Antibiotic Drug Resistance Pattern of Uropathogens in Pediatric Patients in Pakistani Population
Abstract
1. Introduction
2. Results
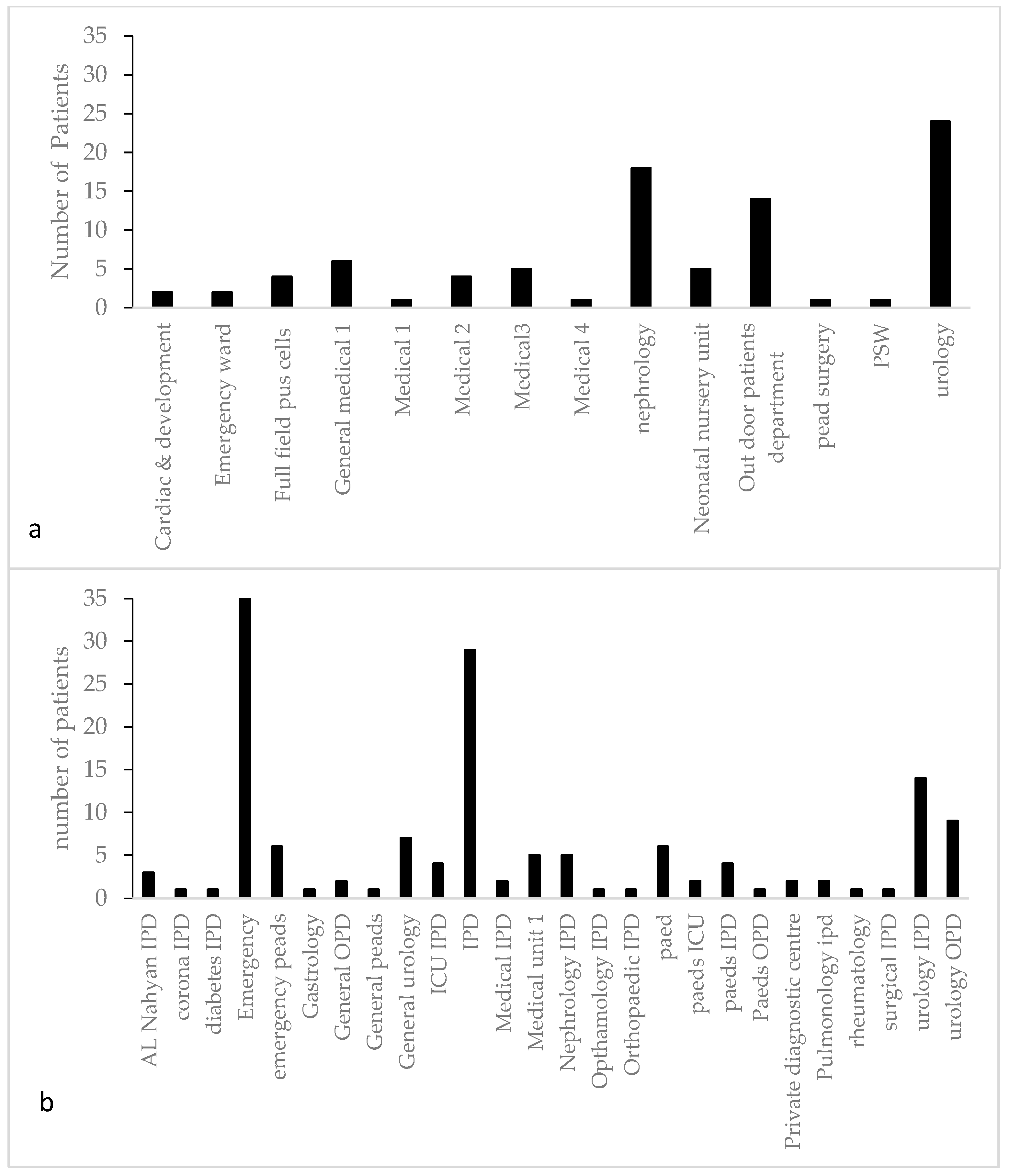
2.1. Sample Collection
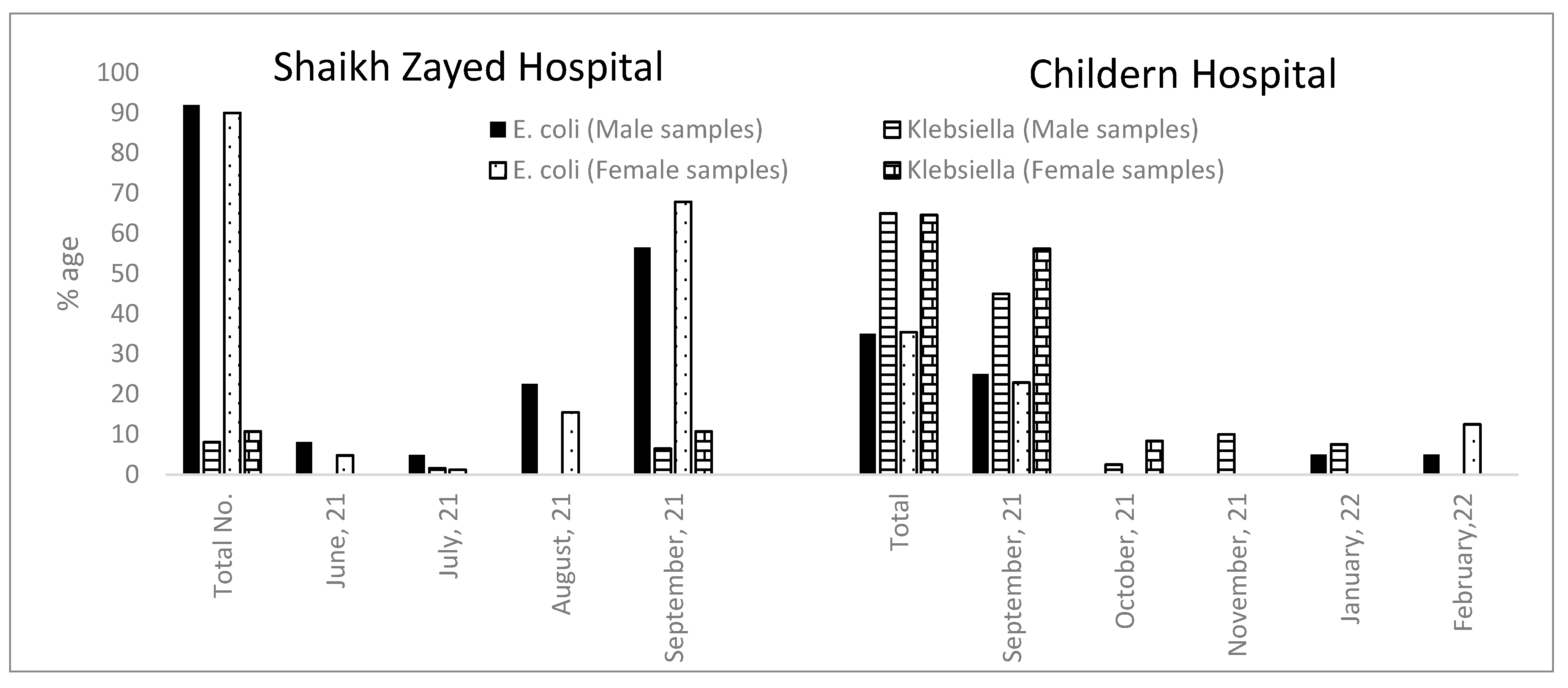
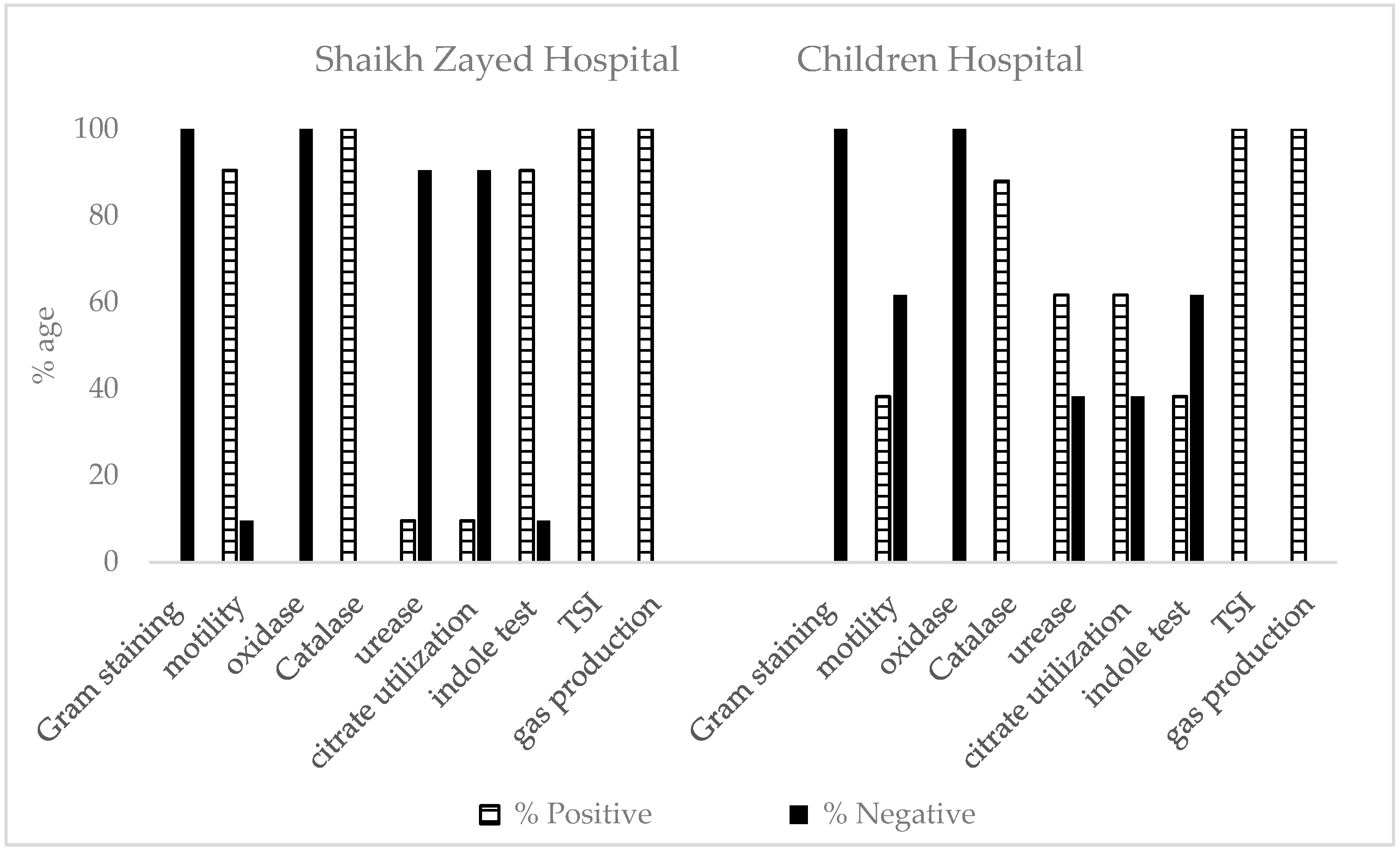
2.2. Morphological and Biochemical Characterization of Antibiotic-Resistant Escherichia and Klebsiella Isolated from UTIs
2.2.1. Shaikh Zayed Hospital
2.2.2. Children Hospital
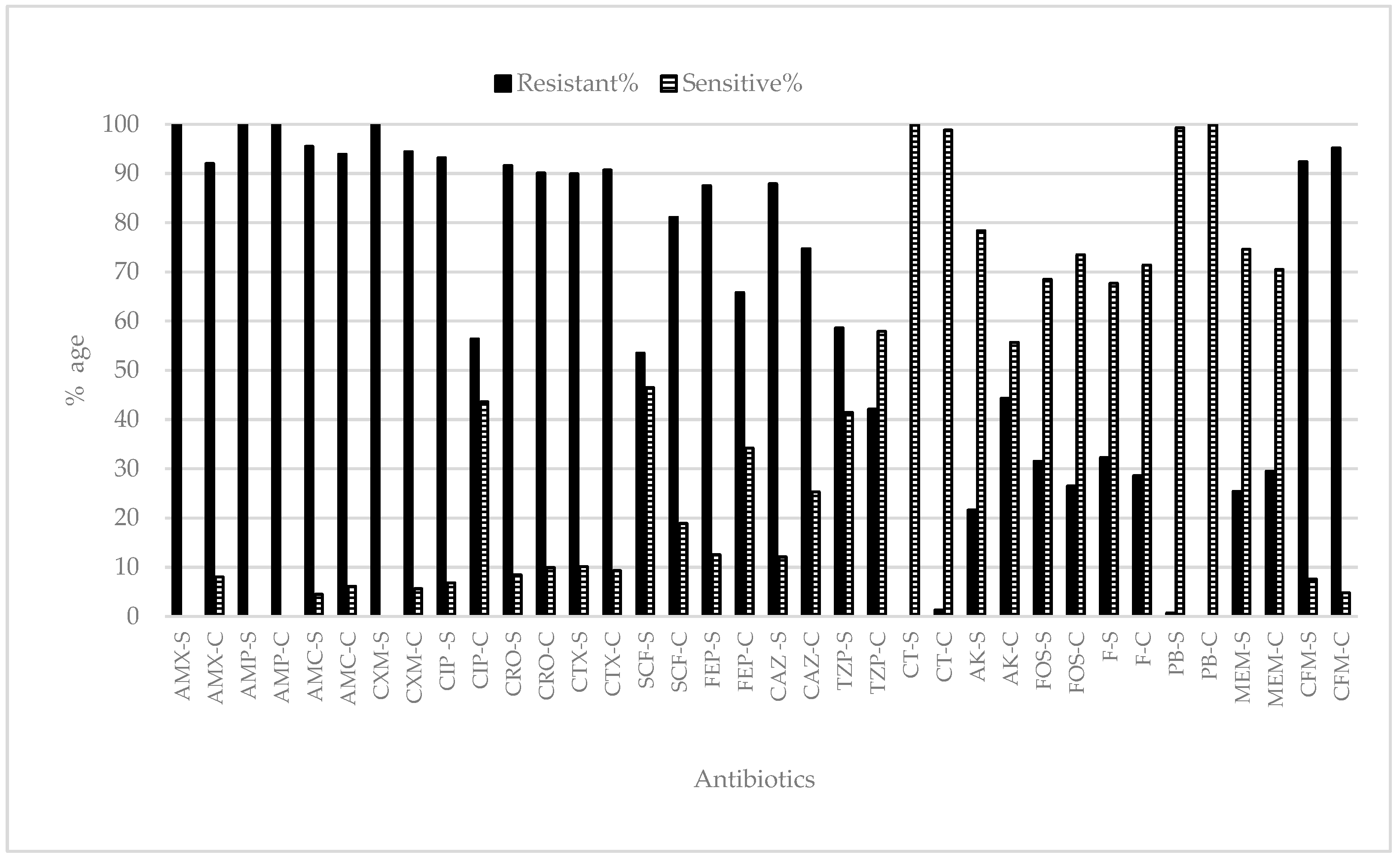
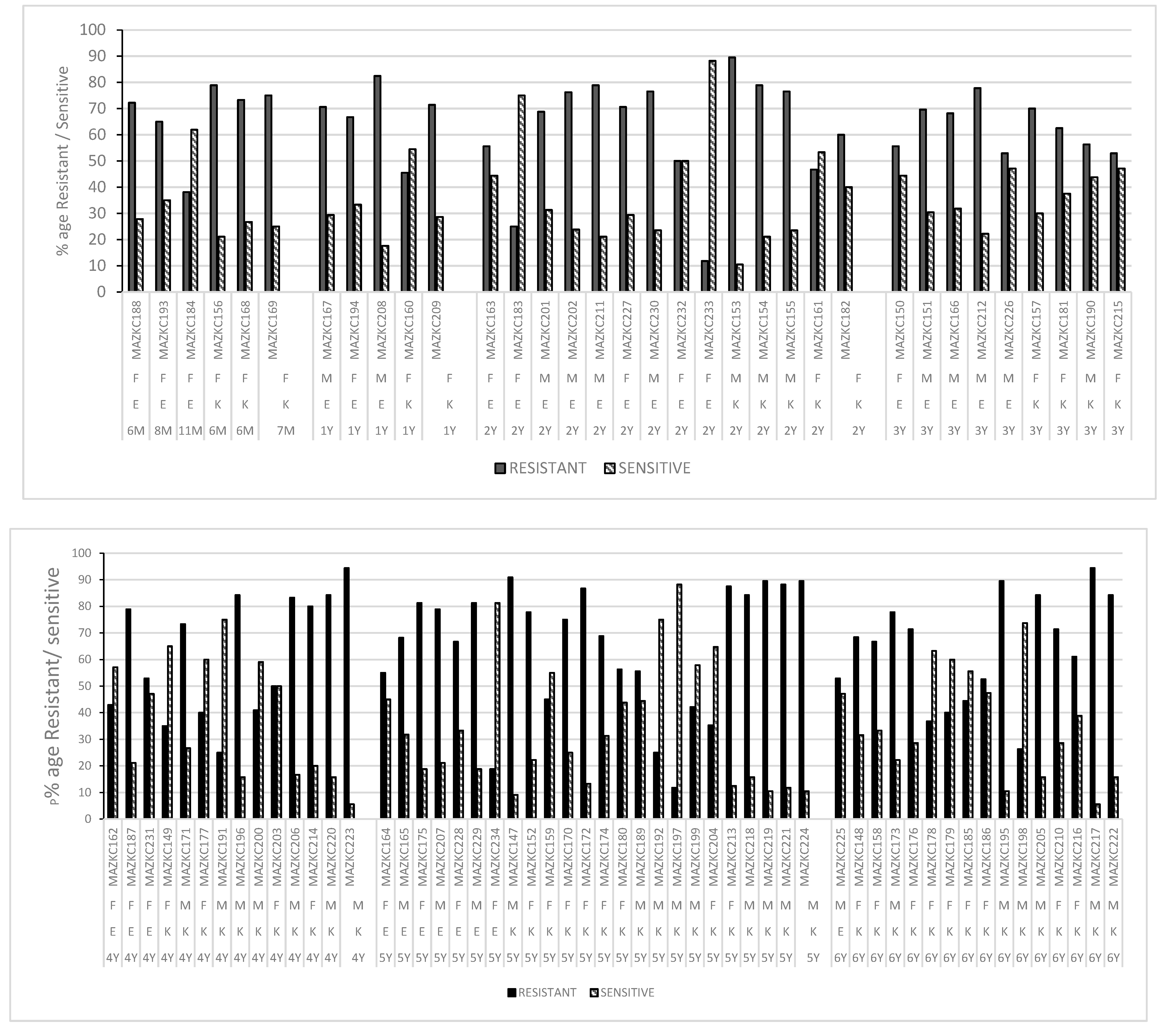
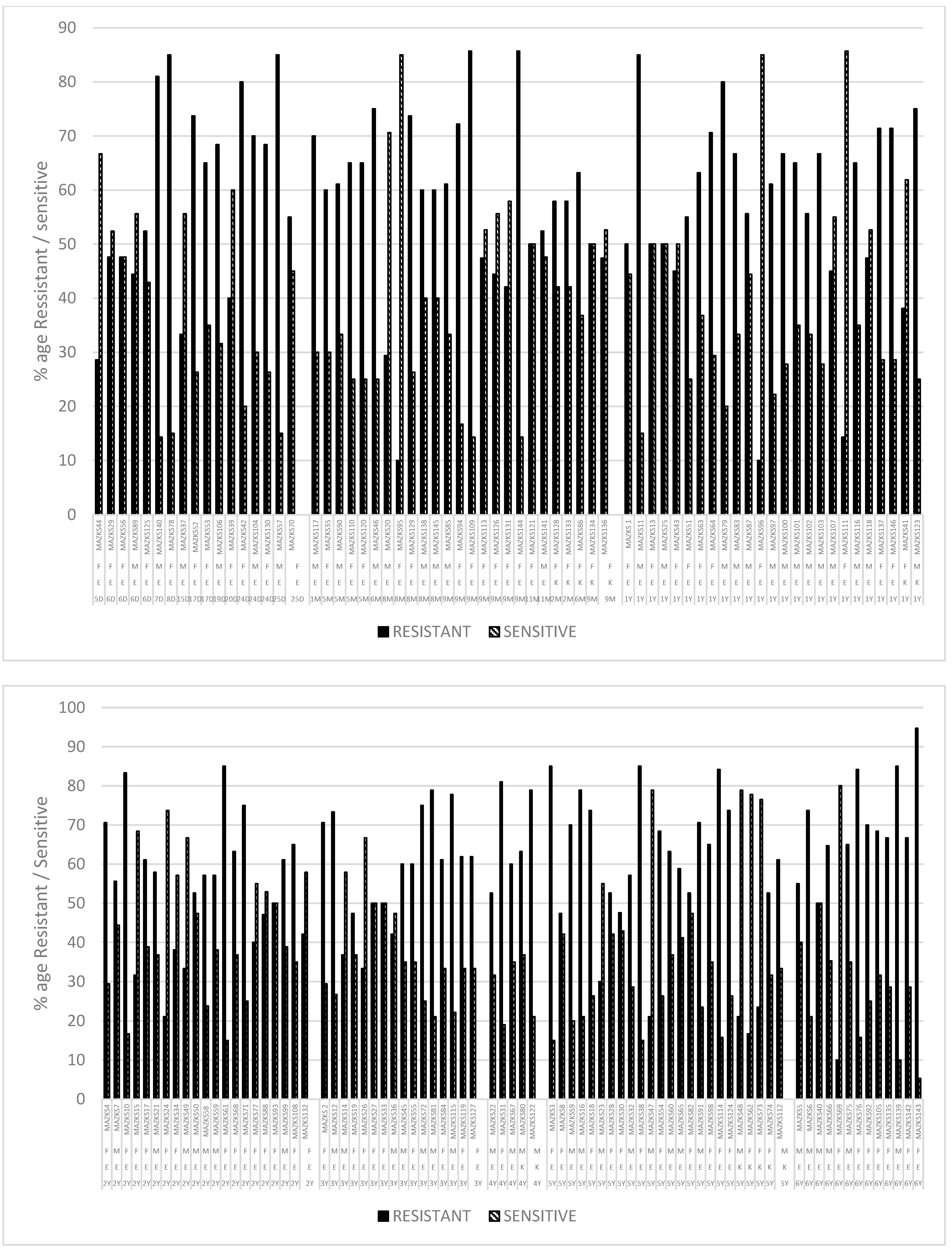
2.3. Antibiotic Resistance/Susceptibility Profile
2.3.1. Shaikh Zayed Hospital
2.3.2. Children Hospital
3. Discussion
4. Materials and Methods
4.1. Study Area
4.2. Inclusion Criteria
4.3. Exclusion Criteria
4.4. Sample Collection
4.5. Morphological and Biochemical Characterization
4.6. Antibiotic Resistance Test
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Garrido, D.; Garrido, S.; Gutierrez, M.; Calvopina, L.; Harrison, A.S.; Fuseau, M.; Salazar Irigoyen, R. Clinical characterization and antimicrobial resistance of Escherichia coli in pediatric patients with urinary tract infection at a third level hospital of Quito, Ecuador. Bol. Med. Hosp. Infant. Mex. 2017, 74, 265–271. [Google Scholar] [CrossRef] [PubMed]
