Potential Strategies to Control the Risk of Antifungal Resistance in Humans: A Comprehensive Review
Abstract
1. Introduction
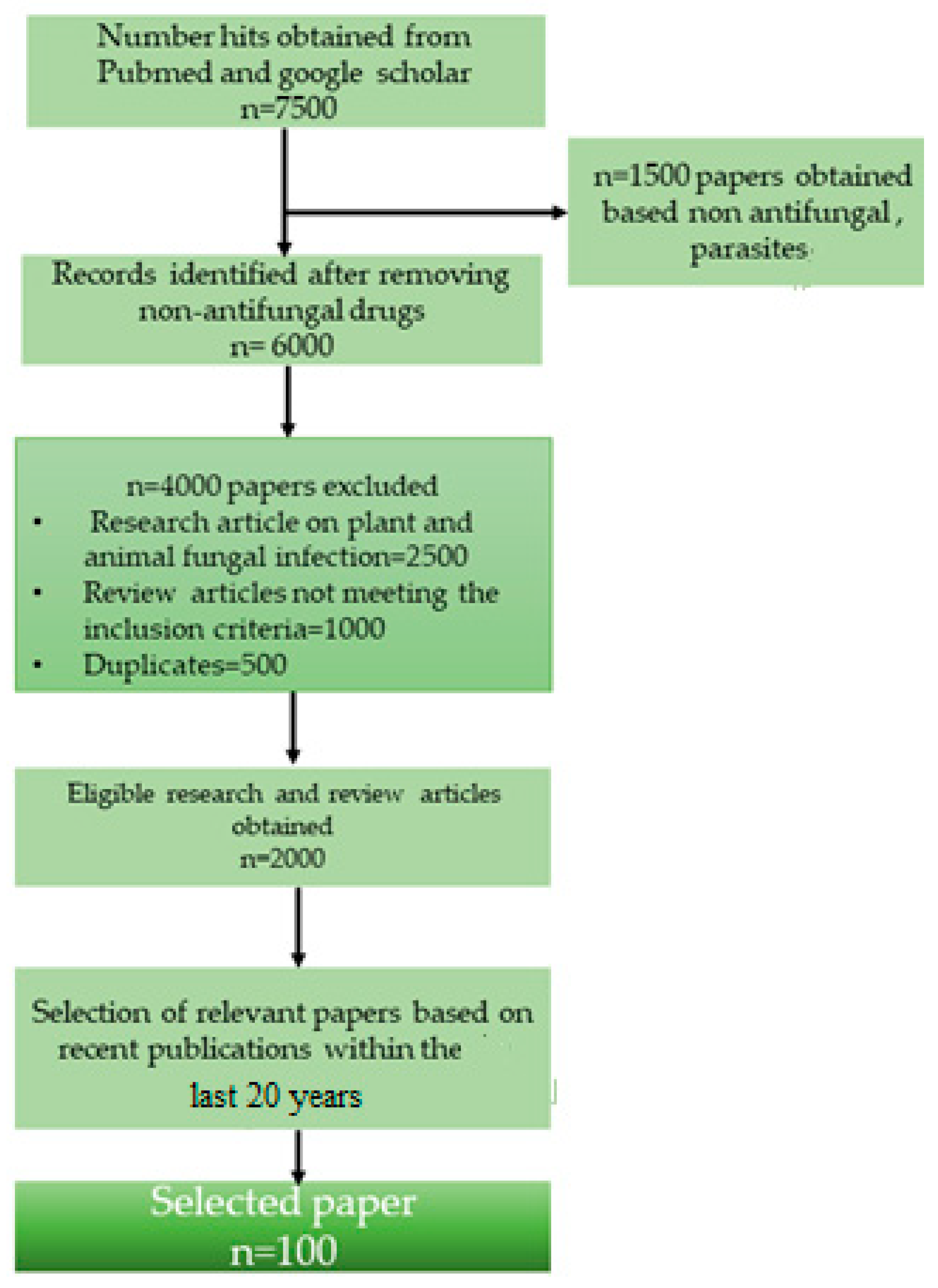
2. Methodology
2.1. Literature Screening
2.2. Exclusion of Articles
2.3. Collection of Data from Relevant Articles
3. Results and Discussion
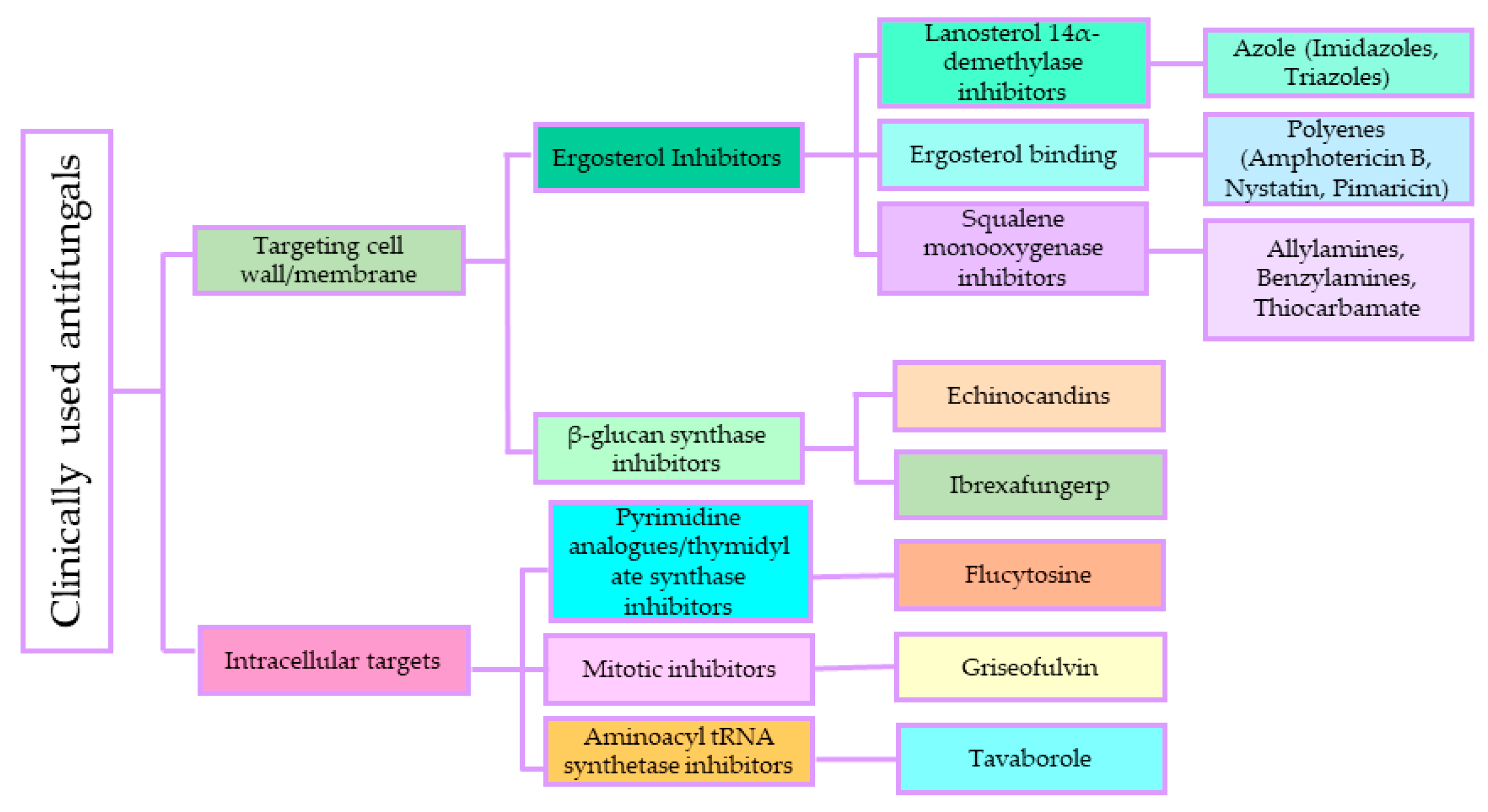
3.1. Antifungal Agents and Their Mode of Action
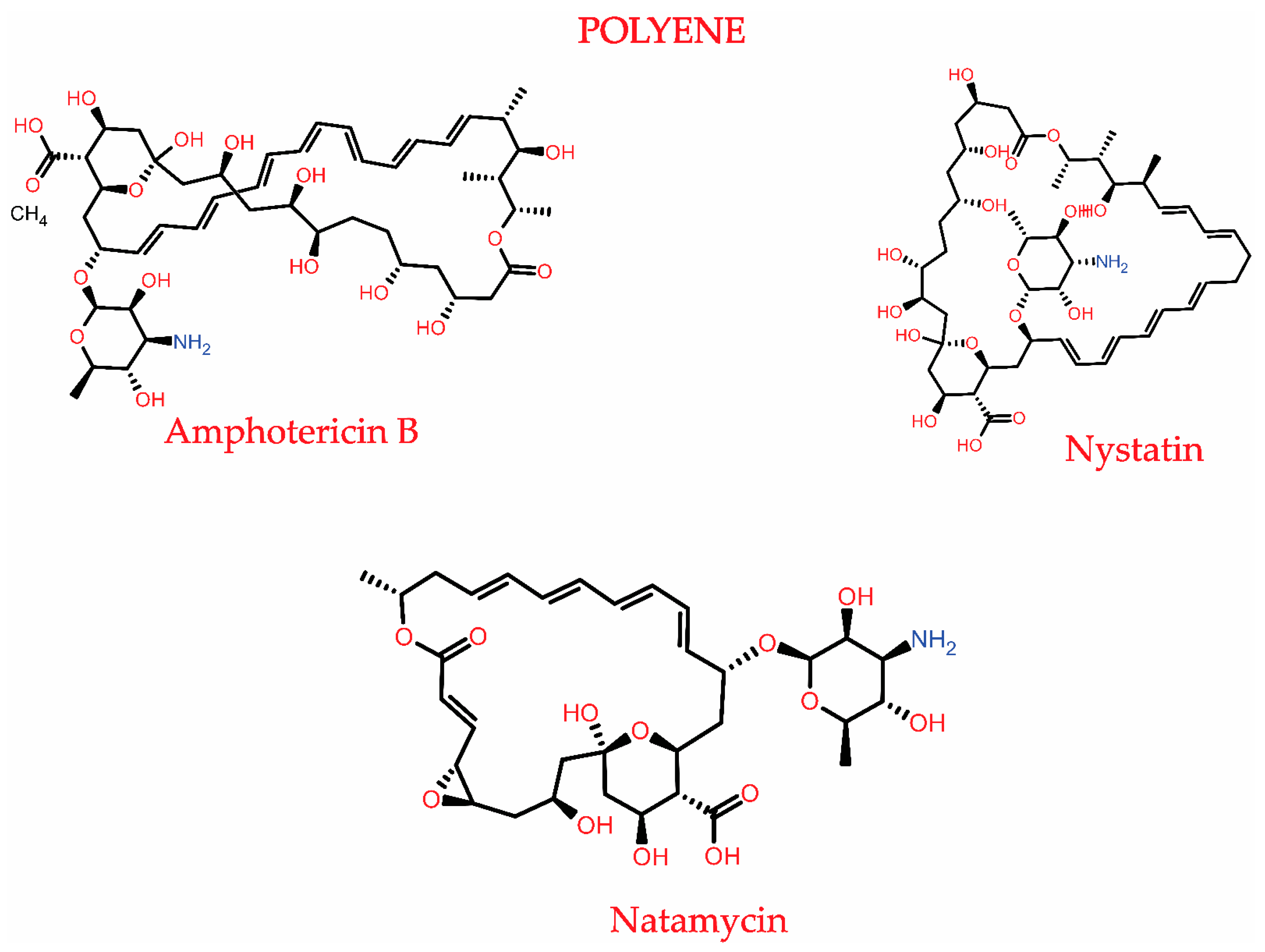
3.1.1. Polyenes
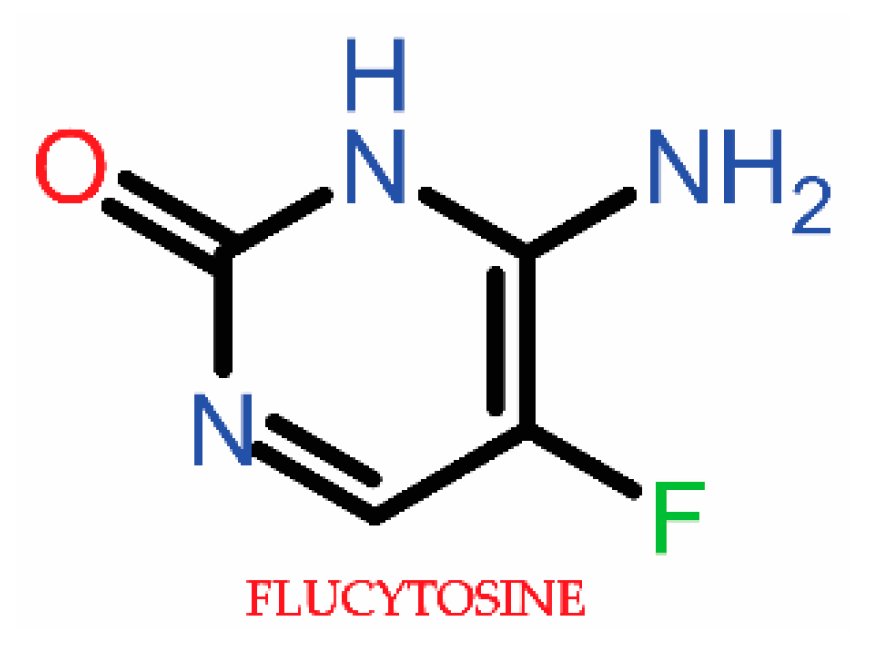
3.1.2. Antimetabolite Agents (5-Fluorocytosine)
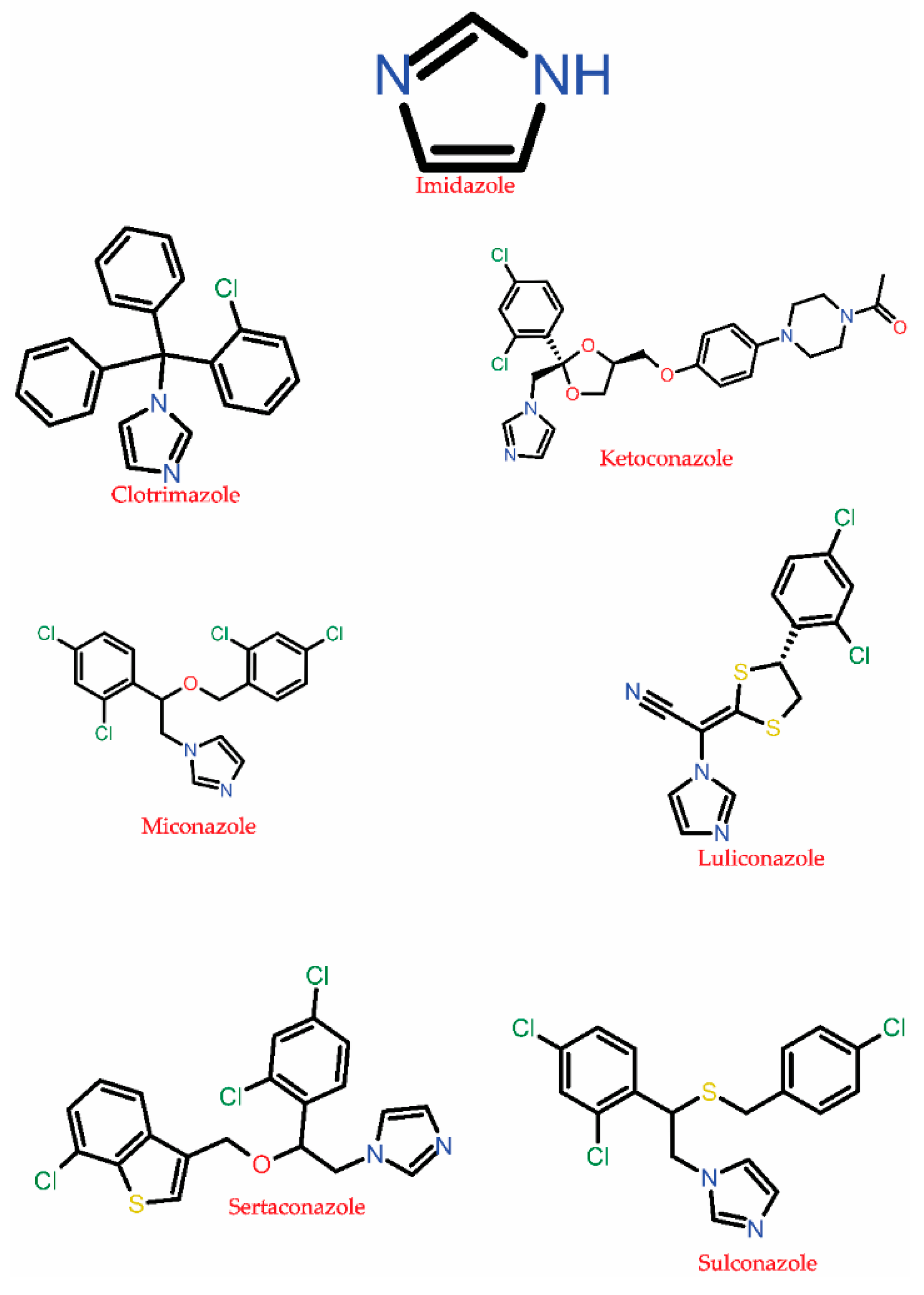
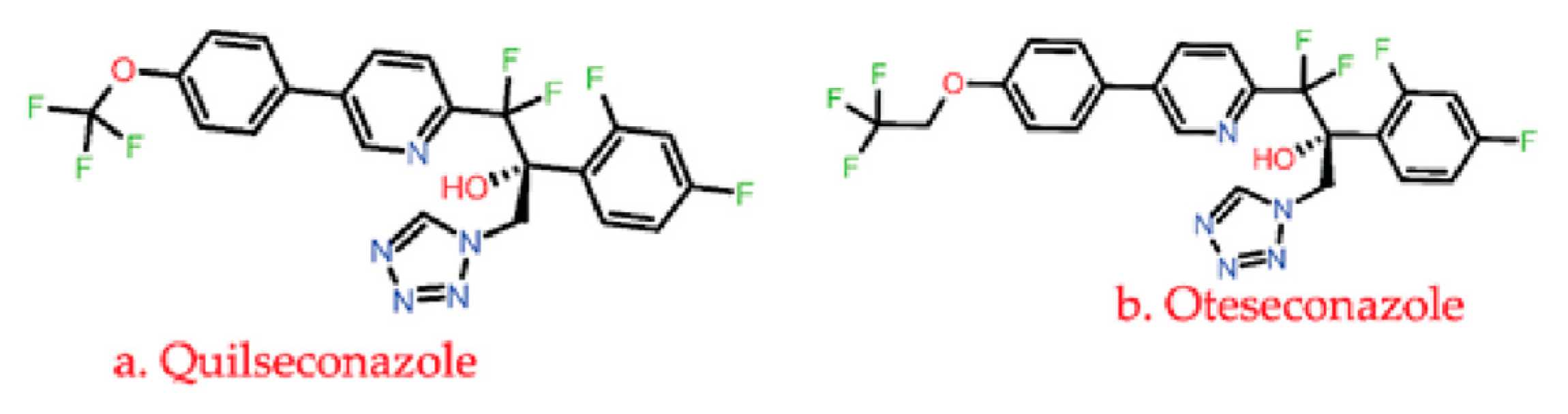
3.1.3. Azole
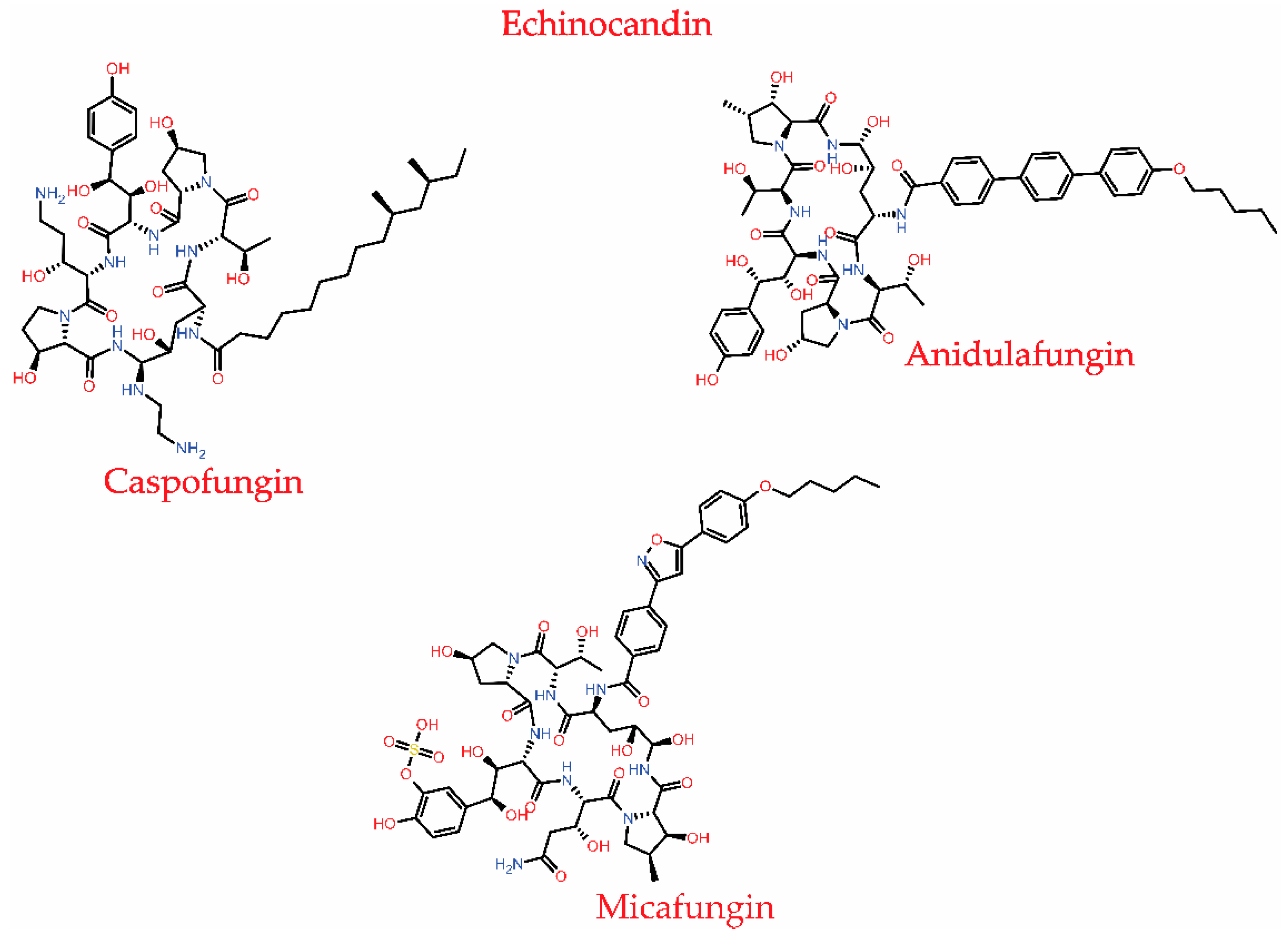
3.1.4. Echinocandins
3.2. Resistance to Antifungal Agents
3.2.1. Polyene Resistance
3.2.2. Antimetabolite Agent Resistance
3.2.3. Azole Resistance
3.2.4. Echinocandin Resistance
| Antifungal Agents | Mode of Action | Example of Antifungal Resistant Species | Resistance Mechanism | Reference |
|---|---|---|---|---|
Polyenes
|
| Candida species, and Aspergillus terreus | Alternation (ERG3, ERG5, and ERG11), deletion (ERG11), and mutation (ERG3, ERG5, and ERG11) genes encoding for C-5 sterol desaturase, C-22 sterol desaturase, and sterol 14-demethylase are responsible for the regulation of ergosterol biosynthesis. | [36,41,93] |
Antimetabolite agent
| Inhibits RNA and protein synthesis by binding 5-fluorouracil(5-FU) to the RNA strand. | Candida spp. | Alternation in the FCY2, FCY1, and FUR1 genes that are responsible for the uptake and conversion of flucytosine. | [54,55,92] |
Azole
| Inhibition of fungal lanosterol 14α-demethylase cytochrome P450 51, P450 3A4, P450 2D6, P450 2C8, P450 2A6, P450 2E1 P450 2C9, etc., that disrupts the ergosterol biosynthesis leading to cell death and lysis. Some of these drugs also targets DNA, bile salt export pump, P-glycoprotein 1, Hydroxycarboxylic acid receptor 2, oxygen-insensitive NADPH nitroreductase. | C. albicans, C. glabrata, C. dubliniensis A. fumigatus, yeast, Trichophyton rubrum | Alternation and overexpression of ERG11, Cyp51A, and Cyp51B encodes for lanosterol 14-alpha-demethylase enzyme. | [65,67,70,71,93,101,102] |
