The Role of Antibiotic Prophylaxis in Anastomotic Leak Prevention during Elective Colorectal Surgery: Systematic Review and Meta-Analysis of Randomized Controlled Trials
Abstract
1. Introduction
2. Results
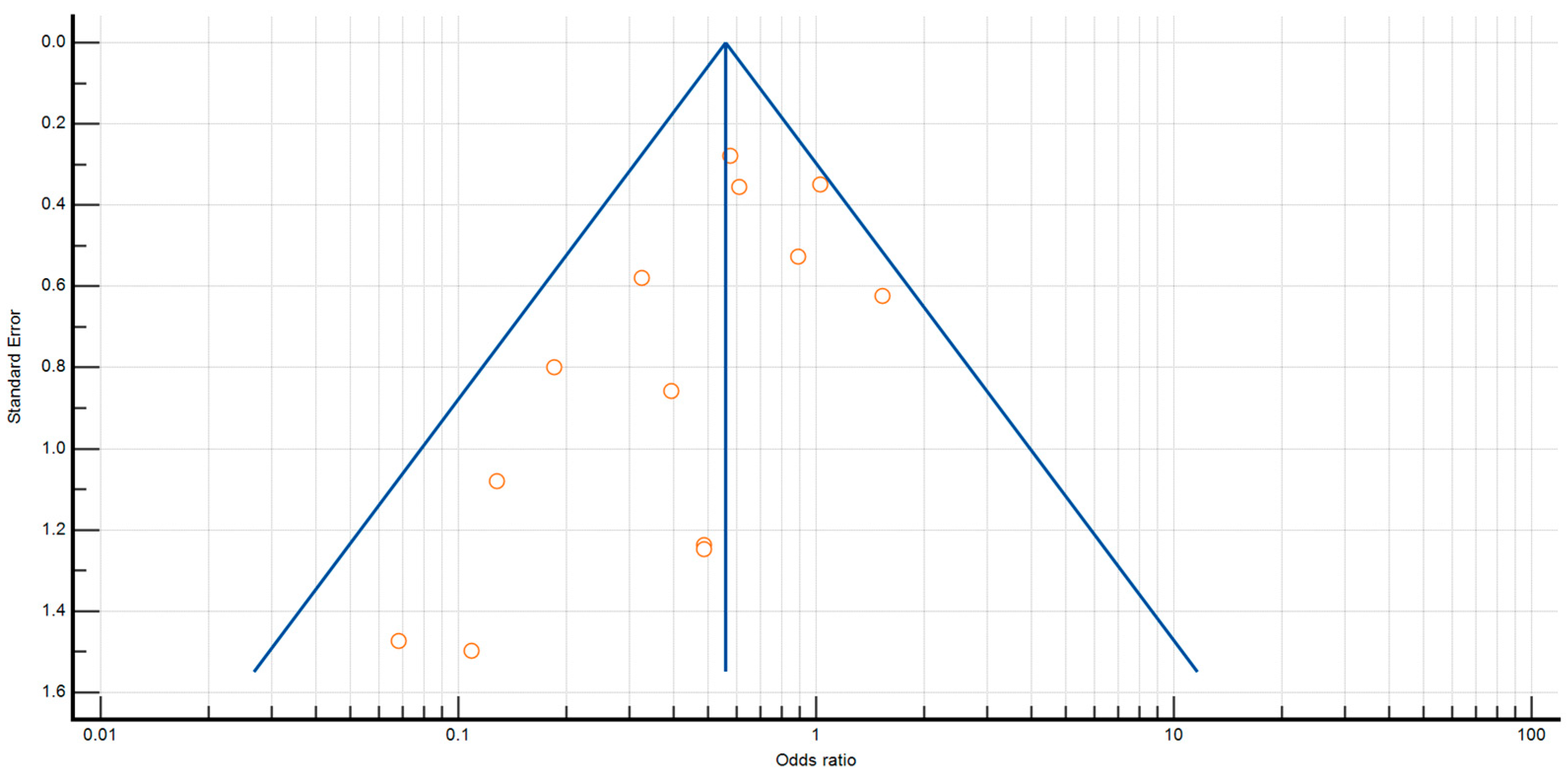
2.1. Methodological Quality Assessment
| Author | Jadad Scores for RCTs |
|---|---|
| Hojer et al. [9] | 2 |
| Matheson et al. [10] | 2 |
| Bartlett et al. [11] | 1 |
| Ishida et al. [12] | 2 |
| Sato et al. [13] | 2 |
| Sadahiro et al. [14] | 2 |
| Hjalmarsson et al. [15] | 3 |
| Anjum et al. [16] | 3 |
| Abis et al. [17] | 3 |
| Koskenvuo et al. [18] | 3 |
| Mulder et al. [19] | 2 |
| Papp et al. [20] | 3 |
| Futier et al. [21] | 4 |
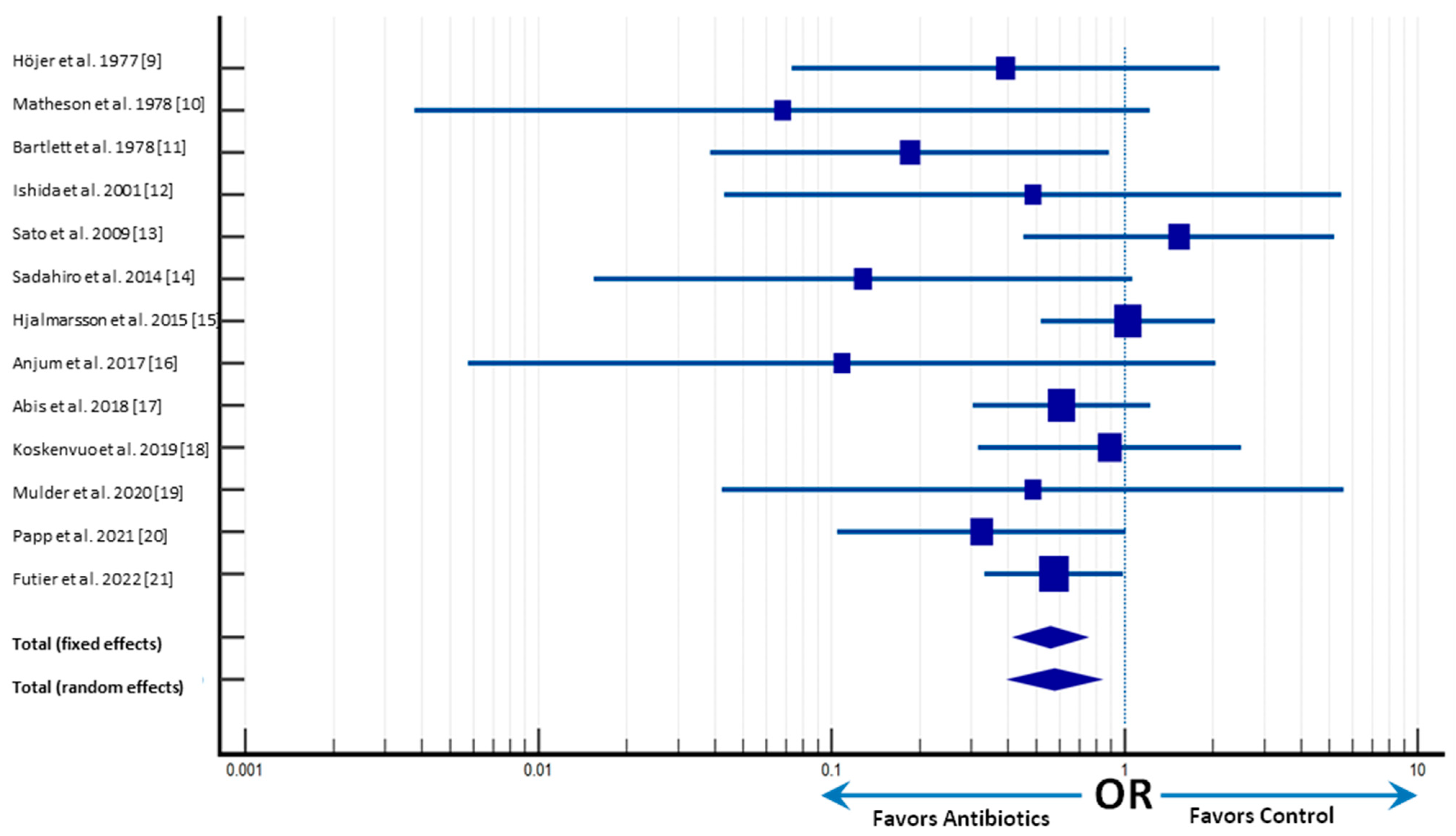
2.2. Primary Outcomes
2.3. Secondary Outcomes
3. Discussion
Study Limitations
4. Materials and Methods
4.1. Search Strategy and Selection of Trials
4.2. Critical Assessment of Trials and Collection of Data
4.3. Outcome Measures
4.4. Risk of Bias Assessment
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chaouch, M.A.; Kellil, T.; Jeddi, C.; Saidani, A.; Chebbi, F.; Zouari, K. How to Prevent Anastomotic Leak in Colorectal Surgery? A Systematic Review. Ann. Coloproctol. 2020, 36, 213–222. [Google Scholar] [CrossRef] [PubMed]
- Daniel, V.T.; Alavi, K.; Davids, J.S.; Sturrock, P.R.; Harnsberger, C.R.; Steele, S.R.; Maykel, J.A. The utility of the delphi method in defining anastomotic leak following colorectal surgery. Am. J. Surg. 2020, 219, 75–79. [Google Scholar] [CrossRef] [PubMed]
- Guyton, K.L.; Hyman, N.H.; Alverdy, J.C. Prevention of Perioperative Anastomotic Healing Complications: Anastomotic Stricture and Anastomotic Leak. Adv. Surg. 2016, 50, 129–141. [Google Scholar] [CrossRef] [PubMed]
- Okada, M.; Bothin, C.; Kanazawa, K.; Midtvedt, T. Experimental study of the influence of intestinal flora on the healing of intestinal anastomoses. Br. J. Surg. 1999, 86, 961–965. [Google Scholar] [CrossRef] [PubMed]
- Guyton, K.; Alverdy, J.C. The gut microbiota and gastrointestinal surgery. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 43–54. [Google Scholar] [CrossRef] [PubMed]
- Shogan, B.D.; Smith, D.P.; Christley, S.; Gilbert, J.A.; Zaborina, O.; Alverdy, J.C. Intestinal anastomotic injury alters spatially defined microbiome composition and function. Microbiome 2014, 2, 35. [Google Scholar] [CrossRef]
