Abstract
Escherichia coli ST141 is one of the ExPEC lineages whose incidence is rising in France, even if no epidemic situation involving multidrug resistant isolates has been reported so far. Nonetheless, in a 2015–2017 monocentric study conducted in our French University hospital, ST141 was the most frequent lineage after ST131 in our collection of phylogroup B2 ESBL-producing E. coli. The genomes of 187 isolates representing ST141 group, including 170 genomes from public databases and 17 from our local collection, of which 13 produced ESBL, were analyzed to infer the maximum likelihood phylogeny SNP-based (Single Nucleotide Polymorphism) free-recombinant tree defining the ST141 population structure. Genomes were screened for genes encoding virulence factors (VFs) and antimicrobial resistance (AMR). We also evaluated the distribution of isolates according to their origin (host, disease, country) and the distribution of VFs or AMR genes. Finally, the phylogenic tree revealed that ST141 isolates clustered into two main sublineages, with low genetic diversity. Contrasting with a highly virulent profile, as many isolates accumulated VFs, the prevalence of AMR was limited, with no evidence of multidrug resistant emerging lineage. However, our results suggest that surveillance of this clonal group, which has the potential to spread widely in the community, would be essential.
1. Introduction
Escherichia coli is the predominant aerobic bacterium in the normal gut microbiota of humans and vertebrates as well as a major human pathogen []. Indeed, E. coli strains can cause both extra-intestinal pathologies (urinary tract infection, intra-abdominal or pulmonary infection as well as newborn meningitis or bacteraemia) and intestinal infections []. Currently, unlike these latter one (Intestinal Pathogenic E. coli, InPEC), for which virulence factors have been clearly identified and for which pathovar classification seems easy, the classification of ExPEC (Extraintestinal Pathogenic E. coli) strains is still subject to discussion, as no disease-specific virulence genes have been identified. It is the combination of different virulence factors, which may be numerous in these strains, that may explain their pathogenicity. Indeed, most, if not all, strains that may cause extra-intestinal infections have different genes encoding for virulence factors, such as adhesins, toxins, protectins and iron capture systems. However, the conditions under which E. coli strains emerge from their intestinal reservoir to cause extraintestinal infections, remain largely unknown as well as the reasons for the success of some pandemic lineages. Indeed, phylogenomic approaches have demonstrated that only four sequence types or sequencing type complexes (STcs), responsible of extraintestinal infections (STc131, STc73 and STc95 belonging to phylogroup B2, and STc69 belonging to phylogroup D) were always observed in epidemiological studies and were thus named “the big four ExPEC clones”. Although not part of the big four ExPEC clones, E. coli ST141 is regularly reported as one of the most represented ExPEC []. Furthermore, among ExPEC, E. coli ST141 is singular: it has recently been hypothesized that the ST141 E. coli lineage genome would be very susceptible to recombinations, making it capable of acquiring and expressing specific genes of InPEC thus conferring a heteropathogen status []. The appearance of such hybrid clones could reshuffle the deck within the pathotype classification widely used to describe E. coli populations. Another matter of concern is the ability of E. coli to gain antibiotic resistance determinants given that E. coli may easily spread, as occurred with the pandemic of highly drug-resistant E. coli sequence type 131 (ST131) H30 sublineage []. To date, no epidemic situation involving multidrug resistant ST141 isolates has been reported []. Nonetheless, in a 2015–2017 monocentric study conducted in our French University hospital, consisting of collecting all non-duplicate extended-spectrum beta-lactamase producing E. coli (ESBLEc), we identified that ST141 was the second most frequent lineage in our collection of phylogroup B2 ESBLEc, accounting for 8.7% of ESBL-producing isolates, while ST131 lineage, the most frequent lineage, accounted for 55.3%, and isolates belonging to other STs each accounted for less than 3% of isolates [].
Here, we used a comprehensive data set of ST141 genomes available in databases to characterize population structure of this clonal group with the aim of identifying the emergence of a multidrug resistant sublineage.
2. Results
2.1. ST141 Collection (Table S1)
- Among the 187 isolates analyzed, the geographic origin was known for 154 (82%): 108 isolates originated from European continent (mainly from France, United Kingdom, and Germany), 40 from American continent (North and South) and 6 from Asian continent. Most of the isolates (72%, 135/187) have been collected from humans, while non-human strains (i.e., environment, animal, and food) accounted for 15% (28/187) of the collection.
- Available genomes came from strains isolated between 1988 and 2019 and the sample date was known for 139 of which. A majority of strains were collected after 2010’s (71.2%, 99/139).