Echinocandin
| Inhibits β-1,3 glucan synthase, an essential enzyme complex responsible for cell wall synthesis in fungi. | C. glabrata and A. fumigatus | Amino acid substitution or point mutation in the FKS1 and FKS2 genes present in the glucan synthase | [75,99] |
3.2.5. Other Mechanisms Involved in Drug Resistance
- (a)
- Biofilm formation
- (b)
- Modification of drug targets
- (c)
- Efflux pump
3.3. Strategies to Overcome Resistance
3.3.1. Development of a New Antifungal Drug
- (a)
- SUBA-itraconazole and VT-1598
- (b)
- Rezafungin and Ibrexafungerp
- (c)
- Olorofim
- (d)
- Amphotericin B Cochleate (CAMB)
- (e)
- MGCD290
- (f)
- Fosmanogepix (APX001)
| New Antifungal Agents Developed | Mode of Action | Developer | Activity Against | Clinical Trial Stage | Reference |
|---|---|---|---|---|---|
| VT-1598 | Inhibits lanosterol 14α-demethylase | Mycovia Pharmaceuticals, Durham, NC, USA | Moulds, Aspergillus spp., Rhizopus arrhizus, and Coccidioides | Phase I | [102,120] |
| SUBA-itraconazole | Enhance the bioavailability of itraconazole by its solid dispersion in a pH-dependent matrix. | Mayne Pharma Ltd., Salisbury South, Australia | Aspergillus spp., Blastomyces dermatitidis | FDA-approved | [118] |
| Rezafungin | Inhibition of 1,3-β-D-glucan synthesis | Cidara Therapeutics, San Diego, CA, USA | Candida spp., Aspergillus spp., and Pneumocystis spp. | FDA-approved as an orphan drug for the treatment of vulvovaginal candidiasis | [119,121] |
| Ibrexafungin | Inhibition of 1,3-β-D-glucan synthesis | SCYNEXIS Inc., Jersey City, NJ, USA | Candida spp. | FDA-approved | [122] |
| Olorofim | Inhibition of dihydroorotate dehydrogenase responsible for pyrimidine biosynthesis. | Shionogi & Co., Ltd. And F2G Ltd., Osaka, Japan and Manchester, UK | Aspergillus and Scedosporium spp. | Phase II | [123] |