- Edomskis, P.; Goudberg, M.R.; Sparreboom, C.L.; Menon, A.G.; Wolthuis, A.M.; D’Hoore, A.; Lange, J.F. Matrix metalloproteinase-9 in relation to patients with complications after colorectal surgery: A systematic review. Int. J. Color. Dis. 2020, 36, 1–10. [Google Scholar] [CrossRef]
- Cohn, I., Jr.; Rives, J.D. Antibiotic Protection of Colon Anastomoses. Ann. Surg. 1955, 141, 707–717. [Google Scholar] [CrossRef]
- Höjer, H.; Wetterfors, J. Systemic Prophylaxis with Doxycycline in Surgery of the Colon and Rectum. Ann. Surg. 1978, 187, 362–368. [Google Scholar] [CrossRef]
- Matheson, D.M.; Arabi, Y.; Baxter-Smith, D.; Alexander-Williams, J.; Keighley, M.R.B. Randomized multicentre trial of oral bowel preparation and antimicrobials for elective colorectal operations. Br. J. Surg. 1978, 65, 597–600. [Google Scholar] [CrossRef]
- Bartlett, J.G.; Condon, R.E.; Gorbach, S.L.; Clarke, J.S.; Nichols, R.L.; Ochi, S. Veterans Administration Cooperative Study on Bowel Preparation for Elective Colorectal Operations: Impact of oral antibiotic regimen on colonic flora, wound irrigation cultures and bacteriology of septic complications. Ann. Surg. 1978, 188, 249–254. [Google Scholar] [CrossRef] [PubMed]
- Ishida, H.; Yokoyama, M.; Nakada, H.; Inokuma, S.; Hashimoto, D. Impact of Oral Antimicrobial Prophylaxis on Surgical Site Infection and Methicillin-Resistant Staphylococcus aureus Infection After Elective Colorectal Surgery. Results of a Prospective Randomized Trial. Surg. Today 2001, 31, 979–983. [Google Scholar] [CrossRef] [PubMed]
- Sato, T.; Takayama, T.; Fujii, M.; Song, K.; Matsuda, M.; Higaki, T.; Okada, S. Systemic use of antibiotics does not prevent postoperative infection in elective colorectal surgery: A randomized controlled trial. J. Infect. Chemother. 2009, 15, 34–38. [Google Scholar] [CrossRef] [PubMed]
- Sadahiro, S.; Suzuki, T.; Tanaka, A.; Okada, K.; Kamata, H.; Ozaki, T.; Koga, Y. Comparison between oral antibiotics and probiotics as bowel preparation for elective colon cancer surgery to prevent infection: Prospective randomized trial. Surgery 2014, 155, 493–503. [Google Scholar] [CrossRef] [PubMed]
- Hjalmarsson, C.; Karlberg, J.; Törnqvist, P.; Arbman, G.; Frisk, B.; Modin, M.; The TSM Study Group. Orally Administered Trimethoprim-Sulfamethoxazole and Metronidazole as Infection Prophylaxis in Elective Colorectal Surgery. Surg. Infect. 2015, 16, 604–610. [Google Scholar] [CrossRef]
- Anjum, N.; Ren, J.; Wang, G.; Li, G.; Wu, X.; Dong, H.; Wu, Q.; Li, J. A Randomized Control Trial of Preoperative Oral Antibiotics as Adjunct Therapy to Systemic Antibiotics for Preventing Surgical Site Infection in Clean Contaminated, Contaminated, and Dirty Type of Colorectal Surgeries. Dis. Colon Rectum 2017, 60, 1291–1298. [Google Scholar] [CrossRef]