- Senel, S.; Karacan, C.; Erkek, N.; Gol, N. A single-center experience of antimicrobial resistance patterns in pediatric urinary tract infection. Med. Princ. Pract. 2010, 19, 359–363. [Google Scholar] [CrossRef] [PubMed]
- Kasanga, M.; Mukosha, R.; Kasanga, M.; Siyanga, M.; Mudenda, S.; Solochi, B.B.; Chileshe, M.; Mwiikisa, M.J.; Gondwe, T.; Kantenga, T.; et al. Antimicrobial resistance patterns of bacterial pathogens their distribution in university teaching hospitals in Zambia. Future Microbiol. 2021, 16, 811–824. [Google Scholar] [CrossRef] [PubMed]
- Zerefaw, G.; Tadesse, S.; Derbie, A. Bacterial Uropathogens, Antimicrobial Susceptibility Profile and Associated Factors among Pediatric Patients in Bahir Dar, Northwest Ethiopia. Ethiop. J. Health Sci. 2022, 32, 81–92. [Google Scholar] [CrossRef] [PubMed]
- Erol, B.; Culpan, M.; Caskurlu, H.; Sari, U.; Cag, Y.; Vahaboglu, H.; Ozumut, S.H.; Karaman, M.I.; Caskurlu, T. Changes in antimicrobial resistance and demographics of UTIs in pediatric patients in a single institution over a 6-year period. J. Pediatr. Urol. 2018, 14, 176.e1–176.e5. [Google Scholar] [CrossRef]
- Celep, G.; Ozcelik, H.B. Evaluation of clinical, etiological and antimicrobial resistance profile of pediatric urinary tract infections in a secondary health care centre. Afr. Health Sci. 2021, 21, 557–565. [Google Scholar] [CrossRef]
