Abstract
Critically ill patients undergo significant pathophysiological changes that affect antibiotic pharmacokinetics. Piperacillin/tazobactam administered by continuous infusion (CI) improves pharmacokinetic/pharmacodynamic (PK/PD) target attainment. This study aimed to characterize piperacillin PK after CI administration of piperacillin/tazobactam in critically ill adult patients with preserved renal function and to determine the empirical optimal dosing regimen. A total of 218 piperacillin concentrations from 106 patients were simultaneously analyzed through the population PK approach. A two-compartment linear model best described the data. Creatinine clearance (CLCR) estimated by CKD-EPI was the covariate, the most predictive factor of piperacillin clearance (CL) interindividual variability. The mean (relative standard error) parameter estimates for the final model were: CL: 12.0 L/h (6.03%); central and peripheral compartment distribution volumes: 20.7 L (8.94%) and 62.4 L (50.80%), respectively; intercompartmental clearance: 4.8 L/h (26.4%). For the PK/PD target of 100% fT>1×MIC, 12 g of piperacillin provide a probability of target attainment > 90% for MIC < 16 mg/L, regardless of CLCR, but higher doses are needed for MIC = 16 mg/L when CLCR > 100 mL/min. For 100% fT>4×MIC, the highest dose (24 g/24 h) was not sufficient to ensure adequate exposure, except for MICs of 1 and 4 mg/L. Our model can be used as a support tool for initial dose guidance and during therapeutic drug monitoring.
1. Introduction
Sepsis in intensive care units (ICUs) is a major health problem worldwide due to the high morbidity and mortality associated with it [1,2,3]. An early and appropriate antimicrobial therapy is a key component for optimizing the chances of survival, with a focus on timely administration, the appropriateness of the antimicrobial spectrum and dosing optimization from the beginning of the treatment [1].
Piperacillin/tazobactam (P/T) is a β-lactam/β-lactamase inhibitor that exhibits activity against a broad range of microorganisms, including gram-positive and gram-negative aerobic and anaerobic bacteria. It is routinely prescribed for the empirical and targeted treatment of infections in critically ill patients with sepsis or septic shock [4]. Its antibacterial activity is time-dependent, so its efficacy is related to the time (T) during which the free drug concentration (f) is maintained above the minimum inhibitory concentration (MIC) (%fT>MIC) [5]. The pharmacokinetic/pharmacodynamic (PK/PD) target is based on this premise, and despite the lack of consensus as to which is optimal, for critically ill patients, treatment is considered adequate when free concentrations of the antibiotic are at least 100% fT>4×MIC [6,7].
Critically ill patients present pathophysiological changes, such as third spacing (fluid extravasation into interstitial space), increased cardiac output, hypoalbuminemia or elevated creatinine clearance that may affect antibiotic pharmacokinetics (PKs), leading to sub-therapeutic antibiotic concentrations [4,8,9,10]. Moreover, the presence of chronic comorbidity can further exacerbate the pathophysiological changes commonly encountered during critical illness [10]. It is worthy of note that such alterations are multifactorial and may not be present in all patients, further decreasing the predictability of PK/PD in critically ill patients.
P/T, as the rest of β-lactam antibiotics, is a hydrophilic antibiotic with moderate protein binding (30%) and mainly cleared by renal excretion (i.e., glomerular filtration and active tubular secretion). These characteristics may cause its PK to be particularly affected by the above-mentioned events [11]. Previous studies have found that administering P/T as extended or continuous infusion (CI) rather than intermittent infusion (II) might improve PK/PD target attainment in these situations [1,11,12,13,14,15]. In fact, the administration of P/T by CI preceded by a loading dose is nowadays a widespread practice in the ICU.
Population PK (PPK) models are powerful descriptive and predictive support tools for the implementation of therapeutic drug monitoring (TDM). While several piperacillin PPK models have been previously published in critically ill adult patients, most of them were the target population undergoing renal replacement therapy (RRT) and are, therefore, not applicable to other population groups [16,17,18,19,20]. Furthermore, in models developed in patients without RRT, CLCR was frequently identified as the strongest covariate with a significant linear relationship to piperacillin clearance [21,22,23,24,25,26,27,28]. However, in the vast majority, P/T administered as II was used to predict PK behavior in CI [22,25,26,27,28]. Caution should be taken when making dose estimates in CI based on models developed in II, as non-linear PKs have been postulated with the latter form of administration due to saturation of piperacillin elimination [29,30]. This phenomenon could make such estimates not fully equivalent since CI-predicted concentrations could be overestimated, leading to underexposure. The development of PPK models based on CI data is, therefore, necessary.
In a previous study by our group [31], statistical analyses showed that standard total daily doses of piperacillin administered by CI were not enough to achieve optimal PK/PD targets when the minimum inhibitory concentration (MIC) was above 8 mg/L. In addition, elevated creatinine clearance (CLCR), followed by neurocritical status and mechanical ventilation, were identified as risk factors associated with subtherapeutic exposure. Therefore, a modeling approach is required to confirm previous results and to enable P/T dose optimization in the target population.
The aims of this study were: (i) to characterize the PK of piperacillin after administration of P/T as a CI in a population of critically ill adult patients with preserved renal function, using a population-based approach; and (ii) to determine the empirical optimal dosing regimen in this population.
2. Results
2.1. Patients and Datasets
A total of 218 samples from 106 patients were available for analysis. Patients’ demographic and clinical characteristics at baseline are reported in Table 1. The patients included were mainly men (67%), the median age was 65 years (interquartile range (IQR) 50–72) and the median CLCR according to the Chronic Kidney Disease Epidemiology Collaboration formula (CLCRCKD-EPI) was 97 mL/min/1.73 m2 (IQR 86–114). Augmented renal clearance (ARC) was identified in 11 patients (10.38%). BMI values were indicative of non-obesity.

Table 1.
Demographic and baseline clinical characteristics.
All patients underwent therapeutic drug monitoring based on the piperacillin measured concentrations, and only on four occasions from three different patients was the dose modified. The piperacillin plasma concentration-time profiles are displayed in Figure 1. The average number of samples per patient was 2.1. The peak and steady-state concentration (Cmax and Css) geometric mean values were 112 mg/L (IQR 69–184) and 39.5 mg/L (27–58), respectively.
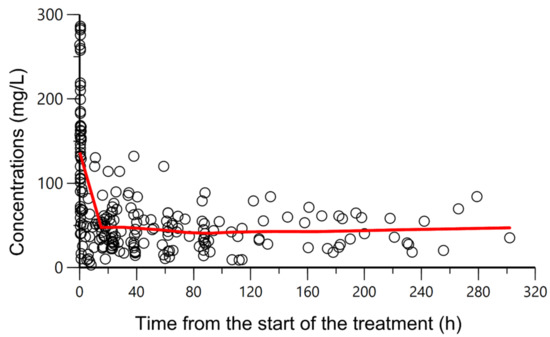
Figure 1.
Overlapping observed piperacillin plasma concentrations (mg/L) (circles) versus time from the start of the treatment (h). A CI of piperacillin was administered, preceded by a loading dose of 4 g given over 30 min. Solid red line: smooth line indicating the general data trend.
