Abstract
Medicinal plants are an essential source of traditional curatives for numerous skin diseases. Polyalthia longifolia (Sonn.) Thwaites (Annonaceae family) is a medicinal plant used to cure skin illnesses. P. longifolia is usually applied in folkloric therapeutical systems to treat skin diseases. The methicillin-resistant Staphylococcus aureus (MRSA) bacteria is among the essential bacteria contributing to skin diseases. Hence, to verify the traditional medicinal claim of P. longifolia usage in skin disease treatment, the current research was performed to study the synergistic antibacterial activity of standardized Polyalthia longifolia methanol leaf extract (MEPL) against MRSA bacteria. The synergistic antimicrobial activity result of ceftriaxone, when mixed with MEPL, against MRSA was investigated by the disc diffusion method, broth microdilution method, checkerboard dilution test, and modulation of mecA gene expression by multiplex polymerase chain reaction (multiplex PCR). The MEPL extract exhibited good synergistic antimicrobial activity against MRSA. Using the checkerboard method, we confirmed the synergistic effect of MEPL from P. longifolia and ceftriaxone (2:1) for MRSA with a marked reduction of the MIC value of the ceftriaxone from 8000 µg/mL to 1000 µg/mL. Moreover, the combination of MEPL with ceftriaxone significantly (p < 0.05) inhibited the presence of the resistant mecA gene in the tested strain. The LC–ESI–MS/MS analysis identified compounds that were reported to exhibit antimicrobial activity. Conclusively, the MEPL extract, an important etiological agent for skin diseases, showed worthy synergistic antimicrobial action against MRSA bacteria, thus supporting the traditional use of P. longifolia.
1. Introduction
Microbial infectious diseases have become the third most crucial reason for mortality and morbidity worldwide. The contagion instigated by methicillin-resistant Staphylococcus aureus (MRSA) has contributed significantly to deadly infections and diseases [1]. There is growing proof that S. aureus is becoming resistant to all the standard antibiotics. Ceftriaxone belongs to a class of drugs identified as cephalosporin antibiotics and is extensively used to treat resistant bacterial strains, including S. aureus infection [2]. Nevertheless, disturbingly, the emergence of ceftriaxone-resistance MRSA bacteria was reported in the literature [3]. Moreover, a genetic mutation was involved in the development of resistance to the antibiotic. The attainment of the mecA gene by horizontal transmission by conjugation was the leading cause of antibiotic resistance in S. aureus [4]. This important mecA gene has contributed to methicillin resistance in S. aureus strains, which encodes a novel penicillin-binding protein 2A (PBP2A) [5]. Therefore, new alternative strategies are needed to address this issue by developing new antimicrobial agents, modifying the existing antibiotic activity with a combination of plant extracts as resistance modifying agents, or using the plant extract combined with existing antibiotics against resistant bacteria to suppress the expression of the mecA gene in MRSA bacteria. Consequently, the increasing incidence of MRSA bacterial infection has drawn the pharmaceutical and scientific community’s attention to studies on the potential antimicrobial activity of plant-derived substances used in traditional medicine in different countries. Scientists from divergent fields are investigating medicinal plants regarding their antimicrobial usefulness. Hence, the development of a new antibacterial against MRSA is of crucial importance.
Consequently, the search for drugs derived from medicinal plants by scientists has accelerated in recent years worldwide. The medicinal plant, a famous healthcare agent, is used daily by billions of people globally for their primary healthcare. The medicinal plant was considered a panacea with various curative values in traditional medicine, including anti-infectious activity. One crucial medicinal plant with multiple curative values is Polyalthia longifolia var. angustifolia Thw. (Annonaceae). P. longifolia is a medicinal plant with linear–lanceolate leaves found in Sri Lanka, India’s tropical parts, and Malaysia. This tree is normally planted along roadsides and gardens due to its beautiful appearance. P. longifolia is one of the most important traditional indigenous medicinal plants commonly used in folk medicine to treat skin diseases, fever, hypertension, helminthiasis, and diabetes [6]. The MRSA bacteria is also one of the important bacteria contributing to skin and soft tissue infection [7], which leads to major illness and death [8], comprising endocarditis, septic shock, bacteremia, and pneumonia [9]. Hence, to verify the traditional medicinal practitioner’s claims on the contribution of P. longifolia to skin disease treatment, the present research studied the synergistic antimicrobial action of P. longifolia leaf extract and ceftriaxone antibiotic against MRSA bacteria.
Until 2019, there was limited experimental evidence of the synergistic activity between P. longifolia leaf extract and synthetic antibiotics against MRSA. Previous experiments have demonstrated the in vitro interaction of ampicillin and P. longifolia leaf ethyl acetate fraction (PLEAF) by checkerboard and microscopic techniques against MRSA [10,11]. That previous study showed that the PLEAF fraction worked synergistically with ampicillin to kill MRSA’s local resistance strain. Moreover, the PLEAF fraction also exhibited excellent antioxidant activity. The combination of the PLEAF fraction with ampicillin also increased Vero cell viability. This critical finding showed the non-toxic nature of ampicillin in the presence of PLEAF in combinational therapy. Further study was also conducted to observe the in situ synergistic antimicrobial effects between PLEAF and ampicillin against a local MRSA isolate using modern scanning electron microscopy (SEM) observation [11]. PLEAF and ampicillin combination exhibited significant antibacterial activity against MRSA by killing the resistant MRSA bacteria, as observed via SEM analysis. However, as a further study in understanding multidrug-resistant bacteria’s challenges, P. longifolia leaf extract antibacterial activity, antibiotic modifying activity, and mutagenic effects combined with different first-line antibiotics commonly used against infectious agents should be investigated. Investigating the synergistic antimicrobial effects of the P. longifolia leaf methanolic extract combined with β-lactam antibiotics, such as ceftriaxone, will enhance the understanding of the synergistic antimicrobial effects of P. longifolia leaf extract, which has never been studied in detail before. In addition, the synergistic effect of ceftriaxone and P. longifolia methanol leaf extract in combination against MRSA bacteria and the mecA gene is still unclear, and few studies were conducted in this line. Therefore, the objective of the current research was to study the action of MEPL from P. longifolia on the regulation of mecA gene presence in the MRSA strain and study the synergistic effect of ceftriaxone and MEPL in this bacterium.
2. Results
2.1. Ceftriaxone and MEPL Antibacterial Activity against MRSA Isolates
Antimicrobial susceptibility of MRSA isolates shows complete resistance to the standard dosage strengths (8 μg/mL, 16 μg/mL, 32 μg/mL, and 64 μg/mL) of ceftriaxone, and no diameter of zone of inhibition was produced by all the different ceftriaxone dosages tested in this study (Table 1). Conversely, the tested MEPL exhibited significant antibacterial activity against MRSA by producing a clear zone of inhibition between 21 mm and 34 mm (Table 1). The negative control 5% dimethyl sulfoxide (DMSO) did not produce any zone of inhibition.

Table 1.
Antimicrobial activity of MEPL against MRSA.
2.2. Determination of the MIC and MBC Concentration of Ceftriaxone and MEPL against the MRSA Isolate
The antibiotic MIC value is an essential aid in evaluating bacterial resistance. According to 2022 CLSI interpretive measures, MRSA is susceptible to ceftriaxone when the MIC value is ≤8 µg/mL, and MRSA is susceptible to ceftriaxone with a MIC value of 32 μg/mL. The MIC of ceftriaxone was obtained using the broth dilution method, and the ceftriaxone MIC value was 8000 µg/mL, visibly inhibiting MRSA growth in the broth. While the ceftriaxone MBC value, where the lowest concentration showed zero growth on sterile NA, was found at 8000 μg/mL. The MIC result demonstrated MRSA growth in a concentration of ≥62.5 μg/mL (the breaking point of ceftriaxone is ≤16 to ≥64 μg/mL). This proves that the MRSA used in this study was highly resistant towards ceftriaxone. The MEPL recorded the MIC value of 16,000 µg/mL. On the other hand, when a volume of 100 µL of inoculum from each tube was plated on fresh sterile NA, the lowest concentration of MEPL where no MRSA growth was observed was at a concentration of 16,000 μg/mL, therefore, indicating the MBC value of the MEPL to be also 16,000 μg/mL. It should be noted that the MIC and MBC results for MEPL against the MRSA strain showed a larger value than ceftriaxone.
2.3. Synergistic Activity of Antibiotic with MEPL
The interrelation effects between ceftriaxone and MEPL against MRSA were tested using the checkerboard technique in association with the MIC value. Ceftriaxone and MEPL combination treatment enhanced the antimicrobial effect and exhibited synergistic activity on MRSA (Table 2). In the combination treatment, the MIC values of ceftriaxone and MEPL against MRSA were reduced to eight times lower (1000 µg/mL and 2000 µg/mL). As predicted, unique antibacterial activity with a lower MIC value was demonstrated by ceftriaxone in the presence of the MEPL in the combination therapy. Coherently, it resulted in a synergistic antibacterial effect against the tested MRSA via the combination therapy of ceftriaxone and MEPL extract.

Table 2.
Synergistic effect of MEPL and ceftriaxone sodium was determined by the checkerboard test.
Calculation of the FIC index of MEPL and ceftriaxone to determine the synergistic effect:
- MIC of ceftriaxone alone = 8000 µg/mL
- MIC of ceftriaxone in combination = 1000 µg/mL
- MIC of MEPL alone = 16,000 µg/mL
- MIC of MEPL in combination = 2000 µg/mL
- FICceftriaxone = 1000 µg/mL ÷ 8000 µg/mL = 0.125
- FICMEPL = 2000 µg/mL ÷ 16000 µg/mL = 0.125
The sum of FIC (ΣFIC) is calculated as follows:
- ΣFIC = FICceftriaxone + FICMEPL
- = 0.125 + 0.125
- = 0.25
In brief, the MIC value of the MEPL and ceftriaxone in the checkerboard test were 2000 µg/mL and 1000 µg/mL, respectively. The FIC index of the combination of MEPL and ceftriaxone was 0.25, which indicates a significant synergistic antimicrobial activity against the MRSA bacteria. The combination is considered synergistic when the ΣFIC index is ≤0.5, and indifference is indicated by an FIC index > 0.5 to ≤4, while antagonism is when the ΣFIC is >4. In addition, the initial MIC values of MEPL (16,000 µg/mL) and ceftriaxone (8000 µg/mL) were found to reduce to 2000 µg/mL for MEPL and 1000 µg/mL for ceftriaxone (p < 0.05), respectively, in the checkerboard test against the MRSA bacteria.