- Among the 135 human-isolated strains, information about source sample was known for 100 isolates: 36% (36/100) came from urine, 33% (33/100) from blood culture, 23% (23/100) from feces (of which 13/23 were responsible of diarrhea) and 8% (8/100) from various clinical samples (of which three came from neonatal infections).
2.2. Whole Genome-Based Typing and SNP-Based Recombination-Free Phylogenetic Tree of ST141 E. coli Strains
- In silico MLST (Multi-Locus Sequence Typing) and phylogrouping confirmed that all selected isolates belonged to ST141 and B2 phylogroup. Within this ST141 clone, we wanted to assess the diversity of serogroup O and the fimH allele, which are two hotspots of recombinations, to be more discriminating []. Surprisingly, the ST141 clone has a low O-serogroupe diversity, as all strains tested belonged to O2:H6 serotype. The fimH allele typing, which is based on minor sequence variations, allowed to identify fimH5 (115/187, 61.5%), fimH14 (48/187, 25.7%) and fimH350 (10/187, 5.3%) as major fimH subtypes. Then, 16 minor fimH subtypes (i.e., shared by two or less isolates) were identified (Table S1, Figure 1) [].
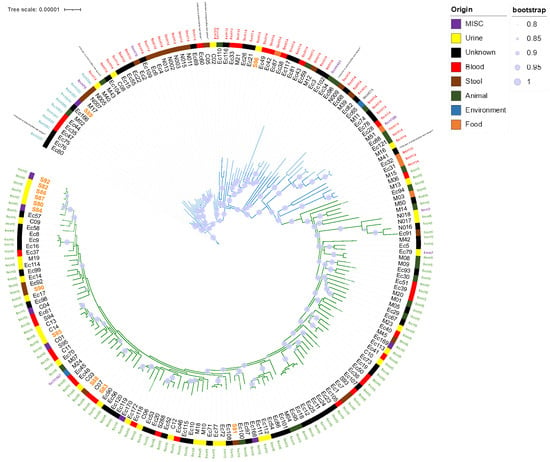 Figure 1. Representation of the two main E. coli ST141 sublineages obtained from maximum likelihood phylogeny SNP-based free-recombinant tree inferred from alignment to a fully sequenced reference strain of E. coli ST141.
Figure 1. Representation of the two main E. coli ST141 sublineages obtained from maximum likelihood phylogeny SNP-based free-recombinant tree inferred from alignment to a fully sequenced reference strain of E. coli ST141. - Sublineages were indicated by edge coloring. Color strip corresponded to source origin. For easy readability, branches with fewer leaf nodes have been displayed on the top. Only bootstraps with value > 80% of confidence were displayed (blue burred nodes). SNP-based analysis displayed two main sublineages (S1 and S2). Whereas S1 (green edges) appeared relatively homogeneous, with highly conserved fimH5 subtype (115/118), S2 (blue edges) fimH subtyping appeared less homogeneous, with predominant fimH14 (48/69) and fimH350 (10/69) subtypes. Isolates from local collection (colored in orange) are scattered throughout the phylogenetic tree. Most of them (15/17) belonged to S1 while only two strains (S89 and S96) belonged to S2.
- The SNP-based recombination-free phylogenetic tree depicted two main sublineages S1 and S2, clustering 118 and 69 isolates, respectively (Figure 1). S1 potentially appeared recently as a result of a fimH subtype conversion (Figure 1). Indeed, all isolates belonging to fimH5 clustered into S1 (115/118, 97.5%). The three remaining S1 isolates belonged to fimH7 (2/118, 1.7%) and fimH1497 (1/118, 0.8%) subtypes.
- Isolates from our local collection mostly belonged to S1 (15/17) and six of them exclusively grouped together. Isolates S86, S87, S80, S82, and S92 were indeed separated to each other by a maximum of 14 SNPs while S84 was distant with 26 SNPs. Finally, only two isolates, S89 and S96, did not belong to S1 (Table S1, Figure 2).