2.2. Population PK Modeling
A two-compartment model with first-order elimination from the central compartment was considered to best describe piperacillin plasma concentration-time profiles. Between-patient variability (BPV) could only be associated with plasma clearance. Residual variability was best described by a proportional error model.
Inclusion of all the candidate covariates in the model, one at each step, identified renal function and administration of vasoactive drugs as the only covariates with a statistically significant effect on piperacillin plasma clearance (CL). CLCR given by CKD-EPI resulted in the highest decrease in the minimum objective function value (MOFV) (∆MOFV = −29.88, p < 0.001), followed by the Cockcroft and Gault formula (CG) (∆MOFV = −27.27, p < 0.001) and then by the Modification of Diet in Renal Disease formula (MDRD-4) (∆MOFV = −18.62, p < 0.001). Between-patient variabilities associated with CL were reduced by 24.90% (CKD-EPI), 24.51% (CG) and 12.25% (MDRD-4). Although vasoactive drugs statistically reduced the MOFV (∆MOFV = −8.83, BPV = 10.67%), the sequential inclusion of the administration of vasoactive drugs on CLCR-models did not result in a significant decrease of the OFV for any of the CLCR models considered.
According to these results, the final model only retained renal function as a covariate of CL, with a linear relationship between the typical value of plasma clearance and CLCRCKDEPI centered at its median value in the target population.
The parameter estimates of the base and final models as the bootstrap results are summarized in Table 2. All the PK parameters were estimated with adequate precision. The mean population values of all the model parameters were within the 95% confidence intervals estimated by the bootstrap method. The relative deviation between the true population value and the median value provided by bootstrap was lower than 10% for all the PK parameters. The condition number of the model was 4.40, suggesting no notable collinearity. Acceptable shrinkage values associated with ω2CL and σ2 were found.

Table 2.
Base model, final model and bootstrap parameter estimates and variabilities from the population pharmacokinetic modeling analysis, including uncertainty and shrinkage.
2.3. Model Evaluation
Goodness-of-fit plots of the final model are displayed in Figure 2. A random distribution around the identity line was defined for observed concentrations (DVs) versus population predicted values (PREDs) and DV versus individual predicted values (IPREDs). Conditional weighted residuals (CWRESs) versus time and individual weighted residuals (IWRESs) versus time also showed a random distribution around zero. All these plots were indicative of no model mis-specification (DV/PRED and CWRES/time) and adequate description of between-patient variability (DV/IPRED) and residual error (IWRES/time).
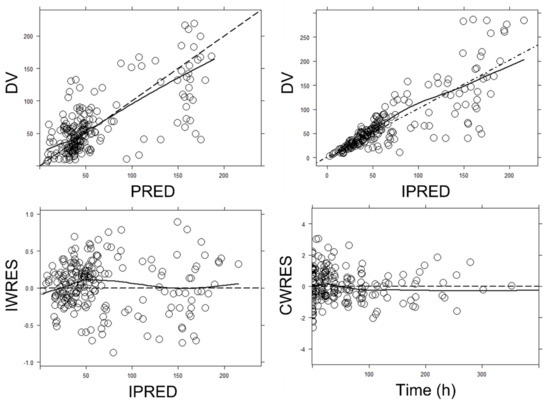
Figure 2.
Goodness-of-fit plots for the final population pharmacokinetic model. Upper left panel: observed (DV) vs. population predicted concentrations (PRED). Upper right panel: DV vs. individual predicted concentrations (IPREDs). Bottom left panel: individual weighted residuals (IWRESs) vs. IPRED. Bottom right panel: population conditional weighted residuals (CWRESs) vs. time from the start of the treatment. Dashed line: identity line; Solid line: smooth line indicating the general data trend. Time was given in hours. Concentrations were given in mg/L.
The visual inspection of the prediction-corrected, visual predictive checks (Figure 3) proved the descriptive and predictive capability of the model. The model adequately described the mean trend of the data. Overall, 50%, 2.5% and 97.5% of the observed data fell within the 95% confidence intervals of the corresponding percentiles of the simulated data, and most of the observed concentrations also fell within the 95% prediction interval.
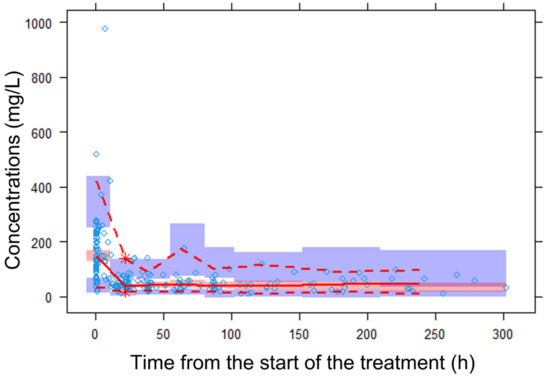
Figure 3.
Prediction-corrected, visual predictive check (Predcorr-vpc) of the final pharmacokinetic model for piperacillin. The diamond represents the observed data. The red dashed lines depict the 2.5th and 97.5th percentiles of the observed concentrations. The solid line corresponds to the 50th percentiles of the observed concentrations. The red and blue bands represent the 95% prediction intervals of the 50% and 2.5% and 97.5% percentiles of the simulated data. Predcorr-vpc showed that most of the observed concentrations fell within the 90% prediction interval of the simulated data and were randomly distributed around the median.
2.4. Probability of Target Attainment and Cumulative Fraction of Response
Figure 4 shows the probability of target attainment (PTA) for each one of the PK/PD evaluated targets (100% fT>1×MIC and 100% fT>4×MIC) for each piperacillin dosage regimen (8, 12, 16, 20 and 24 g given daily as CI), renal function cut-off (from 60 to 200 mL/min/1.73 m2 in steps of 20 mL/min/1.73 m2) and MIC scenarios (from 1 to 16 mg/L).
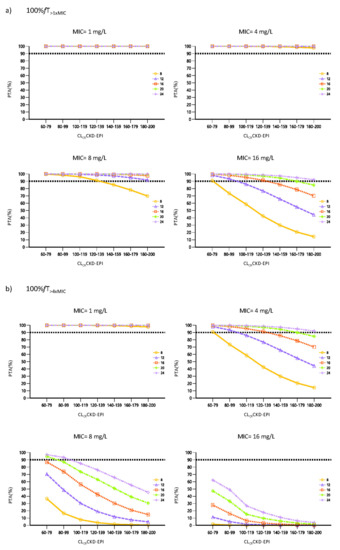
Figure 4.
Probability of Target Attainment (PTA) for free piperacillin plasma concentrations at steady state. The prediction shows the PTA at 5 daily doses levels of 8, 12, 16, 20 and 24 g/24 h given as CI varying renal clearance (cut-offs ranging from 60–79 to 180–200 mL/min/1.73 m2). The MIC values of 1, 4, 8 and 16 mg/L were evaluated considering 2 different PK/PD targets (a) 100% fT>1×MIC, (b) 100% fT>4×MIC. The horizontal dashed line indicates the 90% of PTA.
Cumulative fraction of response (CFR) for susceptible Pseudomonas aeruginosa isolates (MIC ≤ 16 mg/L) for the different piperacillin CI dosage regimens evaluated (preceded by a 4 g piperacillin loading dose) and for each CLCRCKD-EPI cut-off, considering the 2 PK/PD targets (100% fT>1×MIC and 100% fT>4×MIC), are summarized in Table 3.