2.4. Presence of the mecA Gene in MRSA Treated with Different Combinations of MEPL and Ceftriaxone
2.4.1. Purity of Genomic DNA
DNA concentration, purity, and contamination are the three factors that can affect the multiplex PCR test. Nucleic acids are typically quantified (at an absorption ratio of 260 nm/280 nm) to obtain an average DNA concentration and purity necessary to be considered when carrying out PCR (Table S1). All the DNA extracted demonstrated a purity ratio value of 1.8, which indicates low protein contamination. The result was analyzed by electrophoresis on a 0.8% agarose gel followed by ethidium bromide staining to confirm an adequate amount of the DNA present with a clear band for further amplification with primary and targeted band detection. As shown in Figure 1, bands of genomic DNA can be seen on top of the gel. The absence of smearing indicates that the DNA is intact and not degraded.
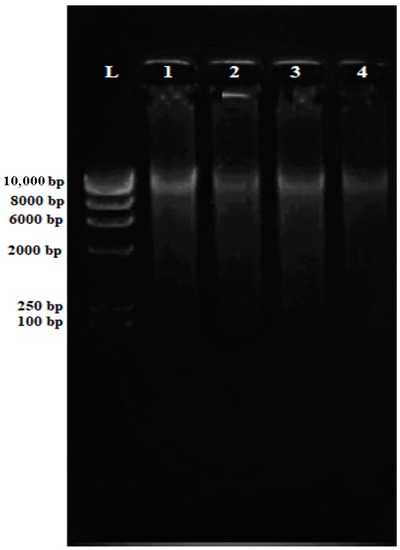
Figure 1.
Electrophoresis gel (0.8% agarose) of the extracted genomic DNA from MRSA isolates. All genomic MRSA DNA (Lane 1–Lane 4) are intact for downstream applications.
2.4.2. Optimization of mecA Gene Amplification
The gradient amplification was performed to obtain the optimum annealing temperature for the multiplex PCR. Isolate 20 with a purity value of 1.7 and DNA concentration of 33.5 ng/µL (Table S1), was used throughout optimization since this isolate shows an enhanced DNA band in extracted product during electrophoresis observation. Four specific temperatures at 55.0 °C, 56.6 °C, 60.0 °C, and 61.0 °C were selected for the gradient PCR. As shown in Figure 2, a clear thick band was visible using the annealing temperature of 60.0 °C. Table S2 shows the relative intensity of the PCR amplicons on the 3% agarose gel. This finding proved that the DNA band present at 60.0 °C annealing temperature was the perfect band for mecA gene amplification via PCR.

Figure 2.
Electrophoresis gel (3% agarose) of the PCR products of the mecA gene for PCR optimization. The amplification optimized using MRSA isolates. Lane L = 1 kb ladder. The amplification of optimized isolate for annealing temperature 55.0 °C, 56.6 °C, 60.0 °C, and 61.0 °C. Lane 1 = amplification at annealing temperature 55.0 °C, Lane 2 = amplification at annealing temperature 56.6 °C, Lane 3 = amplification at annealing temperature 60.0 °C, and Lane 4 = amplification at annealing temperature 61.0 °C. A more apparent band was observed for the 60.0 °C reaction as shown in the yellow box.
2.4.3. Detection of mecA Gene by Multiplex PCR
The multiplex PCR was used to detect mecA gene-encoded ceftriaxone resistance directly from MRSA culture using the mecA gene and specific S. aureus 16S rRNA primers as an internal control for the 16S rRNA gene, which is a conserved region in all prokaryotic bacteria. In the MRSA bacteria, the mecA gene should amplify at 313 bp and the 16S rRNA gene at 528 bp. In comparison, the methicillin-susceptible Staphylococcus Aureus (MSSA) should only amplify the 16S rRNA gene at 528 bp. An MRSA confirmation test was carried out using MRSA and MSSA isolates as the control. As shown in Figure 3, the MRSA isolates successfully amplified the mecA gene (313 bp) and 16S rRNA gene (528 bp), while the MSSA isolates only amplified the 16S rRNA gene (528 bp) as predicted.
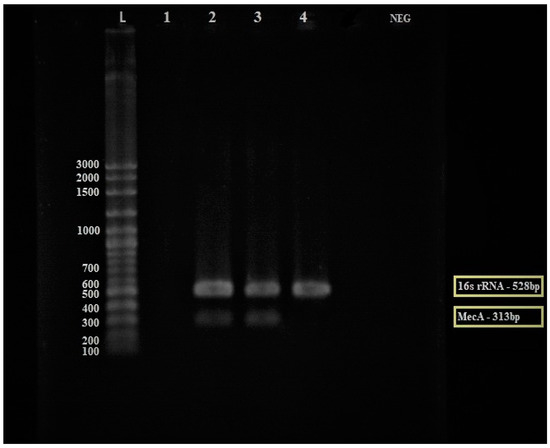
Figure 3.
Electrophoresis gel (3% agarose) of the PCR products of the mecA gene for the discovery of the mecA gene from MRSA (Lane 2–3) and MSSA (Lane 4) isolates. Lane L = 100 bp ladder, Lane 1 = empty lane, Lane 2 and 3 = MRSA isolates, Lane 4 = MSSA control isolate, and Lane NEG = negative control. The yellow box: The mecA gene was expressed in MRSA strain while the 16S rRNA gene was expressed in both MRSA and MSSA strains.
Subsequently, different combinations of MEPL (1000 µg/mL and 2000 µg/mL) with ceftriaxone (1000 µg/mL) were tested against the MRSA isolate to investigate the influences of a different combinations of MEPL with ceftriaxone on the regulation of the mecA gene in the tested MRSA strain. As shown in Figure 4, the mecA gene was present in the MRSA isolate treated with MEPL and ceftriaxone at 1000 µg/mL; however, the combination of MEPL with ceftriaxone at 2000 µg/mL of MEPL and 1000 µg/mL of ceftriaxone successfully suppressed the presence of the mecA gene at 313 bp. In addition, as expected, the mecA gene was not expressed in the tested MSSA isolate.
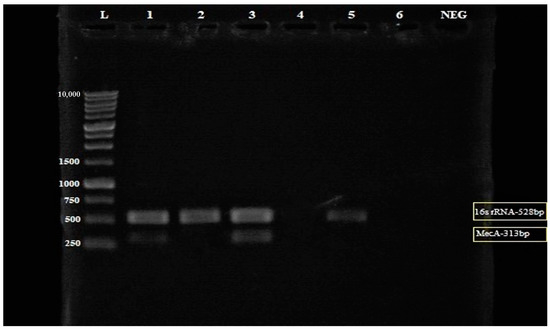
Figure 4.
Electrophoresis gel (3% agarose) of the PCR products of the mecA gene and 16S rRNA for the detection of the mecA gene from MRSA (Lane 2–4) and MSSA (Lane 5) isolates. Lane L = 1 kb ladder, Lane 1 = Treated MRSA isolate (in a combination of 1000 µg/mL PLLME and 1000 µg/mL ceftriaxone), Lane 2 = Treated MRSA isolate (in a combination of 2000 µg/mL PLLME and 1000 µg/mL ceftriaxone), Lane 3 = untreated MRSA isolates, Lane 4 = blank, Lane 5 = MSSA isolate (control), Lane 6 = empty lane and Lane NEG = negative control. The yellow box: The mecA gene was not amplified in MRSA treated with the combination of 2000 µg/mL PLLME and 1000 µg/mL ceftriaxone.
Figure 5 shows the relative intensity (Table S3) of the amplified multiplex PCR products with the mecA gene and 16S rRNA bands formed on the electrophoresis gel using ImageJ software. The ImageJ software analysis of DNA bands can be used to quantify the mecA gene expression in the MRSA isolate. The ImageJ software analysis on the relative intensity of the mecA gene in MRSA provides quantitative data for the convenient evaluation of qualitative electrophoresis gel results. Therefore, with the aid of the ImageJ software, the quantification of the mecA gene band’s relative intensity on the electrophoresis gel was further analyzed. The finding confirmed that the combination treatment of MEPL (2000 µg/mL) with ceftriaxone (1000 µg/mL) against MRSA isolates (Figure 4, Lane 3) displays a zero value for the mecA gene fragment (313 bp), which indicated the complete suppression of the mecA gene in MRSA.
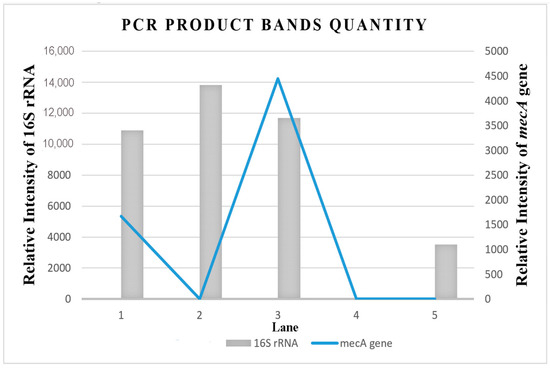
Figure 5.
Relative band intensity by densitometry analysis of electrophoresis (3% agarose) gel of the PCR products of the mecA gene and 16S rRNA performed using ImageJ quantification software. Lane 1 = Treated MRSA isolate (in a combination of 1000 µg/mL MEPL and 1000 µg/mL ceftriaxone); Lane 2 = Treated MRSA isolate (in a combination of 2000 µg/mL MEPL and 1000 µg/mL ceftriaxone); Lane 3 = untreated MRSA isolates, Lane 4 = blank, Lane 5 = MSSA isolate (control).
