Abstract
The environmental release of antimicrobial pharmaceuticals is an imminent threat due to ecological impacts and microbial resistance phenomena. The recent COVID-19 outbreak will likely lead to greater loads of antimicrobials in the environment. Thus, identifying the most used antimicrobials likely to pose environmental risks would be valuable. For that, the ambulatory and hospital consumption patterns of antimicrobials in Portugal during the COVID-19 pandemic (2020–2021) were compared with those of 2019. A predicted risk assessment screening approach based on exposure and hazard in the surface water was conducted, combining consumption, excretion rates, and ecotoxicological/microbiological endpoints in five different regions of Portugal. Among the 22 selected substances, only rifaximin and atovaquone demonstrated predicted potential ecotoxicological risks for aquatic organisms. Flucloxacillin, piperacillin, tazobactam, meropenem, ceftriaxone, fosfomycin, and metronidazole showed the most significant potential for antibiotic resistance in all analysed regions. Regarding the current screening approach and the lack of environmental data, it is advisable to consider rifaximin and atovaquone in subsequent water quality surveys. These results might support the forthcoming monitorisation of surface water quality in a post-pandemic survey.
Keywords:
antimicrobials; antimalarials; antivirals; consumption; COVID-19; environmental risk; water survey 1. Introduction
The environmental release of antimicrobials is of considerable concern due to the potential ecosystem impacts and the development and spread of resistance.
Antimicrobials comprise medicines used to treat infections, and they include antibiotics, antivirals, antifungals and antiparasitics. This pharmacotherapeutic group mainly contains antibacterial compounds and fewer antifungal, antiviral or antimalarial drugs [1].
Currently, the clinical transversal constraints concerning these medicines are the scarcity of new molecules in development and their antimicrobial resistance (AMR), which occurs when bacteria, viruses, fungi and parasites no longer respond to a drug, increasing the risk of disease spread [2,3,4]. For instance, bacteria resistant to the antibiotic colistin have been detected in various regions. This antibiotic is the only last-resort treatment for life-threatening infections caused by the carbapenem-resistant Enterobacteriaceae. According to the World Health Organization (WHO), in 2018, about half a million new cases of rifampicin-resistant tuberculosis (RR-TB) were globally identified [1]. The prevalence of drug-resistant viral, fungal and parasitic infections is also increasing; therefore, for the long-term treatment of chronic infections (e.g., HIV and malaria), efficient therapy is commonly achieved with combination therapy [5,6].
Antimicrobials are not completely metabolised in the body; they are excreted in the urine and the faecal matter as parent compounds and metabolites, pharmacologically active or not, undergoing incomplete removal in conventional wastewater treatment [7]. Consequently, different amounts of such drugs and their metabolites reach aquatic systems with potential ecotoxicological effects on wildlife and AMR onset.
Previous research has focused on antibiotic detection in aquatic systems, including surface and groundwater systems [8,9,10], as well as on identifying resistance genes and resistant bacteria in various environmental compartments [11,12]. However, evidence regarding antiviral and antimalarial medicines’ environmental occurrence and behaviour is scarce and covers only limited geographical regions [5].
Recently, the world underwent a severe and challenging period due to the COVID-19 outbreak; consequently, the consumption of antimicrobials is expected to increase [13,14,15], likely leading to higher loads in the environment. Therefore, it would be valuable to identify the antimicrobials that are most used and likely to pose the most significant environmental risks. In this context, a predicted risk assessment screening approach based on exposure and hazard could be used to support the forthcoming gathering of monitorisation data for a quality survey of post-pandemic surface water. For this purpose, we evaluated the consumption patterns of antimicrobials in Portugal during the emergent COVID-19 pandemic period (2020–2021) dispensed in ambulatory care and used in hospitals, and we compared them with those of 2019. Further, we identified the antimicrobials that pose potential environmental risks by combining consumption, excretion rate, ecotoxic endpoint, and microbiological endpoint data in five different regions of Portugal.
2. Results
2.1. Antimicrobial Consumption—Hospital and Ambulatory Sectors
Over the study period from January 2019 to December 2021, antibiotics comprised most of consumed antimicrobials (80.6%–212.7 t), followed by antivirals (15.2%–40.2 t), antimalarials (2.4%–6.3 t) and antifungals (1.8%–4.8 t).
Besides antimalarials, the pharmacotherapeutic groups demonstrated reductions in their consumption levels during 2020–2021 (COVID-19 pandemic time) compared with 2019 for both the hospital and ambulatory sectors.
The amount of antimicrobials dispensed during ambulatory care during the study period was 193.6 t, about 2.74 times the amount used in hospitals. Regarding the pharmacotherapeutic groups, antivirals were consumed primarily in hospitals (31.5 t versus 8.7 t in ambulatory care); in contrast, antibiotics, antimalarials and antifungals accounted for 174.7, 5.8, and 4.4 t dispensed in ambulatory care and 38.0, 0.56, and 0.45 t used in hospitals, respectively. Comparing the years of 2020 and 2021 to 2019 revealed decreases in antibiotic, antiviral, and antifungal consumption in ambulatory and hospital use. Nevertheless, in hospitals, antibiotic use approached their 2019 values in 2021, while for antivirals, a 2019-like consumption trend was seen in 2020 (Figure 1).
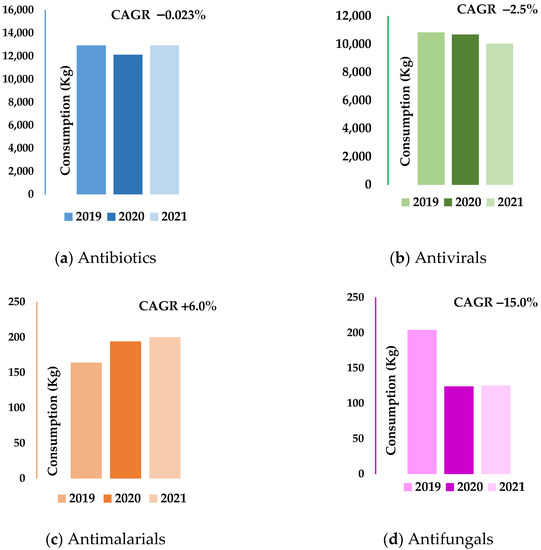
Figure 1.
Annual antibiotic, antiviral, antimalarial and antifungal hospital consumption rates (kg). (a) Antibiotics—hospital; (b) Antivirals—hospital; (c) Antimalarials—hospital; (d) Antifungals—hospital. CAGR—Compound Annual Growth Rate.
Except for antimalarials, negative trends from −2.5% (hospital antivirals) to −15.0% (hospital antifungals) was observed for the compound annual growth rates (CAGRs) for all antimicrobial groups over the study period. However, this tendency was almost imperceptible for the ambulatory consumption of antivirals (CAGR = −0.56%) and the hospital use of antibiotics (CAGR = −0.023%). Figure 1 shows the antibiotic, antiviral, antimalarial, and antifungal consumption rates in the hospital sector and their CAGRs across the study period.
2.2. Consumption Analysis per Therapeutic Class and Its Active Substances
Table 1 illustrates the annual growth rate (AGR) assessment of the active antimicrobial substances during the COVID-19 pandemic (2020 or 2021) and during 2019 used in hospitals and dispensed in ambulatory care.

Table 1.
Assessment of AGRs for the antimicrobials’ active substances during the COVID-19 pandemic period (2020 or 2021) compared with 2019 used in hospitals and dispensed in ambulatory care.
According to the consumption data obtained for 116 active substances, only 22 out of 26 substances showed increase rates with potential environmental concentrations during the pandemic period (2020 and/or 2021) compared with the pre-covid period (2019). The four substances with consumption amounts of below 1.8 kg and without potential environmental concentrations were the antifungals echinocandins (caspofungin, micafungin, and anidulafungin) and voriconazole. The other 22 drugs were distributed in the following classes—antimicrobials: carbapenems, sulfonamides/trimethoprim, and tetracyclines; antivirals: nucleoside and nucleotide reverse transcriptase inhibitors (NRTIs), integrase inhibitors, nucleosides and nucleotides excl. reverse transcriptase inhibitors (NA excl. RTIs); and antimalarials (see Table 1). Considering their active substances, the major determinant for these results was the enhanced usage of ten compounds (meropenem, trimethoprim, doxycycline, minocycline, emtricitabine, lamivudine, dolutegravir, valaciclovir, hydroxychloroquine and atovaquone). Furthermore, this increased consumption trend was preponderant in the first year of COVID-19 for active antiviral and antimalarial substances and some other antibiotic molecules, namely, metronidazole, rifaximin, fosfomycin and ceftriaxone—the first two mainly used in the ambulatory sector and the other two mainly used in the hospital sector. However, the prevalence of active substances with increased consumption was mainly observed in 2021, as the negative consumption trend observed in 2020 cancelled out most of the active substances.
The antibiotic classes—macrolides (e.g., azithromycin and clarithromycin) and quinolones (e.g., ciprofloxacin)—displayed decreased consumption amounts during COVID-19 relative to 2019, with AGRs of roughly −35.0% and −19.2%, respectively.