Table 3.
Cumulative fraction of response for piperacillin over ranges of creatinine clearance values.
3. Discussion
This is the largest study (N = 106 patients) on predictive factors of piperacillin exposure and PK/PD target attainment in critically ill patients with normal renal function after CI administration of P/T. While previous studies on critically ill patients without RRT exist, some were mainly focused on the comparison among different types of administration (II or CI) [11,21]. In other studies, the impact of P/T administration as CI on PK/PD target attainment was investigated, but the target population consisted of patients with a wider range of variation in renal function (from around <30 to >130 mL/min) than in the current study [23,24].
Our large sample size has allowed us to better investigate the influence of new covariates other than those classically explored, such as renal function or weight. Indeed, the influences of neurocritical status, mechanical ventilation or drainage carriage were also evaluated. Moreover, unlike previous studies, the performance of the CKD-EPI formula, compared to others traditionally used (CG and MDRD-4), was assessed.
Although a sparse sample design was applied, our data allowed us to describe the piperacillin PK profile by a two-compartment disposition model with linear elimination. Probably, the sampling design, in which concentrations were measured after the loading dose, benefited the characterization of drug distribution. Hence, the current model resulted in a more adequate description of piperacillin concentrations than the previous one-compartment models reported after CI administration [23,24].
The finding of the first-order elimination agreed with previous studies and confirmed that no saturation of the piperacillin elimination process mediated through tubular secretion takes place during CI administration [23,24]. It should be noted that it has been postulated that saturation could also occur at therapeutic doses during CI administration [21]; nevertheless, a Michaelis–Menten or a parallel first-order/Michaelis–Menten elimination were tested and did not improve the data fit. This was in agreement with other studies that reported that saturable elimination should not be expected to be of major clinical importance in patients given daily doses from 6 to 18 g piperacillin [32].
In line with previous studies, CLCR was identified as the most powerful predictor of piperacillin CL variability, despite the narrower renal function variation of our population [60–180 mL/min/1.73 m2] compared to theirs [23,24]. This finding is supported by the high contribution of renal excretion (70%) to the piperacillin elimination [33]. Due to the controversy as to which formula is the most suitable for predicting renal clearance in critically ill patients, the three most widely accepted (CKD-EPI, CG, MDRD-4) were evaluated in our study [34]. All of them significantly accounted for the variability of CL; however, CKD-EPI provided the best exposure predictions, followed by MDRD-4 and then by CG, confirming the findings of other authors with other drugs also renally excreted [35]. The fact that the IDMS-traceable creatinine was not used for the standardization of creatinine concentrations when the CG module was developed, and that its equation includes body weight instead of body surface area, could be reasons for the overestimation of renal function using this formula. Dhaese et al. also showed that MDRD-4 was superior to CG, but no comparison could be performed with CKD-EPI, as this was the first study in which this equation has been used to predict piperacillin exposure [23].
No influence of any of the other variables on piperacillin concentrations was demonstrated. Mechanical ventilation was able to decrease CL variability, but this was not considered significant. Further larger studies would be necessary to evaluate this association.
The piperacillin CL in our study (12 L/h) was higher than that reported by Dhaese et al. (8.38 L/h) and Klastrup et al. (6.43 L/h) [23,24]. This difference may be explained by the fact that they included patients with renal dysfunction, whereas in our case, in order to obtain a more accurate characterization of piperacillin PK in the subset of patients with preserved renal function, only patients with CLCR ≥ 60 mL/min/1.73 m2 were included. These findings are supported by the results of our previous statistical analysis of the same data [31].
Differences were also encountered between distribution volumes, among studies. The volume of distribution (Vd) of piperacillin was significantly larger in our cohort of patients (83.1 L) than that estimated in two other previous studies (25.54 L and 35.8 L) [23,24]. Furthermore, the central compartment distribution volume (Vc) in our study was larger (20.7 L) than the one found by Roberts et al. in 16 patients treated either with CI or intermittent bolus (7.2 L) [11]. In our analysis, the Cmax values of 53 patients (50%) were included in the model. Considering that Vc estimation relies on Cmax values, the final Vc estimate by our model should be considered reliable. Differences in body weight among studies might also explain these discrepancies. However, neither body weight nor any other size or body composition metrics were statistically significant in any of the parameters of the model. The inclusion of body weight would have allowed a more accurate estimation of PK parameters and exposure predictions; therefore, further studies will be required to investigate the influence of this covariate. On the other hand, several factors lead to an increased Vd in the critically ill patient. The expansion of the interstitial space through capillary leakage, in addition to the intravenous fluid load suffered by these patients, results in a significant increase of the Vd of hydrophilic drugs and thus to a lower antibiotic concentration. This phenomenon is further aggravated by the occurrence of hypoalbuminemia, the presence of which should be considered in our cohort of patients (median albumin concentrations 29 g/L) [36,37].
Conflicting findings have been reported so as to put forward the clinical advantages that P/T CI can provide over other forms of administration [12,13,15,38]. However, a meta-analysis from randomized trials in patients with severe sepsis demonstrated a lower mortality rate in patients treated with beta-lactam by CI with respect to those treated by II [39]. In the same line, two recent meta-analyses comparing the administration of beta-lactams, including P/T, by prolonged versus II demonstrated a reduction in the clinical cure rate and mortality [40,41]. Moreover, there is growing evidence supporting beta-lactam CI over conventional II regimens for a greater achievement of the PK/PD target [11,12,13,14,15]. Thus, considering the need for rapid and adequate antibiotic coverage in the critically ill patient and the absence of adverse effects related to this form of administration, CI of beta-lactams preceded by an initial loading dose is currently a formal recommendation [1].
Since the internal validation endorsed the predictive capability of the model, the impact of elevated renal function and decreasing bacterial susceptibility on piperacillin PK/PD attainment when administered by CI was evaluated. Our simulations indicate that for the target of 100% fT>1×MIC, PTA was greater than 90% when using daily doses of 12 g CI for MICs of 1, 4 and 8 mg/L, regardless of renal function. However, when considering the worst case scenario (MIC of 16 mg/L), standard dosing regimens by CI (12 g/24 h and 16 g/24 h) would be not enough in patients with CLCR greater than 100 and 140 mL/min/1.73 m2, respectively. Only the highest daily doses would provide adequate exposure for patients with ARC, which is in line with previous studies published [23,24]. In the rest of the scenarios, the achievement of a PTA above the cut-off point was closely dependent on renal function. When seeking the higher target fT>4×MIC and for a MIC of 16 mg/L or susceptible Pseudomonas aeruginosa isolates (MIC ≤ 16 mg/L), even the highest dose is far from reaching the cut-off point off 90%. These results confirm those previously found by Dhaese et al., who concluded that the 24 g/24 h dose was insufficient to ensure adequate exposure in patients with CLCR > 90 mL/min/1.73 m2 [23].