2.4.4. Antimicrobial Compounds in MEPL
Ultra High-Performance Liquid Chromatography (UHPLC) analysis was performed to analyze and tentatively annotate the extracted metabolites in the MEPL with the aid of the chemical library of Metlin_AM_PCDL-N-170502.cdb. The UHPLC analysis of the MEPL showed the presence of several antimicrobial phytochemicals. Among these, beta-himachalene (1.9%), 5Z,8Z,11Z,14Z-octadecatetraenoic acid (8.3%), 9Z,12Z,15E-octadecatrienoicc acid (6.1%), and luteolin 7-rhamnosyl(1->6)galactoside (5.7%) were the antimicrobial compounds in MEPL extract. The chemical structures of the antimicrobial phytochemical compounds found in MEPL are presented in Figure 6.
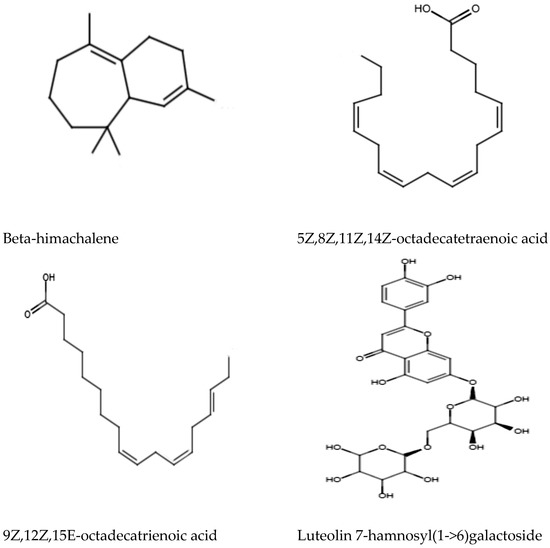
Figure 6.
Antimicrobial phytochemical compounds found in the methanol extract of Polyalthia longifolia Leaf (MEPL) were detected using ultra high-performance liquid chromatography (UHPLC) equipped with the chemical library.
3. Discussion
Methicillin-resistant Staphylococcus aureus (MRSA) infection has become one of the most historic pathogenic bacterial infection associated with health issues in developing countries. Moreover, the crucial mecA gene contributes to methicillin resistance in MRSA strains, which encodes a novel penicillin-binding protein PBP2a. The global trend has represented a rise in MRSA infections with the high emergence of multidrug-resistant strains [12]. This bacterium has shown resistance to various antibiotics such as methicillin, penicillin, and amoxicillin, including ceftriaxone. Ceftriaxone is a third-generation cephalosporin and remains one of the most commonly used antibiotics for antimicrobial therapy due to its efficacy and low therapeutic index [13,14]. It is reported that ceftriaxone has a broad potency spectrum against Gram-positive and Gram-negative bacteria [15]. In addition, ceftriaxone is used frequently to treat MSSA infections [16,17]. It has been used as a first-line treatment against bacteremia alongside other antibiotic combinations [18]. The rise of microorganism resistance towards third-generation cephalosporins is a global burden and has led to antimicrobial treatment failure. The bacterial organism becomes inherently resistant to the increased use of antibiotics at a higher antibiotic dosage [19,20]. Besides, bacterial resistance to an antibiotic can also be attributed to random genetic mutation [21] or the uptake of plasmid DNA (horizontal gene transfer) from foreign cells [22]. Hence, MRSA has become the center of this public health concern due to its high virulence and resistance to a broad spectrum of antibiotics [23]. This widespread organism causes challenges to both the healthcare system and patients due to increased hospitalization costs and notable mortality/morbidity rates [24]. In addition to this complication, antibiotics often produce adverse effects, namely hypersensitivity, immune suppression, and allergic reactions [25,26,27,28]. The need to develop new antimicrobials as an alternative to synthetic antibiotics for MRSA treatment is achieved from various sources. Many developing countries commonly use medicinal plants in the treatment of multiple health complications. The application of medicinal plant extracts rich with pharmacological activity, such as P. longifolia and its associated phytochemicals, can significantly contribute to the treatment of infectious diseases. Hence, the current research was performed to evaluate the synergistic antibacterial activity of natural MEPL in combination with ceftriaxone against MRSA bacteria.
The synergistic antimicrobial action between medicinal plant extracts and conventional antibiotics has been extensively studied to overcome the antibiotic resistance problem [29,30,31]. Synergism takes place when two different molecules interact and strengthen their actions. On the other hand, any reduction in activity from the combination treatment is termed antagonism [32]. The synergistic properties of MEPL with ceftriaxone against MRSA were evaluated in this study. The results indicated positive synergism in the combination treatment of MEPL and ceftriaxone compared to ceftriaxone or MEPL alone against the MRSA bacteria. The MIC and MBC values of ceftriaxone and MEPL decreased in the combination treatment, indicating the synergistic antimicrobial activity of MEPL in combination with ceftriaxone. MEPL may promote synergistic antimicrobial properties by acting as synergistic activity enhancers in combination with ceftriaxone, enhancing the overall antibiotic effect. The advantages associated with the synergistic interactions are that synergism effect increases treatment efficiency, decreases undesirable side effects of the single drug, such as diarrhea, nausea, bloating, and indigestion, increases the bioavailability of free agents, and an adequate therapeutic effect is achieved with comparatively smaller doses when compared with individual synthetic antimicrobials [33]. Many researchers have reported that combination therapy, mainly plant extracts with synthetic antibiotics, exhibited a synergistic effect against S. aureus [34,35,36,37]. Interestingly, a recent study reported impaired cell division, extensive wrinkles, cell shrinkage, and the emergence of rougher cells with fibrous matrix and clustered cells, highlighting the synergistic effect of ethyl acetate P. longifolia in combination with ampicillin against MRSA cells [10,11]. Another study has also suggested that the membrane-disrupting activity of combination therapy between Trp-containing antimicrobial peptides (AMPs) with four classes of traditional chemical antibiotics, namely penicillin, ampicillin, and erythromycin, increases the access of small molecule antibiotics to the cell, which allows the synergistic activity to improve antimicrobial agents’ effectiveness, increasing bacterial killing and prevent resistance development [38]. Moreover, AL-Ali et al. [39] reported the synergistic antimicrobial activity of various plant extracts in combination treatment against multi-drug resistance (MRSA) S. aureus. The combination of four plant extracts, namely Mentha cervina, Mentha longifolia, Ocimum basilicum, and Origanum vulgare showed good synergistic antibacterial activity against the multi-drug resistance (MDR) S. aureus. Besides, another independent study has reported the antimicrobial activities of the methanol, acetone, and 1,4-dioxan fractions of P. longifolia leaves [40]. The tested sample showed better antibacterial activity against Gram-positive bacterial and fungal strains than the Gram-negative bacterial strains studied.
Various secondary metabolites in the MEPL, as reported in the literature, such as flavonoids, alkaloids, and diterpenoids [41], can be responsible for the observed antimicrobial properties of the MEPL. Hence, screening of MEPL was performed to annotate the chemical profiles using UHPLC analysis equipped with the chemical library of Metlin_AM_PCDL-N-170502.cdb to identify the bioactive chemical constituents that could be responsible for the observed antimicrobial activity. UHPLC analysis led to the detection of the various chemical constituents, as shown in Figure 6. Moreover, the presence of himachalene and its derivatives [42], fatty acid octadecatetraenoic (9Z,12Z,15E-octadecatrienoicc acid and 5Z,8Z,11Z,14Z-octadecatetraenoic acid) [43,44], and luteolin and its derivatives [45] compounds were found in MEPL, which were previously reported to show good antimicrobial activity against various microbes including S. aureus, which might have contributed to the observed antimicrobial activity of the MEPL in this study. Besides, rutin was used to standardize the MEPL extract in this study since rutin enhanced the antibacterial activities, as reported in the literature [46]. As observed in this study, rutin also might contribute to the synergistic effect of the MEPL extract.
In addition, various reports in the literature reported the isolation of compounds from P. longifolia with antimicrobial and synergistic antibacterial activity. Interestingly, seven antimicrobial clerodane diterpenoids, namely 16(R and S)-hydroxy-cleroda-3,13(14)Z-dien-15,16-olide, 16-oxo-cleroda-3,13(14)E-dien-15-oic acid, methyl-16-oxo-cleroda-3,13(14)E-dien-15-oate, 2-oxokolavenic acid, 16(R and S) hydroxy-cleroda-3,13(14)Z-dien-15,16-olide-2-one, (4→2)abeo-16(R and S)-hydroxy-cleroda-2, 13(14)Z-dien-15, 16-olide-3-al, and 3β,16α-dihydroxy-cleroda-4(18), 13(14)Z-dien-15,16-olide [47] were isolated from the methanol extract of P. longifolia leaves, which are widely reported for their antibacterial and antifungal properties [48]. Furthermore, diterpenoids induce bacterial membrane disruption [49], which may allow other compounds to enter cells to initiate antibacterial activity in a combination therapy mode. Therefore, the presence of diterpenoids [49] and flavonoids [50] in the MEPL, as reported in the literature, can be hypothesized to be synergistic and enhance the antibiotic function by disrupting the membrane of the MRSA and making it susceptible to ceftriaxone. In particular, the presence of clerodane diterpene 16α-hydroxycleroda-3, 13 (14) Z-dien-15, 16-olide (CD) has been reported to be synergistic against MRSA through the disruption of the cell membrane [51]. In addition, the combination of CD, a bioactive compound in MEPL, reduced the MIC of fluoroquinolones, such as norfloxacin, ciprofloxacin, and ofloxacin, against MRSA through significant inhibition of the efflux pump [52]. Efflux pumps have been cited as the main reason for the emergence of multidrug resistance bacteria towards various antibiotics among Gram-positive and Gram-negative bacteria [53]. It was reported that CD downregulates the expression of efflux pump genes, such as norA, norB, norC, mdeA, and mepA, which are the genes responsible for expelling antibiotics outside the S. aureus cells [54]. Therefore, it can be deduced that the bioactive compounds in the MEPL may play a similar role in inhibiting the efflux pump in S. aureus and synergistically reversing the resistance of MRSA towards ceftriaxone.
