Impact of Nucleic Acid Amplification Test on Clinical Outcomes in Patients with Clostridioides difficile Infection
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
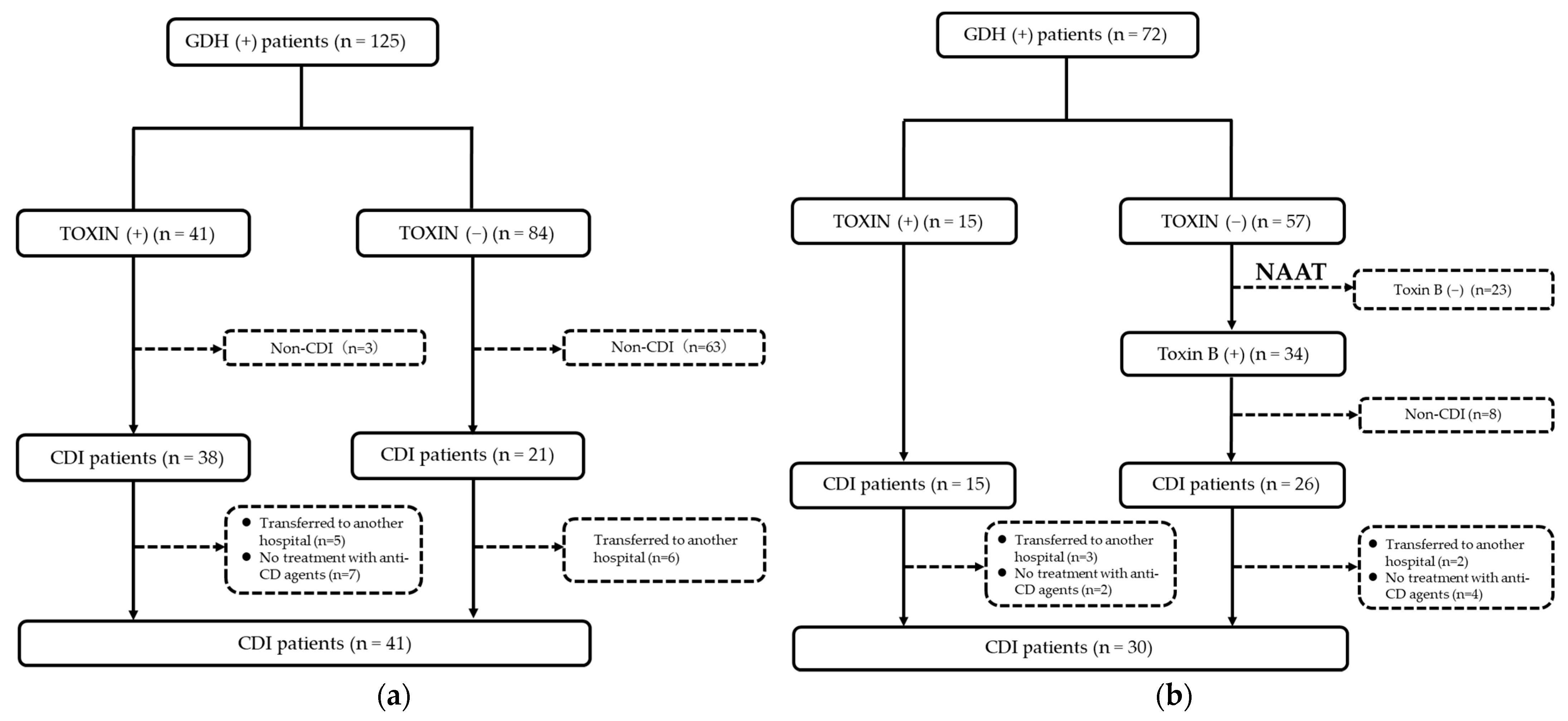
2.2. Tests and Diagnosis of CDI
2.3. Clinical Characteristics
2.4. Clinical Outcome
2.5. Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. Diagnostic Rate of CDI in Patients with GDH(+)
3.3. Comparison of Clinical Outcome between Pre-NAAT Group and Post-NAAT Group
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Badger, V.O.; Ledeboer, N.A.; Graham, M.B.; Edmiston, C.E., Jr. Clostridium difficile: Epidemiology, Pathogenesis, Management, and Prevention of a Recalcitrant Healthcare-Associated Pathogen. JPEN J. Parenter. Enter. Nutr. 2012, 36, 645–662. [Google Scholar] [CrossRef]
- Czepiel, J.; Dróżdż, M.; Pituch, H.; Kuijper, E.J.; Perucki, W.; Mielimonka, A.; Goldman, S.; Wultańska, D.; Garlicki, A.; Biesiada, G. Clostridium Difficile Infection: Review. Eur. J. Clin. Microbiol. Infect. Dis. Off. Publ. Eur. Soc. Clin. Microbiol. 2019, 38, 1211–1221. [Google Scholar] [CrossRef]
- Stabler, R.A.; He, M.; Dawson, L.; Martin, M.; Valiente, E.; Corton, C.; Lawley, T.D.; Sebaihia, M.; Quail, M.A.; Rose, G.; et al. Comparative Genome and Phenotypic Analysis of Clostridium Difficile 027 Strains Provides Insight into the Evolution of a Hypervirulent Bacterium. Genome Biol. 2009, 10, R102. [Google Scholar] [CrossRef]
- Khanna, S.; Pardi, D.S.; Aronson, S.L.; Kammer, P.P.; Orenstein, R.; St Sauver, J.L.; Harmsen, S.W.; Zinsmeister, A.R. The Epidemiology of Community-Acquired Clostridium difficile Infection: A Population-Based Study. Am. J. Gastroenterol. 2012, 107, 150, Erratum in Am. J. Gastroenterol. 2012, 107, 89–95. [Google Scholar] [CrossRef]
- Kwon, J.H.; Olsen, M.A.; Dubberke, E.R. The Morbidity, Mortality, and Costs Associated with Clostridium difficile Infection. Infect. Dis. Clin. N. Am. 2015, 29, 123–134. [Google Scholar] [CrossRef]
- Bloomfield, M.G.; Sherwin, J.C.; Gkrania-Klotsas, E. Risk factors for mortality in Clostridium difficile infection in the general hospital population: A systematic review. J. Hosp. Infect. 2012, 82, 1–12. [Google Scholar] [CrossRef]
- Sbeit, W.; Kadah, A.; Shahin, A.; Abed, N.; Haddad, H.; Jabbour, A.; Said Ahmad, H.; Pellicano, R.; Khoury, T.; Mari, A. Predictors of in-hospital mortality among patients with clostridium difficile infection: A multicenter study. Minerva Med. 2021, 112, 124–129. [Google Scholar] [CrossRef]
- Pepin, J.; Routhier, S.; Gagnon, S.; Brazeau, I. Management and outcomes of a first recurrence of Clostridium difficile-associated disease in Quebec, Canada. Clin. Infect. Dis. 2006, 42, 758–764. [Google Scholar] [CrossRef]
- Johnson, S. Recurrent Clostridium difficile infection: Causality and therapeutic approaches. Int. J. Antimicrob. Agents 2009, 33, S33–S36. [Google Scholar] [CrossRef]
