scholar] [CrossRef]
- Hecht, A.; Glasgow, J.; Jaschke, P.R.; Bawazer, L.A.; Munson, M.S.; Cochran, J.R.; Endy, D.; Salit, M. Measurements of translation initiation from all 64 codons in E. coli. Nucleic Acids Res. 2017, 45, 3615–3626. [Google Scholar] [CrossRef]
- Sanz-Gaitero, M.; Keary, R.; Garcia-Doval, C.; Coffey, A.; Van Raaij, M.J. Crystal structure of the lytic CHAPK domain of the endolysin LysK from Staphylococcus aureus bacteriophage K. Virol. J. 2014, 11, 133. [Google Scholar] [CrossRef]
- Zou, Y.; Hou, C. Systematic analysis of an amidase domain CHAP in 12 Staphylococcus aureus genomes and 44 staphylococcal phage genomes. Comput. Biol. Chem. 2010, 34, 251–257. [Google Scholar] [CrossRef]
- Jayakumar, J.; Kumar, V.A.; Biswas, L.; Biswas, R. Therapeutic applications of lysostaphin against Staphylococcus aureus. J. Appl. Microbiol. 2021, 131, 1072–1082. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.; Kim, J.; Son, B.; Ryu, S. Development of Advanced Chimeric Endolysin to Control Multidrug-Resistant Staphylococcus aureus through Domain Shuffling. ACS Infect. Dis. 2021, 7, 2081–2092. [Google Scholar] [CrossRef] [PubMed]
- Tossavainen, H.; Raulinaitis, V.; Kauppinen, L.; Pentikäinen, U.; Maaheimo, H.; Permi, P. Structural and functional insights into lysostaphin–substrate interaction. Front. Mol. Biosci. 2018, 5, 60. [Google Scholar] [CrossRef]
- Sharif, S.; Singh, M.; Kim, S.J.; Schaefer, J. Staphylococcus aureus peptidoglycan tertiary structure from carbon-13 spin diffusion. J. Am. Chem. Soc. 2009, 131, 7023–7030. [Google Scholar] [CrossRef]
- Donovan, D.M.; Foster-Frey, J. LambdaSa2 prophage endolysin requires Cpl-7-binding domains and amidase-5 domain for antimicrobial lysis of streptococci. FEMS Microbiol. Lett. 2008, 287, 22–33. [Google Scholar] [CrossRef] [PubMed]
- Louzoun Zada, S.; Green, K.D.; Shrestha, S.K.; Herzog, I.M.; Garneau-Tsodikova, S.; Fridman, M. Derivatives of Ribosome-Inhibiting Antibiotic Chloramphenicol Inhibit the Biosynthesis of Bacterial Cell Wall. ACS Infect. Dis. 2018, 4, 1121–1129. [Google Scholar] [CrossRef] [PubMed]
- Yadav, A.K.; Espaillat, A.; Cava, F. Bacterial strategies to preserve cell wall integrity against environmental threats. Front. Microbiol. 2018, 9, 2064. [Google Scholar] [CrossRef] [PubMed]
- Becker, S.C.; Dong, S.; Baker, J.R.; Foster-Frey, J.; Pritchard, D.G.; Donovan, D.M. LysK CHAP endopeptidase domain is required for lysis of live staphylococcal cells. FEMS Microbiol. Lett. 2009, 294, 52–60. [Google Scholar] [CrossRef]
- Park, S.; Jun, S.Y.; Kim, C.-H.; Jung, G.M.; Son, J.S.; Jeong, S.T.; Yoon, S.J.; Lee, S.Y.; Kang, S.H. Characterisation of the antibacterial properties of the recombinant phage endolysins AP50-31 and LysB4 as potent bactericidal agents against Bacillus anthracis. Sci. Rep. 2018, 8, 18. [Google Scholar] [CrossRef] [PubMed]
- Duman, Z.E.; Ünlü, A.; Çakar, M.M.; Ünal, H.; Binay, B. Enhanced production of recombinant Staphylococcus simulans lysostaphin using medium engineering. Prep. Biochem. Biotechnol. 2019, 49, 521–528. [Google Scholar] [CrossRef]
- Varadi, M.; Anyango, S.; Deshpande, M.; Nair, S.; Natassia, C.; Yordanova, G.; Yuan, D.; Stroe, O.; Wood, G.; Laydon, A. AlphaFold Protein Structure Database: Massively expanding the structural coverage of protein-sequence space with high-accuracy models. Nucleic Acids Res. 2022, 50, D439–D444. [Google Scholar] [CrossRef]
- Szweda, P.; Kotłowski, R.; Kur, J. New effective sources of the Staphylococcus simulans lysostaphin. J. Biotechnol. 2005, 117, 203–213. [Google Scholar] [CrossRef]
- Heckman, K.L.; Pease, L.R. Gene splicing and mutagenesis by PCR-driven overlap extension. Nat. Protoc. 2007, 2, 924–932. [Google Scholar] [CrossRef]