This study also attempted to assess whether the combination of MEPL with ceftriaxone influences the presence of the mecA gene by observing the presence of the mecA gene on the agarose gel upon treatment. In this study, MEPL from P. longifolia with ceftriaxone inhibits the manifestation of the resistant mecA gene in the studied strain. In the presence of β-lactam derivatives, the MRSA strains will not demonstrate growth inhibition and can retain their capacity to expand the zone of inhibition [55]. The methicillin-resistant mecA gene in MRSA isolates encodes PBP2a, a transpeptidase that inhibits the antibiotic’s antimicrobial action. Another study has reported that the mecA gene can be a useful molecular marker for MRSA isolates [56]. In contrast, S. aureus isolates lacking the mecA gene can be considered as MSSA strains [57]. The mecA-positive strains differ in the expression levels to methicillin resistance, which may be complex and difficult to diagnose [58]. Therefore, molecular techniques, such as polymerase chain reaction (PCR), are suitable for detecting the methicillin resistance mecA gene. The multiplex PCR technique utilized in this study is a rapid tool and considered the “gold standard” for detecting the methicillin resistance mecA gene due to its efficacy and accuracy [59]. Optimization of the PCR protocol is routine and necessary for better sensitivity and specificity. Adequate DNA templates and optimum annealing temperature are crucial factors for successfully amplifying the mecA gene [60]. This was evidently supported by the current research results, where the positive control MRSA isolate amplification was improved with the appropriate annealing temperature and DNA template.
Besides, the influence of MEPL on the mecA gene in MRSA bacteria was proven by the finding of the checkerboard method conducted in this research to assess the synergistic action of MEPL and ceftriaxone. The checkerboard method results indicate the synergistic effect of ceftriaxone combined with MEPL against MRSA by enhancing the antimicrobial effect. The mecA gene analysis in the MRSA treated with ceftriaxone (1000 µg/mL) combined with MEPL (2000 µg/mL) by multiplex PCR examination showed the absence of the mecA gene band. This finding indicated that the gene-specific primers could not identify and bind to the region coding the mecA gene. This finding disclosed the effective influence of MEPL on inhibiting the presence of the mecA gene in MRSA bacteria. The combination of ceftriaxone and MEPL influenced the presence of the mecA gene in MRSA to make the local strain susceptible to ceftriaxone. Interestingly, several studies report on the influence of medicinal plant extracts on bacterial gene expression, namely T. integrifolia, Eurycoma longifolia Jack, and Helmintostachys zeylanica against Salmonella typhimurium strains via the Ames Test [56,61]. Alkaloids, such as β-carboline, have been a vital influence against bacterial DNA [57,58,62,63]. It was reported that β-carboline alkaloids, such as harman and harmine of Passiflora spp. (Passifloraceae), are responsible for DNA damage of Saccharomyces cerevisiae [59,64]. In another study, the mutagenic properties of the methanolic extract of Byrsonima crassa Niedenzu was reported due to amentoflavone. Plant extracts containing flavonoids, such as Quercitin, have also been implicated in mutagenesis [60,65]. Therefore, flavonoids [50] and alkaloids [61,66] in MEPL might be responsible for the observed suppression of the expression of the mecA gene, which warrants further detailed studies.
The present research studies the antimicrobial effects and modulation of mecA gene expression by MEPL combined with ceftriaxone against an MRSA strain for possible application as a natural product agent. The MEPL combination with ceftriaxone exhibited vigorous antimicrobial activity against the MRSA isolate. Moreover, MEPL showed a synergistic antibacterial effect with ceftriaxone against the tested MRSA strain and suppressed the presence of the resistant mecA gene. From the findings of this research, it was established that MEPL could reinstate the effectiveness of ceftriaxone against MRSA. Consequently, the findings of this research propose that the MEPL and ceftriaxone combination could develop novel natural remedies based on combination antibiotics therapy against MRSA infection. Furthermore, various in vitro and in vivo experiments, such as the genotoxic effect evaluated via plasmid relaxation assay, acute oral toxicity studies in animal models, and the Allium cepa assay [67], showed that MEPL was not toxic and safe in human applications. The in vivo acute oral toxicity study showed that MEPL was safe even at a single dose of 5000 mg/kg body weight in female albino Wistar rats. Besides, the literature also reports that MEPL exhibits various biologically beneficial effects. The antimicrobial activity of P. longifolia leaf extracts were also reported by Chanda and Nair [40] against 91 clinically significant pathogenic microbial strains. The polyphenol-rich MEPL exhibited good antioxidant and hepatoprotective activities against paracetamol-induced oxidative damage [67,68]. Besides, the MEPL also supported the X-ray irradiated mouse survival rate increases compared to 100% mortality in the untreated mice [69], and renoprotection against radiation-induced nephropathy by an anti-oxidative molecular mechanism [70]. These findings highlight that MEPL decreased oxidative stress and nephropathy in rats due to its anti-inflammatory activities. Moreover, MEPL also showed good cytotoxicity against HeLa cancer cells via inducing apoptotic cell death and miRNA regulation [71,72,73,74]. A recent study also showed that MEPL exhibited good antiaging activities in S. cerevisiae by modulating oxidative stress, enhancing GSH content, and increasing SOD and SIRT1 gene expression [75].
4. Materials and Methods
4.1. Polyalthia longifolia Leaf
Mature leaves of P. longifolia were collected (Voucher specimen number: USM/HERBARIUM/11306) from University Sains Malaysia (USM), Pulau Pinang, Malaysia main campus, in February 2020. The leaves were rinsed thoroughly with tap water and air dried under shade inside the laboratory for about 2 weeks until the leaves were dried entirely. Dried leaf parts were homogenized to a fine powder using a regular blender and stored in airtight bottles.
4.2. Preparation of Polyalthia longifolia Leaf Extract
Dried powder P. longifolia leaves then underwent methanol extraction using a cold percolation process on a rotary shaker [76]. A mass of 100 g of dried powder of P. longifolia leaves was added into a conical flask and soaked in 1000 mL of methanol. The flask was sealed with aluminum foil and kept on a rotary shaker (Ohaus, Parsippany, NJ, USA) at 190–220 rpm for 3 days. After 3 days, the content of the flask was filtered at different levels, initiated via 8 layers of muslin cloth followed by Whatman No. 1 filter paper to get the crude extract. The filtrates were then collected and concentrated in a rotary vacuum evaporator (Eyela, Bohemia, NY, USA) [77] at 120 rpm and 200 pi at 41 °C overnight to remove solvents from samples through the evaporation process. The concentrated extract was then collected in a glass Petri dish, kept in the oven (60 °C), and incubated to remove excessive methanol further from the sample. A constant weight of the completely solvent-free filtrates was obtained after incubation in the oven. The filtrates were then stored at 4 °C in air-tight bottles. The final product of the methanol extract of Polyalthia longifolia leaf (MEPL) was used to conduct the antibacterial study. The MEPL stock solution was dissolved and prepared in 5% DMSO at a final 10 mg/mL concentration. The rutin measure in MEPL extract was established on the peak area calculated from the calibration curve equation of commercially (Sigma-Aldrich, St. Louis, MO, USA) available rutin (5, 10, 100, 400, 600, 800, and 1000 µg/mL) compound (standard) (y = 275,885x, r2 = 0.9977). The amount of rutin in the MEPL was found to be 8.96 µg (0.896%) in 1000 µg [69].
4.3. Test Microorganism Collection and Maintenance
The Gram-positive bacterium MRSA and MSSA were collected from the Penang General Hospital Microbiology Unit (GH), Penang, Malaysia. The MRSA and MSSA strains were aseptically removed with an inoculating loop and streaked in a zig-zag pattern onto the freshly prepared nutrient agar (NA) plate. The MRSA and MSSA strains on the NA plate were grown for 24 h at 37 °C. The stock culture was then stored at 4 °C. The stock culture was sub-cultured every 3 weeks to maintain viability.
Antimicrobial Susceptibility Test
Antimicrobial susceptibility testing (AST) of bacterial isolates is a collective and significant technique in most clinical laboratories. In this study, AST was conducted using the Kirby Bauer technique [78] based on the Clinical Laboratory Standard Institutions [79] guidelines on molten Mueller Hinton Agar (MHA). The steps involved in this assay are as follows.
4.4. Culture Media and Inoculum Preparation
The test organisms were grown on molten Mueller Hinton Agar (MHA) at 37 °C during the antibacterial susceptibility test. The molten MHA was prepared according to the manufacturer’s instruction (Oxoid, Basingstoke, UK), autoclaved and poured onto sterile Petri dishes, and solidified at room temperature. An inoculum suspension (1.5 × 108 cells/mL) equal to 0.5 McFarland was prepared by inoculating 5 similar colonies with a wire loop in up to 5 mL of tryptone soya broth (TSB) and incubated at 37 °C for 8 h up until mild-to-moderate turbidity growths could be seen.
4.5. Agar Disc Diffusion Assay of Ceftriaxone
A sterile cotton swab was dipped into the prepared inoculum of the MRSA suspension, which was rotated resolutely against the tube’s upper inside wall to rapidly removed excess fluid and then streaked through the entire surface of MHA plates. The plate was allowed to dry at room temperature with the inoculum, with the lid in place, for about 10 min. Standard antibiotic discs of ceftriaxone (8 μg/mL, 16 μg/mL, 32 μg/mL, and 64 μg/mL), also known as blank cartridges (Oxoid, Basingstoke, UK), were placed on the upper layer of the seeded agar plate and gently pressed on the disc’s handle while making sure all the discs were completely attached to the medium. The plates were incubated for 24 h at 37 °C. The formation of a clear zone of inhibition of ≥21 mm in diameter was considered a significant susceptibility of the organism to the MEPL extract. The experiment was replicated three times, and the mean value is displayed in this study. By measuring the diameter of the zone of inhibition, the antimicrobial activity was determined and recorded in millimeters with the aid of sliding calipers, and the organisms present were classified as sensitive, resistant, or intermediate, referring to CLSI guidelines (Table 4). The 5% DMSO was used as a negative control.