- Abis, G.S.A.; Stockmann, H.B.A.C.; Bonjer, H.J.; van Veenendaal, N.; van Doorn-Schepens, M.L.M.; Budding, A.; Wilschut, J.; van Egmond, M.; Oosterling, S.J.; de Lange, E.S.M.; et al. Randomized clinical trial of selective decontamination of the digestive tract in elective colorectal cancer surgery (SELECT trial). Br. J. Surg. 2019, 106, 355–363. [Google Scholar] [CrossRef]
- Koskenvuo, L.; Lehtonen, T.; Koskensalo, S.; Rasilainen, S.; Klintrup, K.; Ehrlich, A.; Pinta, T.; Scheinin, T.; Sallinen, V. Mechanical and oral antibiotic bowel preparation versus no bowel preparation for elective colectomy (MOBILE): A multicentre, randomised, parallel, single-blinded trial. Lancet 2019, 394, 840–848. [Google Scholar] [CrossRef]
- Mulder, T.; Bergh, M.K.-V.D.; Vlaminckx, B.; Roos, D.; De Smet, A.M.; Cappel, R.D.V.T.N.; Verheijen, P.; Brandt, A.; Smits, A.; Van Der Vorm, E.; et al. Prevention of severe infectious complications after colorectal surgery using oral non-absorbable antimicrobial prophylaxis: Results of a multicenter randomized placebo-controlled clinical trial. Antimicrob. Resist. Infect. Control. 2020, 9, 1–11. [Google Scholar] [CrossRef]
- Papp, G.; Saftics, G.; Szabó, B.E.; Baracs, J.; Vereczkei, A.; Kollár, D.; Oláh, A.; Mészáros, P.; Dubóczki, Z.; Bursics, A. Systemic versus Oral and Systemic Antibiotic Prophylaxis (SOAP) study in colorectal surgery: Prospective randomized multicentre trial. Br. J. Surg. 2021, 108, 271–276. [Google Scholar] [CrossRef]
- Futier, E.; Jaber, S.; Garot, M.; Vignaud, M.; Panis, Y.; Slim, K.; Lucet, J.-C.; Lebuffe, G.; Ouattara, A.; El Amine, Y.; et al. Effect of oral antimicrobial prophylaxis on surgical site infection after elective colorectal surgery: Multicentre, randomised, double blind, placebo controlled trial. BMJ 2022, 379, e071476. [Google Scholar] [CrossRef] [PubMed]
- Baum, M.L.; Anish, D.S.; Chalmers, T.C.; Sacks, H.S.; Smith, H.; Fagerstrom, R.M. A Survey of Clinical Trials of Antibiotic Prophylaxis in Colon Surgery: Evidence against Further Use of No-Treatment Controls. N. Engl. J. Med. 1981, 305, 795–799. [Google Scholar] [CrossRef] [PubMed]
- Nelson, R.L.; Gladman, E.; Barbateskovic, M. Antimicrobial prophylaxis for colorectal surgery. Cochrane Database Syst. Rev. 2014, 2014, CD001181. [Google Scholar] [CrossRef] [PubMed]