| Amphotericin B Cochleate (CAMB) | Cell wall disruption | Matinas BioPharma, Bedminster, NJ, USA. | C. albicans | Phase II | [124] |
| MGCD290 | Inhibits Hos2 fungal histone deacetylase (HDAC) and also affects non-histone protein Hsp90 | Mirati Therapeutics, Inc., San Diego, CA, USA | Candida and Aspergillus spp. | Phase II | [119] |
| Fosmanogepix (APX001) | Inhibition of glycosylphosphatidylinositol. | Amplyx Pharmaceuticals, San Diego, CA, USA | Yeast, moulds, Candida, Cryptococcus, Coccidioides, and Aspergillus spp. | Phase II | [125,126] |
| VL-2397 | Unknown | Vical Pharmaceuticals, San Diego, CA, USA | Aspergillus fumigatus. | Phase II | [128,129] |
| T-2307 | Fungal mitochondrial disruption | Toyama Chemical Co., Tokyo, Japan. | Candida spp. Cryptococcus and Aspergillus spp. | Phase I | [130] |
- (g)
- VL-2397
- (h)
- T-2307
- (i)
- Retinoids and All-trans retinoic acid (ATRA)

3.3.2. Combination Therapy
3.3.3. Antifungal Stewardship
3.3.4. Potential Drug Targets to Overcome Antifungal Resistance
3.3.5. One Health Approach in Combating Antifungal Resistance
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kainz, K.; Bauer, M.A.; Madeo, F.; Carmona-Gutierrez, D. Fungal Infections in Humans: The Silent Crisis. Microb. Cell 2020, 7, 143–145. [Google Scholar] [CrossRef] [PubMed]
- Papon, N.; Bougnoux, M.-E.; d’Enfert, C. Tracing the Origin of Invasive Fungal Infections. Trends Microbiol. 2020, 28, 240–242. [Google Scholar] [CrossRef]
- Firacative, C. Invasive Fungal Disease in Humans: Are We Aware of the Real Impact? Memórias Do Inst. Oswaldo Cruz. 2020, 115, e200430. [Google Scholar] [CrossRef]
- WHO Fungal Priority Pathogens List to Guide Research, Development and Public Health Action. Available online: https://www.who.int/publications-detail-redirect/9789240060241 (accessed on 2 March 2023).