- Figueroa, I.; Johnson, S.; Sambol, S.P.; Goldstein, E.J.C.; Citron, D.M.; Gerding, D.N. Relapse Versus Reinfection: Recurrent Clostridium difficile Infection Following Treatment With Fidaxomicin or Vancomycin. Clin. Infect. Dis. 2012, 55, S104–S109. [Google Scholar] [CrossRef]
- Deshpande, A.; Pasupuleti, V.; Thota, P.; Pant, C.; Rolston, D.D.K.; Hernandez, A.V.; Donskey, C.J.; Fraser, T.G. Risk Factors for Recurrent Clostridium difficile Infection: A Systematic Review and Meta-Analysis. Infect. Control Hosp. Epidemiol. 2015, 36, 452–460. [Google Scholar] [CrossRef]
- van Rossen, T.M.; Ooijevaar, R.E.; Vandenbroucke-Grauls, C.M.J.E.; Dekkers, O.M.; Kuijper, E.J.; Keller, J.J.; van Prehn, J. Prognostic factors for severe and recurrent Clostridioides difficile infection: A systematic review. Clin. Microbiol. Infect. 2022, 28, 321–331. [Google Scholar] [CrossRef]
- Kunishima, H.; Ito, K.; Laurent, T.; Abe, M. Healthcare burden of recurrent Clostridioides difficile infection in Japan: A retrospective database study. J. Infect. Chemother. 2018, 24, 892–901. [Google Scholar] [CrossRef]
- Bartlett, J.G. Detection of Clostridium difficile Infection. Infect. Control. Hosp. Epidemiol. 2010, 31 (Suppl. 1), S35–S37. [Google Scholar] [CrossRef] [PubMed]
- Carroll, K.C.; Mizusawa, M. Laboratory Tests for the Diagnosis of Clostridium Difficile. Clin. Colon Rectal Surg. 2020, 33, 73–81. [Google Scholar] [CrossRef]
- Crobach, M.J.T.; Planche, T.; Eckert, C.; Barbut, F.; Terveer, E.M.; Dekkers, O.M.; Wilcox, M.H.; Kuijper, E.J. European Society of Clinical Microbiology and Infectious Diseases: Update of the Diagnostic Guidance Document for Clostridium difficile Infection. Clin. Microbiol. Infect. Off. Publ. Eur. Soc. Clin. Microbiol. Infect. Dis. 2016, 22 (Suppl. 4), S63–S81. [Google Scholar] [CrossRef]
- McDonald, L.C.; Gerding, D.N.; Johnson, S.; Bakken, J.S.; Carroll, K.C.; Coffin, S.E.; Dubberke, E.R.; Garey, K.W.; Gould, C.V.; Kelly, C.; et al. Clinical Practice Guidelines for Clostridium difficile Infection in Adults and Children: 2017 Update by the Infectious Diseases Society of America (IDSA) and Society for Healthcare Epidemiology of America (SHEA). Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2018, 66, e1–e48. [Google Scholar] [CrossRef] [PubMed]
- Cohen, S.H.; Gerding, D.N.; Johnson, S.; Kelly, C.P.; Loo, V.G.; McDonald, L.C.; Pepin, J.; Wilcox, M.H.; Society for Healthcare Epidemiology of America. Infectious Diseases Society of America Clinical Practice Guidelines for Clostridium difficile Infection in Adults: 2010 Update by the Society for Healthcare Epidemiology of America (SHEA) and the Infectious Diseases Society of America (IDSA). Infect. Control Hosp. Epidemiol. 2010, 31, 431–455. [Google Scholar] [CrossRef]