- Migaly, J.; Bafford, A.C.; Francone, T.D.; Gaertner, W.B.; Eskicioglu, C.; Bordeianou, L.; Feingold, D.L.; Steele, S.R. Clinical Practice Guidelines Committee of the American Society of Colon and Rectal Surgeons The American Society of Colon and Rectal Surgeons Clinical Practice Guidelines for the Use of Bowel Preparation in Elective Colon and Rectal Surgery. Dis. Colon Rectum 2019, 62, 3–8, Erratum in Dis. Colon Rectum 2019, 62, e436. [Google Scholar] [CrossRef]
- Zhang, L.M.; Schuitevoerder, D.; White, M.G.; Feldt, S.; Bs, P.K.; Hyman, N.; Shogan, B.D. Combined mechanical and oral antibiotic bowel preparation is associated with prolonged recurrence-free survival following surgery for colorectal cancer. J. Surg. Oncol. 2021, 124, 1106–1114. [Google Scholar] [CrossRef]
- Koullouros, M.; Khan, N.; Aly, E.H. The role of oral antibiotics prophylaxis in prevention of surgical site infection in colorectal surgery. Int. J. Color. Dis. 2016, 32, 1–18. [Google Scholar] [CrossRef]
- Bellows, C.F.; Mills, K.T.; Kelly, T.N.; Gagliardi, G. Combination of oral non-absorbable and intravenous antibiotics versus intravenous antibiotics alone in the prevention of surgical site infections after colorectal surgery: A meta-analysis of randomized controlled trials. Tech. Coloproctol. 2011, 15, 385–395. [Google Scholar] [CrossRef]
- Bachmann, R.; Leonard, D.; Delzenne, N.; Kartheuser, A.; Cani, P.D. Novel insight into the role of microbiota in colorectal surgery. Gut 2017, 66, 738–749. [Google Scholar] [CrossRef]
- Kiran, R.P.; Murray, A.C.A.; Chiuzan, C.; Estrada, D.; Forde, K. Combined Preoperative Mechanical Bowel Preparation With Oral Antibiotics Significantly Reduces Surgical Site Infection, Anastomotic Leak, and Ileus After Colorectal Surgery. Ann. Surg. 2015, 262, 416–425, discussion 423–425. [Google Scholar] [CrossRef]
- Slim, K.; Vicaut, E.; Panis, Y.; Chipponi, J. Meta-analysis of randomized clinical trials of colorectal surgery with or without mechanical bowel preparation. Br. J. Surg. 2004, 91, 1125–1130. [Google Scholar] [CrossRef]
- Slim, K.; Vicaut, E.; Launay-Savary, M.-V.; Contant, C.; Chipponi, J. Updated Systematic Review and Meta-Analysis of Randomized Clinical Trials on the Role of Mechanical Bowel Preparation Before Colorectal Surgery. Ann. Surg. 2009, 249, 203–209. [Google Scholar] [CrossRef] [PubMed]
- Nichols, R.L.; Broido, P.; Condon, R.E.; Gorbach, S.L.; Nyhus, L.M. Effect of Preoperative Neornycin-Erythromycin Intestinal Preparation on the Incidence of Infectious Complications Following Colon Surgery. Ann. Surg. 1973, 178, 453–462. [Google Scholar] [CrossRef] [PubMed]