The present results highlight the influence of CLCR on piperacillin CL and point out the great difficulty in achieving adequate treatment for critically ill patients without renal impairment when a more demanding PK/PD target is sought. The lack of consensus regarding at what the PK/PD index of beta-lactams in the critically ill patient should be established makes it difficult to provide solid dosing strategy recommendations. In fact, neither the %fT nor the threshold value to be reached are clearly defined, so that the range of targets fixed in the available studies may vary from 20% fT>1×MIC to 100% fT>5×MIC [6]. Available data suggest that maximum bactericidal activity occurs when the dose is maintained between four and five times the MIC. On this basis, beta-lactam treatment of critically ill patients is currently considered optimal when concentrations are at least 100% fT>4×MIC [6,7]. Aware of the difficulties involved in achieving such a demanding objective, we decided to also evaluate the achievement of the 100% fT>1×MIC target, which seems more realistic under certain conditions and has also proven to be effective. In any case, the simulations obtained are consistent with the fact that augmented renal clearance (ARC) is recognized as an important predictor of beta-lactam underexposure [36,42,43]. ARC is being increasingly reported in critically ill patients, and, although its impact on clinical outcome is unclear, it should be identified since patients may benefit from higher antimicrobial doses at the beginning of the antimicrobial treatment [36,43,44,45].
Patients were treated with P/T, but only piperacillin concentrations were measured. There is enough evidence supporting the existence of a high correlation between piperacillin and tazobactam concentrations [28,46,47]. In fact, a recent PK study showed that tazobactam concentrations were above the threshold in 92% of cases in which the piperacillin PK/PD target was achieved [47]. The similarity in the PK of these two compounds means that the determination of tazobactam is often considered dispensable, and consequently, studies in which it is evaluated are scarce. In previous PPK studies carried out in patients undergoing RRT [16,19], simulated tazobactam concentrations were found to be above threshold at all times. In a more recent study in which P/T was administered by II, tazobactam concentrations were predicted in critically ill patients with creatinine clearance up to 200 mL/min [28]. The tazobactam target was reached in 94% and 99% of the patients when doses of 1.5 g/24 h and ≥2 g/24 h were administered, thus P/T efficacy was considered to be uniquely related to piperacillin concentration. Although Wallemburg et al. considered the development of a joint model to adequately predict piperacillin concentrations [28], previous studies showed there was no interaction between them, and the PK/PD analysis was therefore based solely on the simulations performed with the piperacillin model [16,19]. Additional studies assessing tazobactam concentrations are needed to confirm the existence of such an association.
Other limitations may apply to our study. Only total drug concentrations were measured, while pharmacological activity and PK/PD targets are related to free fraction. Since the united fraction is considered to be 30% of the total, the free concentration was estimated [48]. Further, the study explores the likelihood of target attainment using different levels of bacterial susceptibility to optimize empirical dosing regimen, so no conclusions can be drawn regarding the appropriate drug exposure for any specific patient, for which TDM remains critical.
4. Material and Methods
4.1. Study Design
This was an observational prospective study conducted at the ICU of the Bellvitge University Hospital in Barcelona, Spain, during a 4-year period (August 2015–June 2019). Ethical approval was obtained from the local Ethics Committee (SFB-ATB-2014-01) and conducted in accordance with the Declaration of Helsinki. Written informed consent was requested from the patient or the closest relative before inclusion.
4.2. Patient Population
Critically ill patients under treatment with P/T administered as CI and with a preserved renal function (CLCRCKD-EPI ≥ 60 mL/min/1.73 m2) were eligible for the study. Patients receiving renal replacement therapy or extracorporeal membrane oxygenation, pregnant women and patients under 18 years were excluded.
4.3. Dosage, Sampling and Data Collection
Patients received an initial loading dose of P/T 4/0.5 g, administered in 30 min on day 1, followed by a continuous 24 h infusion of piperacillin (500 mg/h), i.e., 12 g piperacillin/1.5 g tazobactam in 150 mL 0.9% sodium chloride (80 mg/mL, stability of 24 h at 25 °C, 1 infusion/day). Patients who had already been on intermittent antibiotic treatment for at least 24 h did not receive the loading dose. This dose was subsequently modified, depending on the plasma concentrations achieved. Two different sampling periods were considered: shortly after the loading dose (30–60 min) and once steady-state conditions were attained (from 6 h after initiation of CI), from which Cmax and Css were measured, respectively.
The patients’ demographic (age, gender, weight, heigh, body mass index (BMI) and race) and clinical data (creatinine, urea, albumin, neurocritical status, mechanical ventilation, treatment with vasoactive drugs, and drainage carriage) were recorded at baseline and on each sampling occasion. CLCR was calculated using three different equations: CKD-EPI, CG and MDRD-4. ARC, defined as CLCRCKD-EPI ≥ 130 mL/min/1.73 m2, was also assessed. The presence of neurological damage, use of mechanical ventilation, use of vasoactive drugs and presence of drainage were also prospectively recorded.
4.4. Bioanalysis
For piperacillin determination, approximately 3 mL of blood were collected in lithium-heparin tubes (Vacuette, Kremsmünster, Austria) and immediately refrigerated at 2–8 °C for a maximum of 30 min. Samples were then centrifuged at 2000× g for 10 min at (4 ± 1) °C, aliquoted and stored at (−75 ± 3) °C until analysis.
We analyzed total plasma concentrations of piperacillin using a previously validated method of ultra-performance liquid chromatography-tandem coupled to mass spectrometry (UHPLC-MS/MS) [49]. Briefly, the inter-day lower limit of quantification (LLOQ) was 0.54 mg/L (S/N ratio of 5.6), and the calibration curve ranged from 0.54 to 175 mg/L (a linear regression curve with a weighting scheme of 1/X).
4.5. Population Pharmacokinetics Analysis
4.5.1. Base Model Development
A simultaneous analysis of all the piperacillin concentration-time data was performed with the PPK approach by means of nonlinear mixed-effects models implemented in NONMEM software, version 7.4 (ICON Development Solutions, Ellicott City, MD, USA) [50]. The first order conditional estimation method with interaction (FOCEI) was used throughout the model-building process. Graphical diagnostics were guided using Xpose version 4.2.1 implemented in the R version 3.3.2 and Perl speaks-NONMEM Toolkit (PsN) version 4.7.0 [51,52]. The Phoenix-WinNonlin version 64 8.2.0.4383 (Certara L.P., 1998–2018) was used for non-compartmental analyses of the data [53].
Models of one and two compartments were fitted to the concentration-time data. The models were parameterized in terms of apparent Vd, distributional clearance (CLD) and CL. First-order, Michaelis–Menten and parallel first-order/Michaelis–Menten kinetics were tested to describe the piperacillin elimination. BPV was evaluated for each PK parameter and modeled exponentially, assuming a log-normal distribution. Additive, proportional and combined (additive + proportional) models were compared to describe the residual error (RE) associated with drug concentrations. To statistically distinguish between nested models, the difference in the MOFV (−2 × log likelihood) was used because this difference is approximately χ2 distributed. A significance level of p < 0.005 equivalent to a difference in MOFV of 7.879 for 1 degree of freedom was considered. For non-hierarchical models, the most parsimonious model with the lowest objective function according to the Akaike information criterion (AIC) was considered [54].