Table 4.
Diameter of inhibition zone interpretative criteria for S. aureus.
4.6. Agar Well Diffusion Method of Antibacterial Susceptibility Test for MEPL
The agar well diffusion method evaluated the antimicrobial activity of the MEPL with certain modifications. MRSA was grown on nutrient broth (NB) and incubated at 37 °C for 24 h. A total of 1600 μL of overnight NB culture was added to 120 mL of molten MHA and mixed well; the mixture was then poured into a sterile Petri dish and set aside to allow the plate to dry at room temperature. A sterile 5 mm in diameter cork-borer was used on the set agar to create wells. Subsequently, 25 μL of diluted plant extract in a sequence of 8 mg/mL, 7 mg/mL, 6 mg/mL, 5 mg/mL, 4 mg/mL, 3 mg/mL, 2 mg/mL, and 1 mg/mL was applied to the wells, and the plates were then incubated at 37 °C overnight. The bacterial growth was assessed based on the diameter of the inhibition zone. The tests were performed in triplicate, and average values were recorded. A 5% DMSO solution was used as a negative control.
4.7. Evaluation of the Minimum Inhibitory Concentration (MIC) and Minimum Bactericidal Concentration (MBC) of the MRSA Isolate against Ceftriaxone and MEPL
4.7.1. Determination of the MIC of Ceftriaxone and MEPL against the MRSA Isolate
To determine the MIC concentration of ceftriaxone and MEPL against the MRSA isolate, the broth dilution method was used under the CLSI guideline. Two-fold serial dilutions of the ceftriaxone at a concentration of (8000—62.5 μg/mL) and the MEPL in the arrangement of 16,000–62.5 μg/mL in 5% DMSO were prepared in sterile capped universal bottles. Subsequently, 2 mL of overnight incubated (37 °C) MRSA suspension was added to 2 mL of each concentration of the antibiotic ceftriaxone and MEPL dilution followed by vortexing and 18 h incubation at 37 °C. The negative control comprised MHB and ceftriaxone antibiotic, while the positive control was MHB and MRSA suspension. Another 2 mL broth prepared in the universal bottle was inoculated MRSA and kept overnight in a refrigerator at 4 °C separately. The tube was used as a standard for determining complete inhibition. The MIC value was determined as the lowest concentration of ceftriaxone and MEPL inhibiting the MRSA by referring to turbidity. Besides, comparing to the standard tube incubated previously in the refrigerator was used to assess the inhibition of the organism’s growth.
4.7.2. Determination of the MBC of Ceftriaxone and MEPL against the MRSA Isolate
Immediately after the MIC determination, the tubes with ceftriaxone and MEPL inhibiting the MRSA growth were used to determine the MBC. Subsequently, about 100 µL of the inoculum was added to a sterile NA media plate and incubated for 24 h in a 37 °C incubator to observe possible bacterial growth. The lowest concentration of ceftriaxone and MEPL in the subculture that showed no bacterial growth on the plate was considered the MBC [80].
4.8. Investigation of the Synergistic Properties of MEPL with Ceftriaxone
4.8.1. Preparation of MEPL and Ceftriaxone for Synergistic Study
Two-fold serial dilutions of the extracts (16,000–62.5 µg/mL) and ceftriaxone (8000–62.5 µg/mL) were prepared. A combination drug was prepared at a ratio of 1:1 of MEPL:ceftriaxone from the highest to lowest concentration to investigate of the synergistic properties of MEPL with Ceftriaxone.
4.8.2. Measurement of the Fractional Inhibitory Concentration (FIC) by Checkerboard Analysis
Ninety-six well microtiter plates were used to measure the FIC concentration for synergistic activity between MEPL and ceftriaxone [81,82]. The inoculum suspension was prepared in MHB. A total volume of 100 µL of two-fold dilution of EPL/ceftriaxone combination (1:1 ratio) was added to 900 µL of the inoculum suspension into each well of the microtiter plates, bringing the final total volume to 1 mL. The ceftriaxone was placed in columns in ascending concentrations starting at zero MIC and ending at two times the MIC. The MEPLs were similarly distributed among the rows. Accordingly, each well of the 96-well microtiter plate had a unique combination of different concentrations of the antibiotic and MEPL. Two control wells were preserved for each test batch. These included the test control (the well containing MEPL/antibiotic and the medium without inoculum) and organism control (the well containing the growth medium and the inoculum). The plate was incubated overnight at 37 °C. The MIC value was determined as the lowest concentration of ceftriaxone and MEPL inhibiting the MRSA by referring to turbidity.
Calculation of the Fractional Inhibitory Concentration (FIC) Index
The ΣFICs were computed with the formulae below [83]:
- FIC of plant extracts = MIC of MEPL in combination/MIC of MEPL alone
- FIC of antibiotic = MIC of antibiotic in combination/MIC of antibiotic alone
FIC index = FIC of MEPL + FIC of antibiotic
Synergy was defined as a FIC index ≤ 0.5.
The additive effect was defined as a FIC index > 0.5 but ≤4.0.
Antagonism was defined as a FIC index > 4.0.
4.9. Presence of the mecA Gene in MRSA Treated with Different Combinations of MEPL and Ceftriaxone
4.9.1. Concentration-Dependent Assay of Ceftriaxone and MEPL against MRSA and MSSA Isolates
The one-day-old cultures of MRSA and MSSA isolates were inoculated in 50 mL MH broth and incubated at 37 °C at 120 rpm agitation. The next day, the MRSA and MSSA isolates were treated with different combinations of plant extract dosages and antibiotics as 1000 μg/mL ceftriaxone with 1000 μg/mL or 2000 μg/mL MEPL. The cultures were incubated at 37 °C at a 120 rpm agitation rate for 24 h. The next day, the culture was pelleted at 0.12× g (120 rpm) speed for 10 min in a tabletop centrifuge. The pellets were then subjected to genomic DNA extraction.
4.9.2. Genomic DNA Extraction
The ready-to-use DNA mini kit from Stratec Molecular GmbH Berlin, Germany, was used to separate bacterial DNA from MRSA and MSSA strains. The genomic DNA of MRSA and MSSA strains was purified using the bacterial DNA purification mini kit (Stratec Molecular, Berlin, Germany) following the manufacturer’s protocol, and stored at −20 °C.
4.9.3. DNA Quantification
The eluted genomic DNA was quantified by measuring UV absorption using a NanoDrop spectrophotometer (BioRad, Hercules, CA, USA). The integrity of each eluted DNA sample was evaluated by subjecting it to 0.8% (w/v) agarose gel electrophoresis analysis, and the DNA samples were kept at −20 °C for future analysis.
4.9.4. Multiplex Polymerase Chain Reaction (PCR)
The genomic DNA was further subjected to multiplex PCR amplification to detect targeted genes (mecA and 16S rRNA). The multiplex PCR amplification was carried out using a Bio-Rad thermal cycler. PCR was carried out in 50 μL reaction mixtures, 25 μL Quick-Load 2X power Taq Master Mix applied with 1 μL reverse primer (1 µM) and 1 μL (1 µM) forward primer and genomic DNA (30 ng/µL). Sterile distilled water was added to bring the total volume to 50 μL. The negative control comprised just the Quick-Load 2X power Taq Master Mix, primers, and sterile water. The list of primers used in this study is listed in Table 5. The conditions of the gradient multiplex PCR (30 cycles) used in this study are as follows: denaturation at 95 °C for 30 s, annealing at 55–61 °C for 30 s, and eventually elongation at 72 °C for 30 s [84]. After the optimization of the annealing temperature, a conventional multiplex PCR was carried out using the same conditions of 95 °C for 30 s, followed by annealing at 60 °C for 30 s, and eventually elongation at 72 °C for 30 s. All PCR products were then assessed using 3% (w/v) gel electrophoresis.

Table 5.
PCR primer sequences for the detection of MRSA.
4.9.5. Agarose Gel Electrophoresis Analysis of the PCR Product
The agarose gel (3%, w/v) was prepared using 3 g gel powder dissolved in 100 mL of TBE buffer before microwave heating for up for 2 min. A 1 μL loading dye ratio to 3 μL PCR liquid (1:3) was used for all reactions. A volume of 4 μL of the sample and appropriate DNA ladder was loaded into each well and ran at a constant 65 V power supply for 40 min. Once the bromophenol blue stain hit more than two-thirds, the gel was examined by staining with ethidium bromide under a UV trans-illuminator (Appleton Woods, Birmingham, UK) to observe the specific band locations of the amplified DNA, which were recorded in an automatic gel documentation scanner. The intensity of the bands was quantified by using the ImageJ software.