The antivirals abacavir, tenofovir, and efavirenz showed consumption amounts above 700 kg. Nevertheless, those values were all lower than those of 2019. The antifungals showed a similar trend. For instance, the most used antifungals of fluconazole, itraconazole, and terbinafine reached average quantities of 200, 178, and 1000 kg, respectively, for 2020–2021, with AGR values in the orders of −32, −13, and −21%, respectively, compared with 2019.
All the active substances included in Table 1 with a positive AGR were selected for analysis in the environmental risk assessment.
2.3. Consumption Analysis per Region Based on Fpen Assessment
The consumption data were separately evaluated for the five regions of Portugal. The Lisbon/Tagus Valley, Centre, and North regions are heavily populated, with 3,106,069, 1,663,772, and 3,586,586 inhabitants, respectively. In contrast, the Alentejo and Algarve regions only have 704,533 and 467,343 inhabitants, respectively. Hence, a penetration factor (Fpen)—the proportion of the population treated daily with a specific antimicrobial active substance—was calculated based on consumption per region, defined daily dose values, and the residential population of each area (Equation (3)). The Fpen was only estimated for the active substances listed in Table 1. As a result, regardless of the number of inhabitants, different patterns were obtained for the distinct locales. For instance, the highest proportions of populations treated daily with carbapenems (meropenem), cephalosporins (ceftriaxone and cefazolin), metronidazole and vancomycin were seen in the Centre region, followed by the North region. However, excluding ceftriaxone (Figure 2a), the Lisbon/Tagus Valley region showed the highest proportion of people treated daily with the remaining substances.
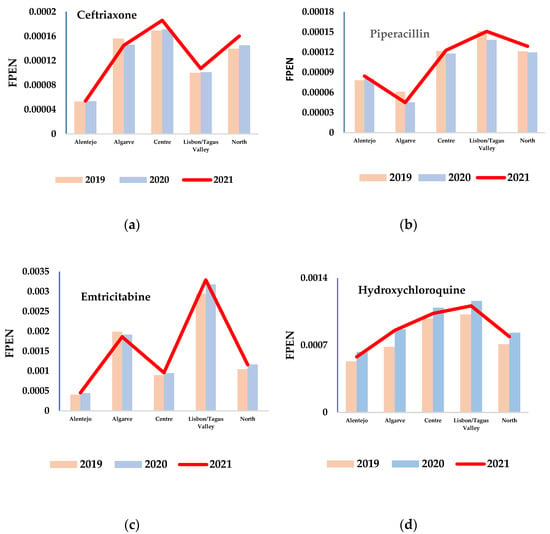
Figure 2.
Different trends and patterns of Fpen for four active substances: (a) ceftriaxone (antibiotic), (b) piperacillin (antibiotic), (c) emtricitabine (antiviral), and (d) hydroxychloroquine (antimalarial) during the study period (2019–2021).
Unexpectedly, an enhanced daily use of antimicrobials (mainly antivirals) in the Algarve region was observed even though it has the fewest inhabitants of the studied areas, with 51% less people than the Alentejo region (the second least populated area), which did not show a similar trend (Figure 2c). In contrast, piperacillin/tazobactam seemed to be the only active substances presenting higher Fpen values in the Alentejo region than in the Algarve region (Figure 2b).
On the other hand, the daily use of antimalarials (e.g., hydroxychloroquine) was found to progressively increase from the Alentejo region to the Algarve, Centre, and Lisbon regions; the North region showed a daily use rate between those registered for the Alentejo and Algarve regions despite having the largest population (Figure 2d).
2.4. Predictive Environmental Risk Assessment of Selected Antimicrobials
2.4.1. Excretion Factor Values
Excretion factor values are displayed in Supplementary Table S1.
Among the 22 selected active substances, only four showed low excretion rates: linezolid (30%), minocycline (43%), trimethoprim (48%), and hydroxychloroquine (25%). When comparing the excretion factors of the other 18 compounds, antivirals showed higher excretion rates, with an average of 92% contrasting with the average of 78% for antibiotics. In addition, the elimination route for most of the substances was found to be renal (e.g., tazobactam—80%; acyclovir—92%), while others were excreted via the bile route and thus appeared in faeces (e.g., rifaximin—97%; atovaquone—≥90%).
It is worth noting that the metabolism and excretion data for these substances are often limited in the literature. Therefore, Summary of Product Characteristics (SPC) information from regulatory authorities was consulted to minimise this constraint.
2.4.2. Predictive Environmental Concentration (PEC) Assessment for the Selected Substances
Predicted effect concentrations (PECs) were assessed using Equation (4) and based on the total (hospital and ambulatory) consumption amount of 2021, which was the year showing the most significant number of substances with positive annual growth rates compared with 2019 (see Table 1). From this perspective, the predictive environmental concentration in surface water was calculated for each region, and these values were refined by considering the different substances’ metabolisms and the proportions of the treated populations.
The analysis of the predictive environmental concentration of active substances in surface water for all regions showed a range of PEC values varying from a maximum of 2.17 µg/L for piperacillin to a minimum of 0.03 µg/L for linezolid. Figure 3 illustrates the characterisation of exposure by region, and Figure 4 shows the total exposure of selected active substances in surface water. The characterisation of exposure disclosed the highest predicted high concentrations of various active substances in the Lisbon/Tagus Valley region, followed by the Centre, North, Algarve, and Alentejo regions (Figure 3). For each region, the substances most representative of and therefore significant for surface water monitorisation were: acyclovir and fosfomycin for all areas (Figure 4), emtricitabine for the Lisbon/Tagus Valley and Algarve regions, lamivudine for the Centre region, and Alentejo and raltegravir for the North region. In addition, the following predicted environmental loads were observed: 6.9 µg/L of antibiotics, 6.5 µg/L of antivirals, and 0.54 µg/L of antimalarials.
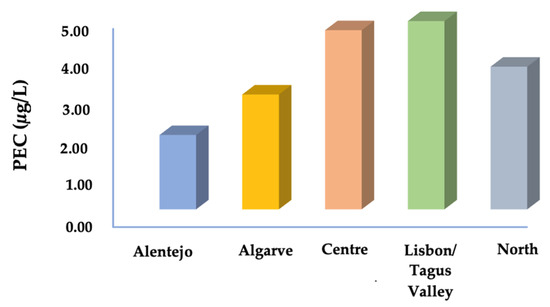
Figure 3.
Predicted environmental concentration (exposure) of surface water in different regions. PEC values were obtained from the sum of each PEC-active substance.
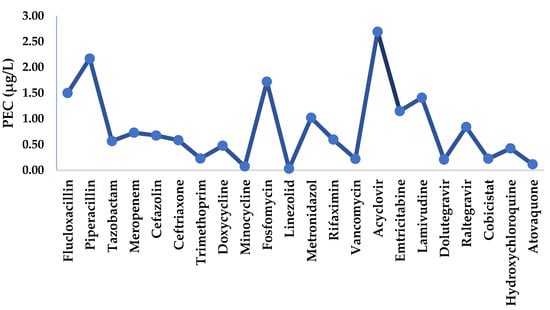
Figure 4.
Predicted environmental concentration (exposure) of surface water to selected active substances.
2.4.3. Predicted No-Effect Concentration Assessment and Risk Characterisation of Selected Substances
Table S2 provides the predicted no-effect concentrations (PNECs) for the ecotoxicological and microbiological risk assessment. Ecotoxicological risk refers to the assessment of hazard potential regarding the aquatic organisms, and microbiological risk refers to resistance selection risk.
Each calculated PNEC was derived from NOECs/EC10/EC50 (PNECECOtox) with sub-MICs (PNECsubMIC) for antibiotics. In analysing the active substances, emtricitabine showed the highest PNEC by fish Pimephales promelas (PNEC 610 µg/L; Table S2), followed by cobicistat (484 µg/L) and raltegravir (380 µg/L), while atovaquone (antimalarial) and rifaximin (antibiotic) showed the lowest PNECs (0.83 ng/L by Ceriodaphnia dubia and 76 ng/L by Anabaena sp., respectively).
The estimated risks calculated for the five studied mainland Portugal regions are reported in Table 2. Among the 22 selected substances that posed predicted potential ecotoxicological risk were rifaximin and atovaquone, with calculated risks for aquatic organisms (RQECOtox) ranging from 1.05 to 2.11 and from 6.63 to 65.1, respectively, depending on region. However, moderate risk was still observed for cefazolin, ceftriaxone and meropenem. Removing the Alentejo region, which only presented a high risk for rifaximin, the predicted risks in the remaining areas were ranked in ascending order as follows: the Centre, Algarve, Lisbon/Tagus Valley, and North regions.

Table 2.
PEC and ecotoxicological/microbiological risk quotients (RQs) for the selected substances in surface water.
The microbial risk quotient (RQMICsub) was assessed regarding the predicted lowest minimal inhibitory concentrations for resistance selection concerning each antibiotic and its exposure to surface water. It was found that most substances showed an elevated-to-moderate risk quotient, with impacts on all regions; flucloxacillin (RQ = 0.67–1.6), piperacillin (RQ = 3.0–10.3), tazobactam (RQ = 0.19–0.64), meropenem (RQ = 0.81–2.9), ceftriaxone (RQ = 1.5–5.3), fosfomycin (RQ = 0.11–0.22), metronidazole (RQ = 0.80–2.08) exhibited the most significant potential for antibiotic resistance. Due to their low predicted surface water concentrations, minocycline, vancomycin, and linezolid did not indicate the possibility of causing risk.