- Brown, G.D.; Denning, D.W.; Gow, N.A.R.; Levitz, S.M.; Netea, M.G.; White, T.C. Hidden Killers: Human Fungal Infections. Sci. Transl. Med. 2012, 4, 165rv13. [Google Scholar] [CrossRef] [PubMed]
- Bongomin, F.; Gago, S.; Oladele, R.O.; Denning, D.W. Global and Multi-National Prevalence of Fungal Diseases—Estimate Precision. J. Fungi 2017, 3, 57. [Google Scholar] [CrossRef] [PubMed]
- Prakash, H.; Chakrabarti, A. Global Epidemiology of Mucormycosis. J. Fungi 2019, 5, 26. [Google Scholar] [CrossRef] [PubMed]
- Verweij, P.E.; Lucas, J.A.; Arendrup, M.C.; Bowyer, P.; Brinkmann, A.J.F.; Denning, D.W.; Dyer, P.S.; Fisher, M.C.; Geenen, P.L.; Gisi, U.; et al. The One Health Problem of Azole Resistance in Aspergillus Fumigatus: Current Insights and Future Research Agenda. Fungal Biol. Rev. 2020, 34, 202–214. [Google Scholar] [CrossRef]
- Rhodes, J.; Fisher, M.C. Global Epidemiology of Emerging Candida Auris. Curr. Opin. Microbiol. 2019, 52, 84–89. [Google Scholar] [CrossRef]
- Bilal, H.; Hou, B.; Shafiq, M.; Chen, X.; Shahid, M.A.; Zeng, Y. Antifungal Susceptibility Pattern of Candida Isolated from Cutaneous Candidiasis Patients in Eastern Guangdong Region: A Retrospective Study of the Past 10 Years. Front. Microbiol. 2022, 13, 981181. [Google Scholar] [CrossRef]
- Bilal, H.; Shafiq, M.; Hou, B.; Islam, R.; Khan, M.N.; Khan, R.U.; Zeng, Y. Distribution and Antifungal Susceptibility Pattern of Candida Species from Mainland China: A Systematic Analysis. Virulence 2022, 13, 1573–1589. [Google Scholar] [CrossRef]
- Janbon, G.; Quintin, J.; Lanternier, F.; d’Enfert, C. Studying Fungal Pathogens of Humans and Fungal Infections: Fungal Diversity and Diversity of Approaches. Microbes Infect. 2019, 21, 237–245. [Google Scholar] [CrossRef]
- Enoch, D.A.; Yang, H.; Aliyu, S.H.; Micallef, C. The Changing Epidemiology of Invasive Fungal Infections. In Human Fungal Pathogen Identification: Methods and Protocols; Lion, T., Ed.; Methods in Molecular Biology; Springer: New York, NY, USA, 2017; pp. 17–65. ISBN 978-1-4939-6515-1. [Google Scholar]
- Song, G.; Liang, G.; Liu, W. Fungal Co-Infections Associated with Global COVID-19 Pandemic: A Clinical and Diagnostic Perspective from China. Mycopathologia 2020, 185, 599–606. [Google Scholar] [CrossRef]
- Pushparaj, K.; Kuchi Bhotla, H.; Arumugam, V.A.; Pappusamy, M.; Easwaran, M.; Liu, W.-C.; Issara, U.; Rengasamy, K.R.R.; Meyyazhagan, A.; Balasubramanian, B. Mucormycosis (Black Fungus) Ensuing COVID-19 and Comorbidity Meets-Magnifying Global Pandemic Grieve and Catastrophe Begins. Sci. Total Environ. 2022, 805, 150355. [Google Scholar] [CrossRef]
- Drissi, C. Black Fungus, the Darker Side of COVID-19. J. Neuroradiol. 2021, 48, 317–318. [Google Scholar] [CrossRef]
- Shapiro, R.S.; Robbins, N.; Cowen, L.E. Regulatory Circuitry Governing Fungal Development, Drug Resistance, and Disease. Microbiol. Mol. Biol. Rev. 2011, 75, 213–267. [Google Scholar] [CrossRef]
- Perlin, D.S.; Rautemaa-Richardson, R.; Alastruey-Izquierdo, A. The Global Problem of Antifungal Resistance: Prevalence, Mechanisms, and Management. Lancet Infect. Dis. 2017, 17, e383–e392. [Google Scholar] [CrossRef]
- Robbins, N.; Caplan, T.; Cowen, L.E. Molecular Evolution of Antifungal Drug Resistance. Annu. Rev. Microbiol. 2017, 71, 753–775. [Google Scholar] [CrossRef] [PubMed]
- Edlind Thomas, D.; Katiyar Santosh, K. Mutational Analysis of Flucytosine Resistance in Candida Glabrata. Antimicrob. Agents Chemother. 2010, 54, 4733–4738. [Google Scholar] [CrossRef] [PubMed]
- Berman, J.; Krysan, D.J. Drug Resistance and Tolerance in Fungi. Nat. Rev. Microbiol. 2020, 18, 319–331. [Google Scholar] [CrossRef] [PubMed]
- Shor, E.; Perlin, D.S. Coping with Stress and the Emergence of Multidrug Resistance in Fungi. PLoS Pathog. 2015, 11, e1004668. [Google Scholar] [CrossRef] [PubMed]
- Meletiadis, J.; Antachopoulos, C.; Stergiopoulou, T.; Pournaras, S.; Roilides, E.; Walsh, T.J. Differential Fungicidal Activities of Amphotericin B and Voriconazole against Aspergillus Species Determined by Microbroth Methodology. Antimicrob. Agents Chemother. 2007, 51, 3329–3337. [Google Scholar] [CrossRef] [PubMed]
- Geißel, B.; Loiko, V.; Klugherz, I.; Zhu, Z.; Wagener, N.; Kurzai, O.; van den Hondel, C.A.M.J.J.; Wagener, J. Azole-Induced Cell Wall Carbohydrate Patches Kill Aspergillus Fumigatus. Nat. Commun. 2018, 9, 3098. [Google Scholar] [CrossRef] [PubMed]
- Patil, A.; Majumdar, S. Echinocandins in Antifungal Pharmacotherapy. J. Pharm. Pharmacol. 2017, 69, 1635–1660. [Google Scholar] [CrossRef] [PubMed]
- Carmona, E.M.; Limper, A.H. Overview of Treatment Approaches for Fungal Infections. Clin. Chest Med. 2017, 38, 393–402. [Google Scholar] [CrossRef]
- Fisher, M.C.; Hawkins, N.J.; Sanglard, D.; Gurr, S.J. Worldwide Emergence of Resistance to Antifungal Drugs Challenges Human Health and Food Security. Science 2018, 360, 739–742. [Google Scholar] [CrossRef]
- Fisher, M.C.; Alastruey-Izquierdo, A.; Berman, J.; Bicanic, T.; Bignell, E.M.; Bowyer, P.; Bromley, M.; Brüggemann, R.; Garber, G.; Cornely, O.A.; et al. Tackling the Emerging Threat of Antifungal Resistance to Human Health. Nat. Rev. Microbiol. 2022, 20, 557–571. [Google Scholar] [CrossRef]
- Revie, N.M.; Iyer, K.R.; Robbins, N.; Cowen, L.E. Antifungal Drug Resistance: Evolution, Mechanisms and Impact. Curr. Opin. Microbiol. 2018, 45, 70–76. [Google Scholar] [CrossRef]
- Zheng, Y.-H.; Ma, Y.-Y.; Ding, Y.; Chen, X.-Q.; Gao, G.-X. An Insight into New Strategies to Combat Antifungal Drug Resistance. Drug Des. Dev. 2018, 12, 3807–3816. [Google Scholar] [CrossRef]
- Pathadka, S.; Yan, V.K.C.; Neoh, C.F.; Al-Badriyeh, D.; Kong, D.C.M.; Slavin, M.A.; Cowling, B.J.; Hung, I.F.N.; Wong, I.C.K.; Chan, E.W. Global Consumption Trend of Antifungal Agents in Humans From 2008 to 2018: Data From 65 Middle- and High-Income Countries. Drugs 2022, 82, 1193–1205. [Google Scholar] [CrossRef]
- Hoenigl, M.; Sprute, R.; Egger, M.; Arastehfar, A.; Cornely, O.A.; Krause, R.; Lass-Flörl, C.; Prattes, J.; Spec, A.; Thompson, G.R.; et al. The Antifungal Pipeline: Fosmanogepix, Ibrexafungerp, Olorofim, Opelconazole, and Rezafungin. Drugs 2021, 81, 1703–1729. [Google Scholar] [CrossRef]
- Perfect, J.R. The Antifungal Pipeline: A Reality Check. Nat. Rev. Drug Discov. 2017, 16, 603–616. [Google Scholar] [CrossRef]
- Ostrosky-Zeichner, L.; Casadevall, A.; Galgiani, J.N.; Odds, F.C.; Rex, J.H. An Insight into the Antifungal Pipeline: Selected New Molecules and Beyond. Nat. Rev. Drug Discov. 2010, 9, 719–727. [Google Scholar] [CrossRef]
- Campoy, S.; Adrio, J.L. Antifungals. Biochem. Pharmacol. 2017, 133, 86–96. [Google Scholar] [CrossRef]
- Odds, F.C.; Brown, A.J.P.; Gow, N.A.R. Antifungal Agents: Mechanisms of Action. Trends Microbiol. 2003, 11, 272–279. [Google Scholar] [CrossRef]
- Zotchev, S.B. Polyene Macrolide Antibiotics and Their Applications in Human Therapy. Curr. Med. Chem. 2003, 10, 211–223. [Google Scholar] [CrossRef]
- Hamilton-Miller, J.M. Chemistry and Biology of the Polyene Macrolide Antibiotics. Bacteriol. Rev. 1973, 37, 166–196. [Google Scholar] [CrossRef]