- Mizuta, M.; Endo, I.; Yamamoto, S.; Inokawa, H.; Kubo, M.; Udaka, T.; Sogabe, O.; Maeda, H.; Shirakawa, K.; Okazaki, E.; et al. Perioperative supplementation with bifidobacteria improves postoperative nutritional recovery, inflammatory response, and fecal microbiota in patients undergoing colorectal surgery: A prospective, randomized clinical trial. Biosci. Microbiota Food Health 2016, 35, 77–87. [Google Scholar] [CrossRef] [PubMed]
- Carlini, M.; Grieco, M.; Spoletini, D.; Menditto, R.; Napoleone, V.; Brachini, G.; Mingoli, A.; Marcellinaro, R. Implementation of the gut microbiota prevents anastomotic leaks in laparoscopic colorectal surgery for cancer:the results of the MIRACLe study. Updat. Surg. 2022, 74, 1253–1262. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Green, S. (Eds.) Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0; The Cochrane Collaboration: Londes, France, 2011. [Google Scholar]
- Jadad, A.R.; Moore, R.A.; Carroll, D.; Jenkinson, C.; Reynolds, D.J.M.; Gavaghan, D.J.; McQuay, H.J. Assessing the quality of reports of randomized clinical trials: Is blinding necessary? Control. Clin. Trials 1996, 17, 1–12. [Google Scholar] [CrossRef]
- Egger, M.; Smith, G.D.; Schneider, M.; Minder, C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997, 315, 629–634. [Google Scholar] [CrossRef]
- Begg, C.B.; Mazumdar, M. Operating Characteristics of a Rank Correlation Test for Publication Bias. Biometrics 1994, 50, 1088–1101. [Google Scholar] [CrossRef]
- MedCalc® Statistical Software, Version 20.211; MedCalc Software Ltd.: Ostend, Belgium, 2023. Available online: https://www.medcalc.org (accessed on 27 December 2022).
- Lau, J.; Ioannidis, J.P.; Schmid, C. Quantitative Synthesis in Systematic Reviews. Ann. Intern. Med. 1997, 127, 820–826. [Google Scholar] [CrossRef]
- Vos, T.; Barber, R.M.; Bell, B.; Bertozzi-Villa, A.; Biryukov, S.; Bolliger, I.; Charlson, F.; Davis, A.; Degenhardt, L.; Dicker, D.; et al. Global, regional, and national incidence, prevalence, and years lived with disability for 301 acute and chronic diseases and injuries in 188 countries, 1990–2013: A systematic analysis for the Global Burden of Disease Study 2013. Lancet 2015, 386, 743–800. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Thompson, S.G. Quantifying heterogeneity in a meta-analysis. Stat. Med. 2002, 21, 1539–1558. [Google Scholar] [CrossRef] [PubMed]