3. Discussion
It is supposed that the greater the antimicrobial usage, the higher the load expected to reach the environment. Thus, we developed an approach based on antimicrobial consumption for systemic use to identify those active substances with increased usage during 2020 and 2021 (pandemic period) compared with 2019, and the predictive ecotoxicological and microbiological risks were evaluated in five different regions of Portugal.
3.1. Antimicrobial Consumption
When we analysed the antimicrobial usage (overall national level), it was readily noticeable that differently from antimalarials, decreases in ambulatory and hospital use were seen for the antibiotic, antiviral, and antifungal groups. These outcomes were evidenced by the negative CAGR trend observed over the study period. Unfortunately, it is impossible to compare our results with previously reported consumption data because these data are often provided in terms of a defined daily dose (DDD), a WHO statistical measure of drug consumption. Nevertheless, our estimated trends provide important insights and can be effectively discussed; for instance, the observed decreased antibiotic use aligns with what was documented for Portugal by the Annual Surveillance Report for 2021 from European Centre for Disease Prevention and Control (ECDC) and by Silva et al. [13,16].
Given the main objective of the present paper, we identified 22 active antimicrobial substances (Table 1) with increased consumption rates during the pandemic that may cause environmental risks. Different pharmaceutical medicine patterns were observed for 2020 and 2021, which may have been related to pandemic evolution. The first year corresponded to the beginning of the pandemic and the first wave of COVID-19. Behavioural interventions such as lockdowns, social distancing, and the usage of masks were then implemented, likely decreasing other infections and access to medical appointments. In addition, recommendations from the WHO narrowed antibiotics use [17,18,19,20,21]. These features are considered possible sources of the perceived significant negative growth rates of macrolides (e.g., azithromycin and clarithromycin) and quinolones (e.g., ciprofloxacin), mostly dispensed in ambulatory care. However, an increasing use trend was observed in 2020, mainly for five antiviral substances (emtricitabine, lamivudine, dolutegravir, raltegravir and cobicistat), four antibiotic substances (ceftriaxone, fosfomycin, metronidazole and rifaximin), and two antimalarial substances (hydroxychloroquine and atovaquone).
Nevertheless, the use of antimicrobials in 2021 cancelled out the negative consumption trend detected in 2020, mainly for the considerable number of active substances used in hospitals, which may have been related to the increased severity of diseases and the need for multiple interventions, especially among intensive care unit patients [22]; even though we do not have data on patients admitted to intensive care, a rise in the number of patients hospitalised in 2021 was reported [23]. Likewise, outpatients with mild symptoms of COVID-19 or other less severe diseases were quarantined at home; this was confirmed by the sequent growth of medicines dispensed in ambulatory care [24]. These data are in line with our data, which demonstrate the increased use of fosfomycin, rifaximin, and tetracyclines in the ambulatory sector during 2021.
Thus, in the present work, the observed increased trend in antimicrobial use seems to be related to repurposed medicines in the context of COVID-19, given that the consumption outcomes matched the higher incidence of COVID-19 infections during the second year of the pandemic. For instance, the used antivirals include substances approved for the treatment of human immunodeficiency virus-1 (HIV-1) (emtricitabine, dolutegravir, and raltegravir), chronic hepatitis B (lamivudine) and herpes (valacyclovir). Furthermore, we observed an enhanced daily use of antivirals in two regions of Portugal (Algarve and Lisboa/Tagus Valley), which may be related to the incidence of HIV-1 in these two regions [25]. Moreover, substances other than commonly applied molecules (e.g., ceftriaxone and meropenem) for community-acquired pneumonia or hospital-acquired/ventilator-associated pneumonia coverage were empirically utilised [26] due to their additional characteristics used to face the great challenge of controlling COVID-19. For instance, fosfomycin demonstrated an immunomodulatory effect on human B cell activation; metronidazole has been shown to decrease the levels of several cytokines, with anti-inflammatory properties; rifaximin seems to have great potential to inhibit the interaction of SARS-CoV-2 with ACE2 (the main human receptor for the entry of SARS-CoV-2 into lower respiratory tract epithelial cells); and tetracyclines present anti-inflammatory, neuroprotective, and even antiviral effects [27,28,29,30,31,32,33].
In this study, antimalarials were the sole therapeutic group that demonstrated evident growth during the study period, particularly the active substances of hydroxychloroquine and atovaquone. Both are used in Portugal for treating malaria due to the country’s connection to African territory. However, during 2020, there was a significant increase in hydroxychloroquine usage (especially in the hospital sector), while in 2021, this trend shifted to atovaquone. Increased ambulatory use was only observed for hydroxychloroquine, but it decreased in the second year of COVID-19. These results suggest that, at least in Portugal, hydroxychloroquine was requested for COVID-19 treatment at the beginning of the pandemic, so it was one of the first medicines to be considered for repurposing. However, hydroxychloroquine’s supposed effectiveness was based on several poorly controlled clinical trials, which led to a lack of use at the late stage of COVID-19, when it was replaced by atovaquone [34,35].
For antifungals, our data showed decreased consumption during 2020–2021. This outcome was unexpected since other authors have reported increased incidences of invasive fungal diseases in COVID-19 patients, with voriconazole the first-line treatment [36]. Nevertheless, we found that the used amount of this antifungal in Portugal displayed a negative rate of −7% for 2020 but a positive rate of 12% for 2021 compared with 2019. Echinocandins additionally had positive consumption rates of 8% and 38% for 2020 and 2021, respectively. However, as their maximum consumption rate was 1.8 kg, they were not considered for the environmental risk assessment because the calculated predicted environmental concentration was very low.
Surprisingly, an enhanced daily use of antimicrobials (mainly antivirals) in the Algarve region was observed even though it has the fewest inhabitants, with 51% less than the Alentejo region (the second least populated area), which did not show a similar trend. The Algarve region, with the lowest resident population, is one of the most touristic areas of Portugal. However, the area’s floating population does not explain the consumption trends observed there, given that tourists were not allowed in Portugal during the lockdown.
3.2. Environmental Risk Assessment
Regarding the environmental risks of antimicrobials, two main issues have been raised in the last few decades. First, antimicrobials in surface water may be toxic to aquatic life, producing adverse effects in aquatic ecosystems such as the inhibition of cell proliferation in aquatic organisms, thus affecting their physiology and morphology, as described by Pomati and colleagues [37]. Likewise, antimicrobials can contribute to the spread of AMR even at low or sub-inhibitory concentrations, posing an actual risk to human health [38,39,40].
Considering its adverse effects, some compounds were included in the last watch list (WL), namely, amoxicillin, ciprofloxacin, erythromycin, clarithromycin, azithromycin, sulfamethoxazole, and trimethoprim. Nevertheless, except for trimethoprim, the consumption of the above-mentioned substances did not increase during the pandemic period in Portugal.
Hospital effluent represents a relevant source of antimicrobial residues and antibiotic-resistant bacteria in wastewater [41]. Moreover, the COVID-19 outbreak has further increased hospital waste generation over the past two years due to the high loads of residues in hospital effluent due to an overload of patients. Thus, antimicrobial medicines may have been used on a larger scale. In fact, the present study found a significant increase in these specific hospital-used antimicrobials, demonstrating the role of hospital settings as environmental hotspots. Additionally, our risk assessment results indicated that flucloxacillin, piperacillin, tazobactam, meropenem, cefazolin, ceftriaxone, fosfomycin, metronidazole, and trimethoprim were associated with microbiological risk (higher than 0.1). Piperacillin had the highest RQ (RQ = 10.3, Table 2) in the Lisbon/Tagus region, followed by ceftriaxone and meropenem (RQ = 5.3 and 2.9, Table 2) in the Centre region of Portugal. High proportions of the population treated daily with carbapenems and cephalosporins were also observed in the Centre region of Portugal.
Ceftriaxone has a low potential for bioaccumulation [42]; nevertheless, it is eliminated unaltered in the urine (by glomerular filtration) and bile. Even though ceftriaxone is one of the most prescribed antibiotics in health facilities, there is little information regarding its occurrence in the environment [43]. A recent study in India found high levels of ceftriaxone (from 1.25 to 29.15 µg/mL) in hospital effluent [44].
Tazobactam is a β-lactamase inhibitor used in combination with piperacillin. This molecule presents high renal excretion rates as an unchanged drug (Table S1) and is considered a very highly mobile hydrophilic substance; therefore, it could move quickly towards surface water. It has already been detected in Portugal [8]. In our study, tazobactam presented an RQMICsub of between 0.19 and 0.64, suggesting a critical microbiological risk.