4.10. LC–ESI–MS/MS Identification of Antimicrobial Compounds in MEPL
Identification of the antimicrobial compounds was carried out by using the Agilent 1200 series Ultra High-Performance Liquid Chromatography (UHPLC) system (Agilent Technologies, Santa Clara, CA, USA), coupled with an Agilent 6520 Accurate-Mass quadrupole-time of flight (Q-TOF) mass spectrometer with a dual electrospray ionization source (ESI). The UHPLC system, equipped with the chemical library of Metlin_AM_PCDL-N-170502.cdb, consisted of a vacuum solvent degassing unit, a capillary pump, and an automatic sample injector. The ESI operated in positive and negative modes with an m/z range from 100–3200. ESI conditions were as follows: fragmentor voltage 125 V; nebulizer pressure 45 psi; capillary voltage 3500 V; gas temperature 300 °C, gas flow 10 L/min, and skimmer 65 V. The chromatography was performed using Agilent Zorbax Eclipse XDB-C18, Narrow-Bore 2.1 × 150 mm, 3.5 microns (Agilent Technologies, Santa Clara, CA, USA). The auto-sampler compartment was maintained at 4 °C, and the mobile phase was 0.1% formic acid in water (A) and 0.1% formic acid in acetonitrile (B). The multi-step linear gradient was applied as follows: 5% solvent B for 5 min and the gradient keep isocratic at 100% solvent B from 20 min to 25 min. The initial condition was held for 5 min before the subsequent analysis. The injection volume was 1 μL, and the flow rate was 0.5 mL/min. The chromatographic separation was performed using C18 column (Agilent Eclipse XDB-C18 Narrow-bore, 150 mm × 2.1 mm, 3.5-micron) and the column temperature was 25 °C. The compounds in MEPL were identified via the Metlin database by using the spectra of chromatograms obtain from liquid chromatography mass spectrometric analysis, which determined the molecular mass of the compounds in the crude extract. The mass spectra of the compounds derived from UHPLC were run against the Metlin_AM_PCDL-N-170502.cdb library for the identification of homologous compounds via Agilent Mass Hunter software. The determination of the novelty of the identified compounds was performed on Scifinder software. Conversely, previously testified compounds were subjected to a literature search for biological activities, specifically for antimicrobial activity.
5. Conclusions
In conclusion, the results obtained in this study demonstrate the potential of MEPL to be a candidate for combination therapy against MRSA bacteria because it has a synergistic antibacterial effect with ceftriaxone in the tested strain. The killing effect of the combinatorial treatment is connected with the inhibition of the presence of the mecA gene in staphylococcal resistance to β-lactams antibiotics. The antimicrobial compound analysis the in MEPL extract showed the presence of several antimicrobial compounds known for their antibacterial activity. These discoveries provided a novel choice for clinicians to use natural MEPL in combination with antibiotics in MRSA infection treatment. Further study is also needed in an animal model to evaluate MEPL and ceftriaxone combination therapy in vivo efficacy.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/antibiotics12030477/s1. Table S1. Extracted MRSA genomic DNA concentration and purity. Table S2. The relative intensity of PCR products of the mecA gene for PCR optimization was obtained using ImageJ quantification software. Table S3: The relative intensity of PCR products of the mecA gene and 16S rRNA was obtained using ImageJ quantification software.
Author Contributions
Conceptualization, V.R., S.S. (Sreenivasan Sasidharan), S.S. (Sumaira Sahreen), Y.C., L.A.A.-K., M.P., M.A., N.A. and S.C.B.G.; methodology, V.R., S.S. (Sreenivasan Sasidharan), S.S. (Sumaira Sahreen), Y.C., L.A.A.-K., M.P., M.A., N.A. and S.C.B.G.; software, V.R.; validation, V.R. and S.S. (Sreenivasan Sasidharan); formal analysis, V.R.; investigation, V.R. and S.S. (Sreenivasan Sasidharan); resources, S.S. (Sreenivasan Sasidharan); data curation, V.R.; writing—original draft preparation, V.R.; writing—review and editing, S.S. (Sreenivasan Sasidharan); visualization, V.R.; supervision, S.S. (Sreenivasan Sasidharan); project administration, S.S. (Sreenivasan Sasidharan); funding acquisition, S.S. (Sreenivasan Sasidharan). All authors have read and agreed to the published version of the manuscript.
Funding
This work was partly funded by the Research University Grants (RUI; Grant No.: 1001/CIPPM/8012229) from the Universiti Sains Malaysia, Malaysia.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
L.A.A. extends her appreciation to Princess Nourah bint Abdulrahman University Researchers Supporting Project Number PNURSP2023R82, Princess Nourah bint Abdulrahman University, Riyadh, Saudi Arabia.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Garoy, E.Y.; Gebreab, Y.B.; Achila, O.O.; Tekeste, D.G.; Kesete, R.; Ghirmay, R.; Kiflay, R.; Tesfu, T. Methicillin-Resistant, Staphylococcus aureus (MRSA): Prevalence and Antimicrobial Sensitivity Pattern among Patients-A Multicenter Study in Asmara, Eritrea. Can. J. Infect. Dis. Med. Microbiol. 2019, 2019, 8321834. [Google Scholar] [CrossRef]
- Fair, R.J.; Tor, Y. Antibiotics and Bacterial Resistance in the 21st Century. Perspect. Med. Chem. 2014, 6, PMC-S14459. [Google Scholar] [CrossRef] [PubMed]
- Kaur, D.C.; Chate, S.S. Study of antibiotic resistance pattern in methicillin resistant Staphylococcus aureus with special reference to newer antibiotic. J. Glob. Infect. Dis. 2015, 7, 78. [Google Scholar] [CrossRef]
- Wielders, C.L.; Fluit, A.C.; Brisse, S.; Verhoef, J.; Schmitz, F.J. mecA gene is widely disseminated in Staphylococcus aureus population. J. Clin. Microbiol. 2002, 40, 3970–3975. [Google Scholar] [CrossRef]
- Stapleton, P.D.; Taylor, P.W. Methicillin resistance in Staphylococcus aureus: Mechanisms and modulation. Sci Prog 2002, 85 Pt 1, 57–72. [Google Scholar] [CrossRef]
- Katkar, K.V.; Suthar, A.C.; Chauhan, V.S. The chemistry, pharmacologic, and therapeutic applications of Polyalthia longifolia. Pharmacogn. Rev. 2010, 4, 62. [Google Scholar] [PubMed]
- Parchman, M.L.; Munoz, A. Risk Factors for Methicillin-Resistant Staphylococcal aureus Skin and Soft Tissue Infections Presenting in Primary Care: A South Texas Ambulatory Research Network (STARNet) Study. J. Am. Board Fam. Med. 2009, 22, 375–379. [Google Scholar] [CrossRef] [PubMed]
- Green, K.W.; Zelbst, P.J.; Meacham, J.; Bhadauria, V.S. Green supply chain management practices: Impact on performance. Supply chain management Supply Chain Manag. 2012, 17, 290–305. [Google Scholar] [CrossRef]
- Dumyati, G.; Stevens, V.; Hannett, G.E.; Thompson, A.D.; Long, C.; MacCannell, D.; Limbago, B. Community-associated Clostridium difficile infections, Monroe County, New York, USA. Emerg. Infect. Dis. 2012, 18, 392. [Google Scholar] [CrossRef]
- Kirubakari, B.; Chen, Y.; Kanwar, J.R.; Shin, L.N.; Sasidharan, S. Studies on In Vitro Interaction of Ampicillin and Polyalthia longifolia Leaf Ethyl Acetate Fraction (PLEAF) by Checkerboard Method Against Methicillin Resistant Staphylococcus aureus (MRSA). Curr. Bioact. Compd. 2020, 16, 1049–1062. [Google Scholar] [CrossRef]
- Kirubakari, B.; Chen, Y.; Sasidharan, S. Synergistic effect of Polyalthia longifolia leaf and antibiotics against clinical isolates of methicillin-resistant Staphylococcus aureus (MRSA) by microscopic technique. Antiinflamm. Antiallergy Agents Med. Chem. 2020, 19, 323–334. [Google Scholar] [CrossRef] [PubMed]
- Van Duin, D.; Paterson, D.L. Multidrug-resistant bacteria in the community: Trends and lessons learned. Dis. Clin. 2016, 30, 377–390. [Google Scholar]
- Gube, A.A.; Gonfa, R.; Tadesse, T. Evaluation of Antibiotic Use in Medical Ward of Fitche District Hospital, North Showa Zone, Oromia Region, Ethiopia. Adv. Pharmacoepidemiol. Drug Saf. 2017, 6, 217. [Google Scholar]
- Lee, H.; Jung, D.; Yeom, J.S.; Son, J.S.; Jung, S.I.; Kim, Y.S.; Kim, C.K.; Chang, H.H.; Kim, S.W.; Ki, H.K.; et al. Evaluation of ceftriaxone utilization at multicenter study. Korean J. Intern. Med. 2009, 24, 374–380. [Google Scholar] [CrossRef] [PubMed]
- Richards, D.G.; Wolz, J.P.; Herman, L.M. Vocal mimicry of computer-generated sounds and vocal labeling of objects by a bottlenosed dolphin, Tursiops truncatus. J. Comp. Psychol. 1984, 98, 10. [Google Scholar] [CrossRef] [PubMed]