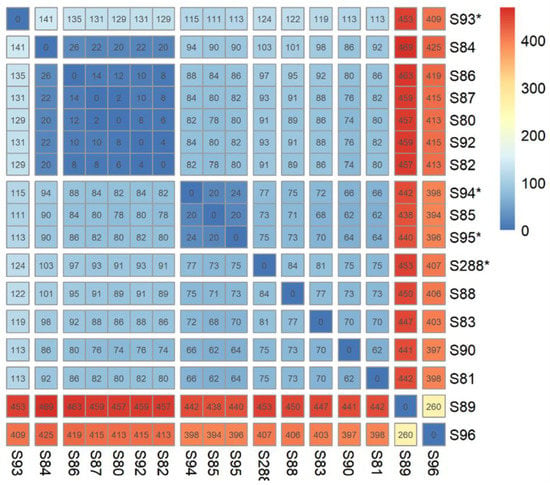 Figure 2. Distance matrix (SNP) of strains from our local collection (n = 17). Isolates identified with «*» symbol had no blaESBL gene. Five of our isolates were less than 15 SNP (S86, S87, S80, S92 and S82) or 26 (S84) from the nearest isolate.
Figure 2. Distance matrix (SNP) of strains from our local collection (n = 17). Isolates identified with «*» symbol had no blaESBL gene. Five of our isolates were less than 15 SNP (S86, S87, S80, S92 and S82) or 26 (S84) from the nearest isolate. - Isolates from urines were more represented in S1 than in S2 (S1: 38%—31/81 vs. S2: 11%—5/47, p < 0.01). Conversely, isolates responsible for diarrhea (S1: 4%, 3/81 vs. S2: 22%—10/47) preferentially belonged to S2 (p < 0.01) (Table 1).
 Table 1. Origin and sublineage distribution of E. coli ST141 isolates (n = 128).
Table 1. Origin and sublineage distribution of E. coli ST141 isolates (n = 128). - Interestingly, 33 isolates of collection and previously studied by Gati et al., we noted a good correlation between the L1/L2 Gati’s lineages and our S1/S2 distribution, as all isolates belonging to L2 clustered in the S1 sublineage and possessed the fimH5 subtype. Conversely, all isolates belonging to L1 clustered in the S2 sublineage, with different fimH subtypes (7 of subtype fimH14; three of subtype fimH350, two of subtype fimH76 and one of subtype fimH 674) [] (Table S1, Figure 1).
2.3. Antimicrobial Resistance Genes in E. coli ST141 (Table S1, Figure 3)
Regarding resistance to β-lactams associated with extended-spectrum β-lactamase, a total of 35 strains (35/187, 18.7%) carried various blaESBL genes. Class A blaCTX m genes were the most prevalent ESBL genes (80.0%, 28/35) with blaCTX-M-14 being the most frequent (7.0%, 13/187) followed by blaCTX-M-15 (3.7%, 7/187), which was most often associated with the broad spectrum β-lactamase blaTEM-1 gene (3/7, 43%), and blaCTX-M-1 2.1% (4/187). Of note, 13 out of these 28 ESBL-producing isolates came from our local collection. No statistical difference relating to the ESBL distribution was observed between S1 (22.9%, 27/118), and S2 (11.2% 8/69, p = 0.08). We did not retrieve blaESBL genes in ST141 isolates recovered from the environment. Additionally, two isolates harbored carbapenemase-encoding genes blaOXA-48 (Ec81) and blaVIM-1 (C04) and one strain (Ec110) isolated from poultry had colistin resistance gene mcr-1 (Table S1, Figure 2).
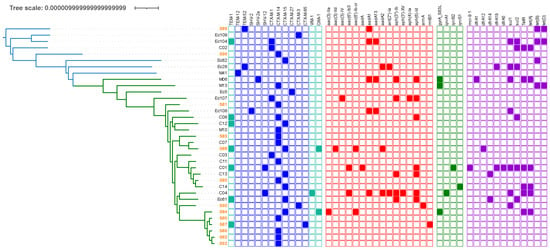
Figure 3.
AMR genes distribution among Escherichia coli ST141 genomes with ESBL gene. Sublineages were indicated by edge coloring (S1, green edges, S2, blue edges). The presence/Absence of genes encoding AMR were indicated by colored squares (blue: β-lactam resistance—ESBL were shown in dark blue; red: aminoglycosides resistance; green: quinolone resistance; purple: miscellaneous).
Resistance genes to aminoglycosides were found in 21.9% (41/187) of the isolates and many of them (82.9%, 34/41) accumulated two or more resistance genes. The aph(6)-Id and aph(3″)-Ib, genes were the most common genes in our collection, as they were present in 15.0% (28/187) and 12.3% (23/187) of the isolates, respectively. We found no difference in the distribution of resistance genes to aminoglycosides between isolates of human and non-human origin (p = 0.6179) or between urine, blood or stool origin. Resistance genes to aminoglycosides were equally distributed between S1 (25.4%, 30/118) and S2 (15.9%, 11/69, p = 0.1462) (Table S1).