In the present study, rifaximin and atovaquone were the most concerning substances regarding ecotoxicological effects (RQECOtox > 1). As stated before, the consumption of rifaximin has increased during the pandemic, and its use poses ecotoxicological risks in all considered regions of Portugal. It has a very low absorption rate (<1%) and is almost completely excreted (97%) as the parent compound in faeces, with only a small proportion of the dose excreted in urine (0.32%). Only one metabolite, 25-diacetyl rifaximin, was identified. However, no data on its behaviour on sewage treatment plants or its environmental occurrence were found in the literature; thus, rifaximin should be integrated into the water quality survey approach. In Portugal, rifaximin is authorised for the treatment of acute infectious diarrhoea caused by susceptible microorganisms and the symptomatic treatment of uncomplicated diverticular disease of the colon when associated with dietary fibre therapy [45].
Atovaquone is an antimalarial drug that is primarily eliminated via the liver (more than 90% of the drug excreted in bile is in the parent form), with almost undetectable amounts (0.6%) being eliminated via the kidneys [46,47], contrariwise to hydroxychloroquine that is barely excreted and has a low RQ. The substance’s LogKow = 5.31 at pH 7 predicts a high potential for bioaccumulation [48]. In the present study, atovaquone was highlighted as having a high risk quotient; however, no environmental occurrence data were found in the literature. Thus, atovaquone should also be integrated into the water quality survey approach.
Lately, antiviral drugs have been recognised as emerging contaminants, and their environmental fate and ecotoxicological effects have been investigated [5,49]. Antiviral drugs are among the most predicted hazardous therapeutic classes regarding their toxicity towards algae, daphnids and fish [50]. Thus, knowing the fate, occurrence, and toxicological effects of antiviral drugs in the aquatic environment is mandatory. The incomplete removal of antiviral drugs from effluent may lead to the development of viral resistance with adverse health effects on humans and harmful effects on the environment [51,52]. In contrast to bacteria, viruses are obligate intracellular parasites that rely on host cellular functions to replicate. Thus, most currently available antivirals target specific viral functions, and many have significant toxicities [53]. Although antiviral resistance is rarely mentioned or considered, it should be a point of concern and was addressed by Laughlin and colleagues [53]. According to a recent study, Portugal has the highest systemic use of antiviral drugs in DDD per 1000 inhabitants per day, with HIV drugs presenting the highest percentage of total use [5]. In the present study, no significant increase in consumption was found during the pandemic period. However, an amount of −2.5 CAGR was observed. Nevertheless, data have confirmed the environmental occurrence of antivirals in countries such as Germany [54,55,56], South Africa [57,58], France [59], Kenya [60] and Finland [61]. In addition, abacavir used for HIV infection treatment was detected in 11 of 13 surface sampling stations and one groundwater sampling station in Portugal in a recent study by our research team [8], highlighting the need for antiviral screening programs at the national and global levels.
In particular, acyclovir (ACV) is widely used as an antiherpetic medication, and it is mainly excreted (92%) as an unchanged parent compound [62]. Recent studies indicated the almost complete removal of acyclovir (97%) and lamivudine (>93)% [54] from sewage treatment plants (STPs). Nevertheless, analyses of different environmental samples revealed the presence of transformation products such as the stable carboxy-acyclovir (carboxy-ACV) in surface and drinking water, with concentrations of up to 3200 ng/L and 40 ng/L, respectively [5,63]. Additionally, carboxy-ACV concentrations should not be neglected since Daphnia magna toxicity was reported [64]. In our study, we must emphasise that no ecotoxicological risk was observed for the five selected antiviral substances.
Cobicistat was one of the substances that showed an increased consumption rate during the pandemic period in Portugal. Cobicistat is an inhibitor of human CYP3A isoforms [65], and it is used as a pharmacokinetic enhancer that inhibits the metabolism of antiretroviral drugs, boosting protease inhibitors (PIs) and integrase inhibitors to prolong their effects in HIV-infected patients. Cobicistat does not have reported activity against HIV [65]. Regarding ecotoxicological risk, this molecule is not easily biodegradable; however, according to the criteria set by the EU, cobicistat should not be considered a persistent, bioaccumulative, and toxic (PBT) substance, and its use is considered to result in negligible environmental risk [66]. Nevertheless, 86% of this substance is excreted in faeces, and 8% is excreted in urine. Furthermore, this molecule may inhibit the CYP activity of marine species and thereby interfere with the hepatic clearance of xenobiotics from those species. Thus, this molecule may also pose an environmental risk similar to those detected with other known CYP inhibitors belonging to the antifungal group [67]. Nevertheless, no risk was observed with the assessment approach used in the present study.
4. Materials and Methods
4.1. Consumption of Antimicrobial Drugs
Information regarding medicine consumption data in terms of packaging, pharmaceutical form, and quantitative composition were obtained from the Department for the Medicine’s Economic Assessment of INFARMED, allowing for the estimation of the consumed amount of active substances for each specific antimicrobial drug/year in kg [68].
The collected data refer to the medicines for systemic use in hospitals and the primary health care sector, i.e., medicines dispensed in ambulatory care, within the Portuguese National Health Service. The study period corresponded to the consecutive years of 2020–2021 and the previous year of 2019, which was used to evaluate changes in antimicrobial consumption trends. The consumption data comprised five regions of Portugal’s mainland ranging from the South to the North: Algarve, Alentejo, Lisbon/Tagus Valley, Centre, and North.
Variables were presented as numbers (kg and tons) and percentages. In addition, compound annual growth rates (CAGRs) and annual growth rates (AGRs) were calculated to illustrate changes in the consumption rates of antimicrobials over the three years of the study period and to visualise the growth of antimicrobial consumption for 2020 and 2021 versus 2019.
In this equation, CONEnd is the consumption of antimicrobials for the last year, CONStart is the consumption of antimicrobials for the first reported year, and N = 3 is the number of years of reporting (2019–2021).
where CONEnd is the consumption of antimicrobials in the last year and CONStart is the consumption of antimicrobials for the year first reported.
The antimicrobial drugs that demonstrated increased consumption—positive annual growth rates—during the pandemic (2020 or/and 2021) compared with the previous year (2019) were selected for environmental risk assessment.
4.2. Assessment of Market Penetration Factor (Fpen)
The fraction of a population receiving the active substance (Fpen) was calculated using the following equation [69]:
where consumption is the amount of antimicrobial drugs used per year (2019–2021), DDD is the defined daily dose [70], hab is the number of inhabitants in Portugal or the different regions of Portugal, and 365 is the number of days per year.
4.3. Metabolisation and Excretion Fraction of the Most Representative Antimicrobials
As the parent compound, the proportion of excretion may contribute to a significant or lesser environmental impact related to the corresponding reported consumption [71]. Therefore, excretion fractions were determined by summing the excreted proportion of the active substance (urine and/or faeces) and the ratio of the parent molecule existing as a glucuronide conjugate. It was assumed that all glucuronide conjugates are cleaved to the parent compound in the environment. If no information was given on the nature of the conjugate, we considered the worst-case scenario, wherein all active conjugates were glucuronide conjugates [72]. When more than one final excretion value was given for the unchanged active substance, the worst-case scenario was again considered; thus, the greater one was reported.
4.4. Predictive Environmental Risk Assessment of Selected Antimicrobials
A risk-based approach was used to evaluate the possible antimicrobial risk to the surface water compartment. Thus, the ratios between the predicted environmental concentrations (PECs) and the predicted no-effect concentrations (PNECs), either environmental or microbiological, were briefly assessed. It was assumed that a risk quotient (RQ) to the aquatic compartment was indicated when the PEC/PNECECOtox ratio was ≥ 1 or PEC/PNECMICsub ratio was ≥0.1 [73].
We categorised risk as critical if RQECOtox ≥ 1 and RQMICsub ≥ 0.1, moderate for RQECOtox between 0.5 ≤ RQ < 1 and RQMICsub between 0.05 ≤ RQ < 0.1, and no risk for RQECOtox < 0.5 and RQMICsub < 0.05.
4.4.1. Calculation of Predicted Environmental Concentrations in Surface Waters (PECs)
The following formula based on EMA guideline [74] was used to estimate the PEC in the surface water:
where DOSEai is the maximum daily dose consumed per inhabitant, Fpen is the fraction of a population receiving the active substance (see Section 4.3), Fexcrete is the proportion of the antimicrobial excreted in urine and/or faeces (see Section 4.2), WASTEWinhab is the amount of wastewater per inhabitant per day (133 L inhabit/day [75]), dilution is the dilution from sewage treatment plant (STP) effluent to surface water (default value: 10), and 365 is the number of days per year.
4.4.2. Calculation of Predicted No-Effect Concentrations (PNECs)
As we studied antimicrobials, both environmental predicted no-effect concentration (PNECECOtox) and PNECs based on minimal inhibitory concentrations (PNECMICsub) were assessed. The estimation was based on the published most sensitive endpoints. Therefore, the PNECECOtox values were calculated by using the lowest value of no-observed effect concentration (NOEC), 10% effect concentration (EC10), or 50% effect concentration (EC50), when the EC10 was not available. Accordingly, an assessment factor was applied [76]. The MICs and minimal selective concentrations were derived from peer-reviewed literature. The PNECMICsub values were then calculated using an assessment factor to account for the differences between MICs and minimal selective concentrations [12,77].
5. Conclusions
Over the study period from January 2019 to December 2021, antibiotics comprised most of the consumed antimicrobials in Portugal, followed by antivirals, antimalarials and antifungals.