- Patel, U.C.; Mckissic, E.L.; Kasper, D.; Lentino, J.R.; Pachucki, C.T.; Lee, T.; Lopansri, B.K. Outcomes of ceftriax-one use compared to standard of therapy in Methicillin Susceptible Staphylococcal aureus (MSSA) blood-stream infections. Int. J. Clin. Pharm. 2014, 36, 1282–1289. [Google Scholar] [CrossRef] [PubMed]
- Kamfose, M.M.; Muriiti, F.G.; Knight, T.; Lasserson, D.; Hayward, G.J.A. Intravenous ceftriaxone versus multiple dosing regimens of intravenous anti-Staphylococcal antibiotics for Methicillin-Susceptible Staphylococcus aureus (MSSA): A Systematic Review. Antibiotic 2020, 9, 39. [Google Scholar] [CrossRef] [PubMed]
- Lother, S.A.; Press, N. Once-Daily Treatments for Methicillin-Susceptible Staphylococcus aureus Bacteremia: Are They Good Enough? Curr. Infect. Dis. Rep. 2017, 19, 43. [Google Scholar] [CrossRef] [PubMed]
- McGowan, J.E. Antimicrobial resistance in hospital organisms and its relation to antibiotic use. Rev. Infect. Dis. 1983, 5, 1033–1048. [Google Scholar] [CrossRef]
- Bbosa, G.; Mwebaza, N.; Odda, J.; Kyegombe, D.; Ntale, M. Antibiotics/antibacterial drug use, their marketing and promotion during the post-antibiotic golden age and their role in emergence of bacterial resistance. Health 2014, 6, 410–425. [Google Scholar] [CrossRef]
- Wooford, N.; Ellington, M.J. The emergence of antibiotic resistance by mutation. Clin. Microbiol. Infect. 2007, 13, 5–18. [Google Scholar] [CrossRef] [PubMed]
- Von Wintersdorff, C.J.; Penders, J.; van Niekerk, J.M.; Mills, N.D.; Majumder, S.; van Alphen, L.B.; Savelkoul, P.H.; Wolffs, P.F. Dissemination of antimicrobial resistance in microbial ecosystems through horizontal gene transfer. Front. Microbiol. 2016, 7, 173. [Google Scholar] [CrossRef] [PubMed]
- Holcomb, H.G.; Durbin, K.J.; Cho, M.; Choi, K.J.; Darling, N.D.; Angerio, A.D. Methicillin-resistant Staphylococcus aureus as a threat to public health: A cellular approach. Georgetown Undergraduate. J. Health. Sci. 2008, 5, 2008. [Google Scholar]
- Al-Zoubi, M.S.; Al-Tayyar, I.A.; Hussein, E.; Jabali, A.A.; Khudairat, S. Antimicrobial susceptibility pattern of Staphylococcus aureus isolated from clinical specimens in Northern area of Jordan. Iran. J. Microbiol. 2015, 7, 265–272. [Google Scholar] [PubMed]
- Davis, J. Inactivation of antibiotics and dissemination of resistance genes. Science 1994, 264, 375–382. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, I.; Mehmood, Z.; Mohammad, F. Screening of Some Indian Medicinal Plants for their Antimicrobial Properties. J. Ethnopharmacol. 1998, 62, 183–193. [Google Scholar] [CrossRef]
- Loper, J.E.; Henkels, M.D.; Roberts, R.G.; Grove, G.G.; Willett, M.J.; Smith, T.J. Evaluation of Streptomycin, Oxytetracycline and Copper Resistance of Erwinia amylavora isolated from pear orchards in Washington State. Plant Dis. 1991, 75, 287–290. [Google Scholar] [CrossRef]
- Service, R.F. Antibiotics That Resist Resistance. Science 1991, 270, 724–727. [Google Scholar] [CrossRef]
- Liu, C.S.; Cham, T.M.; Yang, C.H.; Chang, H.W.; Chen, C.H.; Chuang, L.Y. Antibacterial properties of Chinese herbal medicines against nosocomial antibiotic resistant strains of Pseudomonas aeruginosa in Taiwan. Am. J. Chin. Med. 2007, 35, 1047–1060. [Google Scholar] [CrossRef]
- Alexopoulos, A.; Kimbaris, A.C.; Plessas, S.; Mantzourani, I.; Theodoridou, I.; Stavropoulou, E.; Polissiou, M.G.; Bezirtzoglou, E. Antibacterial activities of essential oils from eight Greek aromatic plants against clinical isolates of Staphylococcus aureus. Anaerobe 2011, 17, 399–402. [Google Scholar] [CrossRef]
- Toroglu, S. In-vitro antimicrobial activity and synergistic/antagonistic effect of interactions between antibiotics and some spice essential oils. J. Environ. Biol. 2011, 32, 23–29. [Google Scholar] [PubMed]
- Chung, P.Y.; Navaratnam, P.; Chung, L.Y. Synergistic antimicrobial activity between pentacyclic triterpenoids and antibiotics against Staphylococcus aureus strains. Ann. Clin. Microbiol. Antimicrob. 2011, 10, 25. [Google Scholar] [CrossRef] [PubMed]
- Inui, T.; Wang, Y.; Deng, S.; Smith, D.C.; Franzblau, S.G.; Paul, G.F. Counter-current chromatography-based analysis of synergy in an anti-tuberculosis ethnobotanical. J. Chromatogr. A 2008, 1151, 211–215. [Google Scholar] [CrossRef] [PubMed]
- Adwan, G.; Mhanna, M. Synergistic effects of plant extracts and antibiotics on Staphylococcus aureus strains isolated from clinical specimen. Asian Pac. J. Trop. Med. 2009, 2, 46–51. [Google Scholar]
- Ahmed, Z.; Khan, S.S.; Khan, M.; Tanveer, A.; Lone, Z.A. Synergistic effect of Salvadora persica extracts, tetracycline and penicillin against Staphylococcus aureus. Afr. J. Basic Appl. Sci. 2010, 2, 25–29. [Google Scholar]
- Aboulmagd, E.; Al-Mohammed, H.I.; Al-Badry, S. Synergism and post-antibiotic effect of green tea ex-tract and imipenem against methicillin-resistant Staphylococcus aureus. Microbiol. J. 2011, 1, 89–96. [Google Scholar] [CrossRef]
- Betoni, J.E.; Mantovani, R.P.; Barbosa, L.N.; Di Stasi, L.C.; Fernandes Junior, A. Synergism between plant ex-tract and antimicrobial drugs used on Staphylococcus aureus diseases. Mem. Inst. Oswaldo Cruz 2006, 101, 387–390. [Google Scholar] [CrossRef] [PubMed]
- Shang, D.; Liu, Y.; Jiang, F.; Ji, F.; Wang, H.; Han, X. Synergistic Antibacterial Activity of Designed Trp-Containing Antibacterial Peptides in Combination with Antibiotics Against Multidrug-Resistant Staphylococcus epidermidis. Front. Microbiol. 2019, 10, 2719. [Google Scholar] [CrossRef]
- AL-Ali, K.; Abdelrazik, M.; Hemeg, H.; Ozbak, H. Antibacterial activity of four herbal extracts against methicillin resistant bacteria isolates collected from Almadinah Hospitals. Saudi Arabia. Int. J. Acad. Res. 2014, 2, 27–34. [Google Scholar]
- Chanda, S.; Nair, R. Antimicrobial activity of Polyalthia longifolia (sonn.) thw. var. pendula leaf extracts against 91 clinically important pathogenic microbial strains. Chin. Med. 2010, 1, 31–38. [Google Scholar] [CrossRef]
- Phadnis, A.P.; Patwardhan, S.A.; Dhaneshwar, N.N. Clerodane diterpenoids from Polyalthia longifolia. Phytochemistry 1988, 27, 2899–2901. [Google Scholar] [CrossRef]
- Chaudhary, A.; Sood, S.; Das, P.; Kaur, P.; Mahajan, I.; Gulati, A.; Singh, B. Synthesis of novel antimicrobial aryl himachalene derivatives from naturally occurring himachalenes. EXCLI J. 2014, 13, 1216. [Google Scholar]
- Silva, A.; Silva, S.A.; Carpena, M.; Garcia-Oliveira, P.; Gullón, P.; Barroso, M.F.; Prieto, M.A.; Simal-Gandara, J. Macroalgae as a Source of Valuable Antimicrobial Compounds: Extraction and Applications. Antibiotics 2020, 9, 642. [Google Scholar] [CrossRef]
- Rahman, M.M.; Ahmad, S.H.; Mohamed, M.T.; Ab Rahman, M.Z. Antimicrobial compounds from leaf extracts of Jatropha curcas, Psidium guajava, and Andrographis paniculata. Sci. World J. 2014, 2014, 635240. [Google Scholar] [CrossRef]
- Qian, W.; Liu, M.; Fu, Y.; Zhang, J.; Liu, W.; Li, J.; Li, X.; Li, Y.; Wang, T. Antimicrobial mechanism of luteolin against Staphylococcus aureus and Listeria monocytogenes and its antibiofilm properties. Microb. Pathog. 2020, 142, 104056. [Google Scholar] [CrossRef] [PubMed]
- Arima, H.; Ashida, H.; Danno, G. Rutin enhanced antibacterial activities of flavonoids against Bacillus cereus and Salmonella enteritidis. Biosci. Biotechnol. Biochem. 2002, 66, 1009–1014. [Google Scholar] [CrossRef]
- Lee, T.H.; Wang, M.J.; Chen, P.Y.; Wu, T.Y.; Wen, W.C.; Tsai, F.Y.; Lee, C.K. Constituents of Polyalthia longifolia var. pendula. J. Nat. Prod. 2009, 72, 1960–1963. [Google Scholar] [CrossRef]
- Murthy, M.M.; Subramanyam, M.; Bindu, M.H.; Annapurna, J. Antimicrobial activity of clerodane diterpenoids from Polyalthia longifolia seeds. Fitoterapia 2005, 76, 336–339. [Google Scholar] [CrossRef]
- Sashidhara, K.V.; Singh, S.P.; Sarkar, J.; Sinha, S.J. Cytotoxic clerodane diterpenoids from the leaves of Polyalthia longifolia. Nat. Prod. Res. 2010, 24, 1687–1694. [Google Scholar] [CrossRef]
- Nahari, D.S.; Prasetyawan, S.; Beltran, M.A.G.; Aulanni’am, A. Separation of flavonoids in the extract Polyalthia longifolia (Sonn.) Thw. leaves from Indonesia and the Philippines. J. Phys. Conf. Ser. 2019, 1374, 012001. [Google Scholar] [CrossRef]
- Gupta, V.K.; Tiwari, N.; Gupta, P.; Verma, S.; Pal, A.; Srivastava, S.K.; Darokar, M.P. A clerodane diterpene from Polyalthia longifolia as a modifying agent of the resistance of methicillin resistant Staphylococcus aureus. Phytomedicine 2016, 23, 654–661. [Google Scholar] [CrossRef] [PubMed]