- Desai, D.J.; Gilbert, B.; McBride, C.A. Paediatric urinary tract infections: Diagnosis and treatment. Aust. Fam. Physician 2016, 45, 558–563. [Google Scholar]
- Sari, E.; YazilitaŞ, F.; ÖZtek ÇElebİ, F.Z.; AkÇAboy, M.; AkiŞOĞLu, Ö.; ŞEnel, S. Antibiotic drug resistance pattern of uropathogens seen in the first episode of community-acquired pediatric urinary tract infections at a Tertiary Care Hospital. Turk. J. Pediatr. Dis. 2022, 16, 138–143. [Google Scholar] [CrossRef]
- Esposito, S.; Biasucci, G.; Pasini, A.; Predieri, B.; Vergine, G.; Crisafi, A.; Malaventura, C.; Casadio, L.; Sella, M.; Pierantoni, L.; et al. Antibiotic Resistance in Paediatric Febrile Urinary Tract Infections. J. Glob. Antimicrob. Resist. 2022, 29, 499–506. [Google Scholar] [CrossRef]
- Alzahrani, M.A.; Sadoma, H.H.M.; Mathew, S.; Alghamdi, S.; Malik, J.A.; Anwar, S. Retrospective analysis of antimicrobial susceptibility of uropathogens isolated from pediatric patients in Tertiary Hospital at Al-Baha Region, Saudi Arabia. Healthcare 2021, 9, 1564. [Google Scholar] [CrossRef]