Only 22 out of the 116 evaluated active substances showed positive increase rates with potential environmental concentrations during the pandemic period (2020 and/or 2021) compared with the pre-covid period (2019). This increased consumption trend was preponderant in the first year of COVID-19 for active antiviral and antimalarial substances and for some other antibiotic molecules, namely, metronidazole, rifaximin, fosfomycin and ceftriaxone. The prevalence of active substances with increased consumption was mainly observed in 2021.
Considering the proportion of the population treated daily with a specific antimicrobial active substance, different patterns were observed for the distinct Portuguese regions regardless of the number of inhabitants. Higher proportions of people treated daily with carbapenems (meropenem), cephalosporins (ceftriaxone and cefazolin), metronidazole, and vancomycin were seen in the Centre region, followed by the North region. However, the Lisbon/Tagus Valley region showed the highest proportion of people treated daily with the remaining substances. Surprisingly, without a reasonable explanation, an enhanced daily use of antimicrobials (mainly antivirals) in the Algarve region was observed even though it has the fewest inhabitants, with 51% less than the Alentejo region (the second least populated area), which did not show a similar trend. Further attention should be given to the antimicrobial consumption trends of the Algarve region. In contrast, piperacillin/tazobactam was found to be the only active substance, with higher Fpen values in the Alentejo region than in the Algarve region.
Among the 22 selected substances, only rifaximin and atovaquone demonstrated predicted potential ecotoxicological risk for aquatic organisms. Except for the Alentejo region, where only rifaximin presented a high risk, predicted risks were observed in the remaining areas, ranked in ascending order as: the Centre, Algarve, Lisbon/Tagus Valley, and North regions. Moderate risk was still observed for cefazolin, ceftriaxone and meropenem. Considering microbial risk, flucloxacillin, piperacillin, tazobactam, meropenem, ceftriaxone, fosfomycin and metronidazole displayed the most significant potential for antibiotic resistance in all five regions of Portugal. On the other hand, due to their low predicted surface water concentration, minocycline, vancomycin, and linezolid did not present probabilities of causing risk. It turns out that if the PEC was not diluted by 10 (surface water), we could obtain a much higher PEC of the STP effluent (PEC effluent), and then microbiological risk would be relevant, especially for minocycline and doxycycline.
The pattern of increased antimicrobial consumption observed during the pandemic might not be maintained in the post-pandemic period. However, according to the present screening approach and the lack of environmental data, it is advisable to consider rifaximin and atovaquone in the groundwork for the forthcoming water quality survey, as these substances have already reached the environment in hazardous amounts. Furthermore, given the potential of all antibiotics evaluated in the present study to promote antimicrobial resistance and the role of hospitals as hotspots, it is essential to promote the monitoring of positive risk-identified antimicrobials in waste surface water.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/antibiotics12040652/s1, Table S1. Excretion rate values for the selected active substances [78,79,80,81,82,83,84,85,86,87,88,89,90,91,92,93,94,95,96,97,98,99,100,101,102,103,104,105]; Table S2. Assessment of PNECECOtox and PNECMICsub for the selected substances [106,107,108,109,110,111,112,113,114,115,116,117,118,119].
Author Contributions
Conceptualisation, L.M., A.A. and C.D.M.-S.; methodology, L.M., A.A., C.D.M.-S. and P.V.; validation, P.V. and R.C.d.S.; formal analysis, P.V. and A.L.; investigation, L.M., A.A., C.D.M.-S. and P.V.; resources, R.C.d.S.; data curation, R.C.d.S.; writing—original draft preparation, R.C.d.S.; writing—review and editing, A.L.; visualisation, R.C.d.S.; supervision, L.M., A.A. and C.D.M.-S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors would like to thank INFARMED for providing data.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Antimicrobial Resistance. Available online: https://www.who.int/news-room/fact-sheets/detail/antimicrobial-resistance (accessed on 24 September 2022).
- Purssell, E. Antimicrobials. In Understanding Pharmacology in Nursing Practice; Hood, P., Khan, E., Eds.; Springer: Cham, Switzerland, 2020; pp. 147–165. [Google Scholar]
- Kräusslich, H.-G.; Bartenschlager, R. Antiviral Strategies. In Handbook of Experimental Pharmacology; Kräusslich, H.-G., Bartenschlager, R., Eds.; Springer: Berlin/Heidelberg, Germany, 2009; Volume 189. [Google Scholar]
- De Clercq, E. Strategies in the Design of Antiviral Drugs. Nat. Rev. Drug Discov. 2002, 1, 13–25. [Google Scholar] [CrossRef] [PubMed]
- Nannou, C.; Ofrydopoulou, A.; Evgenidou, E.; Heath, D.; Heath, E.; Lambropoulou, D. Antiviral Drugs in Aquatic Environment and Wastewater Treatment Plants: A Review on Occurrence, Fate, Removal and Ecotoxicity. Sci. Total Environ. 2020, 699, 134322. [Google Scholar] [CrossRef] [PubMed]
- Tompa, D.R.; Immanuel, A.; Srikanth, S.; Kadhirvel, S. Trends and Strategies to Combat Viral Infections: A Review on FDA Approved Antiviral Drugs. Int. J. Biol. Macromol. 2021, 172, 524–541. [Google Scholar] [CrossRef] [PubMed]
- Burns, E.E.; Carter, L.J.; Snape, J.; Thomas-Oates, J.; Boxall, A.B.A. Application of Prioritization Approaches to Optimize Environmental Monitoring and Testing of Pharmaceuticals. J. Toxicol. Environ. Health 2018, 21, 115–141. [Google Scholar] [CrossRef] [PubMed]
- Viana, P.; Meisel, L.; Lopes, A.; de Jesus, R.; Sarmento, G.; Duarte, S.; Sepodes, B.; Fernandes, A.; dos Santos, M.M.C.; Almeida, A.; et al. Identification of Antibiotics in Surface-Groundwater. A Tool towards the Ecopharmacovigilance Approach: A Portuguese Case-Study. Antibiotics 2021, 10, 888. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Mozaz, S.; Vaz-Moreira, I.; Varela Della Giustina, S.; Llorca, M.; Barceló, D.; Schubert, S.; Berendonk, T.U.; Michael-Kordatou, I.; Fatta-Kassinos, D.; Martinez, J.L.; et al. Antibiotic Residues in Final Effluents of European Wastewater Treatment Plants and Their Impact on the Aquatic Environment. Environ. Int. 2020, 140, 105733. [Google Scholar] [CrossRef] [PubMed]
- Gros, M.; Catalán, N.; Mas-Pla, J.; Čelić, M.; Petrović, M.; Farré, M.J. Groundwater Antibiotic Pollution and Its Relationship with Dissolved Organic Matter: Identification and Environmental Implications. Environ. Pollut. 2021, 289, 117927. [Google Scholar] [CrossRef]
- Schwartz, T.; Kohnen, W.; Jansen, B.; Obst, U. Detection of Antibiotic-Resistant Bacteria and Their Resistance Genes in Wastewater, Surface Water, and Drinking Water Biofilms. FEMS Microbiol. Ecol. 2002, 43, 325–335. [Google Scholar] [CrossRef]
- Kummerer, K. Resistance in the Environment. J. Antimicrob. Chemother. 2003, 54, 311–320. [Google Scholar] [CrossRef]
- Silva, T.M.; Estrela, M.; Gomes, E.R.; Piñeiro-Lamas, M.; Figueiras, A.; Roque, F.; Herdeiro, M.T. The Impact of the COVID-19 Pandemic on Antibiotic Prescribing Trends in Outpatient Care: A Nationwide, Quasi-Experimental Approach. Antibiotics 2021, 10, 1040. [Google Scholar] [CrossRef]
- Chen, X.; Lei, L.; Liu, S.; Han, J.; Li, R.; Men, J.; Li, L.; Wei, L.; Sheng, Y.; Yang, L.; et al. Occurrence and Risk Assessment of Pharmaceuticals and Personal Care Products (PPCPs) against COVID-19 in Lakes and WWTP-River-Estuary System in Wuhan, China. Sci. Total Environ. 2021, 792, 148352. [Google Scholar] [CrossRef] [PubMed]
- Saim, S.; Behira, B. Impact of Chloroquine as Treatment of Pandemic COVID-19 on Environment. Mater. Biomater. Sci. 2021, 4, 100–105. [Google Scholar]
- European Centre for Disease Prevention and Control. Antimicrobial Consumption in the EU/EEA (ESAC-Net); Annual Epidemiological Report; European Centre for Disease Prevention and Control: Stockholm, Sweden, 2021. [Google Scholar]
- Khouja, T.; Mitsantisuk, K.; Tadrous, M.; Suda, K.J. Global Consumption of Antimicrobials: Impact of the WHO Global Action Plan on Antimicrobial Resistance and 2019 Coronavirus Pandemic (COVID-19). J. Antimicrob. Chemother. 2022, 77, 1491–1499. [Google Scholar] [CrossRef]
- Da Silva, R.F.; Macedo, M.; Conceição, J. A Pandemia de COVID-19 Em Portugal: Evolução, Vacinação e Farmacovigilância. RevistaMultidisciplinar 2022, 4, 135–154. [Google Scholar] [CrossRef]
- Pais, R.; Taveira, N. Predicting the Evolution and Control of the COVID-19 Pandemic in Portugal. F1000Res 2020, 9, 283. [Google Scholar] [CrossRef]
- Midão, L.; Almada, M.; Carrilho, J.; Sampaio, R.; Costa, E. Pharmacological Adherence Behavior Changes during COVID-19 Outbreak in a Portugal Patient Cohort. Int. J. Environ. Res. Public Health 2022, 19, 1135. [Google Scholar] [CrossRef]
- Romano, S.; Galante, H.; Figueira, D.; Mendes, Z.; Rodrigues, A.T. Time-Trend Analysis of Medicine Sales and Shortages during COVID-19 Outbreak: Data from Community Pharmacies. Res. Soc. Adm. Pharm. 2021, 17, 1876–1881. [Google Scholar] [CrossRef]
- AlBahrani, S.; Almogbel, F.; Alanazi, W.; Almutairi, S.H.; Alanazi, M.; Maximos, S.; Azaiez, F.; Osman, A.; Almuthen, S.; Jebakumar, A.Z.; et al. Carbapenem Use Correlates with Percentage of Patients with COVID-19 in Intensive Care Units. Infection 2022. [Google Scholar] [CrossRef] [PubMed]
- PORDATA. SNS: Consultas, Internamentos e Urgências Nos Hospitais-Continente. Available online: https://www.pordata.pt/Portugal/SNS+consultas++internamentos+e+urg%C3%AAncias+nos+hospitais+++Continente-159 (accessed on 14 October 2022).