- Kinsky, S.C. Polyene Antibiotics. In Antibiotics: Volume I Mechanism of Action; Gottlieb, D., Shaw, P.D., Eds.; Springer: Berlin/Heidelberg, Germany, 1967; pp. 122–141. ISBN 978-3-662-38439-8. [Google Scholar]
- Bekersky, I.; Fielding, R.M.; Dressler, D.E.; Lee, J.W.; Buell, D.N.; Walsh, T.J. Pharmacokinetics, Excretion, and Mass Balance of Liposomal Amphotericin B (AmBisome) and Amphotericin B Deoxycholate in Humans. Antimicrob. Agents Chemother. 2002, 46, 828–833. [Google Scholar] [CrossRef] [PubMed]
- Mesa-Arango, A.C.; Scorzoni, L.; Zaragoza, O. It Only Takes One to Do Many Jobs: Amphotericin B as Antifungal and Immunomodulatory Drug. Front. Microbiol. 2012, 3, 286. [Google Scholar] [CrossRef] [PubMed]
- Kristanc, L.; Božič, B.; Jokhadar, Š.Z.; Dolenc, M.S.; Gomišček, G. The Pore-Forming Action of Polyenes: From Model Membranes to Living Organisms. Biochim. Et Biophys. Acta (BBA)-Biomembr. 2019, 1861, 418–430. [Google Scholar] [CrossRef] [PubMed]
- Quezada, H.; Martínez-Vázquez, M.; López-Jácome, E.; González-Pedrajo, B.; Andrade, Á.; Fernández-Presas, A.M.; Tovar-García, A.; García-Contreras, R. Repurposed Anti-Cancer Drugs: The Future for Anti-Infective Therapy? Expert Rev. Anti Infect. 2020, 18, 609–612. [Google Scholar] [CrossRef]
- Rojas, E.; Herrera, L.A.; Sordo, M.; Gonsebatt, M.E.; Montero, R.; Rodríguez, R.; Ostrosky-Wegman, P. Mitotic Index and Cell Proliferation Kinetics for Identification of Antineoplastic Activity. Anticancer Drugs 1993, 4, 637–640. [Google Scholar] [CrossRef]
- Longley, D.B.; Harkin, D.P.; Johnston, P.G. 5-Fluorouracil: Mechanisms of Action and Clinical Strategies. Nat. Rev. Cancer 2003, 3, 330–338. [Google Scholar] [CrossRef] [PubMed]
- Moudi, M.; Go, R.; Yien, C.Y.S.; Nazre, M. Vinca Alkaloids. Int. J. Prev. Med. 2013, 4, 1231–1235. [Google Scholar] [PubMed]
- Weaver, B.A. How Taxol/Paclitaxel Kills Cancer Cells. Mol. Biol. Cell 2014, 25, 2677–2681. [Google Scholar] [CrossRef]
- Perfect, J.R.; Dismukes, W.E.; Dromer, F.; Goldman, D.L.; Graybill, J.R.; Hamill, R.J.; Harrison, T.S.; Larsen, R.A.; Lortholary, O.; Nguyen, M.-H.; et al. Clinical Practice Guidelines for the Management of Cryptococcal Disease: 2010 Update by the Infectious Diseases Society of America. Clin. Infect. Dis. 2010, 50, 291–322. [Google Scholar] [CrossRef]
- Cornely, O.A.; Bassetti, M.; Calandra, T.; Garbino, J.; Kullberg, B.J.; Lortholary, O.; Meersseman, W.; Akova, M.; Arendrup, M.C.; Arikan-Akdagli, S.; et al. ESCMID* Guideline for the Diagnosis and Management of Candida Diseases 2012: Non-Neutropenic Adult Patients. Clin. Microbiol. Infect. 2012, 18 (Suppl. 7), 19–37. [Google Scholar] [CrossRef] [PubMed]
- Tobias, J.S.; Wrigley, P.F.; Shaw, E. Combination Antifungal Therapy for Cryptococcal Meningitis. Postgrad. Med. J. 1976, 52, 305–308. [Google Scholar] [CrossRef]
- Vermes, A.; Guchelaar, H.-J.; Dankert, J. Flucytosine: A Review of Its Pharmacology, Clinical Indications, Pharmacokinetics, Toxicity and Drug Interactions. J. Antimicrob. Chemother. 2000, 46, 171–179. [Google Scholar] [CrossRef]
- Heidemann, H.T.; Brune, K.H.; Sabra, R.; Branch, R.A. Acute and Chronic Effects of Flucytosine on Amphotericin B Nephrotoxicity in Rats. Antimicrob. Agents Chemother. 1992, 36, 2670–2675. [Google Scholar] [CrossRef]
- Schwarz, P.; Janbon, G.; Dromer, F.; Lortholary, O.; Dannaoui, E. Combination of Amphotericin B with Flucytosine Is Active In Vitro against Flucytosine-Resistant Isolates of Cryptococcus Neoformans. Antimicrob. Agents Chemother. 2007, 51, 383–385. [Google Scholar] [CrossRef]
- Houšť, J.; Spížek, J.; Havlíček, V. Antifungal Drugs. Metabolites 2020, 10, 106. [Google Scholar] [CrossRef] [PubMed]
- Padda, I.S.; Parmar, M. Flucytosine. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Gsaller, F.; Furukawa, T.; Carr, P.D.; Rash, B.; Jöchl, C.; Bertuzzi, M.; Bignell, E.M.; Bromley, M.J. Mechanistic Basis of PH-Dependent 5-Flucytosine Resistance in Aspergillus Fumigatus. Antimicrob. Agents Chemother. 2018, 62, e02593-17. [Google Scholar] [CrossRef]
- García-García, I.; Borobia, A.M. Current Approaches and Future Strategies for the Implementation of Pharmacogenomics in the Clinical Use of Azole Antifungal Drugs. Expert Opin. Drug Metab. Toxicol. 2021, 17, 509–514. [Google Scholar] [CrossRef] [PubMed]
- Howard, K.C.; Dennis, E.K.; Watt, D.S.; Garneau-Tsodikova, S. A Comprehensive Overview of the Medicinal Chemistry of Antifungal Drugs: Perspectives and Promise. Chem. Soc. Rev. 2020, 49, 2426–2480. [Google Scholar] [CrossRef] [PubMed]
- Brand, S.R.; Degenhardt, T.P.; Person, K.; Sobel, J.D.; Nyirjesy, P.; Schotzinger, R.J.; Tavakkol, A. A Phase 2, Randomized, Double-Blind, Placebo-Controlled, Dose-Ranging Study to Evaluate the Efficacy and Safety of Orally Administered VT-1161 in the Treatment of Recurrent Vulvovaginal Candidiasis. Am. J. Obs. Gynecol. 2018, 218, 624.e1–624.e9. [Google Scholar] [CrossRef] [PubMed]
- Cui, X.; Wang, L.; Lü, Y.; Yue, C. Development and Research Progress of Anti-Drug Resistant Fungal Drugs. J. Infect. Public Health 2022, 15, 986–1000. [Google Scholar] [CrossRef] [PubMed]
- Neochoritis, C.G.; Zhao, T.; Dömling, A. Tetrazoles via Multicomponent Reactions. Chem. Rev. 2019, 119, 1970–2042. [Google Scholar] [CrossRef]
- Monk Brian, C.; Keniya Mikhail, V.; Manya, S.; Wilson Rajni, K.; Graham Danyon, O.; Hassan Harith, F.; Danni, C.; Tyndall Joel, D.A. Azole Resistance Reduces Susceptibility to the Tetrazole Antifungal VT-1161. Antimicrob. Agents Chemother. 2018, 63, e02114-18. [Google Scholar] [CrossRef]
- Hoekstra, W.J.; Garvey, E.P.; Moore, W.R.; Rafferty, S.W.; Yates, C.M.; Schotzinger, R.J. Design and Optimization of Highly-Selective Fungal CYP51 Inhibitors. Bioorganic Med. Chem. Lett. 2014, 24, 3455–3458. [Google Scholar] [CrossRef]
- Warrilow, A.G.S.; Hull, C.M.; Parker, J.E.; Garvey, E.P.; Hoekstra, W.J.; Moore, W.R.; Schotzinger, R.J.; Kelly, D.E.; Kelly, S.L. The Clinical Candidate VT-1161 Is a Highly Potent Inhibitor of Candida Albicans CYP51 but Fails To Bind the Human Enzyme. Antimicrob. Agents Chemother. 2014, 58, 7121–7127. [Google Scholar] [CrossRef]
- Wiederhold, N.P.; Najvar, L.K.; Garvey, E.P.; Brand, S.R.; Xu, X.; Ottinger, E.A.; Alimardanov, A.; Cradock, J.; Behnke, M.; Hoekstra, W.J.; et al. The Fungal Cyp51 Inhibitor VT-1129 Is Efficacious in an Experimental Model of Cryptococcal Meningitis. Antimicrob. Agents Chemother. 2018, 62, e01071-18. [Google Scholar] [CrossRef] [PubMed]
- Sobel, J.D.; Nyirjesy, P. Oteseconazole: An Advance in Treatment of Recurrent Vulvovaginal Candidiasis. Future Microbiol. 2021, 16, 1453–1461. [Google Scholar] [CrossRef]
- Lockhart Shawn, R.; Fothergill Annette, W.; Naureen, I.; Bolden Carol, B.; Grossman Nina, T.; Garvey Edward, P.; Brand Stephen, R.; Hoekstra William, J.; Schotzinger Robert, J.; Elizabeth, O.; et al. The Investigational Fungal Cyp51 Inhibitor VT-1129 Demonstrates Potent In Vitro Activity against Cryptococcus Neoformans and Cryptococcus Gattii. Antimicrob. Agents Chemother. 2016, 60, 2528–2531. [Google Scholar] [CrossRef]
- Kathiravan, M.K.; Salake, A.B.; Chothe, A.S.; Dudhe, P.B.; Watode, R.P.; Mukta, M.S.; Gadhwe, S. The Biology and Chemistry of Antifungal Agents: A Review. Bioorg. Med. Chem. 2012, 20, 5678–5698. [Google Scholar] [CrossRef] [PubMed]