- Shapiro, T.; Dalton, M.; Hammock, J.; Lavery, R.; Matjucha, J.; Salo, D.F. The prevalence of urinary tract infections and sexually transmitted disease in women with symptoms of a simple urinary tract infection stratified by low colony count criteria. Acad. Emerg. Med. 2005, 12, 38–44. [Google Scholar] [CrossRef] [PubMed]
- Usman, N.I.; Abdulwahab, N.M.; Sulaiman, M.J. Multidrug Resistance (MDR), Extensive Drug Resistance (XDR) and Pan Drug Resistance (PDR) Klebsiella Pneumoniae from Clinical Samples. SLU J. Sci. Technol. 2022, 3, 42–50. [Google Scholar] [CrossRef]
- Konca, C.; Tekin, M.; Uckardes, F.; Akgun, S.; Almis, H.; Bucak, I.H.; Genc, Y.; Turgut, M. Antibacterial resistance patterns of pediatric community-acquired urinary infection: Overview. Pediatr. Int. 2017, 59, 309–315. [Google Scholar] [CrossRef] [PubMed]
- Korbel, L.; Howell, M.; Spencer, J.D. The clinical diagnosis and management of urinary tract infections in children and adolescents. Paediatr. Int. Child Health 2017, 37, 273–279. [Google Scholar] [CrossRef]
- Wang, J.; He, L.; Sha, J.; Zhu, H.; Huang, L.; Zhu, X.; Dong, J.; Li, G.; Ge, Z.; Lu, R.; et al. Etiology and antimicrobial resistance patterns in pediatric urinary tract infection. Pediatr. Int. 2018, 60, 418–422. [Google Scholar] [CrossRef]
- Iqbal, Z.; Mumtaz, M.Z.; Malik, A. Extensive drug-resistance in strains of Escherichia coli and Klebsiella pneumoniae isolated from paediatric urinary tract infections. J. Taibah Univ. Med. Sci. 2021, 16, 565–574. [Google Scholar] [CrossRef]