4.5.2. Covariate Model
Once the base model had been developed, the covariate analysis was carried out. Graphical exploration of potential correlations among continuous covariates and of individual PK parameters versus covariates was performed. The most physiologically and clinically relevant covariates were tested, on their respective PK parameters. Firstly, one covariate at a time was included, and then they were entered sequentially by the cumulative forward inclusion/backward elimination procedures. The inclusion of continuous covariates in their respective parameters was done in terms of exponential, linear or power relationships as appropriate. Covariates were centered on their median population value. Specifically, a power relationship was used to test body weight effect by either estimating the exponent or fixing it according to the allometry laws [55]. The statistical and clinical relevance of results of both approaches (estimated and fixed allometric exponents) were considered for model selection.
Significance levels of 5% (reduction in the MOFV of >3.841 units) and 0.1% (increase in the MOFV of >10.8 units) were applied during the forward addition and backward elimination steps. Other parameters considered for model selection were: precision expressed as relative standard error (RSE%), reductions in BPV associated with a specific PK parameter, model completion status (e.g., successful convergence or termination), η- and ε-shrinkage values [56], condition number estimated from the square root of the ratio of the major to the minor eigenvalue and visual inspection of goodness-of-fit plots with Xpose.
The following covariates were considered for screening: weight, BMI, gender, CLCRCKD-EPI, CLCRCG, CLCRMDRD-4, albumin concentration, neurocritical status, occurrence of drainage, occurrence of mechanical ventilation and treatment with vasoactive drugs.
4.5.3. Model Evaluation
Throughout the modeling process, the following goodness-of-fit plots were examined: observed concentrations (DV) versus typical population model-predicted (PRED) or individual Bayesian-predicted concentrations (IPRED), conditional weighted residuals (CWRES) versus time and individual weighted residuals (IWRES) versus IPRED.
The stability and precision of the model were evaluated using a non-parametric bootstrap method [57]. Two hundred resamplings from the original dataset were performed. The median and 95% percentiles of the fixed and random effect parameters were calculated. The bias of each parameter was computed as the ratio of the difference between the median derived from the bootstrap and the final population estimate.
Prediction-corrected VPCs, based on 1000 replicated datasets from the original dataset, were constructed to prove the predictive capability of the model [58]. The 50%, 97.5th and 2.5th percentiles of the observations were checked to be within the non-parametric 95% confidence intervals for the 50%, 2.5th and 97.5th percentiles of the simulated profiles.
4.6. Monte Carlo Simulations
From the final model, Monte Carlo simulations were carried out to generate total concentrations-time profiles for 1000 subjects per dosing regimen. To achieve this, estimated fixed and random parameter values were fixed in the final model. Piperacillin dosage regimens of 8, 12, 16, 20 and 24 g daily given as CI up to the steady state and preceded by a loading dose of 4 g given in 30 min were evaluated. For each dosage, different CLCRCKD-EPI cut-offs, from 60 to 200 mL/min/1.73 m2 in steps of 20 mL/min/1.73 m2, were considered.
Considering 30% of protein binding [48], simulated total plasma concentrations were subsequently transformed into free concentrations (fC) by mathematical calculation as follows: fC = fu × C, where fu is the reported unbound fraction and C the measured piperacillin concentration.
4.7. Probability of Target Attainment and Cumulative Fraction of Response
From Monte Carlo simulations and for each dose and scenario, the PTA of two PK/PD targets, i.e., 100% fT>1×MIC and 100% fT>4×MIC, were assessed. Calculations of PTA for pathogen MICs ranging from 1 to 16 mg/L were performed.
The CFR represents the probability of successful treatment by comparing the PTA with the MIC distribution of a specific population of microorganisms. The wild-type MIC distribution of Pseudomonas aeruginosa was obtained from the 2023 EUCAST database [59]. For each dosing regimen, CLCRCKD-EPI cut-off and PK/PD target, the CFR was calculated by multiplying the PTA found for each MIC by the proportion of isolates found at each MIC, as described previously [60]. Dosing was successful when the CFR was ≥90%.
5. Conclusions
The renal function estimated through CKD-EPI has been identified as the best predictor of inter-individual variability in piperacillin clearance. Our results suggest that in critically ill patients with preserved renal function, the actual dosage regimens have a risk of inadequate piperacillin exposure, which is aggravated with increasing renal function. For the PK/PD target of 100% fT>1×MIC, 12 g of piperacillin provide a PTA > 90% for MIC < 16 mg/L, regardless of CLCR, but higher doses are needed for MIC = 16 mg/L when CLCR > 100 mL/min/1.73 m2. For 100% fT>4×MIC, the highest dose (24 g/24 h) was not sufficient to ensure adequate exposure, except for MICs of 1 and 4 mg/L, regardless of CLCR. To reach the 100%fT>4×MIC target in patients with CLCR above 100 mL/min/1.73 m2, even the dose of 24 g CI would be insufficient to empirically treat susceptible Pseudomonas aeruginosa isolates (MIC ≤ 16 mg/L).
The developed model can be used as a support tool for dose guidance at the start of the treatment and during the TDM, based on renal function and the MIC of the causative pathogen.
Author Contributions
Conception and design: E.E.-P., H.C.-C., V.D.G.-S., S.C.-S., E.S., K.M.-S., J.S.-R., X.L.P.-F., R.R.-B., F.T.-Q., J.C. and A.P.-Z. Data collection: E.E.-P., V.D.G.-S. and K.M.-S. Analysis and interpretation: J.M.-C., H.C.-C. and A.P.-Z. Drafting the manuscript for important intellectual content: J.M.-C., H.C.-C. and A.P.-Z. Revision and final approval: J.M.-C., E.E.-P., H.C.-C., V.D.G.-S., S.C.-S., E.S., K.M.-S., J.S.-R., X.L.P.-F., R.R.-B., F.T.-Q., J.C., H.C.-C. and A.P.-Z. All authors have read and agreed to the published version of the manuscript.
Funding
Part of this study has been financed thanks to the aid for projects of research granted to us by the Sociedad Española de Farmacia Hospitalaria (Convocatoria de Ayudas a Proyectos de Investigación de la SEFH 2013/2014. EPA056/14 ATB-2014-01).
Institutional Review Board Statement
The study was approved by the local Ethics Committee (SFB-ATB-2014-01) and conducted in accordance with the Declaration of Helsinki.
Informed Consent Statement
Written informed consent was requested from the patient or their closest relative before inclusion.