Resistance to quinolones was rare, as mutations in quinolone resistance-determining regions (QRDR) were found in nine isolates (4.8%, 9/187) and Plasmid-Mediated Quinolone-Resistance (PMQR) genes were present in only 2.1% (4/187) for qnr genes and 1.1% (2/187) for aac(6′)-Ib-cr gene. One isolate (S84) harbored both aac(6′)-Ib-cr and gyrA S83L mutation. Globally, the frequency of quinolone resistance (QRDR and PMQR) was not statistically different between S1 (10.2%, 12/118) and S2 (2.9%, 2/69), (p = 0.09) (Table S1).
To note, all 13 ESBL E. coli strains in our collection were tested in vitro (diffusion susceptibility testing according to EUCAST/CA-SFM recommendations in force at the time of the establishment of the strain collection) with a perfect correlation with results obtained in silico.
2.4. Virulence Factors in E. coli ST141 (Table 2 and Table S1)
- Overall, we did not find a strong association between the distribution of VFs and the geographical origin, the origin (human or non-human), and the type of clinical sample. However, some differences in the distribution of VFs in S1 and S2 were statistically significant and displayed in Table 2. Thus, stx2 gene was more frequent in the S2 sublineage (due to the predominance of strains causing diarrhea in this sublineage (S1 = 5/118; S2 = 12/118, p = 0.004)), while the Enteroaggregative E. coli (EAEC) astA gene previously described to exclusively belong to strains of the L2 (i.e., S1 in this study) sublineage was significantly predominant in S1 sublineage []. However, the astA gene was also recovered in two isolates (Ec116 and C08) from our collection, classified as lineage S2, as they belonged to fimH14 subtype. Of note, while this gene is supposed to be typical of EAEC (Enteroadhesive E. coli, responsible of diarrhea), we showed that astA gene was statistically more present in isolates from urine (23/36, 63.9%) than in isolates from stool (5/18, 21.7%, p = 0.0028). Finally, the invasion gene ibeABC was exclusively present in S2 sublineage.
- Assuming that the association of hek, pap, cnf1 and hlyA genes could lead to the hypothesis of the presence of PAI II536-like, we sought to highlight the strains in our collection that possessed these four genes. Finally, a total of 102 isolates (S1 = 69/118, 58.5% and S2 = 33/69, 47.8%, p = 0.17), from various origins (blood = 15/33, 45.5%, urine = 28/36, 77.8%, stool = 17/23, 73.9%, other or unknown = 42/95) could possess the PAI II536-like UPEC virulence factor []. Statistically, PAI II536-like was less frequent in strains isolated in blood than those isolated from urine (p = 0.007). No such difference could be demonstrated between stool and urinary strains (statistically insignificant, p > 0.01) (Table S1).
- Based on the evolutionary model of STEC/UPEC hybrid proposed by Gati et al., we then searched for an association of the gene stx2 with other specific genes (PAI II536-like or EHEC-hly gene) []. Among the four additional isolates owning stx2 and not previously described by Gati et al. (Ec22, Ec23, Ec93 and Ec166), only one of them had both stx2 and PAI II536-like (i.e., Ec22), suggesting that the three others might have lost PAI II536-like (Table S1). However, in our study collection, we have not identified any strain with EHEC-hly gene, except for the strain previously described in Gati’s study (N011) [].

Table 2.
Virulence genes and lineage distribution of E. coli ST141 isolates.
Table 2.
Virulence genes and lineage distribution of E. coli ST141 isolates.