- Donovan, D.M.; Dong, S.; Garrett, W.; Rousseau, G.M.; Moineau, S.; Pritchard, D.G. Peptidoglycan hydrolase fusions maintain their parental specificities. Appl. Environ. Microbiol. 2006, 72, 2988–2996. [Google Scholar] [CrossRef]

| Strains | Origin a | RST b | MRSA Gene | Plate Lysis Test c | Turbidity Reduction Test | Ref | |||
|---|---|---|---|---|---|---|---|---|---|
| 10 µg | 1 µg | 0.1 µg | E. coli d | Cell-Free e | |||||
| PMB2-1 | BM | 10-2 | - | ++ c | + | - | + | - f | [14] |
| PMB4-1 | BM | 22-1 | - | +++ b | +++ | + | + | + f | |
| PMB8-1 | BM | 8-1 | - | +++ | ++ | - | + | + f | |
| PMB46-1 | BM | 10-2 | - | +++ | ++ | + | + | + | |
| PMB61-1-1 | BM | 10-2 | - | ++ | + | + | + | + | |
| PMB64-1 | BM | 10-2 | - | +++ | +++ | ++ | + | + f | |
| PMB66-1 | BM | 14-3 | - | - | - | - | - | - g | |
| PMB67-1 | BM | 5-2 | - | + | - | - | - | - f | |
| PMB81-1 | BM | 10-2 | - | +++ | ++ | ++ | + | + | |
| PMB132-1-1 | BM | 10-2 | - | +++ | ++ | ++ | + | + | |
| PMB146-1 | BM | 11-5 | - | +++ | ++ | - | + | + | |
| PMB177-1 | BM | 14-2 | - | ++ | + | - | + | + | |
| PMB179-1 | BM | 4-1 | - | + | - | - | - | + f | |
| PMB188-1 | BM | 10-3 | - | ++ | + | - | + | - | |
| PMB196-1 | BM | 2-1 | - | +++ | ++ | ++ | + | + g | |
| PMB208-2 | BM | 11-6 | - | - | - | - | - | - | |
| PMB232-1 | BM | 10-2 | - | +++ | ++ | + | + | - | |
| PMB242-1 | BM | 11-4 | - | nt f | nt | nt | - | - | |
| CCARM3805 | HI | 2-1 | + | - | - | - | - | - | |
| CCARM3806 | HI | 10-2 | + | nt | nt | nt | + | + f | |
| CCARM3825 | HI | 2-1 | + | nt | nt | nt | - | - | |
| CCARM3837 | HI | 4-1 | + | - | - | - | - | - g | |
| CCARM3840 | HI | 4-1 | + | nt | nt | nt | - | - g | |
| BP11022 | CI | 3-1 | - | nt | nt | nt | nt | - | [13] |
| BP11069 | CI | 6-3 | + | nt | nt | nt | nt | + g | |
| BP11159 | CI | 3-1 | - | nt | nt | nt | nt | - | |
| BP11214 | CI | 3-1 | - | nt | nt | nt | nt | - | |
| SNU9119 | CI | 3-1 | - | nt | nt | nt | nt | - | |
| SNU11010 | CI | 3-1 | - | nt | nt | nt | nt | - | |
| SNU12007 | CI | 3-1 | - | nt | nt | nt | nt | - | |
| SNU12059 | CI | 3-1 | - | nt | nt | nt | nt | - | |
| SNU12067 | CI | 3-1 | - | nt | nt | nt | nt | - | |
| SNU17044 | CI | 3-1 | - | nt | nt | nt | nt | - | This study |
| SNU17048 | CI | 3-1 | - | nt | nt | nt | nt | - | |
| SNU19039 | CI | 3-1 | - | nt | nt | nt | nt | - | |
| SNU19045 | CI | 3-1 | - | nt | nt | nt | nt | - | |
| SNU20037 | CI | 3-1 | - | nt | nt | nt | nt | - | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, J.-M.; Ko, D.-S.; Kim, H.-S.; Kim, N.-H.; Kim, E.-K.; Roh, Y.-H.; Kim, D.; Kim, J.-H.; Choi, K.-S.; Kwon, H.-J. Rapid Screening and Comparison of Chimeric Lysins for Antibacterial Activity against Staphylococcus aureus Strains. Antibiotics 2023, 12, 667. https://doi.org/10.3390/antibiotics12040667
Park J-M, Ko D-S, Kim H-S, Kim N-H, Kim E-K, Roh Y-H, Kim D, Kim J-H, Choi K-S, Kwon H-J. Rapid Screening and Comparison of Chimeric Lysins for Antibacterial Activity against Staphylococcus aureus Strains. Antibiotics. 2023; 12(4):667. https://doi.org/10.3390/antibiotics12040667
Chicago/Turabian StylePark, Jin-Mi, Dae-Sung Ko, Hee-Soo Kim, Nam-Hyung Kim, Eun-Kyoung Kim, Young-Hye Roh, Danil Kim, Jae-Hong Kim, Kang-Seuk Choi, and Hyuk-Joon Kwon. 2023. "Rapid Screening and Comparison of Chimeric Lysins for Antibacterial Activity against Staphylococcus aureus Strains" Antibiotics 12, no. 4: 667. https://doi.org/10.3390/antibiotics12040667
APA StylePark, J.-M., Ko, D.-S., Kim, H.-S., Kim, N.-H., Kim, E.-K., Roh, Y.-H., Kim, D., Kim, J.-H., Choi, K.-S., & Kwon, H.-J. (2023). Rapid Screening and Comparison of Chimeric Lysins for Antibacterial Activity against Staphylococcus aureus Strains. Antibiotics, 12(4), 667. https://doi.org/10.3390/antibiotics12040667