| Author | 1. Risk of Bias Arising from the Randomization Process | 2.1 Risk of Bias Due to Deviations from the Intended Interventions (Effect of Assignment to Intervention) | 2.2 Risk of Bias Due to Deviations from the Intended Interventions (Effect of Adhering to Intervention) | 3. Risk of Bias Due to Missing Outcome Data | 4. Risk of Bias in the Measurement of the Outcome | 5. Risk of Bias in the Selection of the Reported Result | Overall Risk-of-Bias Judgement |
|---|---|---|---|---|---|---|---|
| Hojer et al. [9] | Low | Low | Low | Some concerns | Low | High | High |
| Matheson et al. [10] | Low | Low | Low | Some concerns | Low | High | High |
| Bartlett et al. [11] | Low | Low | Low | Some concerns | Low | Some concerns | Some concerns |
| Ishida et al. [12] | High | Low | High | Low | Low | High | High |
| Sato et al. [13] | Some concerns | Low | High | Low | Low | High | High |
| Sadahiro et al. [14] | Low | Low | Low | Some concerns | Low | High | High |
| Hjalmarsson et al. [15] | High | Low | High | Low | Low | High | High |
| Anjum et al. [16] | Low | Low | Low | Some concerns | Low | Low | Some concerns |
| Abis et al. [17] | High | Low | Some concerns | Low | Low | High | High |
| Koskenvuo et al. [18] | High | Low | High | Low | Low | High | High |
| Mulder et al. [19] | Low | Low | Low | Some concerns | Low | Some concerns | Some concerns |
| Papp et al. [20] | High | Some concerns | High | Low | Low | High | High |
| Futier et al. [21] | Low | Low | Low | Some concerns | Low | Some concerns | Some concerns |
| Author | Year of Publication | Study Design | Number of Patients | Endpoints | Antibiotic Group | Control Group | Statistical Significance |
|---|---|---|---|---|---|---|---|
| Höjer et al. [9] | 1977 | Single-center, prospective, double-blinded, randomized trial | 118 | 58 | 60 | ||
| Anastomotic Leak | 2 | 5 | p < 0.001 | ||||
| SSI | 5 | 25 | p < 0.001 | ||||
| Matheson et al. [10] | 1978 | Single-center, double-blinded, randomized controlled trial | 110 | 51 | 59 | ||
| Anastomotic Leak | 0 | 7 | p < 0.02 | ||||
| SSI | 9 | 25 | p < 0.01 | ||||
| Bartlett et al. [11] | 1978 | Multicenter, double-blinded, randomized trial | 116 | 56 | 60 | ||
| Anastomotic Leak | 2 | 10 | p = 0.05 | ||||
| SSI | 5 | 21 | p = 0.002 | ||||
| Ishida et al. [12] | 2001 | Single-center, single-blinded, randomized controlled trial | 143 | 72 | 71 | ||
| Anastomotic Leak | 1 | 2 | p = 0.050 | ||||
| SSI | 8 | 17 | p = 0.035 | ||||