- Kunishima, H.; Ohge, H.; Suzuki, H.; Nakamura, A.; Matsumoto, K.; Mikamo, H.; Mori, N.; Morinaga, Y.; Yanagihara, K.; Yamagishi, Y.; et al. Japanese Clinical Practice Guidelines for Management of Clostridioides (Clostridium) difficile infection. J. Infect. Chemother. 2022, 28, 1045–1083. [Google Scholar] [CrossRef]
- Margaret, M.G.; Kristen, B.; Amanda, H.; Susan, B.; Larry, D.; Eric, W. Clinical and economic impact of the introduction of a nucleic acid amplification assay for Clostridium difficile. Ann. Clin. Microbiol. Antimicrob. 2017, 16, 77. [Google Scholar]
- Charlson, M.E.; Pompei, P.; Ales, K.L.; MacKenzie, C.R. A New Method of Classifying Prognostic Comorbidity in Longitudinal Studies: Development and Validation. J. Chronic Dis. 1987, 40, 373–383. [Google Scholar] [CrossRef]
- Yamagishi, Y.; Hagihara, M.; Mikamo, H. Evaluation of Newly Developed Clostridium Difficile Infection Severity Scoring System “MN Criteria” for Clostridium Difficile Infection Patients in Japan. Jpn. Assoc. Anaerob. Infect. Res. 2017, 47, 66–75. [Google Scholar]
- Goldenberg, S.D. Public Reporting of Clostridium Difficile and Improvements in Diagnostic Tests. Infect. Control Hosp. Epidemiol. 2011, 32, 1231–1232. [Google Scholar] [CrossRef] [PubMed]
- Fong, K.S.; Fatica, C.; Hall, G.; Procop, G.; Schindler, S.; Gordon, S.M.; Fraser, T.G. Impact of PCR Testing for Clostridium difficile on Incident Rates and Potential on Public Reporting: Is the Playing Field Level? Infect. Control Hosp. Epidemiol. 2011, 32, 932–933. [Google Scholar] [CrossRef]
- Williamson, D.A.; Basu, I.; Freeman, J.; Swager, T.; Roberts, S.A. Improved Detection of Toxigenic Clostridium difficile Using the Cepheid Xpert C difficile Assay and Impact on C difficile Infection Rates in a Tertiary Hospital: A Double-Edged Sword. Am. J. Infect. Control 2013, 41, 270–272. [Google Scholar] [CrossRef]
- Polage, C.R.; Gyorke, C.E.; Kennedy, M.A.; Leslie, J.L.; Chin, D.L.; Wang, S.; Nguyen, H.H.; Huang, B.; Tang, Y.W.; Lee, L.W.; et al. Overdiagnosis of Clostridium difficile Infection in the Molecular Test Era. JAMA Intern. Med. 2015, 175, 1792–1801. [Google Scholar] [CrossRef]
- Planche, T.D.; Davies, K.A.; Coen, P.G.; Finney, J.M.; Monahan, I.M.; Morris, K.A.; O’Connor, L.; Oakley, S.J.; Pope, C.F.; Wren, M.W.; et al. Differences in Outcome According to Clostridium Difficile Testing Method: A Prospective Multicentre Diagnostic Validation Study of C difficile Infection. Lancet. Infect. Dis. 2013, 13, 936–945. [Google Scholar] [CrossRef]
- Cohen, N.A.; Miller, T.; Na’aminh, W.; Hod, K.; Adler, A.; Cohen, D.; Guzner-Gur, H.; Santo, E.; Halpern, Z.; Carmeli, Y.; et al. Clostridium difficile fecal toxin level is associated with disease severity and prognosis. United Eur. Gastroenterol. J. 2018, 6, 773–780. [Google Scholar] [CrossRef] [PubMed]