- Cadena, J.; Thompson, G.R.; Patterson, T.F. Invasive Aspergillosis: Current Strategies for Diagnosis and Management. Infect. Dis. Clin. 2016, 30, 125–142. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.-H.; Wang, Y. Recent Researches in Triazole Compounds as Medicinal Drugs. Curr. Med. Chem. 2012, 19, 239–280. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.-L.; Yu, S.-J.; Heitman, J.; Wellington, M.; Chen, Y.-L. New Facets of Antifungal Therapy. Virulence 2017, 8, 222–236. [Google Scholar] [CrossRef]
- Lass-Flörl, C. Triazole Antifungal Agents in Invasive Fungal Infections. Drugs 2011, 71, 2405–2419. [Google Scholar] [CrossRef]
- Kernt, M.; Kampik, A. Endophthalmitis: Pathogenesis, Clinical Presentation, Management, and Perspectives. Clin. Ophthalmol. 2010, 4, 121–135. [Google Scholar] [CrossRef]
- Kofla, G.; Ruhnke, M. Pharmacology and Metabolism of Anidulafungin, Caspofungin and Micafungin in the Treatment of Invasive Candidosis—Review of the Literature. Eur. J. Med. Res. 2011, 16, 159. [Google Scholar] [CrossRef]
- Stover, K.R.; Farley, J.M.; Kyle, P.B.; Cleary, J.D. Cardiac Toxicity of Some Echinocandin Antifungals. Expert Opin. Drug Saf. 2014, 13, 5–14. [Google Scholar] [CrossRef]
- Denning, D.W. Echinocandins: A New Class of Antifungal. J. Antimicrob. Chemother. 2002, 49, 889–891. [Google Scholar] [CrossRef]
- Bachmann, S.P.; Patterson, T.F.; López-Ribot, J.L. In Vitro Activity of Caspofungin (MK-0991) against Candida Albicans Clinical Isolates Displaying Different Mechanisms of Azole Resistance. J. Clin. Microbiol. 2002, 40, 2228–2230. [Google Scholar] [CrossRef] [PubMed]
- Gil-Lamaignere, C.; Salvenmoser, S.; Hess, R.; Müller, F.-M.C. Micafungin Enhances Neutrophil Fungicidal Functions against Candida Pseudohyphae. Antimicrob. Agents Chemother. 2004, 48, 2730–2732. [Google Scholar] [CrossRef] [PubMed]
- Pontón, J. [The fungal cell wall and the mechanism of action of anidulafungin]. Rev. Iberoam. Micol. 2008, 25, 78–82. [Google Scholar] [CrossRef] [PubMed]
- Felton, T.; Troke, P.F.; Hope, W.W. Tissue Penetration of Antifungal Agents. Clin. Microbiol. Rev. 2014, 27, 68–88. [Google Scholar] [CrossRef] [PubMed]
- Sucher, A.J.; Chahine, E.B.; Balcer, H.E. Echinocandins: The Newest Class of Antifungals. Ann. Pharm. 2009, 43, 1647–1657. [Google Scholar] [CrossRef]
- Chandrasekar, P.H.; Sobel, J.D. Micafungin: A New Echinocandin. Clin. Infect. Dis. 2006, 42, 1171–1178. [Google Scholar] [CrossRef]
- Bowman, J.C.; Hicks, P.S.; Kurtz, M.B.; Rosen, H.; Schmatz, D.M.; Liberator, P.A.; Douglas, C.M. The Antifungal Echinocandin Caspofungin Acetate Kills Growing Cells of Aspergillus Fumigatus in Vitro. Antimicrob. Agents Chemother. 2002, 46, 3001–3012. [Google Scholar] [CrossRef]
- Kurtz, M.B.; Heath, I.B.; Marrinan, J.; Dreikorn, S.; Onishi, J.; Douglas, C. Morphological Effects of Lipopeptides against Aspergillus Fumigatus Correlate with Activities against (1,3)-Beta-D-Glucan Synthase. Antimicrob. Agents Chemother. 1994, 38, 1480–1489. [Google Scholar] [CrossRef]
- Sanglard, D. Resistance of Human Fungal Pathogens to Antifungal Drugs. Curr. Opin. Microbiol. 2002, 5, 379–385. [Google Scholar] [CrossRef] [PubMed]
- Hull, C.M.; Bader, O.; Parker, J.E.; Weig, M.; Gross, U.; Warrilo, A.G.S.; Kelly, D.E.; Kelly, S.L. Two Clinical Isolates of Candida Glabrata Exhibiting Reduced Sensitivity to Amphotericin B Both Harbor Mutations in ERG2. Antimicrob. Agents Chemother. 2012, 56, 6417–6421. [Google Scholar] [CrossRef] [PubMed]
- Carolus, H.; Pierson, S.; Muñoz, J.F.; Subotić, A.; Cruz, R.B.; Cuomo, C.A.; Van Dijck, P. Genome-Wide Analysis of Experimentally Evolved Candida Auris Reveals Multiple Novel Mechanisms of Multidrug Resistance. mBio 2021, 12, e03333-20. [Google Scholar] [CrossRef]
- Blatzer, M.; Blum, G.; Jukic, E.; Posch, W.; Gruber, P.; Nagl, M.; Binder, U.; Maurer, E.; Sarg, B.; Lindner, H.; et al. Blocking Hsp70 Enhances the Efficiency of Amphotericin B Treatment against Resistant Aspergillus Terreus Strains. Antimicrob. Agents Chemother. 2015, 59, 3778–3788. [Google Scholar] [CrossRef] [PubMed]
- Posch, W.; Blatzer, M.; Wilflingseder, D.; Lass-Flörl, C. Aspergillus Terreus: Novel Lessons Learned on Amphotericin B Resistance. Med. Mycol. 2018, 56, S73–S82. [Google Scholar] [CrossRef] [PubMed]
- Carolus, H.; Pierson, S.; Lagrou, K.; Van Dijck, P. Amphotericin B and Other Polyenes—Discovery, Clinical Use, Mode of Action and Drug Resistance. J. Fungi 2020, 6, 321. [Google Scholar] [CrossRef] [PubMed]
- Delma, F.Z.; Al-Hatmi, A.M.S.; Brüggemann, R.J.M.; Melchers, W.J.G.; de Hoog, S.; Verweij, P.E.; Buil, J.B. Molecular Mechanisms of 5-Fluorocytosine Resistance in Yeasts and Filamentous Fungi. J. Fungi 2021, 7, 909. [Google Scholar] [CrossRef]
- Papon, N.; Noël, T.; Florent, M.; Gibot-Leclerc, S.; Jean, D.; Chastin, C.; Villard, J.; Chapeland-Leclerc, F. Molecular Mechanism of Flucytosine Resistance in Candida Lusitaniae: Contribution of the FCY2, FCY1, and FUR1 Genes to 5-Fluorouracil and Fluconazole Cross-Resistance. Antimicrob. Agents Chemother. 2007, 51, 369–371. [Google Scholar] [CrossRef]
- Burks, C.; Darby, A.; Gómez Londoño, L.; Momany, M.; Brewer, M.T. Azole-Resistant Aspergillus Fumigatus in the Environment: Identifying Key Reservoirs and Hotspots of Antifungal Resistance. PLoS Pathog. 2021, 17, e1009711. [Google Scholar] [CrossRef]
- Sharma, C.; Chowdhary, A. Molecular Bases of Antifungal Resistance in Filamentous Fungi. Int. J. Antimicrob. Agents 2017, 50, 607–616. [Google Scholar] [CrossRef]
- Cowen, L.E.; Sanglard, D.; Howard, S.J.; Rogers, P.D.; Perlin, D.S. Mechanisms of Antifungal Drug Resistance. Cold Spring Harb. Perspect. Med. 2014, 5, a019752. [Google Scholar] [CrossRef] [PubMed]
- Whaley, S.G.; Rogers, P.D. Azole Resistance in Candida Glabrata. Curr. Infect. Dis. Rep. 2016, 18, 41. [Google Scholar] [CrossRef] [PubMed]
- Pinjon, E.; Moran, G.P.; Coleman, D.C.; Sullivan, D.J. Azole Susceptibility and Resistance in Candida Dubliniensis. Biochem. Soc. Trans. 2005, 33, 1210–1214. [Google Scholar] [CrossRef]
- Whaley, S.G.; Berkow, E.L.; Rybak, J.M.; Nishimoto, A.T.; Barker, K.S.; Rogers, P.D. Azole Antifungal Resistance in Candida Albicans and Emerging Non-Albicans Candida Species. Front. Microbiol. 2017, 7, 2173. [Google Scholar] [CrossRef]
- Pfaller, M.A.; Diekema, D.J.; Turnidge, J.D.; Castanheira, M.; Jones, R.N. Twenty Years of the SENTRY Antifungal Surveillance Program: Results for Candida Species From 1997–2016. Open Forum Infect. Dis. 2019, 6, S79–S94. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, C.F.; Rodrigues, M.E.; Henriques, M. Susceptibility of Candida Glabrata Biofilms to Echinocandins: Alterations in the Matrix Composition. Biofouling 2018, 34, 569–578. [Google Scholar] [CrossRef]
- Liu, C.; Shi, C.; Mao, F.; Xu, Y.; Liu, J.; Wei, B.; Zhu, J.; Xiang, M.; Li, J. Discovery of New Imidazole Derivatives Containing the 2,4-Dienone Motif with Broad-Spectrum Antifungal and Antibacterial Activity. Molecules 2014, 19, 15653–15672. [Google Scholar] [CrossRef]