| Sato et al. [13] | 2009 | Multicenter, randomized trial | 100 | 49 | 51 | ||
| Anastomotic Leak | 7 | 5 | p = 0.8293 | ||||
| SSI | 20 | 23 | p = 0.8293 | ||||
| Sadahiro et al. [14] | 2014 | Single-center, double-blinded, randomized trial | 194 | 99 | 95 | ||
| Anastomotic Leak | 1 | 7 | p = 0.014 | ||||
| SSI | 18 | 17 | p = 0.004 | ||||
| Hjalmarsson et al. [15] | 2015 | Prospective, multicenter, single-blinded, randomized controlled trial | 985 | 486 | 499 | ||
| Anastomotic Leak | 17 | 17 | p = 0.95 | ||||
| SSI | 34 | 18 | p = 0.022 | ||||
| Anjum et al. [16] | 2017 | Single-center, double-blinded, prospective, randomized trial | 184 | 91 | 93 | ||
| Anastomotic Leak | 0 | 4 | p = 0.004 | ||||
| SSI | 8 | 26 | p = 0.001 | ||||
| Abis et al. [17] | 2018 | Superiority, open-label, multicenter, randomized trial | 455 | 228 | 227 | ||
| Anastomotic Leak | 14 | 22 | OR 0.61 (0.30–1.22) | ||||
| SSI | 5 | 24 | OR 0.19 (0.07–0.51) | ||||
| Koskenvuo et al. [18] | 2019 | Multicenter, parallel, single-blinded randomized trial | 396 | 196 | 200 | ||
| Anastomotic Leak | 7 | 8 | CI 1.13 (0.40–3.16) | ||||
| SSI | 13 | 21 | CI 1.65 (0.80–3.40) | ||||
| Mulder et al. [19] | 2020 | Multicenter, double-blind, placebo-controlled randomized trial | 78 | 39 | 39 | ||
| Anastomotic Leak | 1 | 2 | RR 0.50 (0.05–5.29) | ||||
| SSI | 4 | 5 | RR 0.80 (0.23–2.78) | ||||
| Papp et al. [20] | 2021 | Multicentre, prospective, randomized, assessor-blinded trial | 529 | 253 | 276 | ||
| Anastomotic Leak | 4 | 13 | p = 0.020 | ||||
| SSI | 8 | 27 | p = 0.001 | ||||
| Futier et al. [21] | 2022 | Multicenter, double-blinded, randomized, placebo-controlled trial | 926 | 463 | 463 | ||
| Anastomotic Leak | 22 | 37 | p = 0.046 | ||||
| SSI | 60 | 100 | p = 0.001 |
| Author | Antibiotic | Administration Regimen | Administration Route |
|---|---|---|---|
| Hojer et al. [9] | Doxycycline 200 mg | - Started 4–6 h preop., single dose - Continued o.d. for 5 days postop. | Oral |
| Matheson et al. [10] | Neomycin 1 g and Metronidazole 200 mg | - Started 2 days preop. t.d.s. | Oral |
| Bartlett et al. [11] | Neomycin 1 g and Erythromycin 1 g | -Started 1 day preop. t.d.s. | Oral |
| Ishida et al. [12] | Metronidazole 400 mg and Kanamycin 500 mg | - Started 2 days preop. b.d. - Continued 3 days postop. b.d. | Oral |
| Sato et al. [13] | Cefotiam | - Started during skin incision - Continued 3 days postop. t.d.s. | Intravenous |
| Sadahiro et al. [14] | Kanamycin 0.5 g + Metronidazole 0.5 g | - Started 1 day preop. t.d.s. | Oral |