- Yoldaş, Ö.; Altındiş, M.; Cufalı, D.; Aşık, G.; Keşli, R. A Diagnostic Algorithm for the Detection of Clostridium difficile-Associated Diarrhea. Balk. Med. J. 2016, 33, 80–86. [Google Scholar] [CrossRef]
- Yamada, Y.; Miyazaki, M.; Kushima, H.; Komiya, Y.; Matsuo, K.; Uchiyama, M.; Nakashima, A.; Kamata, M.; Ishii, H.; Imakyure, O. Association between disease severity according to “MN criteria” and 30-day mortality in patients with Clostridioides difficile infection. J. Infect. Chemother. 2022, 28, 757–761. [Google Scholar] [CrossRef] [PubMed]
- Johnson, S.; Maziade, P.J.; McFarland, L.V.; Trick, W.; Donskey, C.; Currie, B.; Low, D.E.; Goldstein, E.J.C. Is Primary Prevention of Clostridium difficile Infection Possible with Specific Probiotics? Int. J. Infect. Dis. IJID Off. Publ. Int. Soc. Infect. Dis. 2012, 16, e786–e792. [Google Scholar] [CrossRef]
- Gao, X.W.; Mubasher, M.; Fang, C.Y.; Reifer, C.; Miller, L.E. Dose-Response Efficacy of a Proprietary Probiotic Formula of Lactobacillus acidophilus CL1285 and Lactobacillus casei LBC80R for Antibiotic-Associated Diarrhea and Clostridium difficile-Associated Diarrhea Prophylaxis in Adult Patients. Am. J. Gastroenterol. 2010, 105, 1636–1641. [Google Scholar] [CrossRef]
- Ritchie, M.L.; Romanuk, T.N. A Meta-analysis of Probiotic Efficacy for Gastrointestinal Diseases. PLoS ONE 2012, 7, e34938. [Google Scholar] [CrossRef] [PubMed]
- Hempel, S.; Newberry, S.J.; Maher, A.R.; Wang, Z.; Miles, J.N.V.; Shanman, R.; Johnsen, B.; Shekelle, P.G. Probiotics for the Prevention and Treatment of Antibiotic-Associated Diarrhea A Systematic Review and Meta-analysis. JAMA 2012, 307, 1959–1969. [Google Scholar] [PubMed]
- Barker, A.K.; Duster, M.; Valentine, S.; Hess, T.; Archbald-Pannone, L.; Guerrant, R.; Safdar, N. A Randomized Controlled Trial of Probiotics for Clostridium difficile Infection in Adults (PICO). J. Antimicrob. Chemother. 2017, 72, 3177–3180. [Google Scholar] [CrossRef] [PubMed]
- Avni, T.; Babich, T.; Ben-Zvi, H.; Atamna, A.; Yahav, D.; Shepshelovich, D.; Leibovici-Weissman, Y.; Bishara, J. Molecular-Based Diagnosis of Clostridium difficile Infection is Associated with Reduced Mortality. Eur. J. Clin. Microbiol. Infect. Dis. Off. Publ. Eur. Soc. Clin. Microbiol. 2018, 37, 1137–1142. [Google Scholar] [CrossRef] [PubMed]
- Guh, A.Y.; Hatfield, K.M.; Winston, L.G.; Martin, B.; Johnston, H.; Brousseau, G.; Farley, M.M.; Wilson, L.; Perlmutter, R.; Phipps, E.C.; et al. Toxin Enzyme Immunoassays Detect Clostridioides Difficile Infection With Greater Severity and Higher Recurrence Rates. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2019, 69, 1667–1674. [Google Scholar] [CrossRef]