- Gupta, V.K.; Verma, S.; Pal, A.; Srivastava, S.K.; Srivastava, P.K.; Darokar, M.P. In vivo efficacy and synergistic interaction of 16α-hydroxycleroda-3, 13 (14) Z-dien-15, 16-olide, a clerodane diterpene from Polyalthia longifolia against methicillin-resistant Staphylococcus aureus. Appl. Microbiol. Biotechnol. 2013, 97, 9121–9131. [Google Scholar] [CrossRef] [PubMed]
- Mandal, S.M.; Roy, A.; Ghosh, A.K.; Hazra, T.K.; Basak, A.; Franco, O.L. Challenges and future prospects of antibiotic therapy: From peptides to phages utilization. Front. Pharmacol. 2014, 5, 105. [Google Scholar] [CrossRef]
- Li, X.Z.; Nikaido, H. Efflux-mediated drug resistance in bacteria: An update. Drugs 2009, 69, 1555–1623. [Google Scholar] [CrossRef]
- Tang, Y.W.; Kilic, A.; Yang, Q.; McAllister, S.K.; Li, H.; Miller, R.S.; McCormac, M.; Tracy, K.D.; Stratton, C.W.; Han, J.; et al. StaphPlex system for rapid and simultaneous identification of antibiotic resistance determinants and Panton-Valentine leukocidin detection of staphylococci from positive blood cultures. J. Clin. Microbiol. 2007, 45, 1867–1873. [Google Scholar] [CrossRef]
- Moisan, H.; Pruneau, M.; Malouin, F. Binding of ceftaroline to penicillin-binding proteins of Staphylococcus aureus and Streptococcus pneumoniae. J. Antimicrob. Chemother. 2010, 65, 713–716. [Google Scholar] [CrossRef]
- Gradelski, E.; Valera, L.; Aleksunes, L.; Bonner, D.; Fung-Tomc, J. Correlation between genotype and phenotypic categorization of Staphylococci based on methicillin susceptibility and resistance. J. Clin. Microbiol. 2001, 39, 2961–2963. [Google Scholar] [CrossRef] [PubMed]
- Hartman, B.J.; Tomasz, A. Low-affinity penicillin-binding protein associated with beta-lactam resistance in Staphylococcus aureus. J. Bacteriol. 1984, 158, 513–516. [Google Scholar] [CrossRef]
- Özel, G.; Aslan, V.; Erdem, G.B.; Çağatay, M.; Sencan, I.; Mert, A. Comparison of oxacillin, cefoxitin, ceftizoxime, and moxalactam disk diffusion methods for detection of methicillin susceptibility in staphylococci. Mikrobiyoloji Bulteni 2011, 45, 258–265. [Google Scholar]
- Prasad, K.N.; Kumar, R.; Tiwari, D.P.; Mishra, K.K.; Ayyagari, A. Comparison of various conventional methods with a polymerase chain reaction assay for detecting methicillin-resistant & susceptible Staphylococcus aureus strains. Indian J. Med. Res. 2000, 112, 198–202. [Google Scholar]
- Mohd-Fuat, A.R.; Kofi, E.A.; Allan, G.G. Mutagenic and cytotoxic properties of three herbal plants from Southeast Asia. Trop. Biomed. 2007, 24, 49–59. [Google Scholar]
- Allen, J.R.F.; Holmstedt, B.R. The simple β-carboline alkaloids. Phytochemistry 1980, 19, 1573–1582. [Google Scholar] [CrossRef]
- Patel, K.; Gadewar, M.; Tripathi, R.; Prasad, S.; Patel, D.K. A review on medicinal importance, pharmacological activity and bioanalytical aspects of beta-carboline alkaloid “Harmine”. Asian Pac. J. Trop. Biomed. 2012, 2, 660–664. [Google Scholar] [CrossRef]
- Boeira, J.M.; Viana, A.F.; Picada, J.N.; Henriques, J.A. Genotoxic and recombinogenic activities of the two beta-carboline alkaloids harman and harmine in Saccharomyces cerevisiae. Mutat. Res. 2002, 500, 39–48. [Google Scholar] [CrossRef] [PubMed]
- Rietjens, I.M.; Boersma, M.G.; Van Der Woude, H.; Jeurissen, S.M.; Schutte, M.E.; Alink, G.M.J.; Mutagenesis, M.M.O. Flavonoids and alkenylbenzenes: Mechanisms of mutagenic action and carcinogenic risk. Mutat. Res. 2005, 574, 124–138. [Google Scholar] [CrossRef] [PubMed]
- Faizi, S.; Khan, R.A.; Azher, S.; Khan, S.A.; Tauseef, S.; Ahmad, A. New antimicrobial alkaloids from the roots of Polyalthia longifolia var. pendula. Planta Med. 2003, 69, 350–355. [Google Scholar] [CrossRef]
- Jothy, S.L.; Chen, Y.; Kanwar, J.R.; Sasidharan, S. Evaluation of the Genotoxic Potential againstH2O2-Radical-Mediated DNA Damage and Acute Oral Toxicity of Standardized Extract of Polyalthia longifolia Leaf. Evid. Based Complement. Altern. Med. 2013, 2013, 925380. [Google Scholar] [CrossRef]
- Jothy, S.L.; Aziz, A.; Chen, Y.; Sasidharan, S. Antioxidant Activity and Hepatoprotective Potential of Polyalthia longifolia and Cassia spectabilis Leaves against Paracetamol-Induced Liver Injury. Evid. Based Complement. Altern. Med. 2012, 2012, 561284. [Google Scholar] [CrossRef]
- Jothy, S.L.; Saito, T.; Kanwar, J.R.; Chen, Y.; Aziz, A.; Yin-Hui, L.; Sasidharan, S. Radioprotective activity of Polyalthia longifolia standardized extract against X-ray radiation injury in mice. Phys. Med. 2016, 32, 150–161. [Google Scholar] [CrossRef]
- Mostafa, N.M.; Edmond, M.P.; El-Shazly, M.; Fahmy, H.A.; Sherif, N.H.; Singab, A.N.B. Phytoconstituents and renoprotective effect of Polyalthia longifolia leaves extract on radiation-induced nephritis in rats via TGF-β/smad pathway. Nat. Prod. Res. 2021, 36, 4187–4192. [Google Scholar] [CrossRef]
- Vijayarathna, S. Fundamental Studies on the Mechanism of Polyalthia longifolia (Sonn.) Thwaites Polyphenols Action in HeLa Cells in Relation to microRNA Regulation. PhD Thesis, Universiti Sains Malaysia, Pinang, Malaysia, 2017. [Google Scholar]
- Vijayarathna, S.; Chen, Y.; Kanwar, J.R.; Sasidharan, S. Standardized Polyalthia longifolia leaf extract (PLME) inhibits cell proliferation and promotes apoptosis: The anti-cancer study with various microscopy methods. Biomed. Pharmacother. 2017, 91, 366–377. [Google Scholar] [CrossRef] [PubMed]
- Vijayarathna, S.; Oon, C.E.; Chen, Y.; Kanwar, J.R.; Sasidharan, S. Polyalthia longifolia Methanolic Leaf Extracts (PLME) induce apoptosis, cell cycle arrest and mitochondrial potential depolarization by possibly modulating the redox status in hela cells. Biomed. Pharmacother. 2017, 89, 499–514. [Google Scholar] [CrossRef] [PubMed]
- Vijayarathna, S.; Shanmugapriya Khanwar, J.R.; Sasidharan, S. Standardized Polyalthia longifolia methanolic leaf extracts (PLME) inhibits HeLa cells through inducing microRNAs expression and apoptosis. In Proceedings of the International Conference on Traditional & Alternative Medicine, Kerala, India, 27 September 2017. [Google Scholar]
- Hemagirri, M.; Sasidharan, S. In vitro antiaging activity of polyphenol rich Polyalthia longifolia (Annonaceae) leaf extract in Saccharomyces cerevisiae BY611 yeast cells. J. Ethnopharmacol. 2022, 290, 115110. [Google Scholar] [CrossRef]
- Vaghasiya, Y.; Patel, H.; Chanda, S. Antibacterial activity of methanol extract of Mangifera indica against some human pathogens and its phytochemical study. Afr. J. Biotechnol. 2011, 10, 15788–15794. [Google Scholar] [CrossRef]
- Manasa, M.; Vivek, M.N.; Yashoda, K.; Onkarappa, R.; Prashith, K.T.R. Antimicrobial activity of leaf and pericarp extracts of Polyalthia longifolia (Annonaceae). J. Pharm. Sci. 2004, 393, 221–225. [Google Scholar]
- Bauer, A.W.; Kirby, W.M.; Sherris, J.C.; Turck, M. Antibiotic susceptibility testing by a standardized single disk method. Am. J. Clin. Pathol. 1966, 45, 493–496. [Google Scholar] [CrossRef]
- CLSI Standard. M100 Performance Standards for Antimicrobial Susceptibility Testing, 30th ed.; Clinical and Laboratory Standards Institute (CLSI): Wayne, PA, USA, 2020. [Google Scholar]
- Akinyemi, K.O.; Oladapo, O.; Okwara, C.E.; Ibe, C.C.; Fasure, K.A. Screening of crude extracts of six medicinal plants used in South-West Nigerian unorthodox medicine for anti-methicillin resistant Staphylococcus aureus activity. BMC Complement Altern. Med. 2005, 5, 6. [Google Scholar] [CrossRef]
- Andrews, J.M. Determination of minimum inhibitory concentrations. J. Antimicrob. Chemother. 2001, 48, 5–16. [Google Scholar] [CrossRef] [PubMed]
- Sampaio, F.C.; Pereira, M.d.S.V.; Dias, C.S.; Costa, V.C.; Conde, N.C.; Buzalaf, M.A. In vitro antimicrobial activity of Caesalpinia ferrea Martius fruits against oral pathogens. J. Ethnopharmacol. 2009, 124, 289–294. [Google Scholar] [CrossRef]
- Agarwal, A.; Jain, N.; Jain, A. Synergistic effect of cefixime and cloxacillin combination against common bacterial pathogens causing community acquired pneumonia. Indian J. Pharmacol. 2007, 39, 251–252. [Google Scholar]
- Zhao, W.H.; Hu, Z.Q.; Okubo, S.; Hara, Y.; Shimamura, T. Mechanism of Synergy between Epigallocatechin Gallate and b-Lactams against Methicillin-Resistant Staphylococcus aureus. Antimicrob. Agents Chemother. 2001, 45, 1737–1742. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).