| Pathovar | Virulence Genes | S1 1 (%) | S2 1 (%) | p-Value |
|---|---|---|---|---|
| ExPEC 1 | tia/hek | 80 (67.8%) | 51 (73.9%) | 0.41 |
| ExPEC | alpha-hemolysin | 73 (61.9%) | 37 (53.6%) | 0.28 |
| ExPEC | cnf1 | 74 (62.7%) | 36 (52.2%) | 0.17 |
| ExPEC | vat | 99 (83.9%) | 55 (79.7%) | 0.55 |
| ExPEC | Salmochelin system | 114 (96.6%) | 56 (81.2%) | <0.001 * |
| ExPEC | S pilus | 1 (0.8%) | 48 (69.6%) | <0.001 * |
| ExPEC | Type 1 pilus | 118 (100.0%) | 69 (100.0%) | 1 |
| ExPEC | P pilus | 74 (62.7%) | 36 (52.2%) | 0.17 |
| EXPEC | ag43 | 115 (97.5%) | 62 (89.9%) | 0.04 |
| EXPEC | ibeABC | 0 (0%) | 69 (100%) | <0.001 * |
| EXPEC | PAI II536-like | 69 (58.5%) | 33 (47.8%) | 0.17 |
| STEC 1 | Shiga like toxin 2 | 5 (4.2%) | 12 (17.4%) | 0.004 * |
| STEC | iha | 7 (5.9%) | 0 (0.0%) | 0.09 |
| EAEC 1 | astA | 66 (55.9%) | 2 (2.9%) | <0.001 * |
| EAEC | aatA | 1 (0.8%) | 1 (1.4%) | 1 |
| EAEC | pic | 26 (30.5%) | 46 (66.7%) | <0.001 * |
| Capsule | K1 capsule cluster | 111 (94.1%) | 49 (71.0%) | <0.001 * |
| Total | 118 (100%) | 69 (100%) | - |
1 S1, Sublineage 1; S2, Sublineage 2; Misc., Miscellaneous; ExPEC, extra-intestinal pathogenic E. coli; STEC, shiga-toxin-producing E. coli; EAEC, Enteroaggregative E. coli. * Significantly different (p < 0.01). p-values were calculated with Fisher’s exact test. S pilus, Shiga like toxin 2, pic and ibeABC virulence genes were more frequent in S2 (written in bold). Conversely, the frequency of Salmochelin system, astA and K1 capsule were more important in S1 (written in bold).
3. Discussion
We have demonstrated here that the population of E. coli ST141 is organized in two main sublineages S1 and S2, one predominantly associated with urinary tract infection (S1) and the other, more frequently associated with intestinal infections (S2). This confirms results generated from a smaller collection []. Indeed, for 41 isolates of our collection and previously studied by Gati et al., we noted a good correlation between the L1/L2 Gati’s lineages and our S1/S2 distribution, as all isolates belonging to L2 clustered in the S1 sublineage and possessed the fimH5 subtype (Figure 1). Conversely, all isolates belonging to L1 clustered in the S2 sublineage, with different fimH subtypes (seven of subtype fimH14; three of subtype fimH350, two of subtype fimH76 and one of subtype fimH674) [] (Table S1, Figure 1).
The ST141 lineage was highly conserved as all the isolates belonged to B2-phylogroup and had O2:H6 serotype with a restricted number of fimH subtypes. This contrasts with other B2 ExPEC successful lineages such as ST95, ST117 and ST131, which display a diversity of O-serogroups []. Although, E. coli ST141 isolates show various combinations of numerous VFs, as previously observed by Flament et al., we found no clear genomic signature that would indicate an ecological adaptation to a host species (i.e., humans and animals), infections sites, and countries of origin (Table S1, Figure 1) []. However, such diversity of VFs, consistent with the hypothesis that ST141 acts as one of the melting pots within the E. coli population, could be evidence of numerous recombinations and the presence of different PAI (pathogenicity islands), which could be interesting to explore in more details [].
Previously, ST141 had been described as a STEC/UPEC hybrid or an EAEC/UPEC hydrid as some isolates carried stx2 or pic and astA genes, respectively []. However, the majority (13/17) of strains in our ST141 collection (n = 187) which genomes contained stx2 were those described by Gati et al., and only one of the four additional stx2 gene positive strains also possibly had PAI II536-like UPEC virulence factor, questioning the systematic and specifically heteropathogenicity of this clonal group. Moreover, Lindstedt et al. found in a Norwegian collection, a high frequency (64.3%) of E. coli strains combining IPEC and ExPEC virulence-associated genes []. Another German study revealed that 10.6% of strains isolated from UTIs harbored at least one IPEC virulence factor []. Given these conflicting data, the frequency of heteropathogenicity in E. coli remains to be clarified, as well as the involvement of different lineages (notably ST141) in this trait.
The fact remains that the ST141 clone has many virulence factors identified in silico. Taking into account the concept of antagonistic pleiotropy and epistatic interactions, a study aiming to characterize the expression of these, in particular in vivo, in an animal model or in vitro, by the capacity of the strains of our collection to produce biofilm could be envisaged in a future work [].