- Longtin, Y.; Trottier, S.; Brochu, G.; Paquet-Bolduc, B.; Garenc, C.; Loungnarath, V.; Beaulieu, C.; Goulet, D.; Longtin, J. Impact of the Type of Diagnostic Assay on Clostridium difficile Infection and Complication Rates in a Mandatory Reporting Program. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2013, 56, 67–73. [Google Scholar] [CrossRef]
- Beaulieu, C.; Dionne, L.L.; Julien, A.S.; Longtin, Y. Clinical Characteristics and Outcome of Patients With Clostridium Difficile Infection Diagnosed by PCR Versus a Three-Step Algorithm. Clin. Microbiol. Infect. Off. Publ. Eur. Soc. Clin. Microbiol. Infect. Dis. 2014, 20, 1067–1073. [Google Scholar] [CrossRef]
- Zar, F.A.; Bakkanagari, S.R.; Moorthi, K.M.L.S.T.; Davis, M.B. A Comparison of Vancomycin and Metronidazole for the Treatment of Clostridium Difficile—Associated Diarrhea, Stratified by Disease Severity. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2007, 45, 302–307. [Google Scholar] [CrossRef]
- Johnson, S.; Louie, T.J.; Gerding, D.N.; Cornely, O.A.; Chasan-Taber, S.; Fitts, D.; Gelone, S.P.; Broom, C.; Davidson, D.M. Polymer Alternative for CDI Treatment (PACT) Investigators Vancomycin, Metronidazole, or Tolevamer for Clostridium difficile Infection: Results From Two Multinational, Randomized, Controlled Trials. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2014, 59, 345–354. [Google Scholar] [CrossRef] [PubMed]
- Khanafer, N.; Barbut, F.; Eckert, C.; Perraud, M.; Demont, C.; Luxemburger, C.; Vanhems, P. Factors Predictive of Severe Clostridium difficile Infection Depend on the Definition Used. Anaerobe 2016, 37, 43–48. [Google Scholar] [CrossRef] [PubMed]
- Windsor, J.W.; Kaplan, G.G. Evolving Epidemiology of IBD. Curr. Gastroenterol. Rep. 2019, 21, 40. [Google Scholar] [CrossRef] [PubMed]
| Variables | Pre-NAAT (n = 41) | Post-NAAT (n = 30) | p Value |
|---|---|---|---|
| Age, median (IQR) | 81 (71–87.5) | 79.5 (56.5–86.25) | 0.59 |
| Male gender, n (%) | 20 (48.8) | 17 (56.7) | 0.51 |
| Severe, n (%) | 9 (22.0) | 3 (10.0) | 0.22 |
| Proton pump inhibitor, n (%) | 25 (61.0) | 22 (73.3) | 0.28 |
| Charlson comorbidity index, median (IQR) | 2 (1–3.5) | 2 (0–3) | 0.48 |
| Inflammatory bowel diseases, n (%) | 6 (14.6) | 6 (20.0) | 0.55 |
| Chemotherapy for neoplasm, n (%) | 2 (4.9) | 5 (16.7) | 0.12 |
| White blood cell count, median (IQR) | 9800 (7600–17,450) | 9150 (5725–13,500) | 0.24 |
| Estimated glomerular filtration rate, median (IQR) | 80.3 (44.3–108.7) | 70.6 (44.3–106.5) | 0.78 |
| Serum albumin level, median (IQR) | 2.4 (2.0–2.8) | 2.6 (2.1–3.1) | 0.21 |
| Variables | Pre-NAAT (n = 41) | Post-NAAT (n = 30) | p Value |
|---|---|---|---|
| Number of days in hospital, median (IQR) | 33 (25.5–50) | 40 (25.5–58.25) | 0.56 |
| Number of days of treatment with anti-CD agents, median (IQR) | 11 (7.5–14) | 10.0 (7.75–14.0) | 0.92 |
| Clinical cure, n (%) | 20 (48.8) | 23 (76.7) | 0.018 |
| Recurrence of CDI, n (%) | 4 (9.8) | 3 (10.0) | 1.00 |