- Nishimoto Andrew, T.; Wiederhold Nathan, P.; Flowers Stephanie, A.; Zhang, Q.; Kelly Steven, L.; Joachim, M.; Yates Christopher, M.; Hoekstra William, J.; Schotzinger Robert, J.; Garvey Edward, P.; et al. In Vitro Activities of the Novel Investigational Tetrazoles VT-1161 and VT-1598 Compared to the Triazole Antifungals against Azole-Resistant Strains and Clinical Isolates of Candida Albicans. Antimicrob. Agents Chemother. 2019, 63, e00341-19. [Google Scholar] [CrossRef]
- Desai, J.V.; Mitchell, A.P.; Andes, D.R. Fungal Biofilms, Drug Resistance, and Recurrent Infection. Cold Spring Harb. Perspect. Med. 2014, 4, a019729. [Google Scholar] [CrossRef]
- Gebreyohannes, G.; Nyerere, A.; Bii, C.; Sbhatu, D.B. Challenges of Intervention, Treatment, and Antibiotic Resistance of Biofilm-Forming Microorganisms. Heliyon 2019, 5, e02192. [Google Scholar] [CrossRef]
- Rajendran, R.; Sherry, L.; Deshpande, A.; Johnson, E.M.; Hanson, M.F.; Williams, C.; Munro, C.A.; Jones, B.L.; Ramage, G. A Prospective Surveillance Study of Candidaemia: Epidemiology, Risk Factors, Antifungal Treatment and Outcome in Hospitalized Patients. Front. Microbiol. 2016, 7, 915. [Google Scholar] [CrossRef] [PubMed]
- Rajendran, R.; Sherry, L.; Nile, C.J.; Sherriff, A.; Johnson, E.M.; Hanson, M.F.; Williams, C.; Munro, C.A.; Jones, B.J.; Ramage, G. Biofilm Formation Is a Risk Factor for Mortality in Patients with Candida Albicans Bloodstream Infection-Scotland, 2012–2013. Clin. Microbiol. Infect. 2016, 22, 87–93. [Google Scholar] [CrossRef]
- Sherry, L.; Ramage, G.; Kean, R.; Borman, A.; Johnson, E.M.; Richardson, M.D.; Rautemaa-Richardson, R. Biofilm-Forming Capability of Highly Virulent, Multidrug-Resistant Candida Auris. Emerg. Infect. Dis. 2017, 23, 328–331. [Google Scholar] [CrossRef]
- Ramage, G.; Rajendran, R.; Sherry, L.; Williams, C. Fungal Biofilm Resistance. Int. J. Microbiol. 2012, 2012, 528521. [Google Scholar] [CrossRef]
- Perlin, D.S.; Shor, E.; Zhao, Y. Update on Antifungal Drug Resistance. Curr. Clin. Microbiol. Rep. 2015, 2, 84–95. [Google Scholar] [CrossRef] [PubMed]
- Niimi, K.; Maki, K.; Ikeda, F.; Holmes, A.R.; Lamping, E.; Niimi, M.; Monk, B.C.; Cannon, R.D. Overexpression of Candida Albicans CDR1, CDR2, or MDR1 Does Not Produce Significant Changes in Echinocandin Susceptibility. Antimicrob. Agents Chemother. 2006, 50, 1148–1155. [Google Scholar] [CrossRef]
- Garcia-Effron, G.; Lee, S.; Park, S.; Cleary, J.D.; Perlin, D.S. Effect of Candida Glabrata FKS1 and FKS2 Mutations on Echinocandin Sensitivity and Kinetics of 1,3-β-d-Glucan Synthase: Implication for the Existing Susceptibility Breakpoint. Antimicrob. Agents Chemother. 2009, 53, 3690–3699. [Google Scholar] [CrossRef] [PubMed]
- Perlin, D.S. Current Perspectives on Echinocandin Class Drugs. Future Microbiol. 2011, 6, 441–457. [Google Scholar] [CrossRef] [PubMed]
- Morio, F.; Loge, C.; Besse, B.; Hennequin, C.; Le Pape, P. Screening for Amino Acid Substitutions in the Candida Albicans Erg11 Protein of Azole-Susceptible and Azole-Resistant Clinical Isolates: New Substitutions and a Review of the Literature. Diagn. Microbiol. Infect. Dis. 2010, 66, 373–384. [Google Scholar] [CrossRef]
- Sanglard, D.; Coste, A.T. Activity of Isavuconazole and Other Azoles against Candida Clinical Isolates and Yeast Model Systems with Known Azole Resistance Mechanisms. Antimicrob. Agents Chemother. 2015, 60, 229–238. [Google Scholar] [CrossRef]
- Prasad, R.; Banerjee, A.; Shah, A.H. Resistance to Antifungal Therapies. Essays Biochem. 2017, 61, 157–166. [Google Scholar] [CrossRef]
- Holmes, A.R.; Cardno, T.S.; Strouse, J.J.; Ivnitski-Steele, I.; Keniya, M.V.; Lackovic, K.; Monk, B.C.; Sklar, L.A.; Cannon, R.D. Targeting Efflux Pumps to Overcome Antifungal Drug Resistance. Future Med. Chem. 2016, 8, 1485–1501. [Google Scholar] [CrossRef] [PubMed]
- Chang, W.; Liu, J.; Zhang, M.; Shi, H.; Zheng, S.; Jin, X.; Gao, Y.; Wang, S.; Ji, A.; Lou, H. Efflux Pump-Mediated Resistance to Antifungal Compounds Can Be Prevented by Conjugation with Triphenylphosphonium Cation. Nat. Commun. 2018, 9, 5102. [Google Scholar] [CrossRef] [PubMed]
- Abbotsford, J.; Foley, D.A.; Goff, Z.; Bowen, A.C.; Blyth, C.C.; Yeoh, D.K. Clinical Experience with SUBA-Itraconazole at a Tertiary Paediatric Hospital. J. Antimicrob. Chemother. 2021, 76, 249–252. [Google Scholar] [CrossRef] [PubMed]
- Gintjee, T.J.; Donnelley, M.A.; Thompson, G.R. Aspiring Antifungals: Review of Current Antifungal Pipeline Developments. J. Fungi 2020, 6, 28. [Google Scholar] [CrossRef] [PubMed]
- Hargrove, T.Y.; Garvey, E.P.; Hoekstra, W.J.; Yates, C.M.; Wawrzak, Z.; Rachakonda, G.; Villalta, F.; Lepesheva, G.I. Crystal Structure of the New Investigational Drug Candidate VT-1598 in Complex with Aspergillus Fumigatus Sterol 14α-Demethylase Provides Insights into Its Broad-Spectrum Antifungal Activity. Antimicrob. Agents Chemother. 2017, 61, e00570-17. [Google Scholar] [CrossRef] [PubMed]
- Van Daele, R.; Spriet, I.; Wauters, J.; Maertens, J.; Mercier, T.; Van Hecke, S.; Brüggemann, R. Antifungal Drugs: What Brings the Future? Med. Mycol. 2019, 57, S328–S343. [Google Scholar] [CrossRef]
- Davis, M.R.; Donnelley, M.A.; Thompson, G.R., III. Ibrexafungerp: A Novel Oral Glucan Synthase Inhibitor. Med. Mycol. 2020, 58, 579–592. [Google Scholar] [CrossRef]
- Rivero-Menendez, O.; Cuenca-Estrella, M.; Alastruey-Izquierdo, A. In Vitro Activity of Olorofim (F901318) against Clinical Isolates of Cryptic Species of Aspergillus by EUCAST and CLSI Methodologies. J. Antimicrob. Chemother. 2019, 74, 1586–1590. [Google Scholar] [CrossRef]
- Santangelo, R.; Paderu, P.; Delmas, G.; Chen, Z.-W.; Mannino, R.; Zarif, L.; Perlin, D.S. Efficacy of Oral Cochleate-Amphotericin B in a Mouse Model of Systemic Candidiasis. Antimicrob. Agents Chemother. 2000, 44, 2356–2360. [Google Scholar] [CrossRef]
- Alkhazraji, S.; Gebremariam, T.; Alqarihi, A.; Gu, Y.; Mamouei, Z.; Singh, S.; Wiederhold, N.P.; Shaw, K.J.; Ibrahim, A.S. Fosmanogepix (APX001) Is Effective in the Treatment of Immunocompromised Mice Infected with Invasive Pulmonary Scedosporiosis or Disseminated Fusariosis. Antimicrob. Agents Chemother. 2020, 64, e01735-19. [Google Scholar] [CrossRef]
- Shaw, K.J.; Ibrahim, A.S. Fosmanogepix: A Review of the First-in-Class Broad Spectrum Agent for the Treatment of Invasive Fungal Infections. J. Fungi 2020, 6, 239. [Google Scholar] [CrossRef]
- McCarthy, M.W.; Kontoyiannis, D.P.; Cornely, O.A.; Perfect, J.R.; Walsh, T.J. Novel Agents and Drug Targets to Meet the Challenges of Resistant Fungi. J. Infect. Dis. 2017, 216, S474–S483. [Google Scholar] [CrossRef]
- Kovanda Laura, L.; Sullivan Sean, M.; Smith Larry, R.; Desai Amit, V.; Bonate Pete, L.; Hope William, W. Population Pharmacokinetic Modeling of VL-2397, a Novel Systemic Antifungal Agent: Analysis of a Single- and Multiple-Ascending-Dose Study in Healthy Subjects. Antimicrob. Agents Chemother. 2019, 63, e00163-19. [Google Scholar] [CrossRef] [PubMed]
- Anna-Maria, D.; Matthias, M.; Aguiar Mario, M.; Vasyl, I.; David, T.; Joachim, P.; Clemens, D.; Martin, H.; Sullivan Sean, M.; Smith Larry, R.; et al. The Siderophore Transporter Sit1 Determines Susceptibility to the Antifungal VL-2397. Antimicrob. Agents Chemother. 2019, 63, e00807-19. [Google Scholar] [CrossRef]