- Autore, G.; Bernardi, L.; La Scola, C.; Ghidini, F.; Marchetti, F.; Pasini, A.; Pierantoni, L.; Castellini, C.; Gatti, C.; Malaventura, C.; et al. Management of Pediatric Urinary Tract Infections: A Delphi Study. Antibiotics 2022, 11, 1122. [Google Scholar] [CrossRef]
- Ganesh, R.; Shrestha, D.; Bhattachan, B.; Rai, G. Epidemiology of urinary tract infection and antimicrobial resistance in a pediatric hospital in Nepal. BMC Infect. Dis. 2019, 19, 420. [Google Scholar] [CrossRef]
- Masson, P.; Matheson, S.; Webster, A.C.; Craig, J.C. Meta-analyses in Prevention and Treatment of Urinary Tract Infections. Infect. Dis. Clin. N. Am. 2009, 23, 355–385. [Google Scholar] [CrossRef]
- Hafeez, M.M.; Ali, Q.; Rana, M.A.; Javed, Y.; Alyas, S.; Akram, Q.; Ayub, M.; Sajjad, K.; Shahid, M.A.; Sohail, M. Prevalence of Urinary Tract Infection and Drug Resistance among Infants and Children in Pakistan. J. Pharm. Res. Int. 2021, 33, 125–131. [Google Scholar] [CrossRef]
- Fang, P.; Gao, K.; Sun, H.; Shan, Z.; Yang, J.; Wang, Y. Microorganism profile and antimicrobial resistance pattern of pathogenic bacteria in urinary tract infection from a tertiary pediatrics hospital in Henan, China. arXiv 2020. [Google Scholar] [CrossRef]