Data Availability Statement
Main data will be made available on request to the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Evans, L.; Rhodes, A.; Alhazzani, W.; Antonelli, M.; Coopersmith, C.M.; French, C.; Machado, F.R.; Mcintyre, L.; Ostermann, M.; Prescott1, H.C.; et al. Surviving Sepsis Campaign: International Guidelines for Management of Sepsis and Septic Shock 2021. Intensive Care Med. 2021, 47, 1181–1247. [Google Scholar] [CrossRef] [PubMed]
- Karlsson, S.; Ruokonen, E.; Varpula, T.; Ala-Kokko, T.I.; Pettilä, V. Long-term outcome and quality-adjusted life years after severe sepsis. Crit. Care Med. 2009, 37, 1268–1274. [Google Scholar] [CrossRef] [PubMed]
- Fleischmann, C.; Scherag, A.; Adhikari, N.K.; Hartog, C.S. Assessment of global incidence and mortality of hospital-treated sepsis. Current estimates and limitations. Am. J. Respir. Crit. Care Med. 2016, 193, 259–272. [Google Scholar] [CrossRef] [PubMed]
- Claeys, K.C.; Zasowski, E.J.; Trinh, T.D.; Lagnf, A.M.; Davis, S.L.; Rybak, M.J. Antimicrobial Stewardship Opportunities in Critically Ill Patients with Gram-Negative Lower Respiratory Tract Infections: A Multicenter Cross-Sectional Analysis. Infect. Dis. Ther. 2018, 7, 135–146. [Google Scholar] [CrossRef]
- Craig, W.A. Basic pharmacodynamics of antibacterials with clinical applications to the use of β-lactams, glycopeptides, and linezolid. Infect. Dis. Clin. N. Am. 2003, 17, 479–501. [Google Scholar] [CrossRef]
- Delattre, I.K.; Taccone, F.S.; Jacobs, F.; Hites, M.; Dugernier, T.; Spapen, H.; Laterre, P.F.; Wallemacq, P.E.; Van Bambeke, F.; Tulkens, P.M. Optimizing β-lactams treatment in critically-ill patients using pharmacokinetics/pharmacodynamics targets: Are first conventional doses effective? Expert Rev. Anti. Infect. Ther. 2017, 15, 677–688. [Google Scholar] [CrossRef]
- Abdul-Aziz, M.H.; Lipman, J.; Mouton, J.W.; Hope, W.W.; Roberts, J.A. Applying pharmacokinetic/pharmacodynamic principles in critically Ill patients: Optimizing efficacy and reducing resistance development. Semin. Respir. Crit. Care Med. 2015, 36, 136–153. [Google Scholar] [CrossRef]
- Blot, S.I.; Pea, F.; Lipman, J. The effect of pathophysiology on pharmacokinetics in the critically ill patient-Concepts appraised by the example of antimicrobial agents. Adv. Drug Deliv. Rev. 2014, 77, 3–11. [Google Scholar] [CrossRef]
- Hosein, S.; Udy, A.A.; Lipman, J. Physiological Changes in the Critically Ill Patient with Sepsis. Curr. Pharm. Biotechnol. 2011, 12, 1991–1995. [Google Scholar] [CrossRef]
- Roberts, J.A.; Abdul-Aziz, M.H.; Lipman, J.; Mouton, J.W.; Vinks, A.A.; Felton, T.W.; Hope, W.W.; Farkas, A.; Neely, M.N.; Schentag, J.J.; et al. Individualised antibiotic dosing for patients who are critically ill: Challenges and potential solutions. Lancet Infect. Dis. 2014, 14, 498–509. [Google Scholar] [CrossRef]
- Roberts, J.A.; Kirkpatrick, C.M.J.; Roberts, M.S.; Dalley, A.J.; Lipman, J. First-dose and steady-state population pharmacokinetics and pharmacodynamics of piperacillin by continuous or intermittent dosing in critically ill patients with sepsis. Int. J. Antimicrob. Agents 2010, 35, 156–163. [Google Scholar] [CrossRef]
- Dulhunty, J.M.; Roberts, J.A.; Davis, J.S.; Webb, S.A.; Bellomo, R.; Gomersall, C.; Shirwadkar, C.; Eastwood, G.M.; Myburgh, J.; Paterson, D.L.; et al. A Multicenter Randomized Trial of Continuous versus Intermittent β-Lactam Infusion in Severe Sepsis. Am. J. Respir. Crit. Care Med. 2015, 192, 1298–1305. [Google Scholar] [CrossRef]
- Lee, Y.R.; Miller, P.D.; Alzghari, S.K.; Blanco, D.D.; Hager, J.D.; Kuntz, K.S. Continuous Infusion Versus Intermittent Bolus of Beta-Lactams in Critically Ill Patients with Respiratory Infections: A Systematic Review and Meta-analysis. Eur. J. Drug Metab. Pharmacokinet. 2018, 43, 155–170. [Google Scholar] [CrossRef]
- Langgartner, J.; Lehn, N.; Glück, T.; Herzig, H.; Kees, F. Comparison of the pharmacokinetics of piperacillin and sulbactam during intermittent and continuous intravenous infusion. Chemotherapy 2007, 53, 370–377. [Google Scholar] [CrossRef]
- Abdul-Aziz, M.H.; Sulaiman, H.; Mat-Nor, M.B.; Rai, V.; Wong, K.K.; Hasan, M.S.; Abd Rahman, A.N.; Jamal, J.A.; Wallis, S.C.; Lipman, J.; et al. Beta-Lactam Infusion in Severe Sepsis (BLISS): A prospective, two-centre, open-labelled randomised controlled trial of continuous versus intermittent beta-lactam infusion in critically ill patients with severe sepsis. Intensive Care Med. 2016, 42, 1535–1545. [Google Scholar] [CrossRef]
- Asín-Prieto, E.; Rodríguez-Gascón, A.; Trocóniz, I.F.; Soraluce, A.; Maynar, J.; Sánchez-Izquierdo, J.Á.; Isla, A. Population pharmacokinetics of piperacillin and tazobactam in critically ill patients undergoing continuous renal replacement therapy: Application to pharmacokinetic/pharmacodynamic analysis. J. Antimicrob. Chemother. 2014, 69, 180–189. [Google Scholar] [CrossRef]
- Bue, M.; Sou, T.; Okkels, A.S.L.; Hanberg, P.; Thorsted, A.; Friberg, L.E.; Andersson, T.L.; Öbrink-Hansen, K.; Christensen, S. Population pharmacokinetics of piperacillin in plasma and subcutaneous tissue in patients on continuous renal replacement therapy. Int. J. Infect. Dis. 2020, 92, 133–140. [Google Scholar] [CrossRef]
- Roberts, D.M.; Liu, X.; Roberts, J.A.; Nair, P.; Cole, L.; Roberts, M.S.; Lipman, J.; Bellomo, R. A multicenter study on the effect of continuous hemodiafiltration intensity on antibiotic pharmacokinetics. Crit. Care 2015, 19, 84. [Google Scholar] [CrossRef]
- Tamme, K.; Oselin, K.; Kipper, K.; Tasa, T.; Metsvaht, T.; Karjagin, J.; Herodes, K.; Kern, H.; Starkopf, J. Pharmacokinetics and pharmacodynamics of piperacillin/tazobactam during high volume haemodiafiltration in patients with septic shock. Acta Anaesthesiol. Scand. 2016, 60, 230–240. [Google Scholar] [CrossRef]