| Hjalmarsson et al. [15] | Sulfamethoxazole 800 mg/Trimethoprim 160 mg, and three tablets of Metronidazole 400 mg | -Started 2 h preop. single dose | Oral |
| Anjum et al. [16] | Metronidazole 400 mg and Levofloxacin 200 mg | - Started 1 day preop. t.d.s. | Oral |
| Abis et al. [17] | 10 mL suspension containing 5 mL Amphotericin B 500 mg and 5 mL Colistin sulphate 100 mg and Tobramycin 80 mg | - Started 3 days preop. q.i.d. - Continued 3 days postop. | Oral |
| Koskenvuo et al. [18] | Neomycin 2 g and Metronidazole 2 g | - Started 1 day preop. o.d. | Oral |
| Mulder et al. [19] | Tobramycin 16 mg/mL and Colistin sulphate 20 mg/mL | - Started 3 days preop. q.i.d. | Oral |
| Papp et al. [20] | Ceftriaxone 2 g and Metronidazole 500 mg | -Started 1 day preop t.d.s. | Intravenous |
| Futier et al. [21] | Ornidazole 1 g | -Started 12 h preop. o.d. | Oral |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Castagneto-Gissey, L.; Russo, M.F.; Casella-Mariolo, J.; Serao, A.; Marcellinaro, R.; D’Andrea, V.; Carlini, M.; Casella, G. The Role of Antibiotic Prophylaxis in Anastomotic Leak Prevention during Elective Colorectal Surgery: Systematic Review and Meta-Analysis of Randomized Controlled Trials. Antibiotics 2023, 12, 397. https://doi.org/10.3390/antibiotics12020397
Castagneto-Gissey L, Russo MF, Casella-Mariolo J, Serao A, Marcellinaro R, D’Andrea V, Carlini M, Casella G. The Role of Antibiotic Prophylaxis in Anastomotic Leak Prevention during Elective Colorectal Surgery: Systematic Review and Meta-Analysis of Randomized Controlled Trials. Antibiotics. 2023; 12(2):397. https://doi.org/10.3390/antibiotics12020397
Chicago/Turabian StyleCastagneto-Gissey, Lidia, Maria Francesca Russo, James Casella-Mariolo, Angelo Serao, Rosa Marcellinaro, Vito D’Andrea, Massimo Carlini, and Giovanni Casella. 2023. "The Role of Antibiotic Prophylaxis in Anastomotic Leak Prevention during Elective Colorectal Surgery: Systematic Review and Meta-Analysis of Randomized Controlled Trials" Antibiotics 12, no. 2: 397. https://doi.org/10.3390/antibiotics12020397
APA StyleCastagneto-Gissey, L., Russo, M. F., Casella-Mariolo, J., Serao, A., Marcellinaro, R., D’Andrea, V., Carlini, M., & Casella, G. (2023). The Role of Antibiotic Prophylaxis in Anastomotic Leak Prevention during Elective Colorectal Surgery: Systematic Review and Meta-Analysis of Randomized Controlled Trials. Antibiotics, 12(2), 397. https://doi.org/10.3390/antibiotics12020397