- Andaleeb, H.; Zia, W.; Shahid, A.; Iqbal Tarar, Z.; Shams, N.; Haq, K.; Faizan Hamid, M. Spectrum Of Antimicrobial Susceptibility Pattern of Urinary Tract Infection in In Adults. Pak. BioMed. J. 2022, 5, 60–66. [Google Scholar] [CrossRef]
- Bilal, H.; Khan, M.N.; Rehman, T.; Hameed, M.F.; Yang, X. Antibiotic resistance in Pakistan: A systematic review of past decade. BMC Infect. Dis. 2021, 21, 244. [Google Scholar] [CrossRef] [PubMed]
- Dejonckheere, Y.; Desmet, S.; Knops, N. A study of the 20-year evolution of antimicrobial resistance patterns of pediatric urinary tract infections in a single center. Eur. J. Pediatr. 2022, 181, 3271–3281. [Google Scholar] [CrossRef] [PubMed]
- Al Mana, H.; Sundararaju, S.; Tsui, C.K.M.; Perez-Lopez, A.; Yassine, H.; Al Thani, A.; Al-Ansari, K.; Eltai, N.O. Whole-Genome Sequencing for Molecular Characterization of Carbapenem-Resistant Enterobacteriaceae Causing Lower Urinary Tract Infection among Pediatric Patients. Antibiotics 2021, 10, 972. [Google Scholar] [CrossRef] [PubMed]
- Idrees, M.M.; Rasool, M.F.; Imran, I.; Khalid, A.; Saeed, A.; Ahmad, T.; Alqahtani, F. A Cross-Sectional Study to Evaluate Antimicrobial Susceptibility of Uropathogens from South Punjab, Pakistan. Infect. Drug Resist. 2022, 15, 1845–1855. [Google Scholar] [CrossRef] [PubMed]