- Ulldemolins, M.; Martín-Loeches, I.; Llauradó-Serra, M.; Fernández, J.; Vaquer, S.; Rodríguez, A.; Pontes, C.; Calvo, G.; Torres, A.; Soy, D. Piperacillin population pharmacokinetics in critically ill patients with multiple organ dysfunction syndrome receiving continuous venovenous haemodiafiltration: Effect of type of dialysis membrane on dosing requirements. J. Antimicrob. Chemother. 2016, 71, 1651–1659. [Google Scholar] [CrossRef]
- Dhaese, S.A.M.; Colin, P.; Willems, H.; Heffernan, A.; Gadeyne, B.; Van Vooren, S.; Depuydt, P.; Hoste, E.; Stove, V.; Verstraete, A.G.; et al. Saturable elimination of piperacillin in critically ill patients: Implications for continuous infusion. Int. J. Antimicrob. Agents 2019, 54, 741–749. [Google Scholar] [CrossRef]
- Udy, A.A.; Lipman, J.; Jarrett, P.; Klein, K.; Wallis, S.C.; Patel, K.; Kirkpatrick, C.M.J.; Kruger, P.S.; Paterson, D.L.; Roberts, M.S.; et al. Are standard doses of piperacillin sufficient for critically ill patients with augmented creatinine clearance? Crit. Care 2015, 19, 28. [Google Scholar] [CrossRef] [PubMed]
- Dhaese, S.A.M.; Roberts, J.A.; Carlier, M.; Verstraete, A.G.; Stove, V.; De Waele, J.J. Population pharmacokinetics of continuous infusion of piperacillin in critically ill patients. Int. J. Antimicrob. Agents 2018, 51, 594–600. [Google Scholar] [CrossRef] [PubMed]
- Klastrup, V.; Thorsted, A.; Storgaard, M.; Christensen, S.; Friberg, L.E.; Öbrink-Hansen, K. Population Pharmacokinetics of Piperacillin following Continuous Infusion in Critically Ill Patients and Impact of Renal Function on Target Attainment. Antimicrob. Agents Chemother. 2020, 64, e02556-19. [Google Scholar] [CrossRef] [PubMed]
- Öbrink-Hansen, K.; Juul, R.V.; Storgaard, M.; Thomsen, M.K.; Hardlei, T.F.; Brock, B.; Kreilgaard, M.; Gjedsted, J. Population pharmacokinetics of piperacillin in the early phase of septic shock: Does standard dosing result in therapeutic plasma concentrations? Antimicrob. Agents Chemother. 2015, 59, 7018–7026. [Google Scholar] [CrossRef] [PubMed]
- Alobaid, A.S.; Wallis, S.C.; Jarrett, P.; Starr, T.; Stuart, J.; Lassig-Smith, M.; Mejia, J.L.O.; Roberts, M.S.; Roger, C.; Udy, A.A.; et al. Population pharmacokinetics of piperacillin in nonobese, obese, and morbidly obese critically ill patients. Antimicrob. Agents Chemother. 2017, 61, e01276-16. [Google Scholar] [CrossRef]
- Sukarnjanaset, W.; Jaruratanasirikul, S.; Wattanavijitkul, T. Population pharmacokinetics and pharmacodynamics of piperacillin in critically ill patients during the early phase of sepsis. J. Pharmacokinet. Pharmacodyn. 2019, 46, 251–261. [Google Scholar] [CrossRef]
- Wallenburg, E.; ter Heine, R.; Schouten, J.A.; Raaijmakers, J.; ten Oever, J.; Kolwijck, E.; Burger, D.M.; Pickkers, P.; Frenzel, T.; Brüggemann, R.J.M. An Integral Pharmacokinetic Analysis of Piperacillin and Tazobactam in Plasma and Urine in Critically Ill Patients. Clin. Pharmacokinet. 2022, 61, 907–918, Correction in Clin. Pharmacokinet. 2022, 61, 1325–1329. [Google Scholar] [CrossRef]
- Vinks, A.A.; Den Hollander, J.G.; Overbeek, S.E.; Jelliffe, R.W.; Mouton, J.W. Population pharmacokinetic analysis of nonlinear behavior of piperacillin during intermittent or continuous infusion in patients with cystic fibrosis. Antimicrob. Agents Chemother. 2003, 47, 541–547. [Google Scholar] [CrossRef]
- Felton, T.W.; Hope, W.W.; Lomaestro, B.M.; Butterfield, J.M.; Kwa, A.L.; Drusano, G.L.; Lodise, T.P. Population pharmacokinetics of extended-infusion piperacillin-tazobactam in hospitalized patients with nosocomial infections. Antimicrob. Agents Chemother. 2012, 56, 4087–4094. [Google Scholar] [CrossRef]
- Esteve-Pitarch, E.; Gumucio-Sanguino, V.D.; Cobo-Sacristán, S.; Shaw, E.; Maisterra-Santos, K.; Sabater-Riera, J.; Pérez-Fernandez, X.L.; Rigo-Bonnin, R.; Tubau-Quintano, F.; Carratalà, J.; et al. Continuous Infusion of Piperacillin/Tazobactam and Meropenem in ICU Patients Without Renal Dysfunction: Are Patients at Risk of Underexposure? Eur. J. Drug Metab. Pharmacokinet. 2021, 46, 527–538. [Google Scholar] [CrossRef]
- Landersdorfer, C.B.; Bulitta, J.B.; Kirkpatrick, C.M.J.; Kinzig, M.; Holzgrabe, U.; Drusano, G.L.; Stephan, U.; Sörgel, F. Population pharmacokinetics of piperacillin at two dose levels: Influence of nonlinear pharmacokinetics on the pharmacodynamic profile. Antimicrob. Agents Chemother. 2012, 56, 5715–5723. [Google Scholar] [CrossRef]
- Welling, P.G.; Craig, W.A.; Bundtzen, R.W.; Kwok, F.W.; Gerber, A.U.; Madsen, P.O. Pharmacokinetics of Piperacillin in subjects with various degrees of renal function. Antimicrob. Agents Chemother. 1983, 23, 881–887. [Google Scholar] [CrossRef]
- Baptista, J.P.; Neves, M.; Rodrigues, L.; Teixeira, L.; Pinho, J.; Pimentel, J. Accuracy of the estimation of glomerular filtration rate within a population of critically ill patients. J. Nephrol. 2014, 27, 403–410. [Google Scholar] [CrossRef]
- Palacio-Lacambra, M.E.; Comas-Reixach, I.; Blanco-Grau, A.; Suñé-Negre, J.M.; Segarra-Medrano, A.; Montoro-Ronsano, J.B. Comparison of the Cockcroft–Gault, MDRD and CKD-EPI equations for estimating ganciclovir clearance. Br. J. Clin. Pharmacol. 2018, 84, 2120–2128. [Google Scholar] [CrossRef]
- Abdul-Aziz, M.H.; Alffenaar, J.W.C.; Bassetti, M.; Bracht, H.; Dimopoulos, G.; Marriott, D.; Neely, M.N.; Paiva, J.A.; Pea, F.; Sjovall, F.; et al. Antimicrobial therapeutic drug monitoring in critically ill adult patients: A Position Paper#. Intensive Care Med. 2020, 46, 1127–1153. [Google Scholar] [CrossRef]
- Gonçalves-Pereira, J.; Póvoa, P. Antibiotics in critically ill patients: A systematic review of the pharmacokinetics of β-lactams. Crit. Care 2011, 15, R206. [Google Scholar] [CrossRef]
- Dulhunty, J.M.; Roberts, J.A.; Davis, J.S.; Webb, S.A.R.; Bellomo, R.; Gomersall, C.; Shirwadkar, C.; Eastwood, G.M.; Myburgh, J.; Paterson, D.L.; et al. Continuous infusion of beta-lactam antibiotics in severe sepsis: A multicenter double-blind, randomized controlled trial. Clin. Infect. Dis. 2013, 56, 236–244. [Google Scholar] [CrossRef]