Nevertheless, E. coli ST141 is commonly involved in human diseases and its incidence may reach a significant level. Indeed, ST141 lineage belonged to the 12 most frequent STs involved in bacteremia in France in 2014 []. In 2020, ST141 was quoted as one of the most common B2-ExPEC in France []. However, incidence of E. coli ST141 may vary between countries since a higher incidence of ST141-associated infections was found in France, compared to Spain []. In addition, E. coli ST141 has been found as the most frequent E. coli lineage responsible for ventilator-associated pneumonia over the 2012–2014 study period []. Interestingly, in a previous study, Philipps-Houlbracq et al., identified the antigen-43 (Ag43) as significantly associated in pneumonia pathogenesis []. It is important to note that Ag43 is widely represented among our collection (like most of B2 E. coli) as 97.5% (115/118) and 89.9% (62/69) of strains from sublineage 1 and 2, respectively, harbored this gene (Table 2).
Similarly, the panel of antibiotic resistance genes in the lineage ST141 was large with many combinations (Table S1, Figure 3). However, we could not identify a multi-resistant epidemic sub-lineage, such as that was observed with ST131 (i.e., C2-H30 producing CTX-M-15) []. Indeed, our study depicted a situation where E. coli ST141 might only contribute to limited locally spread of ESBL-encoding genes in the community, since the majority of ESBL strains isolated in our hospital form a cluster of strain separated by less than 30 SNPs, suggesting a local limited dissemination of an ESBL clone, although these strains have been isolated from patients with non-obvious epidemiological links (Figure 2) [].
Likewise, although E. coli ST141 was capable of acquiring resistance genes to last resort antibiotics such as carbapenems (blaOXA-48, blaVIM-1) and colistin (mcr-1), we did not find evidence of the spread of such MDR clones.
4. Materials and Methods
4.1. ST141 Genome Collection
The genomes of 187 isolates representing ST141 E. coli group were obtained from various sources collected over a 30-year period (Table S1).
Firstly, 13 non-duplicate ESBL-producing ST141 E. coli isolates, collected in our University hospital, in inpatients, during a previous prospective observational cohort study, between 02/2015 and 01/2017 have been paired-end sequenced with Illumina NextSeq at 2 × 150 bp and included []. Then, four supplemental non-ESBL-producing ST141 E. coli responsible for bloodstream infection, isolated in 2014 in our hospital, have also been fully sequenced and added []. Finally, we collected all the ST 141 E. coli genomes available in public databases in March 2020 (NCBI and ENA).
4.2. Genome Analysis
Raw reads were first trimmed using Sickle and the 187 genomes were de novo assembled from reads using SPADES, as previously described [,]. From these assemblies, we determined in silico: MultiLocus Sequence Typing (MLST) according to the Achtman scheme, O:H serotype, fimH type, and phylogroup [,]. In the same way, we searched and identified antibiotic resistance genes with ResFinder database, and putative virulence factors (VFs) genes using the VFDB database, which compiles most E. coli VFs related to adhesion/invasion, autotransporter system, fimbria or flagella expression, iron uptake, serum resistance and toxicity [,]. A SNP (Single Nucleotide Polymorphism) call variant was performed against a fully sequenced ST141 E. coli genome (NCBI biosample accession number SAMN10740161) used as reference genome using BACTSNP []. After recombination curation with Gubbins, a maximum likelihood phylogenetic tree was then inferred from the resulting SNP-based pseudogenomes using RaxML [,]. A Cluster Picker analysis was processed to identify phylogenetic clusters (Table S1) []. The tree and corresponding metadata information were visualized with iTOL [].
4.3. Statistical Analysis
To evaluate the distribution of isolates according to their origin and the distribution of VF genes, variables were examined by univariate analysis using the Fisher’s exact test. All statistical tests were two tailed, and p-value < 0.01 was considered statistically significant.
5. Conclusions
Using a genome-based methodology, we depicted in our study the population structure of E. coli ST141 group using a large collection of 187 genomes. Our findings did not confirm the initial hypothesis of an emerging ESBL-producing ST141 sublineage, but rather demonstrated the spread of a subgroup of isolates, showing a closer relatedness, on a regional scale. Nonetheless, we found that E. coli ST141 readily acquires and cumulates VF encoding genes and that its prevalence had recently increased either in both extra-intestinal and intestinal diseases. Considering this, there is a need to monitor the spread of this clonal group that has the potential to largely spread in the community and whose involvement in human disease seemed to increase.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/antibiotics12020382/s1, Table S1: Genomes features (origin, VFs, AMR).