- Gironi, L.C.; Damiani, G.; Zavattaro, E.; Pacifico, A.; Santus, P.; Pigatto, P.D.M.; Cremona, O.; Savoia, P. Tetracyclines in COVID-19 Patients Quarantined at Home: Literature Evidence Supporting Real-World Data from a Multicenter Observational Study Targeting Inflammatory and Infectious Dermatoses. Dermatol. Ther. 2021, 34, 14694. [Google Scholar] [CrossRef] [PubMed]
- Instituto Nacional de Saúde Doutor Ricardo Jorge. Infeção VIH e SIDA Em Portugal—2020; Instituto Nacional de Saúde Doutor Ricardo Jorge: Lisboa, Portugal, 2020. [Google Scholar]
- Nestler, M.J.; Godbout, E.; Lee, K.; Kim, J.; Noda, A.J.; Taylor, P.; Pryor, R.; Markley, J.D.; Doll, M.; Bearman, G.; et al. Impact of COVID-19 on Pneumonia-Focused Antibiotic Use at an Academic Medical Center. Infect. Control. Hosp. Epidemiol. 2020, 42, 7. [Google Scholar] [CrossRef]
- Pinte, L.; Ceasovschih, A.; Niculae, C.-M.; Stoichitoiu, L.E.; Ionescu, R.A.; Balea, M.I.; Cernat, R.C.; Vlad, N.; Padureanu, V.; Purcarea, A.; et al. Antibiotic Prescription and In-Hospital Mortality in COVID-19: A Prospective Multicentre Cohort Study. J. Pers. Med. 2022, 12, 877. [Google Scholar] [CrossRef] [PubMed]
- Kalhor, H.; Sadeghi, S.; Abolhasani, H.; Kalhor, R.; Rahimi, H. Repurposing of the Approved Small Molecule Drugs in Order to Inhibit SARS-CoV-2 S Protein and Human ACE2 Interaction through Virtual Screening Approaches. J. Biomol. Struct. Dyn. 2020, 40, 1299–1315. [Google Scholar] [CrossRef]
- Morikawa, K.; Oseko, F.; Morikawa, S. Immunomodulatory effect of fosfomycin on human B-lymphocyte function. Antimicrob Agents Chemother. 1993, 2, 270–275. [Google Scholar] [CrossRef] [PubMed]
- Kazempour, M.; Izadi, H.; Chouhdari, A.; Rezaeifard, M. Anti-Inflammatory Effect of Metronidazole in Hospitalized Patients with Pneumonia Due to COVID-19. Iran. J. Pharm. Res. 2021, 20, 532–540. [Google Scholar] [CrossRef] [PubMed]
- Gharebaghi, R.; Heidary, F.; Moradi, M.; Parvizi, M. Metronidazole a Potential Novel Addition to the COVID-19 Treatment Regimen. Arch. Acad. Emerg. Med. 2020, 8, e40. [Google Scholar] [CrossRef] [PubMed]
- Chaves Filho, A.J.M.; Gonçalves, F.; Mottin, M.; Andrade, C.H.; Fonseca, S.N.S.; Macedo, D.S. Repurposing of Tetracyclines for COVID-19 Neurological and Neuropsychiatric Manifestations: A Valid Option to Control SARS-CoV-2-Associated Neuroinflammation? J. Neuroimmune Pharmacol. 2021, 16, 213–218. [Google Scholar] [CrossRef]
- Sodhi, M.; Etminan, M. Therapeutic Potential for Tetracyclines in the Treatment of COVID-19. Pharmacother. J. Hum. Pharmacol. Drug Ther. 2020, 40, 487–488. [Google Scholar] [CrossRef]
- Schellack, N.; Strydom, M.; Pepper, M.S.; Herd, C.L.; Hendricks, C.L.; Bronkhorst, E.; Meyer, J.C.; Padayachee, N.; Bangalee, V.; Truter, I.; et al. Social Media and COVID-19—Perceptions and Public Deceptions of Ivermectin, Colchicine and Hydroxychloroquine: Lessons for Future Pandemics. Antibiotics 2022, 11, 445. [Google Scholar] [CrossRef]
- Acharya, Y.; Sayed, A. Chloroquine and Hydroxychloroquine as a Repurposed Agent against COVID-19: A Narrative Review. Ther. Adv. Infect. Dis. 2020, 7, 2049936120947517. [Google Scholar] [CrossRef]
- Bienvenu, A.-L.; Bestion, A.; Pradat, P.; Richard, J.-C.; Argaud, L.; Guichon, C.; Roux, S.; Piriou, V.; Paillet, C.; Leboucher, G.; et al. Impact of COVID-19 Pandemic on Antifungal Consumption: A Multicenter Retrospective Analysis. Crit. Care 2022, 26, 384. [Google Scholar] [CrossRef]
- Pomati, F.; Castiglioni, S.; Zuccato, E.; Fanelli, R.; Vigetti, D.; Rossetti, C.; Calamari, D. Effects of a Complex Mixture of Therapeutic Drugs at Environmental Levels on Human Embryonic Cells. Environ. Sci. Technol. 2006, 40, 2442–2447. [Google Scholar] [CrossRef] [PubMed]
- Kümmerer, K.; Henninger, A. Promoting Resistance by the Emission of Antibiotics from Hospitals and Households into Effluent. Clin. Microbiol. Infect. 2003, 9, 1203–1214. [Google Scholar] [CrossRef]
- Sanseverino, I.; Loos, R.; Marinov, D.; Navarro Cuenca, A.; Lettieri, T. State of the Art on the Contribution of Water to Antimicrobial Resistance; EUR 29592 EN; Publications Office of the European Union: Luxembourg, 2018; ISBN 978-92-79-98478-5. [Google Scholar]
- Sandegren, L. Low Sub-Minimal Inhibitory Concentrations of Antibiotics Generate New Types of Resistance. Sustain. Chem. Pharm. 2019, 11, 46–48. [Google Scholar] [CrossRef]
- Varela, A.R.; André, S.; Nunes, O.C.; Manaia, C.M. Insights into the Relationship between Antimicrobial Residues and Bacterial Populations in a Hospital-Urban Wastewater Treatment Plant System. Water Res. 2014, 54, 327–336. [Google Scholar] [CrossRef] [PubMed]
- Rocephalin. Available online: https://www.fass.se/LIF/product?userType=2&nplId=19940902000051 (accessed on 20 November 2022).
- Karungamye, P.; Rugaika, A.; Mtei, K.; Machunda, R. A Review of Methods for Removal of Ceftriaxone from Wastewater. J. Xenobiot. 2022, 12, 223–235. [Google Scholar] [CrossRef]
- Shipingana, L.N.N.; Shivaraju, H.P.; Yashas, S.R. Quantitative Assessment of Pharmaceutical Drugs in a Municipal Wastewater and Overview of Associated Risks. Appl. Water Sci. 2022, 12, 16. [Google Scholar] [CrossRef]
- HPRA. Summary of Product Characteristics-Targaxan. Available online: https://www.hpra.ie/homepage/medicines/medicines-information/find-a-medicine/results/item?pano=PA1336/009/001&t=Targaxan%20550%20mg%20film-coated%20tablets (accessed on 20 November 2022).
- HPRA. Summary of Product Characteristics-Malusone. Available online: https://www.hpra.ie/img/uploaded/swedocuments/LicenseSPC_PA0126-233-001_28082017170036.pdf (accessed on 20 November 2022).