- Alfuraiji, N.; Al-Hamami, A.; Ibrahim, M.; Rajab, H.K.; Hussain, B.W. Uropathogenic Escherichia coli virulence characteristics and antimicrobial resistance amongst pediatric urinary tract infections. J. Med. Life 2022, 15, 650–654. [Google Scholar] [CrossRef]
- Shrestha, L.B.; Baral, R.; Poudel, P.; Khanal, B. Clinical, etiological and antimicrobial susceptibility profile of pediatric urinary tract infections in a tertiary care hospital of Nepal. BMC Pediatr. 2019, 19, 36. [Google Scholar] [CrossRef]
- Shaikh, N.; Morone, N.E.; Bost, J.E.; Farrell, M.H. Prevalence of urinary tract infection in childhood: A meta-analysis. Pediatr. Infect. Dis. J. 2008, 27, 302–308. [Google Scholar] [CrossRef]
- Simões e Silva, A.C.; Oliveira, E.A.d.; Mak, R.H. Urinary tract infection in pediatrics: An overview. J. Pediatr. 2019, 96, 65–79. [Google Scholar] [CrossRef]
- Cappuccino, J.G.; Sherman, N. Microbiology: A Laboratory Manual; Benjamin Cummings: San Francisco, CA, USA, 2002. [Google Scholar]
- CLSI. Performance Standards for Antimicrobial Susceptibility Testing: 32nd ed. CLSI Supplement M100. Clinical and Laboratory Standards Institute, USA, 2022. Available online: https://clsi.org/media/wi0pmpke/m100ed32_sample.pdf (accessed on 29 November 2022).