| 30-day mortality, n (%) | 9 (22.0) | 3 (10.0) | 0.22 |
| Variables | Clinical Non-Cure (n = 28) | Clinical Cure (n = 43) | p Value | Multivariable Analysis | p Value | ||
|---|---|---|---|---|---|---|---|
| OR | 95% CI | ||||||
| Age, median (IQR) | 76.5 (61–88.5) | 81.0 (73–87) | 0.59 | - | - | - | |
| Male gender, n (%) | 15 (53.6) | 22 (51.2) | 0.84 | - | - | - | |
| Severe CDI, n (%) | 5 (17.9) | 7 (16.3) | 0.86 | - | - | - | |
| NAAT introduction, n (%) | 7 (25.0) | 23 (53.5) | 0.018 | 3.31 | 1.18–10.03 | 0.022 | |
| Proton pump inhibitor, n (%) | 17 (60.7) | 30 (69.8) | 0.43 | - | - | - | |
| Probiotics, n (%) | 16 (57.1) | 32 (74.4) | 0.13 | - | - | - | |
| The number of days to start treatment | 2 (1.0–3.0) | 3 (1.0–4.0) | 0.11 | - | - | - | |
| Treatment with anti-CD agents, n (%) | 28 (100.0) | 43 (100.0) | 0.40 | - | - | - | |
| metronidazole | 24 (85.7) | 32 (74.4) | - | - | - | ||
| vancomycin | 3 (10.7) | 10 (23.3) | - | - | - | ||
| metronidazole + vancomycin | 1 (3.6) | 1 (2.3) | - | - | - | ||
| Charlson comorbidity index, median (IQR) | 2 (0–3.75) | 2 (1–3) | 0.73 | - | - | - | |
| Inflammatory bowel diseases, n (%) | 6 (21.4) | 6 (14.0) | 0.41 | - | - | - | |
| Chemotherapy for neoplasm, n (%) | 3 (10.7) | 4 (9.3) | 1.00 | - | - | - | |
| White blood cell count, median (IQR) | 9250 (7550–16,800) | 9800 (6000–16,000) | 0.76 | - | - | - | |
| Estimated glomerular filtration rate, median (IQR) | 83.5 (46.6–111.7) | 67.7 (41.2–106.2) | 0.54 | - | - | - | |
| Serum albumin level, median (IQR) | 2.4 (1.9–2.7) | 2.4 (2.0–3.0) | 0.52 | - | - | - | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yamada, Y.; Miyazaki, M.; Kushima, H.; Komiya, Y.; Nakashima, A.; Ishii, H.; Imakyure, O. Impact of Nucleic Acid Amplification Test on Clinical Outcomes in Patients with Clostridioides difficile Infection. Antibiotics 2023, 12, 428. https://doi.org/10.3390/antibiotics12030428
Yamada Y, Miyazaki M, Kushima H, Komiya Y, Nakashima A, Ishii H, Imakyure O. Impact of Nucleic Acid Amplification Test on Clinical Outcomes in Patients with Clostridioides difficile Infection. Antibiotics. 2023; 12(3):428. https://doi.org/10.3390/antibiotics12030428
Chicago/Turabian StyleYamada, Yota, Motoyasu Miyazaki, Hisako Kushima, Yukie Komiya, Akio Nakashima, Hiroshi Ishii, and Osamu Imakyure. 2023. "Impact of Nucleic Acid Amplification Test on Clinical Outcomes in Patients with Clostridioides difficile Infection" Antibiotics 12, no. 3: 428. https://doi.org/10.3390/antibiotics12030428
APA StyleYamada, Y., Miyazaki, M., Kushima, H., Komiya, Y., Nakashima, A., Ishii, H., & Imakyure, O. (2023). Impact of Nucleic Acid Amplification Test on Clinical Outcomes in Patients with Clostridioides difficile Infection. Antibiotics, 12(3), 428. https://doi.org/10.3390/antibiotics12030428