- Nixon, G.L.; Moss, D.M.; Shone, A.E.; Lalloo, D.G.; Fisher, N.; O’Neill, P.M.; Ward, S.A.; Biagini, G.A. Antimalarial Pharmacology and Therapeutics of Atovaquone. J. Antimicrob. Chemother. 2013, 68, 977–985. [Google Scholar] [CrossRef] [PubMed]
- Wellvone. Available online: https://www.fass.se/LIF/product?userType=2&nplId=19970327000107 (accessed on 2 November 2022).
- Ncube, S.; Madikizela, L.M.; Chimuka, L.; Nindi, M.M. Environmental Fate and Ecotoxicological Effects of Antiretrovirals: A Current Global Status and Future Perspectives. Water Res. 2018, 145, 231–247. [Google Scholar] [CrossRef]
- Sanderson, H.; Johnson, D.J.; Reitsma, T.; Brain, R.A.; Wilson, C.J.; Solomon, K.R. Ranking and Prioritization of Environmental Risks of Pharmaceuticals in Surface Waters. Regul. Toxicol. Pharmacol. 2004, 39, 158–183. [Google Scholar] [CrossRef]
- Kümmerer, K. Pharmaceuticals in the Environment: Sources, Fate, Effects and Risks, 3rd ed.; Kümmerer, K., Ed.; Springer: Berlin/Heidelberg, Germany, 2008. [Google Scholar]
- Jain, S.; Kumar, P.; Vyas, R.K.; Pandit, P.; Dalai, A.K. Occurrence and Removal of Antiviral Drugs in Environment: A Review. Water Air Soil Pollut. 2013, 224, 1410. [Google Scholar] [CrossRef]
- Laughlin, C.; Schleif, A.; Heilman, C.A. Addressing Viral Resistance through Vaccines. Future Virol. 2015, 10, 1011–1022. [Google Scholar] [CrossRef]
- Prasse, C.; Schlüsener, M.P.; Schulz, R.; Ternes, T.A. Antiviral Drugs in Wastewater and Surface Waters: A New Pharmaceutical Class of Environmental Relevance? Environ. Sci. Technol. 2010, 44, 1728–1735. [Google Scholar] [CrossRef]
- Funke, J.; Prasse, C.; Ternes, T.A. Identification of Transformation Products of Antiviral Drugs Formed during Biological Wastewater Treatment and Their Occurrence in the Urban Water Cycle. Water Res. 2016, 98, 75–83. [Google Scholar] [CrossRef]
- Boulard, L.; Dierkes, G.; Ternes, T. Utilization of Large Volume Zwitterionic Hydrophilic Interaction Liquid Chromatography for the Analysis of Polar Pharmaceuticals in Aqueous Environmental Samples: Benefits and Limitations. J. Chromatogr. A 2018, 1535, 27–43. [Google Scholar] [CrossRef] [PubMed]
- Abafe, O.A.; Späth, J.; Fick, J.; Jansson, S.; Buckley, C.; Stark, A.; Pietruschka, B.; Martincigh, B.S. LC-MS/MS Determination of Antiretroviral Drugs in Influents and Effluents from Wastewater Treatment Plants in KwaZulu-Natal, South Africa. Chemosphere 2018, 200, 660–670. [Google Scholar] [CrossRef]
- Mosekiemang, T.T.; Stander, M.A.; de Villiers, A. Simultaneous Quantification of Commonly Prescribed Antiretroviral Drugs and Their Selected Metabolites in Aqueous Environmental Samples by Direct Injection and Solid Phase Extraction Liquid Chromatography—Tandem Mass Spectrometry. Chemosphere 2019, 220, 983–992. [Google Scholar] [CrossRef]
- Aminot, Y.; Le Menach, K.; Pardon, P.; Etcheber, H.; Budzinski, H. Inputs and Seasonal Removal of Pharmaceuticals in the Estuarine Garonne River. Mar. Chem. 2016, 185, 3–11. [Google Scholar] [CrossRef]
- K’oreje, K.O.; Vergeynst, L.; Ombaka, D.; De Wispelaere, P.; Okoth, M.; Van Langenhove, H.; Demeestere, K. Occurrence Patterns of Pharmaceutical Residues in Wastewater, Surface Water and Groundwater of Nairobi and Kisumu City, Kenya. Chemosphere 2016, 149, 238–244. [Google Scholar] [CrossRef] [PubMed]
- Ngumba, E.; Kosunen, P.; Gachanja, A.; Tuhkanen, T. A Multiresidue Analytical Method for Trace Level Determination of Antibiotics and Antiretroviral Drugs in Wastewater and Surface Water Using SPE-LC-MS/MS and Matrix-Matched Standards. Anal. Methods 2016, 8, 6720–6729. [Google Scholar] [CrossRef]
- King, D.H. History, Pharmacokinetics, and Pharmacology of Acyclovir. J. Am. Acad. Dermatol. 1988, 18, 176–179. [Google Scholar] [CrossRef] [PubMed]
- Prasse, C.; Wagner, M.; Schulz, R.; Ternes, T.A. Biotransformation of the Antiviral Drugs Acyclovir and Penciclovir in Activated Sludge Treatment. Environ. Sci. Technol. 2011, 45, 2761–2769. [Google Scholar] [CrossRef]
- Schlüter-Vorberg, L.; Prasse, C.; Ternes, T.A.; Mückter, H.; Coors, A. Toxification by Transformation in Conventional and Advanced Wastewater Treatment: The Antiviral Drug Acyclovir. Environ. Sci. Technol. Lett. 2015, 2, 342–346. [Google Scholar] [CrossRef]
- Sherman, E.M.; Worley, M.V.; Unger, N.R.; Gauthier, T.P.; Schafer, J.J. Cobicistat: Review of a Pharmacokinetic Enhancer for HIV Infection. Clin. Ther. 2015, 37, 1876–1893. [Google Scholar] [CrossRef]
- Rezolsta. Available online: https://www.fass.se/LIF/product?userType=2&nplId=20131031000074 (accessed on 26 October 2022).
- Pihlaja, T.L.M.; Niemissalo, S.M.; Sikanen, T.M. Cytochrome P450 Inhibition by Antimicrobials and Their Mixtures in Rainbow Trout Liver Microsomes In Vitro. Environ. Toxicol. Chem. 2022, 41, 663–676. [Google Scholar] [CrossRef]
- INFARMED. Estatística Do Medicamento Produtos Saúde 2017. Lisboa, Portugal. 2018. Available online: https://www.infarmed.pt/documents/15786/1229727/Estat%C3%ADstica+do+Medicamento+2017/c759b946-9dcb-4b0a-b10b-6287bf76c114?version=1.0 (accessed on 2 November 2022).
- Besse, J.-P.; Kausch-Barreto, C.; Garric, J. Exposure Assessment of Pharmaceuticals and Their Metabolites in the Aquatic Environment: Application to the French Situation and Preliminary Prioritization. Hum. Ecol. Risk Assess. Int. J. 2008, 14, 665–695. [Google Scholar] [CrossRef]
- Definition and General Considerations. Available online: https://www.whocc.no/ddd/definition_and_general_considera/ (accessed on 10 May 2022).
- Almeida, A.; Duarte, S.; Nunes, R.; Rocha, H.; Pena, A.; Meisel, L. Human and Veterinary Antibiotics Used in Portugal—A Ranking for Ecosurveillance. Toxics 2014, 2, 188–225. [Google Scholar] [CrossRef]
- Guo, J.; Sinclair, C.J.; Selby, K.; Boxall, A.B.A. Toxicological and Ecotoxicological Risk-based Prioritization of Pharmaceuticals in the Natural Environment. Environ. Toxicol. Chem. 2016, 35, 1550–1559. [Google Scholar] [CrossRef] [PubMed]
- Schmitt, H.; ter Laak, T.; Duis, K. Development and Dissemination of Antibiotic Resistance in the Environment under Environmentally Relevant Concentrations of Antibiotics and Its Risk Assessment; Literature Study; Umweltbundesamt: Dessau-Roßlau, Germany, 2017. [Google Scholar]
- Committee for Medicinal Products for Human Use. Guideline on the Environmental Risk Assessment of Medicinal Products for Human Use; European Medicines Agency: Amsterdam, The Netherlands, 2006. [Google Scholar]
- Pereira, A.M.P.T.; Silva, L.J.G.; Lino, C.M.; Meisel, L.M.; Pena, A. A Critical Evaluation of Different Parameters for Estimating Pharmaceutical Exposure Seeking an Improved Environmental Risk Assessment. Sci. Total Environ. 2017, 603, 226–236. [Google Scholar] [CrossRef]
- Guidance on Information Requirements and Chemical Safety Assessment Chapter R.10: Characterisation of Dose [Concentration]-Response for Environment, 2008. Available online: https://echa.europa.eu/documents/10162/17224/information_requirements_r10_en.pdf/bb902be7-a503-4ab7-9036-d866b8ddce69?t=1322594768638 (accessed on 17 February 2023).
- Bengtsson-Palme, J.; Larsson, D.G.J. Concentrations of Antibiotics Predicted to Select for Resistant Bacteria: Proposed Limits for Environmental Regulation. Environ. Int. 2016, 86, 140–149. [Google Scholar] [CrossRef]
- Cefazoline. Available online: https://go.drugbank.com/drugs/DB01327 (accessed on 23 October 2022).