- Magiorakos, A.P.; Srinivasan, A.; Carey, R.B.; Carmeli, Y.; Falagas, M.E.; Giske, C.G.; Harbarth, S.; Hindler, J.F.; Kahlmeter, G.; Olsson-Liljequist, B.; et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: An international expert proposal for interim standard definitions for acquired resistance. Clin. Microbiol. Infect. 2012, 18, 268–281. [Google Scholar] [CrossRef] [PubMed]
| Name of Hospital | Shaikh Zayed Hospital | Children Hospital | ||||
|---|---|---|---|---|---|---|
| Sampling duration | Samples collected in four months | Samples collected in five months | ||||
| Number of samples | 15,000 | 24,750 | ||||
| Exclusion criteria | Excluded by microscopy < 5 WBC under high power field | |||||
| Number of samples excluded | 10,800 | 16,500 | ||||
| Inclusion criteria | Total number of samples cultured on CLED agar (≥5 WBC high power field) | |||||
| Number of samples included | 4200 | 8250 | ||||
| Total number of bacterial growth shown on CLED agar | ||||||
| Number of plates with positive growth | 600 | 750 | ||||
| Selection criteria | Total number of positive samples for Escherichia sp. and Klebsiella sp. on CLED agar | |||||
| Number of samples selected | 146 | 88 | ||||
| Gender-wise distribution of samples | Male n (%) | Female n (%) | Male n (%) | Female n (%) | ||
| 62 (42.46) | 84 (57.53) | 40 (45.45) | 48 (54.54) | |||
| Bacterial count | Bacterial count in samples n (%) | |||||
| >104 CFU/mL | 05 (8.06) | 16 (19.04) | 05 (12.5) | 02 (4.16) | ||
| >105 CFU/mL | 57(91.93) | 68 (80.95) | 35 (87.5) | 46 (95.83) | ||
| Name of Hospital | Co-Ordinates (DMS) |
|---|---|
| Shaikh Zayed Hospital | 31°30′31″ N, 74°18′31″ E |
| Children Hospital | 31°28′48.36″ N, 74°20′34.8″ E |
| Antimicrobial Agents Used with Concentrations | |||
|---|---|---|---|
| Aminoglycosidases Amikacin (AK, 30 μg Gentamicin (CN, 120 μg) Tobramycin (TOB, 10 μg) | Quinolones Ciprofloxacin (CP, 5 μg) | Polymyxin Polymyxin B (PB, 300 units) Collistein sulphate (CT, 25 μg) | |
| Β-lactam | |||
| Penicillins | Cephalosporins | ||
| Co-Amoxiclav (AMC, 20/10 μg) Amoxicillin/clavulanic acid (AUG, 30 μg) Piperacillin/Tazobactam (TZP, 100/10 μg) Amoxicillin (AMX 10 μg) | Ist generation Ampicillin (AMP- 10 μg) 2nd generation Cefuroxime (CXM, 30 μg) 4th generation Cefepime (FEP, 30 μg) | 3rd generation Cefixime (CFM, 5 μg) Cefotaxime (CTX, 30 μg) Ceftazidime (CAZ, 30 μg) Ceftriaxone (CRO, 30 μg) Cefoperazone/Sulbactam (SCF, 10/5 μg) | Carbepenem (10 μg each) Imipenem (IPM) Meropenem (MEM) Ertapenem (ETP) |
| Cotrimoxazole ( sulphamethoxazole and trimethoprim) -SXT, 1.25/23 μg | Fosfomycin (FOS, 50/200 μg) | Nitrofurantoin (F, 300 μg) | |
| Norfloxaxin (NOR, 10 μg) | Pipedemic acid (PIP, 20 μg) | Nalidixic acid (NA, 30 μg) | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iqbal, Z.; Sheikh, A.S.; Basheer, A.; Hafsa, H.t.; Ahmed, M.; Sabri, A.N.; Shahid, S. Antibiotic Drug Resistance Pattern of Uropathogens in Pediatric Patients in Pakistani Population. Antibiotics 2023, 12, 395. https://doi.org/10.3390/antibiotics12020395
Iqbal Z, Sheikh AS, Basheer A, Hafsa Ht, Ahmed M, Sabri AN, Shahid S. Antibiotic Drug Resistance Pattern of Uropathogens in Pediatric Patients in Pakistani Population. Antibiotics. 2023; 12(2):395. https://doi.org/10.3390/antibiotics12020395
Chicago/Turabian StyleIqbal, Zakia, Ahsan Sattar Sheikh, Anwaar Basheer, Hadiqa tul Hafsa, Mehboob Ahmed, Anjum Nasim Sabri, and Samiah Shahid. 2023. "Antibiotic Drug Resistance Pattern of Uropathogens in Pediatric Patients in Pakistani Population" Antibiotics 12, no. 2: 395. https://doi.org/10.3390/antibiotics12020395
APA StyleIqbal, Z., Sheikh, A. S., Basheer, A., Hafsa, H. t., Ahmed, M., Sabri, A. N., & Shahid, S. (2023). Antibiotic Drug Resistance Pattern of Uropathogens in Pediatric Patients in Pakistani Population. Antibiotics, 12(2), 395. https://doi.org/10.3390/antibiotics12020395