Author Contributions
Conceptualization, A.E. and X.B.; Data curation, A.E. and R.B.; Formal analysis, A.E. and R.B.; Methodology, A.E., D.H., R.B. and X.B.; Project administration, X.B.; Supervision, X.B.; Validation, D.H., R.B. and X.B.; Writing—original draft, A.E. and X.B.; Writing—review and editing, D.H., R.B., A.E. and X.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
All information associated with the genome data were anonymized, thus identification of individuals is not possible. Therefore, ethical approval was not required.
Informed Consent Statement
Not applicable.
Data Availability Statement
All ST141 genomes from strains recovered from Besançon (local collection, n = 17) are publicy available through the NCBI BioProject PRJNA667655.
Acknowledgments
We thank B. Valot and A. Birer for technical assistance in WGS analysis.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Tenaillon, O.; Skurnik, D.; Picard, B.; Denamur, E. The population genetics of commensal Escherichia coli. Nat. Rev. Genet. 2010, 8, 207–217. [Google Scholar] [CrossRef]
- Denamur, E.; Clermont, O.; Bonacorsi, S.; Gordon, D. The population genetics of pathogenic Escherichia coli. Nat. Rev. Genet. 2020, 19, 37–54. [Google Scholar] [CrossRef]
- Van Der Mee-Marquet, N.L.; Blanc, D.S.; Gbaguidi-Haore, H.; Dos Santos Borges, S.; Viboud, Q.; Bertrand, X.; Quentin, R. Marked increase in incidence for bloodstream infections due to Escherichia coli, a side effect of previous antibiotic therapy in the elderly. Front. Microbiol. 2015, 6, 646. [Google Scholar] [CrossRef] [PubMed]
- Gati, N.S.; Middendorf-Bauchart, B.; Bletz, S.; Dobrindt, U.; Mellmann, A. Origin and Evolution of Hybrid Shiga Toxin-Producing and Uropathogenic Escherichia coli Strains of Sequence Type 141. J. Clin. Microbiol. 2019, 58, e01309-19. [Google Scholar] [CrossRef] [PubMed]
- Nicolas-Chanoine, M.-H.; Bertrand, X.; Madec, J.-Y. Escherichia coli ST131, an Intriguing Clonal Group. Clin. Microbiol. Rev. 2014, 27, 543–574. [Google Scholar] [CrossRef] [PubMed]
- Brisse, S.; Diancourt, L.; Laouénan, C.; Vigan, M.; Caro, V.; Arlet, G.; Drieux, L.; Leflon-Guibout, V.; Mentré, F.; Jarlier, V.; et al. Phylogenetic Distribution of CTX-M- and Non-Extended-Spectrum-β-Lactamase-Producing Escherichia coli Isolates: Group B2 Isolates, Except Clone ST131, Rarely Produce CTX-M Enzymes. J. Clin. Microbiol. 2012, 50, 2974–2981. [Google Scholar] [CrossRef]
- Muller, A.; Gbaguidi-Haore, H.; Cholley, P.; Hocquet, D.; Sauget, M.; Bertrand, X. Hospital-diagnosed infections with Escherichia coli clonal group ST131 are mostly acquired in the community. Sci. Rep. 2021, 11, 5702. [Google Scholar] [CrossRef]
- Roer, L.; Tchesnokova, V.; Allesøe, R.; Muradova, M.; Chattopadhyay, S.; Ahrenfeldt, J.; Thomsen, M.C.F.; Lund, O.; Hansen, F.; Hammerum, A.M.; et al. Development of a Web Tool for Escherichia coli Subtyping Based on fimH Alleles. J. Clin. Microbiol. 2017, 55, 2538–2543. [Google Scholar] [CrossRef]
- Flament-Simon, S.-C.; Nicolas-Chanoine, M.-H.; García, V.; Duprilot, M.; Mayer, N.; Alonso, M.P.; García-Meniño, I.; Blanco, J.E.; Blanco, M.; Blanco, J. Clonal Structure, Virulence Factor-encoding Genes and Antibiotic Resistance of Escherichia coli, Causing Urinary Tract Infections and Other Extraintestinal Infections in Humans in Spain and France during 2016. Antibiotics 2020, 9, 161. [Google Scholar] [CrossRef]