- Roberts, J.A.; Abdul-Aziz, M.-H.; Davis, J.S.; Dulhunty, J.M.; Cotta, M.O.; Myburgh, J.; Bellomo, R.; Lipman, J. Continuous versus intermittent beta-lactam infusion in severe sepsis: A meta-analysis of individual patient data from randomized trials. Am. J. Respir. Crit. Care Med. 2016, 194, 681–691. [Google Scholar] [CrossRef]
- Kondo, Y.; Ota, K.; Imura, H.; Hara, N.; Shime, N. Prolonged versus intermittent β-lactam antibiotics intravenous infusion strategy in sepsis or septic shock patients: A systematic review with meta-Analysis and trial sequential analysis of randomized trials. J. Intensive Care 2020, 8, 77. [Google Scholar] [CrossRef]
- Vardakas, K.Z.; Voulgaris, G.L.; Maliaros, A.; Samonis, G.; Falagas, M.E. Prolonged versus short-term intravenous infusion of antipseudomonal β-lactams for patients with sepsis: A systematic review and meta-analysis of randomised trials. Lancet Infect. Dis. 2018, 18, 108–120. [Google Scholar] [CrossRef] [PubMed]
- Udy, A.A.; Varghese, J.M.; Altukroni, M.; Briscoe, S.; McWhinney, B.C.; Ungerer, J.P.; Lipman, J.; Roberts, J.A. Subtherapeutic initial β-lactam concentrations in select critically Ill patients: Association between augmented renal clearance and low trough drug concentrations. Chest 2012, 142, 30–39. [Google Scholar] [CrossRef] [PubMed]
- Huttner, A.; Von Dach, E.; Renzoni, A.; Huttner, B.D.; Affaticati, M.; Pagani, L.; Daali, Y.; Pugin, J.; Karmime, A.; Fathi, M.; et al. Augmented renal clearance, low β-lactam concentrations and clinical outcomes in the critically ill: An observational prospective cohort study. Int. J. Antimicrob. Agents 2015, 45, 385–392. [Google Scholar] [CrossRef] [PubMed]
- Sime, F.B.; Udy, A.A.; Roberts, J.A. Augmented renal clearance in critically ill patients: Etiology, definition and implications for beta-lactam dose optimization. Curr. Opin. Pharmacol. 2015, 24, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Udy, A.A.; Dulhunty, J.M.; Roberts, J.A.; Davis, J.S.; Webb, S.A.R.; Bellomo, R.; Gomersall, C.; Shirwadkar, C.; Eastwood, G.M.; Myburgh, J.; et al. Association between augmented renal clearance and clinical outcomes in patients receiving β-lactam antibiotic therapy by continuous or intermittent infusion: A nested cohort study of the BLING-II randomised, placebo-controlled, clinical trial. Int. J. Antimicrob. Agents 2017, 49, 624–630. [Google Scholar] [CrossRef]
- Occhipinti, D.J.; Pendland, S.L.; Schoonover, L.L.; Rypins, E.B.; Danziger, L.H.; Rodvold, K.A. Pharmacokinetics and Pharmacodynamics of Two Multiple-Dose Piperacillin-Tazobactam Regimens. Antimicrob. Agents Chemother. 1997, 41, 2511–2517. [Google Scholar] [CrossRef]
- Zander, J.; Döbbeler, G.; Nagel, D.; Scharf, C.; Huseyn-Zada, M.; Jung, J.; Frey, L.; Vogeser, M.; Zoller, M. Variability of piperacillin concentrations in relation to tazobactam concentrations in critically ill patients. Int. J. Antimicrob. Agents 2016, 48, 435–439. [Google Scholar] [CrossRef]
- Roberts, J.A.; Roberts, M.S.; Robertson, T.A.; Dalley, A.J.; Lipman, J. Piperacillin penetration into tissue of critically ill patients with sepsis-bolus versus continuous administration? Crit. Care Med. 2009, 37, 926–933. [Google Scholar] [CrossRef]
- Rigo-Bonnin, R.; Ribera, A.; Arbiol-Roca, A.; Cobo-Sacristán, S.; Padullés, A.; Murillo, Ò.; Shaw, E.; Granada, R.; Pérez-Fernández, X.L.; Tubau, F.; et al. Development and validation of a measurement procedure based on ultra-high performance liquid chromatography-tandem mass spectrometry for simultaneous measurement of β-lactam antibiotic concentration in human plasma. Clin. Chim. Acta 2017, 468, 215–224. [Google Scholar] [CrossRef]
- Bauer, R. NONMEM User’s Guide; Icon Development Solutions: Ellicot City, MD, USA, 2011. [Google Scholar]
- Jonsson, E.N.; Karlsson, M.O. Xpose-An S-PLUS based population pharmacokinetic/pharmacodynamic model building aid for NONMEM. Comput. Methods Programs Biomed. 1998, 58, 51–64. [Google Scholar] [CrossRef]
- Lindbom, L.; Pihlgren, P.; Jonsson, N. PsN-Toolkit-A collection of computer intensive statistical methods for non-linear mixed effect modeling using NONMEM. Comput. Methods Programs Biomed. 2005, 79, 241–257. [Google Scholar] [CrossRef]
- Phoenix-WinNonlin. 1998–2020. Certara LP. Ver 64 8.3.4. 295. Available online: https://www.certara.com/software/phoenix-winnonlin/ (accessed on 15 January 2023).
- Yamaoka, K.; Nakagawa, T.; Uno, T. Application of Akaike’s information criterion (AIC) in the evaluation of linear pharmacokinetic equations. J. Pharmacokinet. Biopharm. 1978, 6, 165–175. [Google Scholar] [CrossRef]
- Anderson, B.J.; Holford, N.H.G. Mechanistic basis of using body size and maturation to predict clearance in humans. Drug Metab. Pharmacokinet. 2009, 24, 25–36. [Google Scholar] [CrossRef]
- Savic, R.M.; Karlsson, M.O. Importance of shrinkage in empirical bayes estimates for diagnostics: Problems and solutions. AAPS J. 2009, 11, 558–569. [Google Scholar] [CrossRef]
- Efron, B. Bootstrap methods: Another look at the jackknife. Ann. Stat. 1979, 7, 1–26. [Google Scholar] [CrossRef]
- Bergstrand, M.; Hooker, A.C.; Wallin, J.E.; Karlsson, M.O. Prediction-corrected visual predictive checks for diagnosing nonlinear mixed-effects models. AAPS J. 2011, 13, 143–151. [Google Scholar] [CrossRef]
- European Committe on Antimicrobial Susceptibility Testing (EUCAST). 2022. Clinical Breakpoints. Available online: https://www.eucast.org/ (accessed on 15 January 2023).
- Mouton, J.W.; Dudley, M.N.; Cars, O.; Derendorf, H.; Drusano, G.L. Standardization of pharmacokinetic/pharmacodynamic (PK/PD) terminology for anti-infective drugs: An update. J. Antimicrob. Chemother. 2005, 55, 601–607. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).