- EMA. Annex III-Summary of Product Characteristics, Labelling and Package leaflet-Rocephin and Associated Names. Available online: https://www.ema.europa.eu/en/documents/referral/rocephin-article-30-referral-annex-iii_en.pdf (accessed on 26 October 2022).
- Agwuh, K.N.; MacGowan, A. Pharmacokinetics and Pharmacodynamics of the Tetracyclines Including Glycylcyclines. J. Antimicrob. Chemother. 2006, 58, 256–265. [Google Scholar] [CrossRef] [PubMed]
- Flucloxacillin. Available online: https://www.medicines.org.uk/emc/product/545/smpc#gref (accessed on 20 November 2022).
- HPRA. Summary of Product Characteristics-Monuril. 2020. Available online: https://www.hpra.ie/img/uploaded/swedocuments/Licence_PA1441-002-002_24072020094950.pdf (accessed on 20 November 2022).
- Linezolid. Available online: https://go.drugbank.com/drugs/DB00601 (accessed on 21 October 2022).
- EMA. Annex I-Summary of Product Characteristics-Vaborem. 2018. Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/vaborem#product-information-section (accessed on 20 November 2022).
- Flagyl. Available online: https://go.drugbank.com/drugs/DB00916 (accessed on 20 October 2022).
- Minocycline. Available online: https://go.drugbank.com/drugs/DB01017 (accessed on 2 October 2022).
- HPRA. Summary of Product Characteristics-Tazocin. Available online: https://www.hpra.ie/img/uploaded/swedocuments/LicenseSPC_PA0822-098-001_22072016100058.pdf (accessed on 20 November 2022).
- Su, C.G.; Aberra, F.; Lichtenstein, G.R. Utility of the Nonabsorbed (<0.4%) Antibiotic Rifaximin in Gastroenterology and Hepatology. Gastroenterol. Hepatol. 2006, 2, 186–197. [Google Scholar]
- Taylor, D.N.; McKenzie, R.; Durbin, A.; Carpenter, C.; Haake, R.; Bourgeois, A.L. Systemic Pharmacokinetics of Rifaximin in Volunteers with Shigellosis. Antimicrob. Agents Chemother. 2008, 52, 1179–1181. [Google Scholar] [CrossRef]
- Trimethoprim. Available online: https://go.drugbank.com/drugs/DB00440 (accessed on 2 November 2022).
- Türk, D.; Hanke, N.; Lehr, T. A Physiologically-Based Pharmacokinetic Model of Trimethoprim for MATE1, OCT1, OCT2, and CYP2C8 Drug–Drug–Gene Interaction Predictions. Pharmaceutics 2020, 12, 1074. [Google Scholar] [CrossRef]
- Vancomycin. Available online: https://go.drugbank.com/drugs/DB00512 (accessed on 12 October 2022).
- Acyclovir. Available online: https://go.drugbank.com/drugs/DB00787 (accessed on 14 October 2022).
- Abdalla, S.; Briand, C.; Oualha, M.; Bendavid, M.; Béranger, A.; Benaboud, S.; Tréluyer, J.-M.; Zheng, Y.; Capito, C.; Demir, Z.; et al. Population Pharmacokinetics of Intravenous and Oral Acyclovir and Oral Valacyclovir in Pediatric Population To Optimize Dosing Regimens. Antimicrob. Agents Chemother. 2020, 64, e01426-20. [Google Scholar] [CrossRef]
- Lamivudine. Available online: https://go.drugbank.com/drugs/DB00709 (accessed on 20 October 2022).
- EMA. Annex I-Summary of Product Characteristics-Epivir. 2022. Available online: https://www.ema.europa.eu/en/documents/product-information/epivir-epar-product-information_en.pdf (accessed on 20 October 2022).
- EMA. Annex I-Summary of Product Characteristics-Emtriva. Available online: https://www.ema.europa.eu/en/documents/product-information/emtriva-epar-product-information_en.pdf (accessed on 22 October 2022).
- Modrzejewski, K.A.; Herman, R.A. Emtricitabine: A Once-Daily Nucleoside Reverse Transcriptase Inhibitor. Ann. Pharmacother. 2004, 38, 1006–1014. [Google Scholar] [CrossRef] [PubMed]
- EMA. Annex I-Summary of Product Characteristics-Isentress. 2022. Available online: https://www.ema.europa.eu/en/documents/product-information/isentress-epar-product-information_en.pdf (accessed on 20 October 2022).
- Burger, D.M. Raltegravir: A Review of Its Pharmacokinetics, Pharmacology and Clinical Studies. Expert Opin. Drug Metab. Toxicol. 2010, 6, 1151–1160. [Google Scholar] [CrossRef]
- EMA. Annex I-Summary of Product Characteristics-Tivicay. 2022. Available online: https://www.ema.europa.eu/en/documents/product-information/tivicay-epar-product-information_en.pdf (accessed on 20 October 2022).
- Castellino, S.; Moss, L.; Wagner, D.; Borland, J.; Song, I.; Chen, S.; Lou, Y.; Min, S.S.; Goljer, I.; Culp, A.; et al. Metabolism, Excretion, and Mass Balance of the HIV-1 Integrase Inhibitor Dolutegravir in Humans. Antimicrob. Agents Chemother. 2013, 57, 3536–3546. [Google Scholar] [CrossRef] [PubMed]
- Nicol, M.R.; Joshi, A.; Rizk, M.L.; Sabato, P.E.; Savic, R.M.; Wesche, D.; Zheng, J.H.; Cook, J. Pharmacokinetics and Pharmacological Properties of Chloroquine and Hydroxychloroquine in the Context of COVID-19 Infection. Clin. Pharmacol. Ther. 2020, 108, 1135–1149. [Google Scholar] [CrossRef] [PubMed]
- Hydroxychloroquine. Available online: https://go.drugbank.com/drugs/DB01611 (accessed on 21 October 2022).
- Baggish, A.L.; Hill, D.R. Antiparasitic Agent Atovaquone. Antimicrob. Agents Chemother. 2002, 46, 1163–1173. [Google Scholar] [CrossRef]
- Wess, R.A.; Schmidt, T.; Höger, S. Challenges of Regulatory Environmental Risk Assessment for Human Pharmaceuticals with Focus on Antibiotics. Chimia 2020, 74, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Piperacillin/Tazobactam. Available online: https://www.fass.se/LIF/product?userType=2&nplId=20070720000011 (accessed on 25 October 2022).
- Meropenem. Available online: https://www.fass.se/LIF/product?userType=2&nplId=20090613000065&docType=78&scrollPosition=901.69873046875 (accessed on 21 October 2022).
- Le Page, G.C. Environmental Risk Assessment of Antibiotics: Investigations into Cyanobacteria Interspecies Sensitivities and Establishing Appropriate Protection Limits. Ph.D. Thesis, University of Exeter, Exeter, UK, 2018. [Google Scholar]
- Guo, J.; Selby, K.; Boxall, A.B.A. Comparing the Sensitivity of Chlorophytes, Cyanobacteria, and Diatoms to Major-Use Antibiotics: Algal Sensitivity to Antibiotics. Environ. Toxicol. Chem. 2016, 35, 2587–2596. [Google Scholar] [CrossRef] [PubMed]
- Brain, R.A.; Johnson, D.J.; Richards, S.M.; Sanderson, H.; Sibley, P.K.; Solomon, K.R. Effects Of 25 Pharmaceutical Compounds to Lemna Gibba Using a Seven-Day Static-Renewal Test. Environ. Toxicol. Chem. 2004, 23, 371–382. [Google Scholar] [CrossRef] [PubMed]
- Stoichev, T.; Baptista, M.S.; Basto, M.C.P.; Vasconcelos, V.M.; Vasconcelos, M.T.S.D. Effects of Minocycline and Its Degradation Products on the Growth of Microcystis Aeruginosa. Ecotoxicol. Environ. Saf. 2011, 74, 219–224. [Google Scholar] [CrossRef] [PubMed]
- Xifaxan. Available online: https://www.fass.se/LIF/product?userType=2&nplId=20110923000033 (accessed on 15 November 2022).
- Havelkova, B.; Beklova, M.; Kovacova, V.; Hlavkova, D.; Pikula, J. Ecotoxicity of Selected Antibiotics for Organisms of Aquatic and Terrestrial Ecosystems. Neuroendocrinol. Lett. 2016, 37, 38–44. [Google Scholar] [PubMed]
- Emtricitabine/Tenofovir Disoproxil Sandoz. Available online: https://www.fass.se/LIF/product?userType=2&nplId=20160202000087 (accessed on 27 November 2022).
- Abacavir/Lamivudine Sandoz. Available online: https://www.fass.se/LIF/product?userType=2&nplId=20141230000119 (accessed on 27 November 2022).
- Dovato. Available online: https://www.fass.se/LIF/product?userType=2&nplId=20180914000069 (accessed on 15 November 2022).
- Isentress. Available online: https://www.fass.se/LIF/product?userType=2&nplId=20070602000016 (accessed on 15 November 2022).
- Plaquenil. Available online: https://www.fass.se/LIF/product?userType=2&nplId=19610412000017 (accessed on 21 October 2022).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).