- Lindstedt, B.-A.; Finton, M.D.; Porcellato, D.; Brandal, L.T. High frequency of hybrid Escherichia coli strains with combined Intestinal Pathogenic Escherichia coli (IPEC) and Extraintestinal Pathogenic Escherichia coli (ExPEC) virulence factors isolated from human faecal samples. BMC Infect. Dis. 2018, 18, 544. [Google Scholar] [CrossRef]
- Toval, F.; Köhler, C.-D.; Vogel, U.; Wagenlehner, F.; Mellmann, A.; Fruth, A.; Schmidt, M.A.; Karch, H.; Bielaszewska, M.; Dobrindt, U. Characterization of Escherichia coli Isolates from Hospital Inpatients or Outpatients with Urinary Tract Infection. J. Clin. Microbiol. 2014, 52, 407–418. [Google Scholar] [CrossRef] [PubMed]
- La Combe, B.; Clermont, O.; Messika, J.; Eveillard, M.; Kouatchet, A.; Lasocki, S.; Corvec, S.; Lakhal, K.; Billard-Pomares, T.; Fernandes, R.; et al. Pneumonia-Specific Escherichia coli with Distinct Phylogenetic and Virulence Profiles, France, 2012–2014. Emerg. Infect. Dis. 2019, 25, 710–718. [Google Scholar] [CrossRef] [PubMed]
- Phillips-Houlbracq, M.; Ricard, J.-D.; Foucrier, A.; Yoder-Himes, D.; Gaudry, S.; Bex, J.; Messika, J.; Margetis, D.; Chatel, J.; Dobrindt, U.; et al. Pathophysiology of Escherichia coli pneumonia: Respective contribution of pathogenicity islands to virulence. Int. J. Med. Microbiol. 2018, 308, 290–296. [Google Scholar] [CrossRef] [PubMed]
- Joshi, N.A.; Fass, J.N. Sickle: A Sliding-Window, Adaptive, Quality-Based Trimming Tool for FastQ Files; Version 1.33; 2011. Available online: https://scholar.google.com/scholar?hl=zh-CN&as_sdt=0,5&cluster=15670407978335035877 (accessed on 14 January 2023).
- Bankevich, A.; Nurk, S.; Antipov, D.; Gurevich, A.A.; Dvorkin, M.; Kulikov, A.S.; Lesin, V.M.; Nikolenko, S.I.; Pham, S.; Prjibelski, A.D.; et al. SPAdes: A new genome assembly algorithm and its applications to single-cell sequencing. J. Comput. Biol. 2012, 19, 455–477. [Google Scholar] [CrossRef]
- Beghain, J.; Bridier-Nahmias, A.; Le Nagard, H.; Denamur, E.; Clermont, O. ClermonTyping: An easy-to-use and accurate in silico method for Escherichia genus strain phylotyping. Microb. Genom. 2018, 4, e000192. [Google Scholar] [CrossRef]
- Zankari, E.; Hasman, H.; Cosentino, S.; Vestergaard, M.; Rasmussen, S.; Lund, O.; Aarestrup, F.M.; Larsen, M.V. Identification of acquired antimicrobial resistance genes. J. Antimicrob. Chemother. 2012, 67, 2640–2644. [Google Scholar] [CrossRef]
- Chen, L.; Yang, J.; Yu, J.; Yao, Z.; Sun, L.; Shen, Y.; Jin, Q. VFDB: A reference database for bacterial virulence factors. Nucleic Acids Res. 2005, 33, D325–D328. [Google Scholar] [CrossRef]
- Yoshimura, D.; Kajitani, R.; Gotoh, Y.; Katahira, K.; Okuno, M.; Ogura, Y.; Hayashi, T.; Itoh, T. Evaluation of SNP calling methods for closely related bacterial isolates and a novel high-accuracy pipeline: BactSNP. Microb. Genom. 2019, 5, e000261. [Google Scholar] [CrossRef]
- Croucher, N.J.; Page, A.J.; Connor, T.R.; Delaney, A.J.; Keane, J.A.; Bentley, S.D.; Parkhill, J.; Harris, S.R. Rapid phylogenetic analysis of large samples of recombinant bacterial whole genome sequences using Gubbins. Nucleic Acids Res. 2015, 43, e15. [Google Scholar] [CrossRef]
- Stamatakis, A. RAxML version 8: A tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics 2014, 30, 1312–1313. [Google Scholar] [CrossRef]
- Ragonnet-Cronin, M.; Hodcroft, E.; Hué, S.; Fearnhill, E.; Delpech, V.; Brown, A.J.L.; Lycett, S. Automated analysis of phylogenetic clusters. BMC Bioinform. 2013, 14, 317. [Google Scholar] [CrossRef] [PubMed]
- Letunic, I.; Bork, P. Interactive Tree of Life (iTOL) v4: Recent updates and new developments. Nucleic Acids Res. 2019, 47, 256–259. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).