- Nishikawa, H.; Yamada, E.; Shibata, T.; Uchihashi, S.; Fan, H.; Hayakawa, H.; Nomura, N.; Mitsuyama, J. Uptake of T-2307, a Novel Arylamidine, in Candida Albicans. J. Antimicrob. Chemother. 2010, 65, 1681–1687. [Google Scholar] [CrossRef]
- Mitsuyama, J.; Nomura, N.; Hashimoto, K.; Yamada, E.; Nishikawa, H.; K, M.; Kimura, A.; Todo, Y.; Narita, H. In Vitro and In Vivo Antifungal Activities of T-2307, a Novel Arylamidine. Antimicrob. Agents Chemother. 2008, 52, 1318–1324. [Google Scholar] [CrossRef]
- Wiederhold, N.P. Review of T-2307, an Investigational Agent That Causes Collapse of Fungal Mitochondrial Membrane Potential. J. Fungi 2021, 7, 130. [Google Scholar] [CrossRef]
- Campione, E.; Gaziano, R.; Marino, D.; Orlandi, A. Fungistatic Activity of All-Trans Retinoic Acid against Aspergillus Fumigatus and Candida Albicans. Drug Des. Dev. 2016, 10, 1551–1555. [Google Scholar] [CrossRef] [PubMed]
- Cosio, T.; Gaziano, R.; Zuccari, G.; Costanza, G.; Grelli, S.; Di Francesco, P.; Bianchi, L.; Campione, E. Retinoids in Fungal Infections: From Bench to Bedside. Pharmaceuticals 2021, 14, 962. [Google Scholar] [CrossRef]
- Campione, E.; Cosio, T.; Lanna, C.; Mazzilli, S.; Ventura, A.; Dika, E.; Gaziano, R.; Dattola, A.; Candi, E.; Bianchi, L. Predictive Role of Vitamin A Serum Concentration in Psoriatic Patients Treated with IL-17 Inhibitors to Prevent Skin and Systemic Fungal Infections. J. Pharmacol. Sci. 2020, 144, 52–56. [Google Scholar] [CrossRef] [PubMed]
- Hill, J.A.; Cowen, L.E. Using Combination Therapy to Thwart Drug Resistance. Future Microbiol. 2015, 10, 1719–1726. [Google Scholar] [CrossRef] [PubMed]
- Livengood, S.J.; Drew, R.H.; Perfect, J.R. Combination Therapy for Invasive Fungal Infections. Curr. Fungal Infect. Rep. 2020, 14, 40–49. [Google Scholar] [CrossRef]
- Evans, E.G.V. The Rationale for Combination Therapy. Br. J. Dermatol. 2001, 145, 9–13. [Google Scholar] [CrossRef] [PubMed]
- Ruhnke, M. Antifungal Stewardship in Invasive Candida Infections. Clin. Microbiol. Infect. 2014, 20, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Johnson, M.D.; Lewis, R.E.; Dodds Ashley, E.S.; Ostrosky-Zeichner, L.; Zaoutis, T.; Thompson, G.R., III; Andes, D.R.; Walsh, T.J.; Pappas, P.G.; Cornely, O.A.; et al. Core Recommendations for Antifungal Stewardship: A Statement of the Mycoses Study Group Education and Research Consortium. J. Infect. Dis. 2020, 222, S175–S198. [Google Scholar] [CrossRef] [PubMed]
- Valerio, M.; Muñoz, P.; Rodríguez-González, C.; Sanjurjo, M.; Guinea, J.; Bouza, E. Training Should Be the First Step toward an Antifungal Stewardship Program. Enferm. Infecc. Y Microbiol. Clínica 2015, 33, 221–227. [Google Scholar] [CrossRef]
- Urbancic, K.F.; Thursky, K.; Kong, D.C.M.; Johnson, P.D.R.; Slavin, M.A. Antifungal Stewardship: Developments in the Field. Curr. Opin. Infect. Dis. 2018, 31, 490–498. [Google Scholar] [CrossRef]
- Micallef, C.; Aliyu, S.H.; Santos, R.; Brown, N.M.; Rosembert, D.; Enoch, D.A. Introduction of an Antifungal Stewardship Programme Targeting High-Cost Antifungals at a Tertiary Hospital in Cambridge, England. J. Antimicrob. Chemother. 2015, 70, 1908–1911. [Google Scholar] [CrossRef]
- Valerio, M.; Muñoz, P.; Rodríguez, C.G.; Caliz, B.; Padilla, B.; Fernández-Cruz, A.; Sánchez-Somolinos, M.; Gijón, P.; Peral, J.; Gayoso, J.; et al. Antifungal Stewardship in a Tertiary-Care Institution: A Bedside Intervention. Clin. Microbiol. Infect. 2015, 21, 492.e1–492.e9. [Google Scholar] [CrossRef]
- Leach, M.D.; Klipp, E.; Cowen, L.E.; Brown, A.J.P. Fungal Hsp90: A Biological Transistor That Tunes Cellular Outputs to Thermal Inputs. Nat. Rev. Microbiol. 2012, 10, 693–704. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.D.; Robbins, N.; Zaas, A.K.; Schell, W.A.; Perfect, J.R.; Cowen, L.E. Hsp90 Governs Echinocandin Resistance in the Pathogenic Yeast Candida Albicans via Calcineurin. PLoS Pathog. 2009, 5, e1000532. [Google Scholar] [CrossRef]
- Lamoth, F.; Juvvadi, P.R.; Gehrke, C.; Steinbach, W.J. In Vitro Activity of Calcineurin and Heat Shock Protein 90 Inhibitors against Aspergillus Fumigatus Azole- and Echinocandin-Resistant Strains. Antimicrob. Agents Chemother. 2013, 57, 1035–1039. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-L.; Lehman, V.N.; Lewit, Y.; Averette, A.F.; Heitman, J. Calcineurin Governs Thermotolerance and Virulence of Cryptococcus Gattii. G3 (Bethesda) 2013, 3, 527–539. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-L.; Brand, A.; Morrison, E.L.; Silao, F.G.S.; Bigol, U.G.; Malbas, F.F.; Nett, J.E.; Andes, D.R.; Solis, N.V.; Filler, S.G.; et al. Calcineurin Controls Drug Tolerance, Hyphal Growth, and Virulence in Candida Dubliniensis. Eukaryot. Cell 2011, 10, 803–819. [Google Scholar] [CrossRef] [PubMed]
- One Health: Fungal Pathogens of Humans, Animals, and Plants: Report on an American Academy of Microbiology Colloquium Held in Washington, DC, on 18 October 2017; American Academy of Microbiology Colloquia Reports; American Society for Microbiology: Washington, DC, USA, 2019.
- Schneider, M.C.; Munoz-Zanzi, C.; Min, K.; Aldighieri, S. “One Health” From Concept to Application in the Global World; Oxford Research Encyclopedia, Global Public Health; Oxford University Press: Oxford, UK, 2018. [Google Scholar]
- Chowdhary, A.; Meis, J. Emergence of Azole Resistant Aspergillus Fumigatus and One Health: Time to Implement Environmental Stewardship. Environ. Microbiol. 2018, 20, 1299–1301. [Google Scholar] [CrossRef]
- Banerjee, S.; Denning, D.W.; Chakrabarti, A. One Health Aspects & Priority Roadmap for Fungal Diseases: A Mini-Review. Indian J. Med. Res. 2021, 153, 311–319. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rabaan, A.A.; Sulaiman, T.; Al-Ahmed, S.H.; Buhaliqah, Z.A.; Buhaliqah, A.A.; AlYuosof, B.; Alfaresi, M.; Al Fares, M.A.; Alwarthan, S.; Alkathlan, M.S.; et al. Potential Strategies to Control the Risk of Antifungal Resistance in Humans: A Comprehensive Review. Antibiotics 2023, 12, 608. https://doi.org/10.3390/antibiotics12030608
Rabaan AA, Sulaiman T, Al-Ahmed SH, Buhaliqah ZA, Buhaliqah AA, AlYuosof B, Alfaresi M, Al Fares MA, Alwarthan S, Alkathlan MS, et al. Potential Strategies to Control the Risk of Antifungal Resistance in Humans: A Comprehensive Review. Antibiotics. 2023; 12(3):608. https://doi.org/10.3390/antibiotics12030608
Chicago/Turabian StyleRabaan, Ali A., Tarek Sulaiman, Shamsah H. Al-Ahmed, Zainab A. Buhaliqah, Ali A. Buhaliqah, Buthina AlYuosof, Mubarak Alfaresi, Mona A. Al Fares, Sara Alwarthan, Mohammed S. Alkathlan, and et al. 2023. "Potential Strategies to Control the Risk of Antifungal Resistance in Humans: A Comprehensive Review" Antibiotics 12, no. 3: 608. https://doi.org/10.3390/antibiotics12030608
APA StyleRabaan, A. A., Sulaiman, T., Al-Ahmed, S. H., Buhaliqah, Z. A., Buhaliqah, A. A., AlYuosof, B., Alfaresi, M., Al Fares, M. A., Alwarthan, S., Alkathlan, M. S., Almaghrabi, R. S., Abuzaid, A. A., Altowaileb, J. A., Al Ibrahim, M., AlSalman, E. M., Alsalman, F., Alghounaim, M., Bueid, A. S., Al-Omari, A., & Mohapatra, R. K. (2023). Potential Strategies to Control the Risk of Antifungal Resistance in Humans: A Comprehensive Review. Antibiotics, 12(3), 608. https://doi.org/10.3390/antibiotics12030608







